Introduction
Mesenchymal stromal cells (MSCs) are a population of
heterogeneous multipotent cells that differentiate into diverse
cell types. Stromal cells contain various populations, including
stem cells (1,2). MSCs have self-renewal ability,
immunomodulation and multi-directional differentiation potential,
and have become a practical source of cells in the field of gene
therapy. MSCs have been extensively used for the treatment of
several diseases including immune diseases and hematological
diseases, based on their biological and therapeutic effects
(3). MSCs can be isolated from
different tissues, including bone marrow (4), adipose tissue (5), the umbilical cord (6), umbilical cord blood (UCB) (7), menstrual blood (8), the placenta (9), amniotic membrane (10), dental pulp (11) and tissue from the central nervous
system (12). Compared with other
sources of MSCs, human UCB-derived MSCs (hUCB-MSCs) have many
advantages, such as extensive sources, convenient collection,
strong proliferation and differentiation abilities, low
immunogenicity and the lack of ethical issues surrounding their use
(13–15). There are currently different
methods for the in vitro isolation and culture of hUCB-MSCs,
however, it is still difficult to obtain hUCB-MSCs in large numbers
(16). Therefore, a simple,
practical and low-cost method to obtain the largest proportion of
MSCs from UCB is urgently needed.
Previously, hUCB-MSCs were cultured in media
including DMEM/F12, DMEM, and α-MEM (17,18).
Some methods added supplements including hydrocortisone,
granulocyte-macrophage colony stimulating factor and insulin, while
others used only MesenCult™ medium (19,20).
However, these methods result in hUCB-MSCs that have a low success
rate and are not suitable for scientific research, and clinical
applications that require large numbers of hUCB-MSCs. Moreover,
many commercially available special stem cell culture media are
costly. Finally, the growth rate of osteoclast-like cells is also
high during the primary culture process (21), which is not conducive to the
subculture of hUCB-MSCs, and thus also reduces the culture success
rate of hUCB-MSCs.
Due to the unique characteristics of hUCB-MSCs,
there are several methods for their isolation and culture; however,
there is no consensus on a standard method. In the present study, a
simple, efficient and economical method for isolating and culturing
high-quality hUCB-MSCs is described. Once this method becomes the
accepted standard, it can be used in scientific research, clinical
medicine and cell banking.
Materials and methods
Isolation and culture of UCB
mononuclear cells (MNCs)
All UCB samples (age, 24–38 years) were collected
from the Third Affiliated Hospital of Xinxiang Medical University,
Xinxiang, China between February 2018 and December 2018. Typically,
30–60 ml of UCB samples were collected through the umbilical cord
by way of gravity drainage. UCB was collected from the umbilical
cord vein with written informed consent of the mother and in strict
accordance with the ethical standards of the local ethics
committee. All blood samples were processed 4–6 h after collection.
To isolate and expand MSCs from the UCB, MNCs were isolated using a
lymphocyte separation medium (ρ=1.077 g/l; TBDscience). D-Hanks
balanced salt solution (Beijing Solarbio Science & Technology
Co., Ltd.) was used to dilute 15–30 ml of fresh UCB at a 1:1 ratio,
then the diluted blood was slowly added to the lymphocyte
separation medium (taking care not to disturb the liquid surface).
The diluted UCB was mixed with the lymphocyte separation media with
a volume ratio of 2:1. After centrifugation at 1,317 × g for 20 min
at room temperature, the white cloud-like MNCs in the interface
layer were carefully aspirated, centrifuged as aforementioned,
washed twice with PBS at 750 × g for 10 min at room temperature,
and washed once with the DMEM (Gibco; Thermo Fisher Scientific,
Inc.) at the same speed. After centrifugation, DMEM supplemented
with 10% FBS (Hangzhou Sijiqing Biological Engineering Materials
Co., Ltd.) and 1% penicillin/streptomycin was added to the
collected cells to prepare a single cell suspension. The cells were
counted and inoculated into an FBS-coated T25 cell culture flask
(area of 25 cm2 required 1–1.5 ml FBS) at a density of
1×107 cells/ml and incubated at 37°C in a humidified
atmosphere with 5% CO2 (19). Upon reaching 70–80% confluency, the
cells were digested with 0.1% trypsin and further subcultured. The
growth and morphological characteristics of the primary cells were
observed daily under an inverted light microscope (Leica
Microsystems GmbH; magnification, ×200).
