Introduction
Hepatic fibrosis (HF), a reversible
pathophysiological process, is characterized by an increased and
altered deposition of extracellular matrix (ECM) proteins that
results in excessive tissue scarring and promotes chronic liver
injury (1,2). Due to its end-stage outcome
(cirrhosis), it is responsible for high morbidity and mortality
(3). Over the past several years,
great efforts have been made to provide insight into the molecular
mechanisms underlying HF (4,5).
However, the precise molecular mechanisms still remain to be
determined (6). Therefore,
elucidating the pathogenesis of liver fibrosis and identifying
novel treatment strategies, including potential biomarkers and
therapeutic targets to combat HF, are of great importance.
Accumulating evidence suggests that although they
have no or little protein-coding capacity, various non-coding RNAs
(ncRNAs) serve as master regulators that affect expression levels
of dozens or even hundreds of target genes (7,8).
Long (L)ncRNAs are defined as RNA molecules that are >200
nucleotides in length, transcribed by RNA polymerase II and lacking
an open reading frame (9). LncRNAs
have been found to be associated with the pathogenesis of different
types of diseases by regulating diverse biological processes, such
as transcription, splicing and translation (10–13).
To date, >50,000 LncRNAs have been cloned and identified in the
human genome; however, only a small number of these have been
functionally annotated (14).
Improved knowledge has suggested that LncRNAs can regulate microRNA
(miRNA) abundance by binding and sequestering them, thereby
regulating the expression of target mRNAs (15). Thus, it has been demonstrated that
an efficient way to the infer potential function of LncRNAs is by
studying associated miRNAs and/or mRNAs whose functions have been
annotated. This association has been demonstrated in several
diseases, but has not been studied in HF (16–18).
The present study reconstructed a competitive
endogenous RNA (ceRNA) network using the data from National Center
for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/) Gene Expression Omnibus
(GEO; http://www.ncbi.nlm.nih.gov/geo/), based on the ceRNA
theory, a new RNA language that mRNAs, transcribed pseudogenes, and
LncRNAs ‘talk’ to each other using miRNA response elements
(19). Subsequently, Gene Ontology
(GO; http://geneontology.org/) and Kyoto
Encyclopedia of Genes and Genomes (KEGG; http://www.kegg.jp/) pathway analysis displayed that a
number of processes overrepresented in HF were related to the
regulation of cytokine and collagen, and the transforming growth
factor-β (TGF-β) and Toll-like receptor (TLR) signaling pathways.
Furthermore, the ceRNA network analysis identified that 4 LncRNAs
(NONMMUT036242, XR_877072, XR_378619 and XR_378418) were highly
related to HF, suggesting that these LncRNAs were key LncRNAs, and
could be used as potential diagnosis markers and treatment targets.
Thus, the present study may help expand the understanding of the
possible mechanism of HF from the perspective of LncRNAs, and
provide some new potential LncRNA markers for the clinical
diagnosis and treatment of HF.
Materials and methods
Raw data
Mouse miRNA expression data were downloaded from
NCBI GEO (GSE77271) (20), based
on the SurePrint G3 Mouse miRNA Microarray. A total of 6 samples
were included in the dataset: 3 samples were the control group and
3 samples were the liver fibrosis group, which was induced using
carbon-tetrachloride (CCl4). Mouse LncRNA and mRNA
expression data were also downloaded from NCBI GEO (GSE80601)
(21), generated using an
Affymetrix GeneChip Mouse Exon 1.0 ST Array. A total of 10 samples
were included in this dataset: 5 samples were the control group and
5 samples were the liver fibrosis group, which was also induced by
CCl4.
Gene chip probe re-annotation
Based on the LncRNA classification pipeline
constructed in our previous study (22), a number of LncRNAs represented on
the Affymetrix microarrays were identified. First, the latest
version of the NetAffx™ Annotation File (MoEx-1_0-st-v1 Probeset
Annotations, CSV Format, release 36; uploaded 7/6/16) was obtained
from the Affymetrix official website (http://www.affymetrix.com/support/technical/byproduct.affx?product=moexon-st).
This annotation file was mapped to the MoEx-1_0-st-v1 probe sets
ID. Second, the probe sets were annotated in the Refseq database
(https://www.ncbi.nlm.nih.gov/refseq/); those IDs
beginning with ‘NR’ were retained, and transcript IDs labeled with
‘NP’ were deleted. Probe sets from the Ensembl database (https://www.ensembl.org/index.html) and the
online software BioMart v95 (https://www.ensembl.org/info/data/biomart/index.html)
were applied to convert Affymetrix microarray IDs to Ensembl IDs,
together with the corresponding gene type, and only RNAs type which
were annotated as ‘lincRNA’, ‘sense_intronic’,
‘processed_transcript’, ‘antisense’, ‘sense_overlapping’,
‘3prime_overlapping_ncrna’ or ‘misc_RNA’ were retained. The probe
sets that annotated in Noncode v4 (http://www.noncode.org/) were all retained. Next,
based on the above two steps, probe set IDs annotated as
‘microRNA’, ‘snoRNAs’, ‘pseudogenes’ and other small RNAs were
removed.
Screening of differentially expressed
LncRNAs, miRNAs and mRNAs
First, differential probes of GSE77271 and GSE80601
were selected with thresholds of fold change (FC) >1.5 and
P<0.05 using GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/). Second,
differential probes were matched to miRNAs in GSE77271, and LncRNAs
and mRNAs in GSE80601. Then, the differentially expressed LncRNAs
(DELs), miRNAs (DEMis) and mRNAs (DEMs) were identified.
