Introduction
The kidney has the ability to recover from ischemic
or toxic injury. During acute tubular cell injury, the basement
membrane of the tubules becomes rough, suggesting that normal cells
migrate to the injured basement membrane. However, the migrated
cells often lose their polarity and change in structure. Renal
proximal tubular cells (RPTCs) are the primary cell type
responsible for regeneration of tubular epithelial cells during
acute kidney injury (AKI) (1).
Renal tubular epithelial cell migration was decreased following
treatment with dimethyloxallyl glycine, which upregulates
hypoxia-inducible factor expression (2), suggesting that cell migration may be
affected by hypoxia or its associated regulatory factors.
Heat shock transcription factor 1 (HSF1) is a
well-studied regulatory factor that is active during AKI. In our
previous study, HSF1 was markedly activated and exhibited a
protective effect against cisplatin-induced AKI (3). However, during ischemic renal injury,
HSF1 activation may contribute to early injury (4). The effect of HSF1 on cell migration
is hypothesized to be different in different cell lines. Several
studies have suggested that HSF1 promoted cell migration,
particularly in cancer cells, including pancreatic cancer (5), osteosarcoma (6), hepatocellular carcinoma (7,8) and
melanoma cells (9). However, in
lung epithelium, activation of heat shock response decreased the
rate of wound closure (10). The
precise role of HSF1 in kidney tubular cell migration and
self-repairing remains largely unknown.
In addition to HSF1, transforming growth factor-β1
(TGF-β1) also serves a role in cell migration. TGF-β1 may be
cleaved by proteolytic enzymes to form a 112-amino acid peptide,
which binds to its receptors and transduce signals through
conserved Smad proteins. The TGF-β-Smad signaling pathway was
demonstrated to be activated during gastric cancer (11) and lung adenocarcinoma (12) cell migration and invasion, and in
the process of epithelial-mesenchymal transition (EMT). TGF-β may
trigger EMT and increase the migratory and invasive capacities of
nasopharyngeal carcinoma cells (13) and breast carcinoma MCF-7 and
MDA-MB-231 cell lines (14,15).
The stimulation of TGF-β1 enhanced podocyte adhesion and migration
(16). In renal carcinoma 769-P
and OSRC cell lines, TGF-β1 also facilitates migration and
invasion, which is primarily associated with the regulation of the
extracellular signal-regulated kinase (ERK) and c-Jun N-terminal
kinase (JNK) signaling pathways (17). In vascular smooth muscle cells
(VSMCs), proliferation and migration were inhibited by
microRNA-145-5p, which was regulated by TGF-β signaling pathway
proteins, including Smad2, Smad3 and TGF-β (16). The same phenomenon was observed in
cervical cancer cells, whose proliferation and migration were
decreased by C glycoprotein, and this process was accompanied by
decreased expression levels of TGF-β1, matrix metalloproteinase
(MMP)-2 and MMP-9 (18). However,
in a chemical model of RPTC cell injury and regeneration, TGF-β1
treatment inhibited the self-repair capability of the injured
monolayers (19). TGF-β receptor
inhibitors did not affect redifferentiation of rat kidney proximal
tubular cells (RPTCs) following H2O2 injury (20). To date, the precise role of the
TGF-β signaling pathway and HSF1 activation in renal tubular cell
repair remain unknown.
In the present study, the functional role of HSF1
and associated signaling events in RPTC cell migration were
investigated. Our results demonstrated that HSF1 decreased the
activation of the TGF-β-Smad2/3 pathway, and thereby inhibited the
RPTC cell migration and invasion processes.
Materials and methods
Cell culture
RPTCs were originally obtained from Dr. Hopfer (Case
Western Reserve University). RPTC HSF1 knockdown and scramble cell
lines were constructed as described in our previous study (3). The HSF1 short hairpin (shRNA)
plasmids were purchased from Qiagen, Inc. (cat. no. 336312
KR47022H). All shRNA plasmids were used at a concentration of 50
ng/ml. The scramble and shRNA target sequences for rat HSF1 were
5′-GGAATCTCATTCGATGCATAC-3′ and 5′-TGTCAACAAGCTCATCCAATT-3′,
respectively. Lipofectamine® 2000 was purchased from
Thermo Fisher Scientific, Inc. RPTCs were plated at a density of
1×106 cells per dish in 35 mm dishes. The cells reached
100% confluency within 24 h. The cells were subcultured or plated
for subsequent experiments 72 h after transfection. RPTCs were
cultured in Ham's F-12-Dulbecco's modified Eagle's medium (DMEM;
12400024; Thermo Fisher Scientific, Inc.) containing transferrin (5
µg/ml), insulin (5 µg/ml), epidermal growth factor (EGF; 1 ng/ml),
dexamethasone (4 µg/ml), 10% FBS and 1% Antibiotic-Antimycotic
(15240062; Thermo Fisher Scientific, Inc.).