Effects of different media on the
culture of hUCB-MSCs
In the present study, the culture efficiency of
hUCB-MSCs was compared in two types of media. DMEM supplemented
with 10% FBS (n=16; DMEM group) and DMEM supplemented with
mesenchymal stem cell growth supplement (ScienCell Research
Laboratories, Inc.) and 10% FBS [n=22; the mesenchymal stem cell
medium (MSCM) group]. To reduce costs, a sequential culture method
consisting of two media was used in the MSCM group. hUCB-MSCs in
the MSCM group were cultured in DMEM supplemented with 10%
mesenchymal stem cell growth supplement (ScienCell Research
Laboratories, Inc.) and 10% FBS for three passages, and on the
fourth passage the culture media was replaced with DMEM
supplemented with 10% FBS. The isolated hUCB-MSCs were inoculated
into an FBS-coated T25 cell culture flask at a density of
1×107/ml (5 ml/flask) and cultured at 37°C in a
humidified atmosphere with 5% CO2. The growth and
morphological characteristics of the primary cells were observed
daily under an inverted microscope and images were captured. The
numbers of hUCB-MSCs in the two groups were counted on the 5, 7 and
14th day under the same magnification using a light microscope.
After uniform spindle fibroblast-like cells, which grew in a
whirled manner, were cultured successfully, the culture success
rates and effects of the different types of media on the culture of
hUCB-MSCs were compared.
Effects of different inoculation
densities on the culture of hUCB-MSCs
MNCs from nine blood samples were divided equally
into three subgroups and were inoculated in T25 cell culture flasks
at cell densities of 1×106, 1×107 and
1×108/ml (with each density representing one subgroup).
The cells were cultured in DMEM supplemented with mesenchymal stem
cell growth supplement and 10% FBS. The growth of hUCB-MSCs was
observed daily under an inverted microscope, and the extension time
and primary culture time were recorded.
Effects of the first medium changes on
the culture of hUCB-MSCs
MNCs from nine blood samples were divided equally
and inoculated in a T25 cell culture flask at a cell density of
1×107/ml; the cells were cultured in DMEM supplemented
with mesenchymal stem cell growth supplement and 10% FBS. The
medium was changed on the 3, 4, 5 and 7th day after inoculation.
The media was transferred to a new culture flask for >5 days and
further observed to determine whether MSCs could grow, and to
determine the time of first medium change.
Growth curves
Passage 3 and 10 of hUCB-MSCs were prepared into
single cell suspensions, the cell concentrations were adjusted and
the cells were inoculated into a 24-well plate. The number of cells
added to each well was 1×103. Cells were cultured in
DMEM supplemented with 10% FBS in a 37°C humidified atmosphere with
5% CO2. Cells were counted twice per day and the mean
was used as the final cell number. Any increase in the number of
cells was calculated for 12 consecutive days. Growth curves of
hUCB-MSCs were plotted according to the number of cells, and the
population doubling time was calculated from the growth curves. All
experiments were performed in triplicate.
Detection of cell surface markers
using flow cytometry
hUCB-MSCs from passage 3 and 10 were digested with
0.1% trypsin. After centrifugation at 750 × g for 10 min at room
temperature, the supernatant was removed and washed with PBS.
Aliquots of ~1×106 cells for each antibody were
obtained. The harvested cells were stained with phycoerythrin-mouse
anti-human CD29 (cat. no. 561795; 20 µl/106 cells; BD
Biosciences), FITC mouse anti-human CD44 (cat. no. 560977; 20
µl/106 cells; BD Biosciences) and FITC mouse anti-human
CD45 (cat. no. 560976; 20 µl/106 cells; BD Biosciences).
After 30 min of incubation at room temperature in the dark, the
cells were centrifuged at 750 × g for 5 min at room temperature and
the unconjugated antibody was removed. The stained cells were
resuspended in 500 µl PBS and analyzed using a flow cytometer
(Beckman-Coulter, Inc.), and CytExpert software 2.0
(Beckman-Coulter, Inc.) was used for data analysis. All experiments
were performed in triplicate.
Reverse transcription (RT)-PCR
hUCB-MSCs (1×106) from passage 3 and 10
were harvested and the total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions, and the RNA concentrations were
determined. RNA sample (500 ng) was reverse transcribed into cDNA
using a PrimeScript™ RT Reagent kit (Takara Biotechnology Co.,
Ltd.). The temperature protocol was as follows: 37°C for 30 min and
then 85°C for 5 sec. Primers were designed according to the
sequences of octamer-binding transcription factor 4 (Oct4), Sex
determining region Y-box 2 (Sox2) and the homeobox protein Nanog
(Table I). The PCR protocol was as
follows: 94°C for 5 min, followed by 35 cycles of 94°C for 30 sec,
60°C for 30 sec and 72°C for 40 sec. The reaction was completed
with a final extension at 72°C for 5 min. GAPDH was used as a
positive control. PCR products were visualized with ethidium
bromide on a 1% agarose gel.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Gene | Sequence
(5′-3′) |
|---|
| Oct4 | Forward:
AGTGAGAGGCAACCTGGAGA |
|
| Reverse:
GTGAAGTGAGGGCTCCCATA |
| Nanog | Forward:
CAGAAGGCCTCAGCACCTAC |
|
| Reverse:
GAATTTGGCTGGAAGTGCAT |
| Sox2 | Forward:
ACCAGCTCGCAGACCTACAT |
|
| Reverse:
GGTAGTGCTGGGACATGTGA |
| GAPDH | Forward:
TGGTGAAGACGCCAGTGGA |
|
| Reverse:
GCACCGTCAAGGCTGAGAAC |
RT-quantitative (q)PCR
The cDNA from passage 3 and 10 of the hUCB-MSCs was
used for RT-qPCR using the SYBR Premix Ex Taq (Vazyme) using the
ABI 7500 real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction mixture (20 µl) consisted of 2 µl
template (50 ng/µl), 10 µl 2X SYBR mix (Vazyme), 0.5 µl each primer
(10 µmol/l) and 7 µl deionized water. The primer sequences were the
same and are listed in Table I.