Reconstruction ceRNA network
The LncRNA-miRNA-mRNA network was reconstructed
based on ceRNA theory as described previously (7). DEL sequence were obtained from
Refseq, Noncode and Ensemble databases, whereas DEMi sequences were
obtained from miRBase (http://www.mirbase.org/). Then, the target LncRNAs of
miRNAs were predicted by MiRanda (http://www.microrna.org, release 2010) (23). The target mRNAs of miRNAs were
predicted by MiRanda and TargetScan v7.2 (http://www.targetscan.org) (24). mRNAs and LncRNAs that were targeted
and negatively co-expressed with a certain common miRNA were
identified as LncRNA-miRNA-mRNA co-expression competing triplets.
The ceRNA network was reconstructed by assembling all co-expression
competing triplets, and was visualized using Cytoscape v3.2.8
(25) software. Simultaneously,
all node degrees of the LncRNA-miRNA-mRNA network were
calculated.
Functional enrichment analysis
To assess functional enrichment, GO (26,27)
and KEGG pathway (28,29) analyses of mRNAs in the ceRNA
network were performed using Database for Annotation,
Visualization, and Integration Discovery (v6.8; http://david.ncifcrf.gov/). Then, a false discovery
rate (FDR) was calculated to correct the P-value, and FDR <0.05
was selected as the threshold.
Reconstruction of the key
LncRNA-miRNA-mRNA sub-networks
In order to evaluate the key LncRNAs, several
topological properties, such as the hub nodes and relationship
pairs, were considered. First, the hub nodes of LncRNAs with node
degree >5 were selected. Second, the first relationship pairs of
LncRNA-miRNA and the secondary relationship pairs of miRNA-mRNA
were calculated. The LncRNAs whose total relationship pairs were in
the top 4 were selected as key LncRNAs. Then, key LncRNA-miRNA-mRNA
sub-networks were visualized using Cytoscape software. For further
analysis, GO and KEGG pathway annotations for each of the key
LncRNAs were analyzed by using their original mRNA neighbors in the
key LncRNA-miRNA-mRNA sub-networks.
Results
Identification of DELs, DEMis and
DEMs
Based on the cut-off criteria, a total of 254 DELs
(107 significantly up- and 147 downregulated), 25 DEMis (12
significantly up- and 13 downregulated) and 472 DEMs (308
significantly up- and 164 downregulated) in the GSE77271 and
GSE80601 dataset were identified.
LncRNA-miRNA-mRNA network
To further understand how LncRNAs regulate mRNAs
through interactions with miRNAs in HF, a ceRNA network was
reconstructed based on the above data and visualized. As presented