Western blot analysis
HSF1 knockdown and scramble cells were cultured in a
3.5 cm dish. The cells were treated with the TGF-β1 inhibitor
SB431542 or with DMSO at room temperature for 24 h. Proteins were
extracted with SDS lysis solution containing 1% protease inhibitor
cocktail (cat. no. 78430; Thermo Fisher Scientific, Inc.) and were
then quantified using a BCA assay. A total of ~30 µg protein was
subjected to 12% SDS-PAGE and transferred to PVDF membranes. The
membranes were incubated with primary antibodies at 4°C overnight,
including rabbit polyclonal anti-HSF1 (1:1,000; cat. no. 4356S;
Cell Signaling Technology, Inc.), rabbit polyclonal anti-TGF-β1
(1:500; cat. no. ab66043; Abcam), rabbit monoclonal anti-Smad2/3
(D7G7; 1:1,000; cat. no. 8685; Cell Signaling Technology, Inc.),
rabbit monoclonal anti-phospho-Smad2 (Ser465/467)/Smad3
(Ser423/425) (D27F4; 1:1,000; cat. no. 8828; Cell Signaling
Technology, Inc.), mouse monoclonal anti-Ki67 (8D5; 1:500; cat. no.
NBP2-22112; Novus Biologicals, LLC.) and mouse monoclonal
anti-GAPDH (1:10,000; cat. no. AC002; ABclonal Biotech Co., Ltd.)
antibodies. The corresponding secondary antibodies were horseradish
peroxidase-conjugated goat anti-rabbit (1:5,000; cat. no.
111-035-003; Jackson ImmunoResearch Laboratories, Inc.) and goat
anti-mouse (1:5,000; cat. no. 115-035-003; Jackson ImmunoResearch
Laboratories, Inc.) antibodies. The signal intensities were
visualized via enhanced chemiluminescence and quantified with
ImageJ 1.51 software (National Institutes of Health). GAPDH was
used as an internal control. The protein expression levels in the
experimental or control groups were normalized to GAPDH.
Immunofluorescence
HSF1 knockdown and scramble cells were cultured in
35 mm dishes at 37°C until 100% confluent, scratched with a 1 ml
pipette tip across the maximum diameter, and cultured at 37°C for
additional 6, 12 or 24 h. The expression and cellular localization
of TGF-β1 was determined by immunofluorescence with a polyclonal
antibody against TGF-β1, as described previously (21). Following wound healing, cells were
washed with PBS, fixed in 4% paraformaldehyde (Tianjin Zhiyuan
Chemical Reagent Co., Ltd.) in PBS at room temperature for 15 min,
permeabilized with −20°C precooled 100% methanol for 10 min and
then washed with PBS. Following blocking in 5% BSA (Sigma-Aldrich;
Merck KGaA) buffer at room temperature for ~1 h, the cells were
incubated with rabbit polyclonal anti-TGF-β1 antibody (1:500; cat.
no. ab66043; Abcam) overnight at 4°C and then washed with PBS.
Next, Alexa Fluor 488-conjugated goat anti-rat IgG secondary
antibody (1:500; cat. no. A-11006; Thermo Fisher Scientific, Inc.)
was used in PBS buffer containing 1% BSA for 1 h at room
temperature. Coverslips were mounted with Prolong Gold Antifade
Mountant with DAPI (cat. no. P36941; Thermo Fisher Scientific,
Inc.) and viewed using fluorescence microscopy (magnification,
×100). Images were analyzed using ImageJ 1.51 software (National
Institutes of Health).
Wound healing assay
The wound healing ability of RPTCs was evaluated as
described previously (22). HSF1
knockdown (KD) and scramble negative control (NC) cells were seeded
in 35 mm dishes at a density of 1ⅹ106 cells per dish.
The next day, wounds were created with a 1-ml pipette tip and were
allowed to heal for 6, 12 or 24 h. Images of the cells were
captured at the indicated time points (6, 12 and 24 h after
scratching) with an Olympus CKX41 microscope (magnification; ×100;
Olympus Corporation). The healing distance was measured using Leica
Application Suite V4.2 microscope software (Leica Microsystems,
Inc.). Data were calculated as percentages of healing distance
relative to the initial wound width. To evaluate the effect of
TGF-β1 inhibitor on the migration speed of RPTC HSF1 knockdown and
scramble cells, the cells were incubated in culture medium with the
TGF-β1 inhibitor SB431542 or with DMSO prior to the wound healing
assay. The percentages of healing distance were calculated as
described above. The percentage difference upon treatment with or
without TGF-β1 inhibitor in the NC group was compared with that in
the KD group in the 0–6 or 6–12 h time frame.
Transwell assay
To evaluate the effect of HSF1 on the invasion
ability of RPTCs, HSF1 KD and scramble cells (8×104)
were seeded in serum-free DMEM-F12 in the upper compartment of a
Matrigel Invasion Chamber with 8 µm pore size (cat. no.,
08-774-121; Thermo Fisher Scientific, Inc.). The lower chamber was
loaded with DMEM-F12 containing 10% FBS. After 12 h incubation at
37°C, the cells on the lower surface were fixed with 75% cold
ethanol for 10 min and stained with 1% crystal violet for 5 min at
room temperature. Cells in 6 randomly selected fields were observed
under an Olympus CKX41 microscope (Olympus Corporation) at
magnification, ×100. The experiments were repeated 3 times.