The thermocycling conditions were as follows: 95°C for 5 min,
followed by 40 cycles of 9°C for 10 sec and 60°C for 30 sec. The
relative gene expression level was determined using the
2−ΔΔCq method (22).
GAPDH was used for normalization.
Adipogenic differentiation
hUCB-MSCs from passage 3 and 10 were used for
adipogenic differentiation. Briefly, for the differentiation assay,
passage 3 and 10 of hUCB-MSCs were seeded in a 6-well plate at a
density of 2×104 cells/well and grown to 80% confluence.
Subsequently, the growth medium was substituted for adipogenic
differentiation medium (Cyagen Biosciences, Inc.) containing 1
µmol/l dexamethasone (Sigma-Aldrich; Merch KGaA), 10 µg/ml insulin
(Sigma-Aldrich; Merck KGaA), 200 µmol/l indomethacin
(Sigma-Aldrich; Merck KGaA) and 0.5 mmol/l
3-isobutyl-1-methylxanthine (Sigma-Aldrich; Merck KGaA), the medium
was replaced every 3 days. After 14 days, the cells were washed
with PBS and fixed using 4% formaldehyde for 30 min at room
temperature. Adipogenic differentiation was determined using 0.3%
Oil Red O staining for 30 min at room temperature. All experiments
were performed in triplicate.
Statistical analysis
All experiments were performed in triplicate. All
data were analysed using SPSS 22.0 software (IBM Corp.). Data were
expressed as the mean ± SD. Student's t-tests were performed to
compare differences between groups, whereas the differences among
multiple groups were determined using one-way ANOVA, followed by
post hoc Duncan's tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of the effects of different
media on the culture efficiency of hUCB-MSCs
Using the same isolation procedure, 5 of 16 samples
in the DMEM group obtained a uniform culture of hUCB-MSCs,
indicating a culture success rate of 31.25%, whereas 18 of 22
samples in the MSCM group obtained a uniform culture of hUCB-MSCs,
indicating a culture success rate of 81.81%, which was
significantly higher than the DMEM group (P<0.05). The numbers
of hUCB-MSCs in the MSCM group on the 5, 7 and 14th days were
significantly higher than the DMEM group on the same days
(P<0.05), and the hUCB-MSCs grew rapidly in a whirled manner in
the MSCM group (Table II). In the
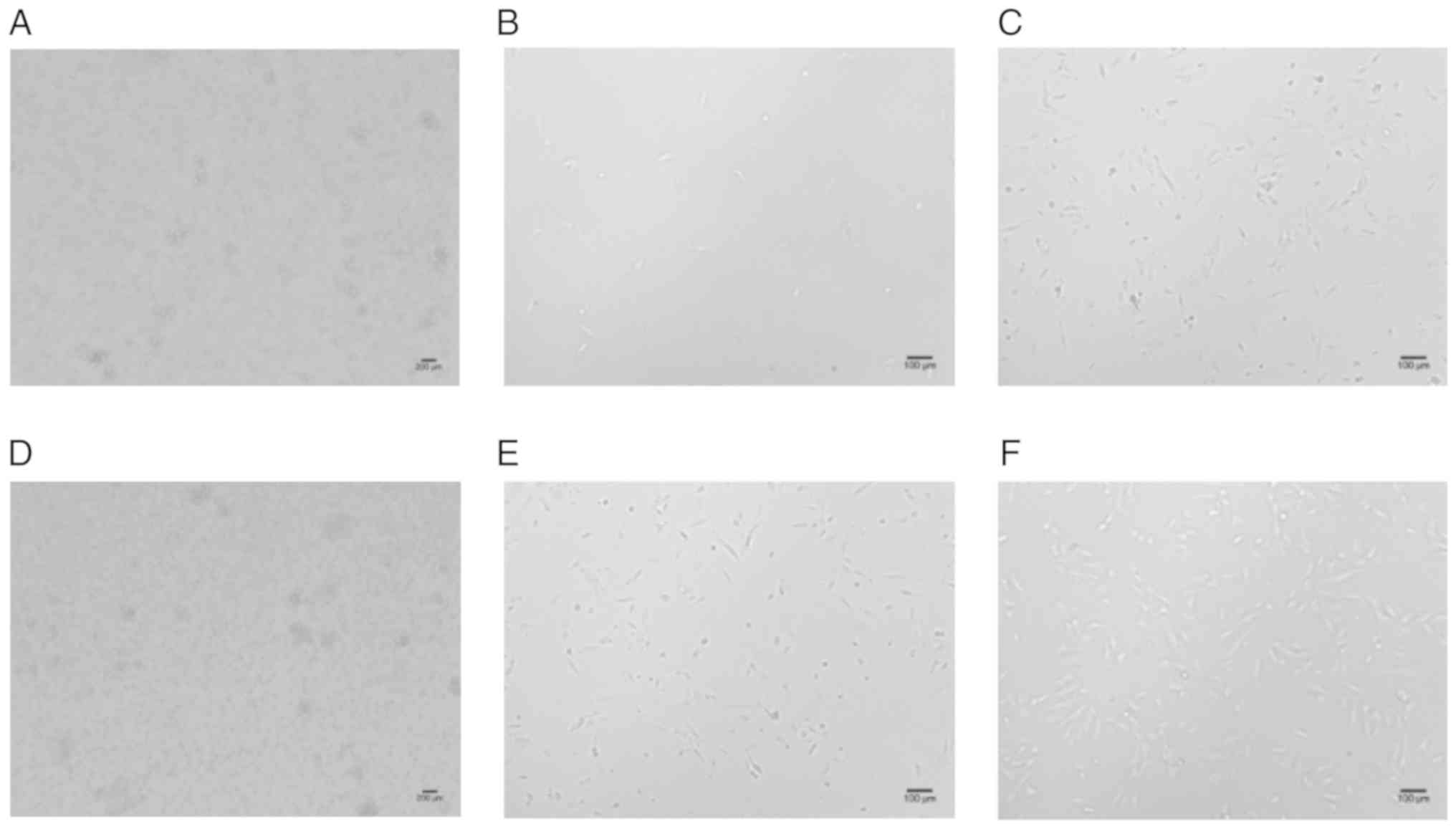
DMEM group, the MNCs were round on the first day (Fig. 1A), after 7 days, the hUCB-MSCs