in Fig. 1, the ceRNA network was
composed of 220 LncRNA nodes, 24 miRNA nodes, 164 mRNA nodes and
1,149 edges. Base on the LncRNA-miRNA-mRNA network, it was found
that one LncRNA can interact with multiple miRNAs and mRNAs, one
miRNA can interact with multiple LncRNAs and mRNAs, and one mRNA
can interact with multiple LncRNAs and miRNAs; this indicated that
the expression profiles of LncRNAs, miRNAs and mRNAs were
significantly associated.
Functional enrichment analysis of
LncRNAs based on the ceRNA network
To explore the biological functions of LncRNAs
during the development of HF, GO and KEGG analyses were conducted.
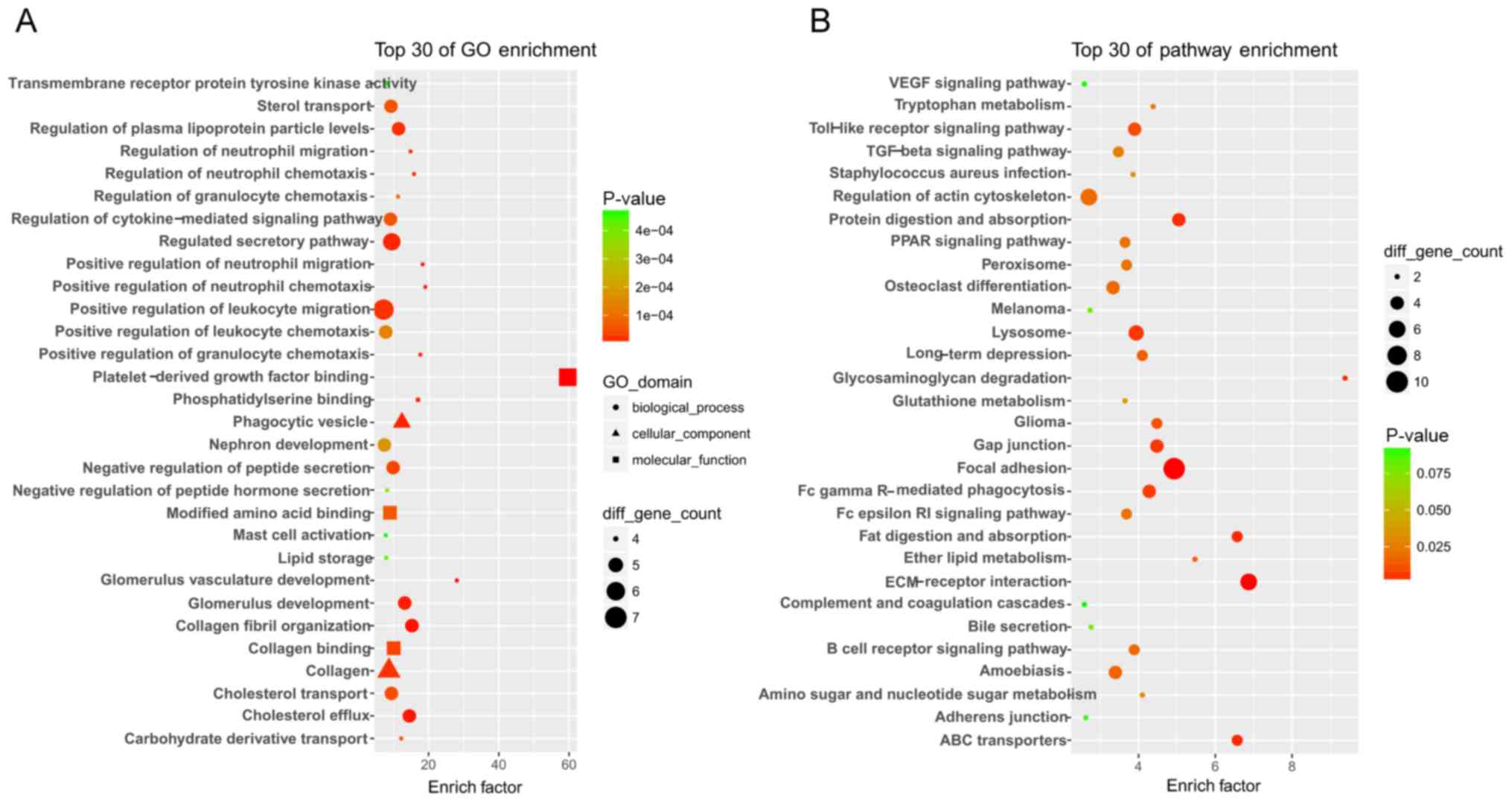
GO analysis revealed that a total of 338 GO terms, including
platelet-derived growth factor binding, and regulation of cytokine
and collagen, were significantly altered. The top 30 significantly
GO terms are shown in Fig. 2A.
KEGG pathway analysis indicated that 25 pathways were significantly
enriched, including the TGF-β, TLR and peroxisome
proliferator-activated receptor signaling pathways. The top 30
pathways were demonstrated in Fig.
2B.
Topological analysis of the ceRNA
network
In general, a higher degree indicates that a node in
the ceRNA network was a hub that participated in more ceRNA
interactions. According to a previous study by Han et al
(30), in which they defined a hub
as a node degree >5, the present study found that 77 nodes could
be chosen as hub nodes, including 43 LncRNAs, 24 miRNAs and 10
mRNAs. The results are shown in Fig.
3. In addition, the number of first relationship LncRNA-miRNA
pairs and secondary relationship miRNA-mRNA pairs of hubs were
calculated. The results were demonstrated in Table I. On the basis of total
relationship pairs, 4 LncRNAs (NONMMUT036242, XR_877072, XR_378619
and XR_378418) were selected as the key LncRNAs.
 | Table I.LncRNA-miRNA and miRNA-mRNA pairs of
network hubs. |
Table I.
LncRNA-miRNA and miRNA-mRNA pairs of
network hubs.
| Number | Gene name | LncRNA-miRNA
pairs | miRNA-mRNA
pairs | Total number |
|---|
| 1 | NONMMUT036242 | 8 | 203 | 211 |
| 2 | XR_877072 | 10 | 199 | 209 |
| 3 | XR_378619 | 7 | 187 | 194 |
| 4 | XR_378418 | 7 | 171 | 178 |
| 5 | NONMMUT025112 | 8 | 161 | 169 |
| 6 | NONMMUT069361 | 8 | 159 | 167 |
| 7 | NONMMUT041965 | 7 | 152 | 159 |
| 8 | NONMMUT039701 | 7 | 144 | 151 |
| 9 | NONMMUT067035 | 7 | 133 | 140 |
| 10 | NONMMUT030757 | 6 | 127 | 133 |
| 11 | NONMMUT043221 | 6 | 113 | 119 |
| 12 | NONMMUT010366 | 6 | 106 | 112 |