Cell proliferation assay
RPTC HSF1 KD and scramble cells (5×105)
were incubated in 35 mm dishes at 37°C. After 0, 12, 24 and 48 h,
cells were washed with PBS 3 times and then stained with 0.4%
trypan blue solution (cat. no., T8154; Sigma-Aldrich; Merck KGaA).
Cell proliferation was measured by counting the number of stained
cells. Cells were observed under an Olympus CKX41 microscope
(magnification, ×200; Olympus Corporation). Relative cell
proliferation was calculated from 5 fields of view. A total of ≥3
independent repeat experiments were performed.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean of 3 independent experiments and analyzed with GraphPad
Prism v.7.00 software (GraphPad Software, Inc.). The statistical
difference between two groups was analyzed by Student's t-test. The
statistical differences between multiple groups were analyzed by
two-way analysis of variance followed by a Bonferroni post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
HSF1 knockdown promotes RPTCs
migration
To determine the effect of HSF1 on the rate of RPTC
cell migration, HSF1 KD and scramble NC cell lines were constructed
by stably transfecting HSF1 shRNA or scramble sequence in RPTCs.
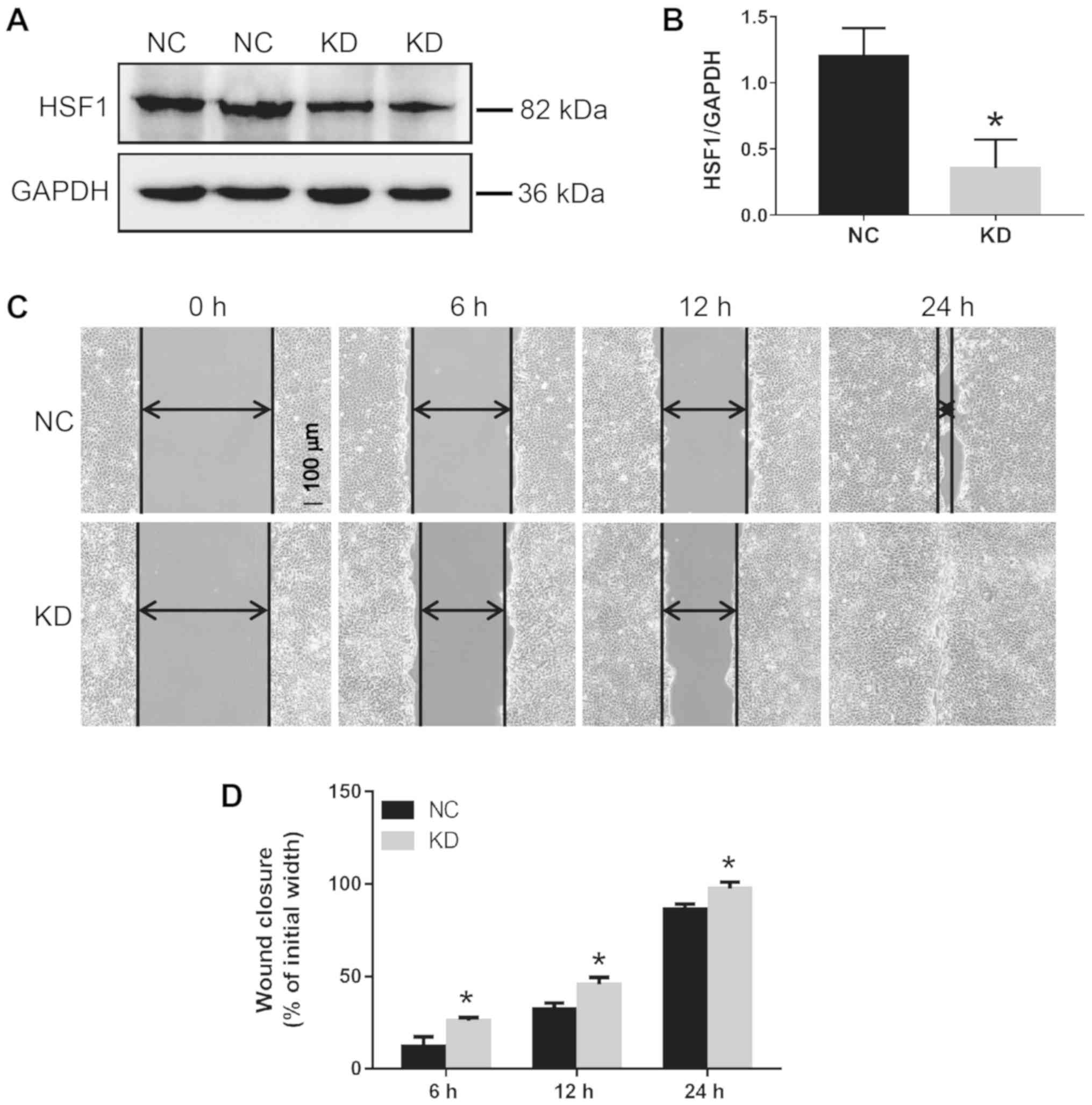
The HSF1 expression level in KD cells was decreased by ~70.5%
compared with NC transfected cells (Fig. 1A and B). HSF1 KD and NC cells were
scratched with a 1 ml pipette tip and then cultured for 6, 12 or 24
h. The HSF1 KD cells exhibited an increased migration rate compared
with the HSF1 NC cells. The wound closure rates in the KD and NC
cells were 25.9 and 12.1% at 6 h, 45.7 and 32.3% at 12 h, and 97.4
and 86.6% at 24 h, respectively (Fig.