began to expand (Fig. 1B); the
primary cultured cells reached a confluency of 10–20% on day 14
(Fig. 1C) and 80–90% on day 40. In
the MSCM group, the MNCs were round on the first day (Fig. 1D), then after five days, the
hUCB-MSCs began to expand; the primary cultured cells reached a
confluency of 10–15% on day 7 (Fig.
1E), 30–40% on day 14 (Fig.
1F) and 80–90% on day 22. The cultured cells were passaged
twice to obtain a more uniform culture of hUCB-MSCs at passage 3.
The UCB samples that were not cultured successfully contained a
small numbers of MSCs. In addition, the volume of MSCs in the
unsuccessful cultures was not as large as the volume of
osteoclast-like cells, and a small number of MSCs were mixed with
the osteoclast-like cells and could not expand normally.
Osteoclast-like cells occupied the bottom of the culture flask and
reached 80% confluence after 3–4 weeks; the cells could not be
trypsinized from the base of the flask. After passaging, MSCs in
the cultures initially determined to be unsuccessful could no
longer be cultured, ultimately leading to cell death.
 | Table II.Comparison of cell numbers in the two
types of media during the culture of human umbilical cord
blood-derived mesenchymal stromal cells. |
Table II.
Comparison of cell numbers in the two
types of media during the culture of human umbilical cord
blood-derived mesenchymal stromal cells.
|
| Culturing time
(day) |
|---|
|
|
|
|---|
| Group | 5 | 7 | 14 |
|---|
| MSCM |
6.3±1.5a |
20.4±3.1a |
42.6±5.7a |
| DMEM | 2.1±1.3 | 10.8±2.7 | 19.3±3.4 |
Comparison of different inoculation
densities on the culture efficiency of hUCB-MSCs
DMEM supplemented with mesenchymal stem cell growth
supplement was used to compare the culture efficiency using three
inoculation densities. As shown in Table III, an inoculation density of
1×107 cells/ml (2×106 cells/cm2)
resulted in a shorter extension time and primary culture time than
the other densities tested; the difference was statistically
significant (P<0.05), indicating that this density was good for
the culture of hUCB-MSCs.
 | Table III.Effect of the inoculation densities
of the isolated cells from human umbilical cord blood on the growth
of human umbilical cord blood-derived mesenchymal stromal
cells. |
Table III.