| 13 | NONMMUT053659 | 6 | 87 | 93 |
| 14 | Gm34394 | 12 | 77 | 89 |
| 15 | Gm41622 | 11 | 77 | 88 |
| 16 | Gm35562 | 11 | 77 | 88 |
| 17 | NONMMUT022066 | 8 | 74 | 82 |
| 18 | Gm29966 | 8 | 74 | 82 |
| 19 | NONMMUT013707 | 8 | 72 | 80 |
| 20 | NONMMUT051651 | 7 | 72 | 79 |
| 21 | NONMMUT058428 | 7 | 66 | 73 |
| 22 | NONMMUT033508 | 8 | 64 | 72 |
| 23 | NONMMUT033142 | 8 | 63 | 71 |
| 24 | Gm32861 | 6 | 60 | 66 |
| 25 | NONMMUT051738 | 8 | 58 | 66 |
| 26 | NONMMUT048637 | 8 | 56 | 64 |
| 27 | NONMMUT010786 | 6 | 57 | 63 |
| 28 | NONMMUT059817 | 4 | 58 | 62 |
| 29 | NONMMUT050190 | 7 | 55 | 62 |
| 30 | NONMMUT041236 | 8 | 54 | 62 |
| 31 | 1700001L05Rik | 7 | 54 | 61 |
| 32 | NONMMUT068223 | 6 | 54 | 60 |
| 33 | NONMMUT064230 | 6 | 54 | 60 |
| 34 | NONMMUT044605 | 6 | 53 | 59 |
| 35 | NONMMUT057586 | 8 | 48 | 56 |
| 36 | NONMMUT053611 | 6 | 46 | 52 |
| 37 | NONMMUT036431 | 7 | 45 | 52 |
| 38 | NONMMUT039492 | 6 | 43 | 49 |
| 39 | NONMMUT042546 | 6 | 41 | 47 |
| 40 | Gm32374 | 6 | 36 | 42 |
| 41 | NONMMUT022800 | 6 | 27 | 33 |
| 42 | NONMMUT045026 | 6 | 23 | 29 |
| 43 | NONMMUT025285 | 6 | 11 | 17 |
Key LncRNA-miRNA-mRNA
sub-networks
The new key LncRNA-mRNA sub-networks were
reconstructed by extracting the key LncRNAs and their linked miRNAs
and mRNAs from the triple global network. As presented in Fig. 4, the LncRNA
NONMMUT036242-miRNA-mRNA sub-network was composed of 8 miRNA nodes,
112 mRNA nodes and 211 edges. The LncRNA XR_877072-miRNA-mRNA
sub-network was composed of 10 miRNA nodes, 120 mRNA nodes and 220
edges. The LncRNA XR_378619-miRNA-mRNA sub-network was composed of
7 miRNA nodes, 109 mRNA nodes and 197 edges. The LncRNA
XR_378418-miRNA-mRNA sub-network was composed of 7 miRNA nodes, 100
mRNA nodes and 178 edges.
Functional prediction of key
LncRNAs-miRNA-mRNA sub-networks
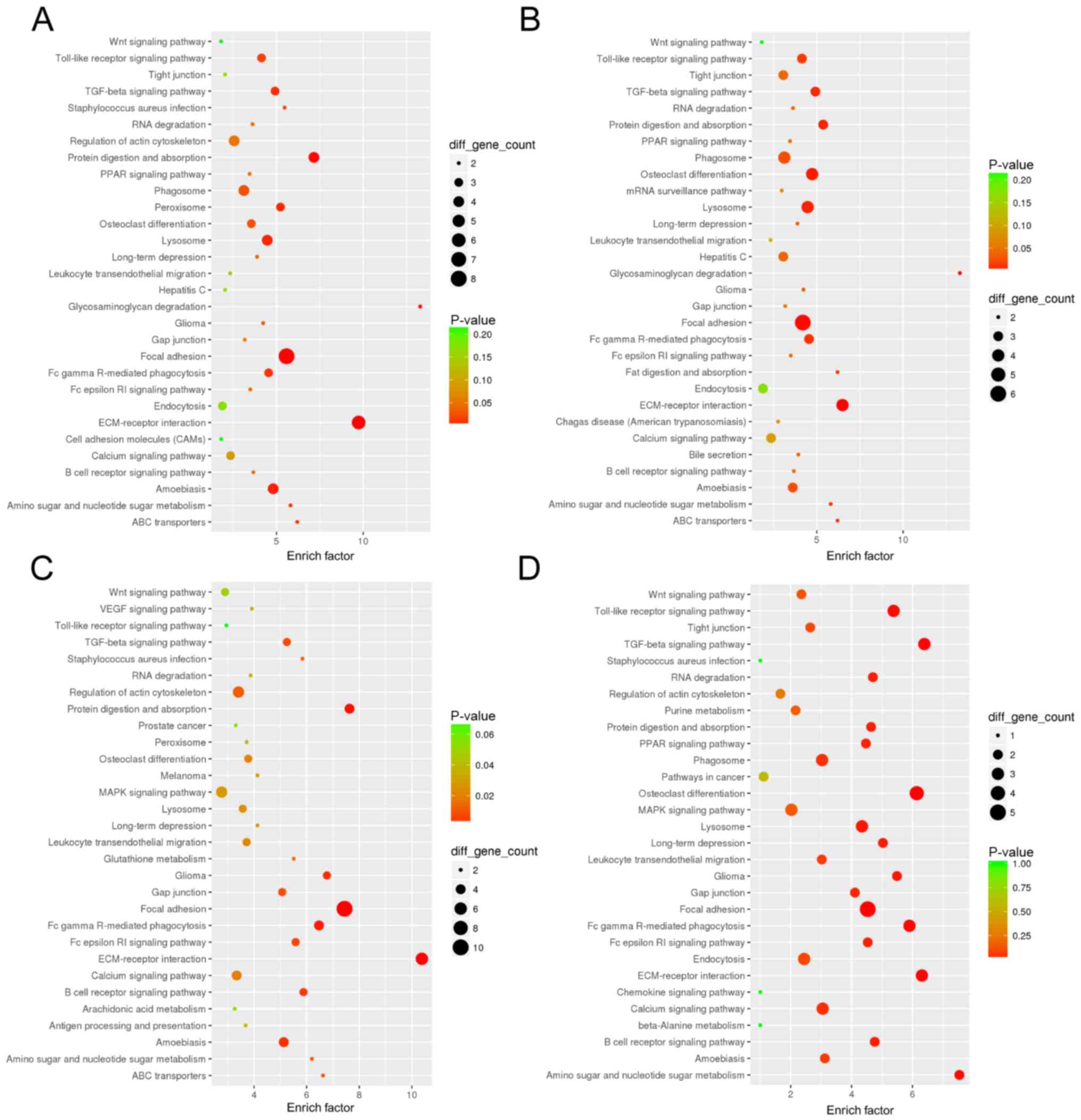
GO and KEGG pathway annotations were further
performed for the 4 key LncRNAs. The results revealed that 335 GO
terms and 21 pathways were significantly enriched in the LncRNA
NONMMUT036242-miRNA-mRNA sub-network, 230 GO terms and 23 pathways
were significantly enriched in the LncRNA XR_877072-miRNA-mRNA
sub-network, 399 GO terms and 27 pathways were significantly
enriched in the LncRNA XR_378619-miRNA-mRNA sub-network, 200 GO
terms and 18 pathways were significantly enriched in the LncRNA
XR_378418-miRNA-mRNA sub-network. The top 30 significant GO terms
are shown in Fig. 5 and the top 30
enriched pathways shown in Fig. 6.