1C and D).
HSF1 inhibits TGF-β signaling during
wound healing
The fluorescence intensity of TGF-β1 immediately
following wounding was weak, and after 12 and 24 h, the
fluorescence intensity became more marked. Notably, TGF-β1
expression was predominantly distributed in cells at the edge of
the wound (Fig. 2A). Compared with
HSF1 NC cells, HSF1 KD cells exhibited more marked fluorescence
intensity at each time point during wound healing, indicating that
TGF-β1 expression was significantly increased compared with the
corresponding control (Fig. 2B).
The increased expression level of TGF-β1 upon HSF1 knockdown was
additionally confirmed by western blot analysis (Fig. 2C and D). In addition, the
phosphorylation of Smad2/3, which is downstream of the TGF-β
signaling pathway, was also upregulated in HSF1 KD cells. Notably,
the TGF-β1 inhibitor SB431542 exerted a significantly suppressive
effect on Smad2/3 phosphorylation (Fig. 2C and D), and was used in subsequent
experiments.
TGF inhibitor suppresses RPTC wound
healing ability
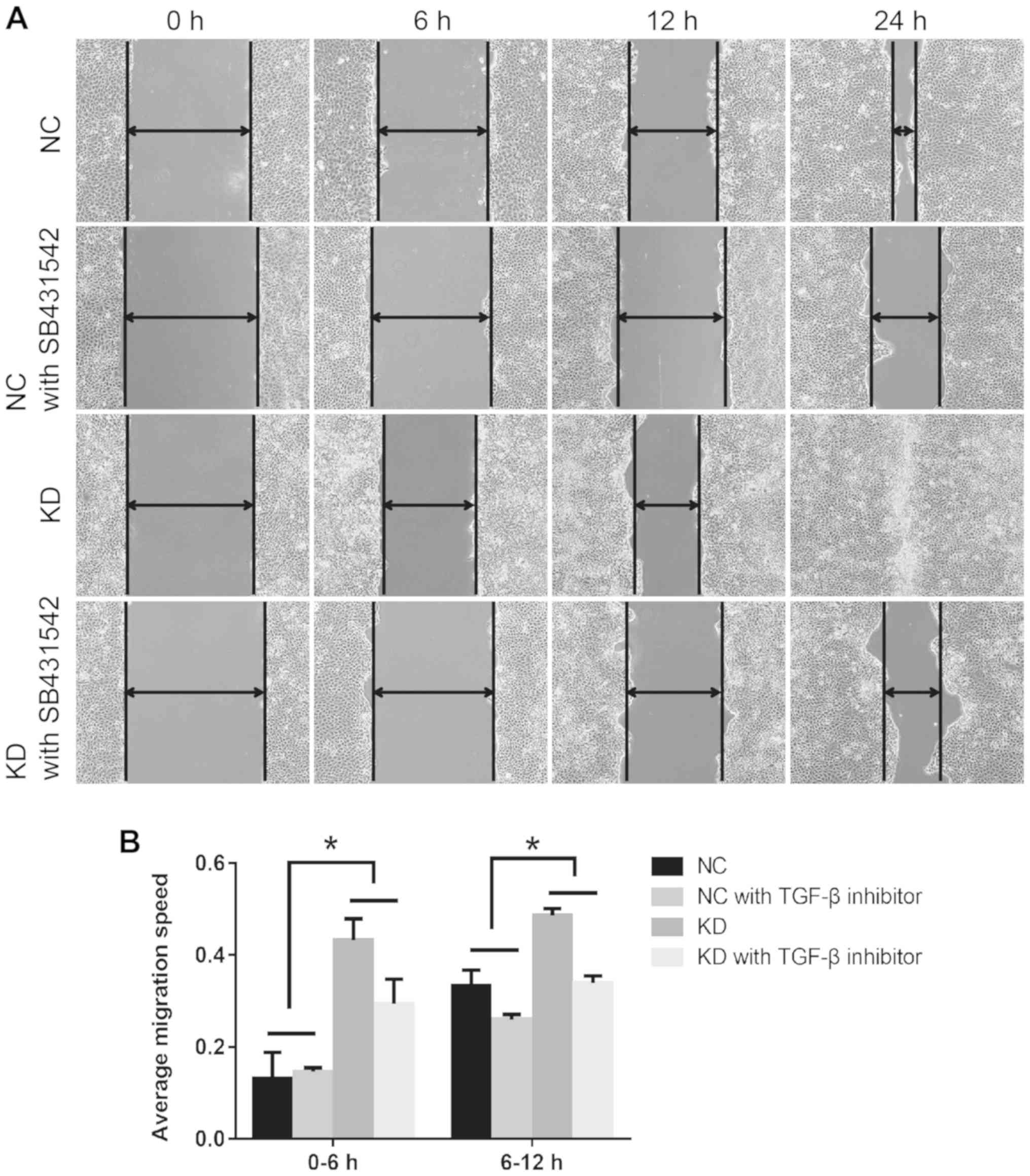
To additionally verify the effect of HSF1 on TGF-β1
activation during wound healing, the TGF-β1 inhibitor SB431542 was
applied prior to the wounding process. The percentage difference of
migration in the NC group was compared with that in the KD group in
the 0–6 and 6–12 h time frames. As indicated in Fig. 3A, SB431542 treatment markedly
decreased the rate of wound healing. Compared with the HSF1 NC
cells, SB431542 treatment significantly augmented the decrease in
migration rate in KD cells, particularly between 0 and 6 h
(Fig. 3B). Upon treatment with
SB431542, the migration rate decreased by 32.2% in KD cells and by
9.47% in NC cells at 0–6 h, and by 30.3% in KD cells and 22.1% in
NC cells at 6–12 h.