Effect of the inoculation densities
of the isolated cells from human umbilical cord blood on the growth
of human umbilical cord blood-derived mesenchymal stromal
cells.
| Inoculation density
(/ml) | Extension time
(h) | Primary culture
time (day) |
|---|
|
1×106 | 126.3±6.1 | 31.3±3.5 |
|
1×107 |
93.5±5.4a,b |
22.5±2.1a,b |
|
1×108 | 128.6±6.3 |
28.1±3.7a |
Comparison of the first medium changes
on the culture efficiency of hUCB-MSCs
The medium was changed for the first time on the 3,
4, 5 and 7th day after inoculation. Changing the media on the 7th
day did not result in the growth of MSCs in the media removed; only
a small number of MSCs grew in the new culture flask on the 5th
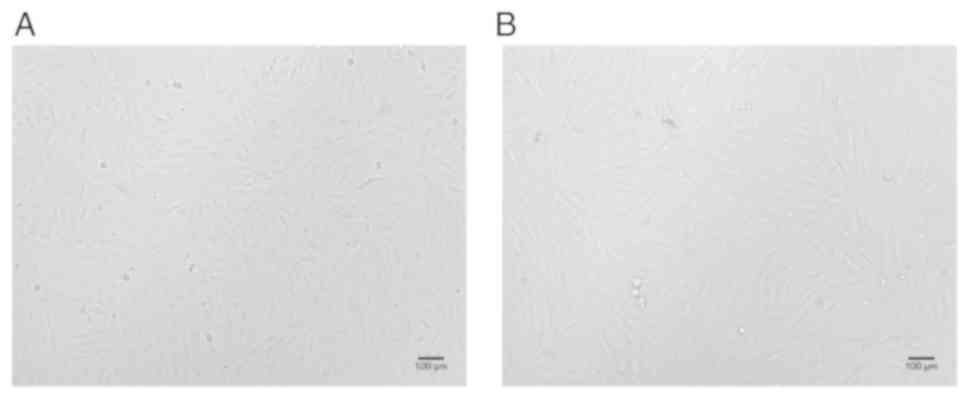
day, and achieving a high cell density was difficult (Fig. 2A). When media was changed on the
3rd or 4th day MSCs could be obtained following long-term culture,
therefore, the first medium change was set at four days after
inoculation (Fig. 2B). Compared
with changing the media on day 5 after inoculation, the
subculturing efficiency was significantly higher at day 4 after
inoculation, with a more uniform MSC population obtained when
subcultured at passage 3 (Fig.
2C).
Constructing the growth curve of
hUCB-MSCs
The cell expansion rate of hUCB-MSCs before passage
3 was slower than that of passage 4 or of later passages. As
hUCB-MSCs were gradually purified, the osteoclast-like cells were
removed, and the doubling rate of each passage became more stable.
Furthermore, to reduce costs, starting with passage 4, conventional
DMEM was also used to subculture hUCB-MSCs in the MSCM group. After
several rounds of passaging, the growth rate of cells was almost
equivalent as in the stem cell media and there were no significant
differences in the morphological characteristics of the cells
(Fig. 3). The growth curves of
hUCB-MSCs showed that the growth latency was 1–3 days. Starting on
the 4th day, cells entered the logarithmic phase of growth and
began to proliferate, the cell density increased rapidly. The
proliferation rate reached a peak on the 10th day and began to
slow, and plateaued. The population doubling time for the entire
hUCB-MSCs population was 68 h. There were no significant
differences in the proliferation rate and growth cycle of hUCB-MSCs
between passages 3 and 10 (Fig.
4).
Analysis of cell surface markers of
hUCB-MSCs
Flow cytometry was used to detect the cell surface
markers of hUCB-MSCs. hUCB-MSCs from passage 3 showed little to no
expression of the hematopoietic marker CD45 (0.06%), however, the
cells did stably express the stem cell markers CD29 (98.81%) and
CD44 (98.41%). hUCB-MSCs stably expressed CD29 (99.29%) and CD44
(98.59%) until passage 10, and did not express CD45 (0.10%;
Fig. 5). The expression levels of
the cell surface markers of the passage 10 cells was almost
equivalent to the passage 3 cells, which is consistent with a
previous study investigating bone marrow derived mesenchymal stem
cells (BM-MSCs) by Nagamura-Inoue and He (15).
Gene expression of Oct4, Sox2 and
Nanog
RT-PCR was used to detect the gene expression levels
of the embryonic stem cell-specific genes Oct4, Sox2 and Nanog in
hUCB-MSCs. It was found that Oct4, Sox2 and Nanog mRNAs were
present in the cells from passage 3 (Fig. 6A) and 10 (Fig. 6B). Moreover, RT-qPCR was used to
analyze the gene expression levels of Oct4, Sox2 and Nanog in
hUCB-MSCs. As shown in Fig. 6C,
the gene expression levels of Oct4, Sox2 and Nanog showed no
significant difference between passage 3 and 10 (P>0.05),
indicating that the hUCB-MSCs had stem cell characteristics.