It is notable that some common GO terms such as ‘regulation of
cytokine-mediated signaling pathway’, ‘regulation of plasma
lipoprotein particle levels’ and ‘collagen’, and some common
pathways such as ‘Wnt signaling pathway, ‘TGF-β signaling pathway’
and ‘Toll-like receptor signaling pathway’ were enriched for all 4
sub-networks.
Discussion
HF is well recognized as a wound-healing response
that occurs in liver following any type of acute or chronic injury
(31–33). It is hypothesized that liver
fibrosis is reversible; however, the resulting structural damage to
liver lobules and vasculature leads to cirrhosis, which is
irreversible (34–36). Therefore, there is an increasing
requirement for diagnostic and prognostic biomarkers of HF.
Increasing evidence has demonstrated that LncRNAs
are involved in numerous complex diseases by communicating with
mRNAs and miRNAs, and with each other (37). Compared with protein-coding genes,
LncRNAs have clear advantages as diagnostic and prognostic
biomarkers. Exploiting interactions between LncRNAs and
mRNAs/miRNAs to LncRNA functional similarity is an effective method
to explore function of LncRNAs (38–40).
The present study, on the basis of the ceRNA hypothesis, used
interaction data generated using datasets from GEO to reconstruct a
triple global network. The LncRNA-miRNA-mRNA network consisted of
220 LncRNAs, 24 miRNAs, 164 mRNAs and 1,149 interactions.
Furthermore, to investigate the functions of
HF-related genes, these genes were annotated via GO and KEGG
pathway analysis, and it was found that the most significant
enriched terms and pathways were regulation of cytokine and
collagen, and TGF-β and TLR signaling. It is well known that
activated hepatic stellate cells (HSCs) serve a role in liver
fibrosis (41). Several studies
have demonstrated that TGF-β plays a critical role in HSC
activation, and that the TGF-β signaling pathway could be a
potential therapeutic target for HF (42–44).
This classic signaling pathway is activated by TGF-β binding to its
receptors located on the cell membrane. The downstream proteins,
including Smad2 and Smad3, are activated by phosphorylation, which
further promotes the transcription of genes encoding ECM
components, thereby accelerating the development of liver fibrosis
(45).
Previous studies have demonstrated that hub nodes,
characterized by their high degree of connectivity to other nodes,
can be used as topological properties of the network to evaluate
the importance of genes (46,47).
In general, a LncRNA that has more edges indicates that the LncRNA
is a hub, which participates in more ceRNA interactions. Thus, the
LncRNA is essential in network organization and plays a critical
role in a network. In the present study, according to the node
degree and relationship pairs, 4 LncRNAs (NONMMUT036242, XR_877072,
XR_378619 and XR_378418) were selected as the key LncRNAs, which
may be used as potential novel biomarkers for the clinical
diagnosis and treatment of HF.
Several LncRNAs, which are altered during HF and
could affect HSC activation, have already been reported, including
liver fibrosis-associated LncRNA1 (21), metastasis-associated lung
adenocarcinoma transcript 1 (48),
LncRNA-p21 (49) and maternally
expressed 3 (50). However,
functional characterization of the 4 key LncRNAs (NONMMUT036242,
XR_877072, XR_378619 and XR_378418) is still in its infancy. In
order to infer the potential functions of LncRNAs, according to the
ceRNA theory, the related miRNAs and/or mRNAs, whose functions have
been annotated, can be studied. While the LncRNA-miRNA-mRNA could
provide a global view of all possible ceRNA interactions that could
be used to investigate the regulatory properties of the LncRNAs,
the key LncRNA-miRNA-mRNA sub-network revealed a more detailed
picture of how the key LncRNAs synergized with competing mRNAs
(51). According to the key
LncRNA-miRNA-mRNA sub-networks, the LncRNA XR_877072, a seldom
reported LncRNA, interacted with numerous mRNAs, including CD74,
discoidin domain receptor 2 and tissue inhibitor of
metallopeptidases 2, which were highly associated with HF (52–54).