HSF1 knockdown promotes RPTC
invasion
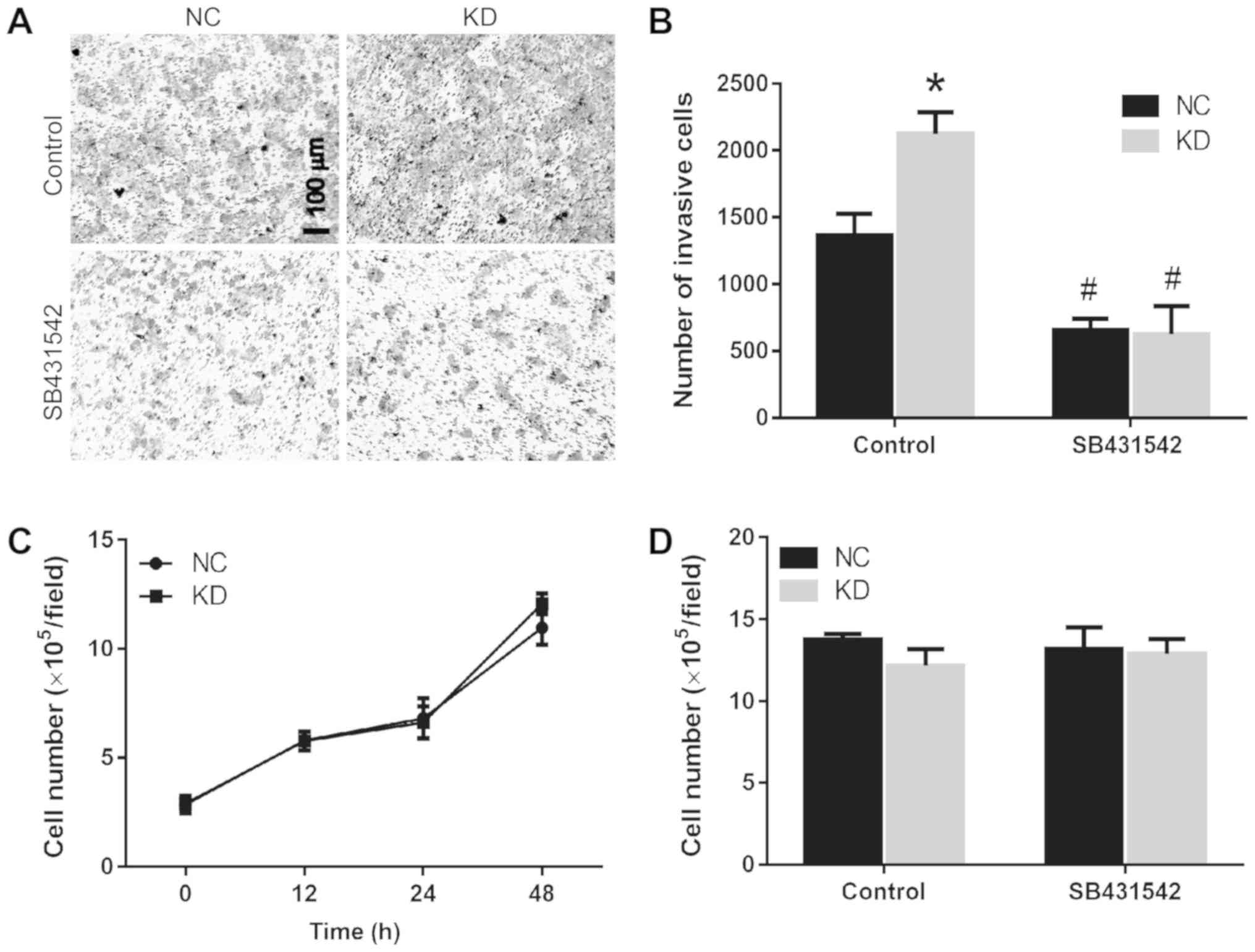
As HSF1 negatively affected the RPTC cell migration,
and that this inhibitory effect was demonstrated to occur via
regulation of the TGF-β1 signaling pathway, the invasive ability of
HSF1 KD and NC cells was then evaluated. Following knockdown of
HSF1 in RPTCs, the number of cells penetrating the Matrigel was
markedly increased (Fig. 4A). Upon
treating with 10 mM TGF-β1 inhibitor SB431542, the RPTC invasive
ability of the HSF1 KD and scramble groups was decreased by 70.7
and 52%, respectively (Fig. 4B).