Adipogenic differentiation
An adipogenic differentiation assay confirmed that
hUCB-MSCs from passage 3 and 10 could undergo adipogenic
differentiation after being exposed to specific induction media.
The formation of lipid droplets was observed under an inverted
microscope following Oil Red O staining (Fig. 7), further indicating that the
hUCB-MSCs had multi-directional differentiation potential.
Discussion
The cellular components of UCB are complex, and the
primary cultures present as heterogeneous cell populations. There
are two main types of adherent cells in UCB: Spindle
fibroblast-like MSCs and round osteoclast-like cells (23). Osteoclast-like cells predominate in
many UCB samples, with only a small number of MSCs found with the
osteoclast-like cells; these MSCs cannot expand normally and
achieve higher confluence, and, therefore, cannot be passaged
(24). Moreover, other factors may
affect the adherence of MSCs. For example, a large number of red
blood cells will occupy the bottom of the culture flask, and as the
cells grow in a mass, this makes it difficult for MSCs to come into
contact with the culture flask (25). Therefore, some interfering factors
during initial cell adherence should be eliminated in order to
promote adherence and facilitate the growth of MSCs.
The medium is an important factor in cultivating
hUCB-MSCs. Most researchers have adopted DMEM (low or high glucose)
supplemented with 5–20% FBS, though the success rate is low
(17,18,26).
Bieback et al (19) used
MesenCult™ medium to improve culture results. MesenCult™ medium is
an acidic medium that includes basal medium and supplements, such
as pre-separated serum and glutamine, and does not require the
addition of other cytokines. As MesenCult™ can promote the
proliferation of hUCB-MSCs and inhibit the growth of other adherent
cells, it is a suitable medium for growing hUCB-MSCs (19). However, this medium is expensive
and MSCs have a long culture period, which creates high costs for
the average laboratory.
In the present study, a sequential culture method
was introduced that uses two types of culture media. First, DMEM
supplemented with mesenchymal stem cell growth supplement was used
to improve the primary culture success rate of hUCB-MSCs. After
removing the heterogeneous cell population, standard DMEM was
adopted from the 4th passage. hUCB-MSCs can be passaged multiple
times and the cell population expands rapidly; these features allow
the biological characteristics of MSCs to be maintained and greatly
reduces the cost of culture. In addition, FBS-coated culture flasks
were used in the present study, which may also have contributed to
cell adherence and growth (27).
The reason for this enhanced adherence and growth may be a result
of FBS covering the surface of heterogeneous antigens in the
culture flasks, thus facilitating the adherence of MSCs and
allowing them to adapt to the environment quicker. The FBS coating
may also provide additional growth and adherence factors, favoring
the growth of MSC-like cells (27).
To provide an efficient and practical method for
isolating hUCB-MSCs, the effects of different inoculation densities
and different timings for first media change on culture efficiency
were investigated. The proportion of MSCs in UCB is small and the
inoculation density is an important factor affecting cell culture.
During the process of primary culture and subculture, hUCB-MSCs
show density dependence (28). If
the cell density is very low, the few cells present are unable to
form the microenvironment required for cell growth, therefore,
osteoclast-like cells become the dominant cell type, and MSCs
gradually undergo cell death due to aging. Moreover, if the cell
density is very high, this may affect the expansion of adherent
cells (29). The present study
showed that the optimal cell inoculation density of the primary
culture was 1×107 cells/ml (2×106
cells/cm2); this was the density at which the expansion
ability of hUCB-MSCs was significantly increased.
The adherence time of hUCB-MSCs is longer than that
of BM-MSCs (30). Therefore, the
appropriate time when the medium is first changed is an important
factor for ensuring a high yield of hUCB-MSCs. If the medium is
changed too early, this may cause unnecessary cell loss. If the
medium is changed too late, the nutrient deficiency in the culture
medium will hinder normal growth. According to the results of the
present study, the optimal time for the first medium change was 4
days after inoculation. This time-point preserved the maximum
number of active cells without affecting cell growth, and allowed a
high yield of hUCB-MSCs to be obtained.