In addition, this LncRNA could directly interact with a number of
miRNAs, including miR-378a-3p and miR-212-3p, which have been
verified as important factors in the development of HF. For
example, Hyun et al (55)
reported that expression of miR-378a-3p declined in mice models of
liver fibrosis. In activated HSCs transfected with miR-378a-3p
mimic, the profibrotic gene expression of Vimentin, α-smooth muscle
actin and collagen Iα1 decreased. In addition, the levels of Gli3,
a critical target gene of the Hedgehog signaling pathway that
promotes HF (56,57), were also downregulated (55). Zhu et al (58) confirmed that overexpression of
miR-212-3p can induce the expression of HSC markers, including
α-SMA, and collagens by activating the TGF-β signaling pathway.
In conclusion, based on the ceRNA theory, the
present study constructed an HF-associated LncRNA-miRNA-mRNA
network to explore the biological functions of LncRNAs during the
development of HF at a system-wide level. Notably, 4 LncRNAs
(NONMMUT036242, XR_877072, XR_378619 and XR_378418) were selected
that participated in the most ceRNA interactions, and may play
important roles in HF. The present study uncovered a ceRNA network
that could further the understanding of the mechanisms underlying
HF progression and provide novel potential markers for clinical
diagnosis and targets for treatment. However, there were still
certain limitations to the present study. First, the 4 key LncRNAs
need to be verified in liver tissue specimens from patients with
liver fibrosis via reverse transcription-quantitative PCR analysis.
Second, further experiments are required to validate their effects
and mechanisms in HF. Finally, the homology of the 4 key LncRNAs,
which were identified based on expression in mice, needs to be
validated to support their use as potential biomarkers and
therapeutic targets in clinical settings.
Acknowledgements
The authors would like to thank Mr Qiang Fan (Ao Ji
Bio-tech Co., Ltd., Shanghai, China) for providing help with data
analysis.
Funding
The present study was supported by Leading Talents
Introduction and Cultivation Plan Project of Colleges in Anhui
Province (grant no. gxfxZD2016118) and the National Natural Science
Foundation of China (grant no. 81973648).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and JG made substantial contributions to the
conception and design of the study. HJ, FW and NJ contributed to
data acquisition, and data analysis and interpretation. HJ and JG
drafter and revised the manuscript critically for important
intellectual content. All authors agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ni MM, Wang YR, Wu WW, Xia CC, Zhang YH,
Xu J, Xu T and Li J: Novel Insights on Notch signaling pathways in
liver fibrosis. Eur J Pharmacol. 826:66–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li H, Lan J, Han C, Guo K, Wang G, Hu J,
Gong J, Luo X and Cao Z: Brg1 promotes liver fibrosis via
activation of hepatic stellate cells. Exp Cell Res. 364:191–197.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zhang Z, Yao Z, Wang L, Zhang F,
Shao J, Chen A and Zheng S: Activation of autophagy is required for
Oroxylin A to alleviate carbon tetrachloride-induced liver fibrosis
and hepatic stellate cell activation. Int Immunopharmacol.
56:148–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panebianco C, Oben JA, Vinciguerra M and
Pazienza V: Senescence in hepatic stellate cells as a mechanism of
liver fibrosis reversal: A putative synergy between retinoic acid
and PPAR-gamma signalings. Clin Exp Med. 17:269–280. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomita K, Teratani T, Suzuki T, Shimizu M,
Sato H, Narimatsu K, Okada Y, Kurihara C, Irie R, Yokoyama H, et
al: Free cholesterol accumulation in hepatic stellate cells:
Mechanism of liver fibrosis aggravation in nonalcoholic
steatohepatitis in mice. Hepatology. 59:154–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He Y, Jin L, Wang J, Yan Z, Chen T and
Zhao Y: Mechanisms of fibrosis in acute liver failure. Liver Int.
35:1877–1885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang H, Ma R, Zou S, Wang Y, Li Z and Li
W: Reconstruction and analysis of the lncRNA-miRNA-mRNA network
based on competitive endogenous RNA reveal functional lncRNAs in
rheumatoid arthritis. Mol Biosyst. 13:1182–1192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu Z and Adelson DL: Evolutionary
conservation and functional roles of ncRNA. Front Genet. 3:2052012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng B, Jeong S, Zhu Y, Chen L and Xia Q:
miRNA and lncRNA as biomarkers in cholangiocarcinoma (CCA).
Oncotarget. 8:100819–100830. 2017.PubMed/NCBI
|
|
10
|
Huang GW, Xue YJ, Wu ZY, Xu XE, Wu JY, Cao
HH, Zhu Y, He JZ, Li CQ, Li EM and Xu LY: A three-lncRNA signature
predicts overall survival and disease-free survival in patients
with esophageal squamous cell carcinoma. BMC Cancer. 18:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding L, Wang M, Sun D and Li A: TPGLDA:
Novel prediction of associations between lncRNAs and diseases via
lncRNA-disease-gene tripartite graph. Sci Rep. 8:10652018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye Y, Gao X and Yang N: LncRNA ZFAS1
promotes cell migration and invasion of fibroblast-like
synoviocytes by suppression of miR-27a in rheumatoid arthritis. Hum
Cell. 31:14–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grelet S, Link LA, Howley B, Obellianne C,
Palanisamy V, Gangaraju VK, Diehl JA and Howe PH: A regulated PNUTS
mRNA to lncRNA splice switch mediates EMT and tumour progression.