In addition, no significant difference was observed in the invasive
abilities between the HSF1 shRNA1 and shRNA2 groups (data not
shown).
HSF1 does not affect RPTC
proliferation
The changes in cell migration may be due to
differing proliferative abilities during the wound healing process.
Therefore, the present study compared the numbers of living cells
in the HSF1 KD and scramble cell lines following culture for 0, 12,
24 and 48 h. The results indicated that their growth curves were
similar (Fig. 4C). In addition,
the expression of proliferation marker protein Ki-67 was assessed.
As demonstrated in Fig. S1, there
was no marked difference between HSF1 knockdown and control cells,
whether in 0 or 24 h after culture. Following treatment with the
TGF-β1 inhibitor SB431542 for 24 h, no significant difference in
the cell number in the 2 cell lines was observed (Fig. 4D), indicating that the effect of
HSF1 or TGF-β1 signaling on cell migration was not mediated through
an inhibition of proliferation.
Discussion
In the present study, the effects of HSF1 on kidney
tubular cell migration and invasion were investigated. HSF1 was
demonstrated to be protective in kidney epithelial cells during
cisplatin-induced AKI in our previous study (3). In addition, the migration of vascular
endothelial (23) or macrophage
(24) cells contributes to the
repair of cisplatin-induced AKI. Therefore, we hypothesized that
HSF1 may serve a protective role against injury by promoting cell
migration. Unexpectedly, HSF1 knockdown promoted kidney tubular
cell migration and invasion. In addition, the regulatory effects of
HSF1 on cell migration were associated with the induced expression
of the TGF-β1/phosphorylated (p)-Smad2/3 pathway proteins. These
data suggest that, in addition to serving a protective role against
AKI, HSF1 also has the effect of inhibiting kidney tubular cell
migration.
The effect of HSF1 on the migration process appears
to vary in different cell lines. For example, HSF1 was demonstrated
to be able to stimulate osteosarcoma cell migration and upregulate
EGF receptor 1 expression (6).
HSF1 activation was also suggested to be associated with poor
outcomes in patients with breast and lung cancer (25). In a separate study, it was revealed
that HSF1 promoted the inhibition of EMT-associated migration in
hepatocellular carcinoma cells and the upregulation of epithelial
cadherin (E-cadherin) expression (8). These data suggest that HSF1 may
affect cell migration by regulating different signaling molecules
in different disease models.
It has been established that cell motility is
closely associated with the activation of the TGF-β1 signaling
pathway (16,17). As cell motility was enhanced in the
HSF1 KD cell line, we hypothesized that certain TGF-β1 pathway
proteins may be highly expressed in RPTC HSF1 KD cells. The results
of the present study demonstrated that Smad2/3 phosphorylation was
induced in HSF1 KD cells. Therefore, TGF-β1/Smad2/3 signaling
contributes at least partially to promoting cell migration. Of
note, TGF-β1 expression was significantly upregulated at the edge
of the wound, which is important for cell migration. Notably, it
was previously demonstrated that treatment of human kidney 2 cells
with TGF-β1 protein may lead to a marked inhibition of migration
(26); Supplementation with
exogenous TGF-β1 also significantly inhibited proliferation and
migration of oral mucosal fibroblasts (27); an additional in vivo study
indicated that overexpressing TGF-β1 exacerbated renal injury while
latent TGF-β1 inhibited renal inflammation and fibrosis (28). Therefore, it is possible that
latent TGF-β1 promotes cell migration, while excess TGF-β1 has the
opposite effect. In addition, HSF1 knockdown may induce endogenous
TGF-β1 signaling pathway activation.
HSF1 knockdown causes the promotion of kidney
tubular cell migration by regulating the TGF-β1 signaling pathway.
Our first hypothesis was that the promotive effect may also occur
through regulating E-cadherin expression, as cell migration usually
is associated with EMT. In addition, it has been demonstrated
previously that HSF1 promoted the inhibition of EMT-associated
migration in hepatocellular carcinoma cells by downregulating
E-cadherin expression (8).
However, there was no induction of E-cadherin or vimentin
expression in the present study, thereby suggesting that the
enhanced cell migration caused by HSF1 knockdown is not associated
with cell adhesion proteins. E-cadherin is primarily expressed in
renal distal tubular cells, whereas RPTCs contain mainly neural
cadherin (29), therefore,
E-cadherin may not be involved in the process of HSF1-mediated
regulation of RPTC migration.
A potential mechanism by which HSF1 knockdown leads
to the activation of the TGF-β1 signaling pathway is that HSF1
decreases TGF-β1 activation by regulating heat shock protein (HSP)
expression. For example, HSP70 and HSP90 regulate TGF-β signaling
through opposing mechanisms (30).