MSCs are a mixed cell population and their
expression of cell surface markers is not uniform (31). The integrin family member CD29 is
considered as an important cell surface marker on MSCs (7). In the present study, flow cytometry
analysis showed that hUCB-MSCs did not express the hematopoietic
precursor cell surface marker CD45 (0.06%), however, they did
stably express CD29 (98.81%) and CD44 (98.41%). Compared with
BM-MSCs, hUCB-MSCs stably expressed CD29 (99.29%) and CD44 (98.59%)
until passage 10, and their morphological characteristics and
proliferative activity did not change significantly, which was
supported by the growth curves of hUCB-MSCs. In addition, mRNA for
Oct4, Sox2 and Nanog was present in the hUCB-MSCs from passage 3
and 10, and the expression levels showed no significant
differences. Oct4, Sox2 and Nanog are embryonic stem cell-specific
genes (32). An adipogenic
differentiation assay showed that hUCB-MSCs from passage 3 and 10
could undergo adipogenic differentiation. The aforementioned
results indicated that compared with BM-MSCs, hUCB-MSCs are more
naïve, have stronger proliferative ability and differentiation
potential, which is consistent with the findings previously
reported by Baksh et al (33). Several MSC populations, for example
BM-MSCs, are being tested in the field of immunotherapy, however,
donor variance, ex vivo expansion, senescence and
immunogenicity are among the main factors influencing the
effectiveness of hUCB-MSCs (2,34),
in order to develop more standardized culture methods and
procedures further studies are required.
In summary, the present study described a sequential
culture method that uses two types of culture media to optimize the
isolation and culture of hUCB-MSCs. This method has a short culture
period and produces a high cell purity with a low economic cost. It
also provides an important experimental basis for the large-scale
cultivation and clinical applications of hUCB-MSCs in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants
from the Fundamental Research Funds for the Central Universities
(grant no. GK201704009), the National Natural Science Foundation of
China (grant no. 81773265) and the Key Research and Development
Plan of Shaanxi Province (grant no. 2018SF-106).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HX and JHZ designed, analyzed the experiments and
wrote the manuscript. JLZ performed the experiment for cultured
cells. QM analyzed some experimental data and co-wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the declaration of Helsinki. The present study was conducted with
approval from the Ethics Committee of Shaanxi Normal University.
Written informed consent was obtained from all participants.
Patient consent for publication
All participants within this study provided consent
for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Squillaro T, Peluso G and Galderisi U:
Clinical trials with mesenchymal stem cells: An update. Cell
Transplant. 25:829–848. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Wolf C, van de Bovenkamp M and
Hoefnagel M: Regulatory perspective on in vitro potency assays for
human mesenchymal stromal cells used in immunotherapy. Cytotherapy.
19:784–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miura Y: Human bone marrow mesenchymal
stromal/stem cells: Current clinical applications and potential for
hematology. Int J Hematol. 103:122–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang HX, Li ZY and Guo ZK and Guo ZK:
Easily-handled method to isolate mesenchymal stem cells from
coagulated human bone marrow samples. World J Stem Cells.
7:1137–1144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Francis SL, Duchi S, Onofrillo C, Di Bella
C and Choong PFM: Adipose-derived mesenchymal stem cells in the use
of cartilage tissue engineering: The need for a rapid isolation
procedure. Stem Cells Int. 2018:89475482018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bharti D, Shivakumar SB, Park JK, Ullah I,
Subbarao RB, Park JS, Lee SL, Park BW and Rho GJ: Comparative
analysis of human Wharton's jelly mesenchymal stem cells derived
from different parts of the same umbilical cord. Cell Tissue Res.
372:51–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pham PV, Vu NB, Pham VM, Truong NH, Pham
TL, Dang LT, Nguyen TT, Bui AN and Phan NK: Good manufacturing
practice-compliant isolation and culture of human umbilical cord
blood-derived mesenchymal stem cells. J Transl Med. 12:562014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossignoli F, Caselli A, Grisendi G,
Piccinno S, Burns JS, Murgia A, Veronesi E, Loschi P, Masini C,
Conte P, et al: Isolation, characterization, and transduction of
endometrial decidual tissue multipotent mesenchymal stromal/stem
cells from menstrual blood. Biomed Res Int. 2013:9018212013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vellasamy S, Sandrasaigaran P, Vidyadaran
S, George E and Ramasamy R: Isolation and characterisation of
mesenchymal stem cells derived from human placenta tissue. World J
Stem Cells. 4:53–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Magatti M, Pianta S, Silini A and Parolini
O: Isolation, culture, and phenotypic characterization of
mesenchymal stromal cells from the amniotic membrane of the human
term placenta. Methods Mol Biol. 1416:233–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perry BC, Zhou D, Wu X, Yang FC, Byers MA,
Chu TM, Hockema JJ, Woods EJ and Goebel WS: Collection,
cryopreservation, and characterization of human dental pulp-derived
mesenchymal stem cells for banking and clinical use. Tissue Eng
Part C Methods. 14:149–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marshall GP II, Laywell ED, Zheng T,
Steindler DA and Scott EW: In vitro-derived ‘neural stem cells’
function as neural progenitors without the capacity for
self-renewal. Stem Cells. 24:731–738. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung DJ, Choi CB, Lee SH, Kang EH, Lee
JH, Hwang SH, Han H, Lee JH, Choe BY, Lee SY, et al:
Intraarterially delivered human umbilical cord blood-derived
mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res.