Nat Cell Biol. 19:1105–1115. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szafranski P, Dharmadhikari AV, Brosens E,
Gurha P, Kolodziejska KE, Zhishuo O, Dittwald P, Majewski T, Mohan
KN, Chen B, et al: Small noncoding differentially methylated
copy-number variants, including lncRNA genes, cause a lethal lung
developmental disorder. Genome Res. 23:23–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li F, Huang C, Li Q and Wu X: Construction
and comprehensive analysis for dysregulated long non-coding RNA
(lncRNA)-associated competing endogenous RNA (ceRNA) network in
gastric cancer. Med Sci Monit. 24:37–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang X, Wu X, Chen F, He W, Chen X, Liu L
and Tang H: The profiles and networks of miRNA, lncRNA, mRNA, and
circRNA in benzo(a)pyrene-transformed bronchial epithelial cells. J
Toxicol Sci. 43:281–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue WH, Fan ZR, Li LF, Lu JL, Ma BJ, Kan
QC and Zhao J: Construction of an oesophageal cancer-specific ceRNA
network based on miRNA, lncRNA, and mRNA expression data. World J
Gastroenterol. 24:23–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao Y, Liu R, Zhou H, Yin S, Zhao Q, Ding
X and Wang H: Transcriptome analysis of miRNA-lncRNA-mRNA
interactions in the malignant transformation process of gastric
cancer initiation. Cancer Gene Ther. 24:267–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hyun J, Park J, Wang S, Kim J, Lee HH, Seo
YS and Jung Y: MicroRNA expression profiling in
CCl4-induced liver fibrosis of Mus musculus. Int J Mol
Sci. 17:9612016. View Article : Google Scholar :
|
|
21
|
Zhang K, Han X, Zhang Z, Zheng L, Hu Z,
Yao Q, Cui H, Shu G, Si M, Li C, et al: The liver-enriched
lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch
pathways. Nat Commun. 8:1442017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao JR, Qin XJ, Jiang H, Gao YC, Guo MF
and Jiang NN: Potential role of lncRNAs in contributing to
pathogenesis of chronic glomerulonephritis based on microarray
data. Gene. 643:46–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Betel D, Koppal A, Agius P, Sander C and
Leslie C: Comprehensive modeling of microRNA targets predicts
functional non-conserved and non-canonical sites. Genome Biol.
11:R902010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
25
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
The Gene Ontology Consortium, . The Gene
Ontology Resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47:D590–D595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han JD, Bertin N, Hao T, Goldberg DS,
Berriz GF, Zhang LV, Dupuy D, Walhout AJ, Cusick ME, Roth FP and
Vidal M: Evidence for dynamically organized modularity in the yeast
protein-protein interaction network. Nature. 430:88–93. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao H, Li S, Xie R, Xu N, Qian Y, Chen H,
Hu Q, Quan Y, Yu Z, Liu J and Xiang M: Exploring the mechanism of
dangguiliuhuang decoction against hepatic fibrosis by network
pharmacology and experimental validation. Front Pharmacol.
9:1872018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li P, Li Y, Zhu L, Yang Z, He J, Wang L,
Shang Q, Pan H, Wang H, Ma X, et al: Targeting secreted cytokine
BMP9 gates the attenuation of hepatic fibrosis. Biochim Biophys
Acta Mol Basis Dis. 1864:709–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuda M, Tsurusaki S, Miyata N, Saijou
E, Okochi H, Miyajima A and Tanaka M: Oncostatin M causes liver
fibrosis by regulating cooperation between hepatic stellate cells
and macrophages in mice. Hepatology. 67:296–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chi C, Liu XY, Hou F, Yu XZ, Li CY, Cui
LJ, Liu RX and Yin CH: Herbal compound 861 prevents hepatic
fibrosis by inhibiting the TGF-β1/Smad/SnoN pathway in bile
duct-ligated rats. BMC Complement Altern Med. 18:522018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Omar R, Yang J, Liu H, Davies NM and Gong
Y: Hepatic stellate cells in liver fibrosis and siRNA-based
therapy. Rev Physiol Biochem Pharmacol. 172:1–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pascher A, Nebrig M and Neuhaus P:
Irreversible liver failure: Treatment by transplantation: Part 3 of
a series on liver cirrhosis. Dtsch Arztebl Int. 110:167–173.
2013.PubMed/NCBI
|
|
37
|
Cheng L, Shi H, Wang Z, Hu Y, Yang H, Zhou
C, Sun J and Zhou M: IntNetLncSim: An integrative network analysis
method to infer human lncRNA functional similarity. Oncotarget.
7:47864–47874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Wang H, Wu C, Yan M, Wu H, Wang J,
Yang X and Shao Q: Construction and investigation of
lncRNA-associated ceRNA regulatory network in papillary thyroid
cancer. Oncol Rep. 39:1197–1206. 2018.PubMed/NCBI
|
|
39
|
Zhou M, Wang X, Shi H, Cheng L, Wang Z,
Zhao H, Yang L and Sun J: Characterization of long non-coding
RNA-associated ceRNA network to reveal potential prognostic lncRNA
biomarkers in human ovarian cancer. Oncotarget. 7:12598–12611.
2016.PubMed/NCBI
|
|
40
|
Xu JH, Chang WH, Fu HW, Yuan T and Chen P:
The mRNA, miRNA and lncRNA networks in hepatocellular carcinoma: An
integrative transcriptomic analysis from Gene Expression Omnibus.