TGF-β1-induced cell motility was also suggested to be associated
with HSP27 activation in prostate epithelial cells (31). An additional explanation why HSF1
is able to regulate TGF-β1 activation may be due to proteins not
associated with the heat shock response. For example, HSF1 was
suggested to be able to inhibit the activation of a variety of
transcription factors, including NF-κB (32). It is possible, therefore, that the
enhancement of cell migration in RPTC HSF1 KD cells may be caused
by changes in expression of certain HSPs or the NF-κB signaling
pathway.
In conclusion, the present study demonstrated that
HSF1 suppressed RPTC migration by regulating the TGF-β1/p-Smad2/3
signaling pathway. The results of the present study suggested that
HSF1 may serve an inhibitory role during renal fibrosis and provide
a novel strategy for the treatment of kidney injury.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank professor Zheng Dong
(Augusta University) for his academic advice and technical
support.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81970592 and U1404826), Key
Scientific and Technological Project of Henan Province (grant no.
192102310314), Research Fund of Henan University and Shanghai
Pujiang Program (grant no. 17PJ1411100).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL conceived and designed the current study. YLi
performed the experiments. YLi, QL, BH, YLiu, YZ, JH and YM
analyzed the data. YLi drafted the manuscript, and YLi, QL, BH,
YLiu, YZ, JH and YM edited and revised the manuscript. All authors
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RPTC
|
rat kidney proximal tubular cells
|
|
HSF1
|
heat shock transcription factor 1
|
|
TGF-β1
|
transforming growth factor- β1
|
|
AKI
|
acute kidney injury
|
|
EMT
|
epithelial-mesenchymal transition
|
|
NC
|
negative control
|
|
KD
|
HSF1 knockdown
|
References
|
1
|
Duffield JS, Park KM, Hsiao LL, Kelley VR,
Scadden DT, Ichimura T and Bonventre J: Restoration of tubular
epithelial cells during repair of the postischemic kidney occurs
independently of bone marrow-derived stem cells. J Clin Invest.
115:1743–1755. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Müller S, Djudjaj S, Lange J, Iacovescu M,
Goppelt-Struebe M and Boor P: HIF stabilization inhibits renal
epithelial cell migration and is associated with cytoskeletal
alterations. Sci Rep. 8:94972018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lou Q, Hu Y, Ma Y and Dong Z: Heat shock
factor 1 induces crystallin-αB to protect against cisplatin
nephrotoxicity. Am J Physiol Renal Physiol. 311:F94–F102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sreedharan R, Chen S, Miller M, Haribhai
D, Williams CB and Van Why SK: Mice with an absent stress response
are protected against ischemic renal injury. Kidney Int.
86:515–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen K, Qian W, Li J, Jiang Z, Cheng L,
Yan B, Cao J, Sun L, Zhou C, Lei M, et al: Loss of AMPK activation
promotes the invasion and metastasis of pancreatic cancer through
an HSF1-dependent pathway. Mol Oncol. 11:1475–1492. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Z, Li Y, Jia Q, Wang Z, Wang X, Hu J
and Xiao J: Heat shock transcription factor 1 promotes the
proliferation, migration and invasion of osteosarcoma cells. Cell
Prolif. 50:2017. View Article : Google Scholar :
|
|
7
|
Li Y, Xu D, Bao C, Zhang Y, Chen D, Zhao
F, Ding J, Liang L, Wang Q, Liu L, et al: MicroRNA-135b, a HSF1
target, promotes tumor invasion and metastasis by regulating RECK
and EVI5 in hepatocellular carcinoma. Oncotarget. 6:2421–2433.
2015.PubMed/NCBI
|
|
8
|
Liu D, Sun L, Qin X, Liu T, Zhang S, Liu
Y, Li S and Guo K: HSF1 promotes the inhibition of EMT-associated
migration by low glucose via directly regulating Snail1 expression
in HCC cells. Discov Med. 22:87–96. 2016.PubMed/NCBI
|
|
9
|
Toma-Jonik A, Widlak W, Korfanty J, Cichon
T, Smolarczyk R, Gogler-Piglowska A, Widlak P and Vydra N: Active
heat shock transcription factor 1 supports migration of the
melanoma cells via vinculin down-regulation. Cell Signal.
27:394–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scheraga RG, Thompson C, Tulapurkar ME,
Nagarsekar AC, Cowan M, Potla R, Sun J, Cai R, Logun C, Shelhamer
J, et al: Activation of heat shock response augments fibroblast
growth factor-1 expression in wounded lung epithelium. Am J Physiol
Lung Cell Mol Physiol. 311:L941–L955. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang JK, Wang WJ, Cai HY, Du BB, Mai P,
Zhang LJ, Ma W, Hu YG, Feng SF and Miao GY: MFAP2 promotes
epithelial-mesenchymal transition in gastric cancer cells by
activating TGF-β/SMAD2/3 signaling pathway. Onco Targets Ther.