87:3554–3567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon
SJ, Chang JW, Choi SJ, Oh W, Yang YS, et al: Low immunogenicity of
allogeneic human umbilical cord blood-derived mesenchymal stem
cells in vitro and in vivo. Biochem Biophys Res Commun.
446:983–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagamura-Inoue T and He H: Umbilical
cord-derived mesenchymal stem cells: Their advantages and potential
clinical utility. World J Stem Cells. 6:195–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bieback K and Netsch P: Isolation,
culture, and characterization of human umbilical cord blood-derived
mesenchymal stromal cells. Methods Mol Biol. 1416:245–258. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laitinen A, Lampinen M, Liedtke S,
Kilpinen L, Kerkelä E, Sarkanen JR, Heinonen T, Kogler G and
Laitinen S: The effects of culture conditions on the functionality
of efficiently obtained mesenchymal stromal cells from human cord
blood. Cytotherapy. 18:423–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujii S, Miura Y, Iwasa M, Yoshioka S,
Fujishiro A, Sugino N, Kaneko H, Nakagawa Y, Hirai H, Takaori-Kondo
A, et al: Isolation of mesenchymal stromal/stem cells from
cryopreserved umbilical cord blood cells. J Clin Exp Hematop.
57:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bieback K, Kern S, Klüter H and Eichler H:
Critical parameters for the isolation of mesenchymal stem cells
from umbilical cord blood. Stem Cells. 22:625–634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan X, Liu T, Liu Y, Ma X and Cui Z:
Optimization of primary culture condition for mesenchymal stem
cells derived from umbilical cord blood with factorial design.
Biotechnol Prog. 25:499–507. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Zhang ZG, Chen B, Liu XZ, Liu ZL,
Liu HL, Li G, Su ZG, Wang JF and Hui GZ: Brain-derived neurotrophic
factor induces neuron-like cellular differentiation of mesenchymal
stem cells derived from human umbilical cord blood cells in vitro.
Neural Regen Res. 6:972–977. 2011.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Schauwer C, Meyer E, Cornillie P, De
Vliegher S, van de Walle GR, Hoogewijs M, Declercq H, Govaere J,
Demeyere K, Cornelissen M and Van Soom A: Optimization of the
isolation, culture, and characterization of equine umbilical cord
blood mesenchymal stromal cells. Tissue Eng Part C Methods.
17:1061–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erices A, Conget P and Minguell JJ:
Mesenchymal progenitor cells in human umbilical cord blood. Br J
Haematol. 109:235–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manca MF, Zwart I, Beo J, Palasingham R,
Jen LS, Navarrete R, Girdlestone J and Navarrete CV:
Characterization of mesenchymal stromal cells derived from
full-term umbilical cord blood. Cytotherapy. 10:54–68. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ibrahim AM, Elgharabawi NM, Makhlouf MM
and Ibrahim OY: Chondrogenic differentiation of human umbilical
cord blood-derived mesenchymal stem cells in vitro. Microsc Res
Tech. 78:667–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nielsen H: Isolation and functional
activity of human blood monocytes at adherence to plastic surfaces:
Comparison of different detachment methods. Acta Pathol Microbiol
Immunol Scand C. 95:81–84. 1987.PubMed/NCBI
|
|
28
|
Revencu T. Trifan V, Nacu L, Gutium T,
Globa L, Motoc AG and Nacu V: Collection, isolation and
characterization of the stem cells of umbilical cord blood. Rom J
Morphol Embryol. 54:291–297. 2013.PubMed/NCBI
|
|
29
|
Gang EJ, Hong SH, Jeong JA, Hwang SH, Kim
SW, Yang IH, Ahn C, Han H and Kim H: In vitro mesengenic potential
of human umbilical cord blood-derived mesenchymal stem cells.
Biochem Biophys Res Commun. 321:102–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsieh JY, Fu YS, Chang SJ, Tsuang YH and
Wang HW: Functional module analysis reveals differential osteogenic
and stemness potentials in human mesenchymal stem cells from bone
marrow and Wharton's jelly of umbilical cord. Stem Cells Dev.
19:1895–1910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh W, Kim DS, Yang YS and Lee JK:
Immunological properties of umbilical cord blood-derived
mesenchymal stromal cells. Cell Immunol. 251:116–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing
L, Zhang Y, Ling EA, Gao J and Hao A: Expression profile of
embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human
gliomas. Histopathology. 59:763–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Capasso S, Alessio N, Squillaro T, Di
Bernardo G, Melone MA, Cipollaro M, Peluso G and Galderisi U:
Changes in autophagy, proteasome activity and metabolism to
determine a specific signature for acute and chronic senescent
mesenchymal stromal cells. Oncotarget. 6:39457–39468. 2015.
View Article : Google Scholar : PubMed/NCBI
|