Mol Med Rep. 17:6472–6482. 2018.PubMed/NCBI
|
|
41
|
Wei S, Wang Q, Zhou H, Qiu J, Li C, Shi C,
Zhou S, Liu R and Lu L: miR-455-3p alleviates hepatic stellate cell
activation and liver fibrosis by suppressing HSF1 expression. Mol
Ther Nucleic Acids. 16:758–769. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen N, Geng Q, Zheng J, He S, Huo X and
Sun X: Suppression of the TGF-β/Smad signaling pathway and
inhibition of hepatic stellate cell proliferation play a role in
the hepatoprotective effects of curcumin against alcohol-induced
hepatic fibrosis. Int J Mol Med. 34:1110–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang K, Tang Y, Yan F, Zhu J and Li J:
Potent inhibition of TGF-β signaling pathway regulator Abl:
Potential therapeutics for hepatic fibrosis. J Recept Signal
Transduct Res. 35:410–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu A, Li Y, Zhao W, Hou F, Li X, Sun L,
Chen W, Yang A, Wu S, Zhang B, et al: PHP14 regulates hepatic
stellate cells migration in liver fibrosis via mediating TGF-β1
signaling to PI3Kγ/AKT/Rac1 pathway. J Mol Med (Berl). 96:119–133.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou L, Dong X, Wang L, Shan L, Li T, Xu
W, Ding Y, Lai M, Lin X, Dai M, et al: Casticin attenuates liver
fibrosis and hepatic stellate cell activation by blocking
TGF-β/Smad signaling pathway. Oncotarget. 8:56267–56280.
2017.PubMed/NCBI
|
|
46
|
Zhang Y, Xu Y, Feng L, Li F, Sun Z, Wu T,
Shi X, Li J and Li X: Comprehensive characterization of lncRNA-mRNA
related ceRNA network across 12 major cancers. Oncotarget.
7:64148–64167. 2016.PubMed/NCBI
|
|
47
|
Zhou M, Diao Z, Yue X, Chen Y, Zhao H,
Cheng L and Sun J: Construction and analysis of dysregulated
lncRNA-associated ceRNA network identified novel lncRNA biomarkers
for early diagnosis of human pancreatic cancer. Oncotarget.
7:56383–56394. 2016.PubMed/NCBI
|
|
48
|
Leti F, Legendre C, Still CD, Chu X,
Petrick A, Gerhard GS and DiStefano JK: Altered expression of
MALAT1 lncRNA in nonalcoholic steatohepatitis fibrosis regulates
CXCL5 in hepatic stellate cells. Transl Res. 190:25.e21–39.e21.
2017. View Article : Google Scholar
|
|
49
|
Yu F, Lu Z, Chen B, Dong P and Zheng J:
Identification of a novel lincRNA-p21-miR-181b-PTEN signaling
cascade in liver fibrosis. Mediators Inflamm. 2016:98565382016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen MJ, Wang XG, Sun ZX and Liu XC:
Diagnostic value of LncRNA-MEG3 as a serum biomarker in patients
with hepatitis B complicated with liver fibrosis. Eur Rev Med
Pharmacol Sci. 23:4360–4367. 2019.PubMed/NCBI
|
|
51
|
Cao Y, Wang P, Ning S, Xiao W, Xiao B and
Li X: Identification of prognostic biomarkers in glioblastoma using
a long non-coding RNA-mediated, competitive endogenous RNA network.
Oncotarget. 7:41737–41747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Heinrichs D, Knauel M, Offermanns C,
Berres ML, Nellen A, Leng L, Schmitz P, Bucala R, Trautwein C,
Weber C, et al: Macrophage migration inhibitory factor (MIF) exerts
antifibrotic effects in experimental liver fibrosis via CD74. Proc
Natl Acad Sci USA. 108:17444–17449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang XH, Yan M, Liu L, Wu TJ, Ma LL and
Wang LX: Expression of discoidin domain receptors (DDR2) in
alcoholic liver fibrosis in rats. Arch Med Res. 41:586–592. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang J, Zheng J, Wu L, Shi M, Zhang H,
Wang X, Xia N, Wang D, Liu X, Yao L, et al: NDRG2 ameliorates
hepatic fibrosis by inhibiting the TGF-β1/Smad pathway and altering
the MMP2/TIMP2 ratio in rats. PLoS One. 6:e277102011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hyun J, Wang S, Kim J, Rao KM, Park SY,
Chung I, Ha CS, Kim SW, Yun YH and Jung Y: MicroRNA-378 limits
activation of hepatic stellate cells and liver fibrosis by
suppressing Gli3 expression. Nat Commun. 7:109932016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang F, Hao M, Jin H, Yao Z, Lian N, Wu
L, Shao J, Chen A and Zheng S: Canonical hedgehog signalling
regulates hepatic stellate cell-mediated angiogenesis in liver
fibrosis. Br J Pharmacol. 174:409–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chung SI, Moon H, Ju HL, Cho KJ, Kim DY,
Han KH, Eun JW, Nam SW, Ribback S, Dombrowski F, et al: Hepatic
expression of Sonic Hedgehog induces liver fibrosis and promotes
hepatocarcinogenesis in a transgenic mouse model. J Hepatol.
64:618–627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhu J, Zhang Z, Zhang Y, Li W, Zheng W, Yu
J, Wang B, Chen L, Zhuo Q, Chen L, et al: MicroRNA-212 activates
hepatic stellate cells and promotes liver fibrosis via targeting
SMAD7. Biochem Biophys Res Commun. 496:176–183. 2018. View Article : Google Scholar : PubMed/NCBI
|