11:4001–4017. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng G, Chen L, Zhang Y, Fan S, Li W, Lu J
and Chen X: Fucosyltransferase 2 induced epithelial-mesenchymal
transition via TGF-β/Smad signaling pathway in lung
adenocarcinaoma. Exp Cell Res. 370:613–622. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang G, Du MY, Zhu H, Zhang N, Lu ZW,
Qian LX, Zhang W, Tian X, He X and Yin L: MiRNA-34a reversed
TGF-β-induced epithelial-mesenchymal transition via suppression of
SMAD4 in NPC cells. Biomed Pharmacother. 106:217–224. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Wang JX, Huang D, Wang B, Li LL, Li
XX, Ni P, Dong XL, Xia W, Yu CX, et al: PMLIV overexpression
promotes TGF-β-associated epithelial-mesenchymal transition and
migration in MCF-7 cancer cells. J Cell Physiol. 233:9575–9583.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang R, Liang J, Xu GX, Ding LM, Huang HM,
Su QZ, Yan J and Li YC: Human cytomegalovirus glycoprotein B
inhibits migration of breast cancer MDA-MB-231 cells and impairs
TGF-β/Smad2/3 expression. Oncol Lett. 15:7730–7738. 2018.PubMed/NCBI
|
|
16
|
Chen CA, Chang JM, Chang EE, Chen HC and
Yang YL: TGF-β1 modulates podocyte migration by regulating the
expression of integrin-β1 and -β3 through different signaling
pathways. Biomed Pharmacother. 105:974–980. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Zhang N, Gao R, Zhu Y, Zhang Z, Xu
X, Wang J, Li Z, Liu X, Li Z, et al: TGF-β1 induced fascin1
expression facilitates the migration and invasion of kidney
carcinoma cells through ERK and JNK signaling pathways. Biochem
Biophys Res Commun. 501:913–919. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang DG, Li TM and Liu X: RHCG suppresses
cervical cancer progression through inhibiting migration and
inducing apoptosis regulated by TGF-β1. Biochem Biophys Res Commun.
503:86–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kays SE, Nowak G and Schnellmann RG:
Transforming growth factor-beta 1 inhibits regeneration of renal
proximal tubular cells after oxidant exposure. J Biochem Toxicol.
11:79–84. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hallman MA, Zhuang S and Schnellmann RG:
Regulation of dedifferentiation and redifferentiation in renal
proximal tubular cells by the epidermal growth factor receptor. J
Pharmacol Exp Ther. 325:520–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long M, Cai L, Li W, Zhang L, Guo S, Zhang
R, Zheng Y, Liu X, Wang M, Zhou X, et al: DPP-4 inhibitors improve
diabetic wound healing via direct and indirect promotion of
epithelial-mesenchymal transition and reduction of scarring.
Diabetes. 67:518–531. 2018.PubMed/NCBI
|
|
22
|
Zhou X, Zhang W, Yao Q, Zhang H, Dong G,
Zhang M, Liu Y, Chen JK and Dong Z: Exosome production and its
regulation of EGFR during wound healing in renal tubular cells. Am
J Physiol Renal Physiol. 312:F963–F970. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Yin L, Zhang B, Shi H, Sun Y, Ji
C, Chen J, Wu P, Zhang L, Xu W and Qian H: Resveratrol improves
human umbilical cord-derived mesenchymal stem cells repair for
cisplatin-induced acute kidney injury. Cell Death Dis. 9:9652018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Tang Y, Tang PMK, Huang XR,
Carlsson-Skwirut C, Da Costa L, Aspesi A, Fröhlich S, Szczęśniak P,
et al: Blocking macrophage migration inhibitory factor protects
against cisplatin-induced acute kidney injury in mice. Mol Ther.
26:2523–2532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scherz-Shouval R, Santagata S, Mendillo
ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M,
Stemmer SM, Whitesell L and Lindquist S: The reprogramming of tumor
stroma by HSF1 is a potent enabler of malignancy. Cell.
158:564–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian YC and Phillips AO:
TGF-beta1-mediated inhibition of HK-2 cell migration. J Am Soc
Nephrol. 14:631–640. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dally J, Khan JS, Voisey A, Charalambous
C, John HL, Woods EL, Steadman R, Moseley R and Midgley AC:
Hepatocyte growth factor mediates enhanced wound healing responses
and resistance to transforming growth factor-β1-driven
myofibroblast differentiation in oral mucosal fibroblasts. Int J
Mol Sci. 18:E18432017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kroening S, Neubauer E, Wullich B, Aten J
and Goppelt-Struebe M: Characterization of connective tissue growth
factor expression in primary cultures of human tubular epithelial
cells: Modulation by hypoxia. Am J Physiol Renal Physiol.
298:F796–F806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang Y, Xu X, Duan X, Guo J, Wang Y, Ren
F, He D and Chang Z: Hsp70 and Hsp90 oppositely regulate TGF-β
signaling through CHIP/Stub1. Biochem Biophys Res Commun.
446:387–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di K, Wong YC and Wang X: Id-1 promotes
TGF-beta1-induced cell motility through HSP27 activation and
disassembly of adherens junction in prostate epithelial cells. Exp
Cell Res. 313:3983–3999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wirth D, Bureau F, Melotte D, Christians E
and Gustin P: Evidence for a role of heat shock factor 1 in
inhibition of NF-kappaB pathway during heat shock response-mediated
lung protection. Am J Physiol Lung Cell Mol Physiol. 287:L953–L961.
2004. View Article : Google Scholar : PubMed/NCBI
|