Introduction
Inner medullary collecting duct (IMCD) is the last
part of the collecting duct system, which connects the nephrons to
the ureter (1). The main function
of IMCD is maintenance of body water homeostasis through regulation
of electrolyte and fluid balance by managing the reabsorption and
excretion of sodium ions (Na+) and water along with
hormonal regulation (2–4). However surprisingly, IMCD also plays
a pivotal role in inflammatory responses of the kidney, because it
is a preferred site for Escherichia coli adherence (5). Previous studies have reported that
pro-inflammatory mediators such as inducible nitric oxide synthase
(iNOS), cyclooxygenase-2 (COX-2), and cytokines are produced by
IMCD through various stimuli (6–8).
However, regulation of inflammatory responses of IMCD cells during
renal injury has not been studied in depth.
Berberine (BBR) is an isoquinoline alkaloid and the
major compound of Coptidis rhizoma and Cortex
Phellodendri. Both these plants have been used to treat
gastroenteritis, secretory diarrhea, febrile illness, and
hepatobiliary diseases in Chinese traditional medicine (9,10).
BBR has been reported to have biochemical and pharmacological
effects such as antidiabetic, anticancer, anti-fibrotic,
antibacterial, antioxidant, and anti-inflammatory (11–15).
Recent studies have reported that BBR ameliorates renal conditions
such as podocyte injury, nephrotoxicity, acute hepato-renal
toxicity, and type 2 diabetic nephropathy (9,16–18).
Additionally, BBR inhibits LPS-induced inflammation in rat
glomerular mesangial cells and LPS-induced oxidative stress in rat
kidneys (19,20). However, the beneficial properties
of BBR in LPS-induced inflammatory responses in mouse IMCD-3 cells
have not been reported.
Therefore, we firstly examined the potential effects
of BBR on LPS-treated mIMCD-3 cells. So, we investigated the
production of inflammatory mediators such as iNOS, COX-2, IL-1β,
IL-6, and TNF-α in LPS-exposed IMCD cells. Secondly, we examined
the regulating mechanisms of BBR including NF-κB and MAPKs against
LPS in mIMCD-3 cells.
Materials and methods
Chemicals and reagents
Dulbecco's Modified Eagle's Medium/Ham's Nutrient
Mixture F-12 (DMEM/F-12) (cat. no. 11330-032), fetal bovine serum
(FBS) (cat. no. 26140-079) and antibiotics (cat. no. 15140122) were
obtained from Gibco; Thermo Fisher Scientific, Inc.
N-acetylcysteine (NAC) (cat. no. A7250), Bay 11-7082 (cat. no.
B5556), Parthenolide (cat. no. P0667) BBR (cat. no. B3412; Purity:
99%), LPS from Escherichia coli (cat. no. L2880; serotype
055:B5) and paraformaldehyde (cat. no. 158127) were purchased from
Sigma-Aldrich; Merck KGaA. Tris-HCl (cat. no. #161-0798; #161-0799)
and pre-stained sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) marker (cat. no. 1610395) were purchased
from Bio-Rad Laboratories, Inc. Sodium dodecyl sulfate (cat. no.
18220) was purchased from Affymetrix; Thermo Fisher Scientific,
Inc. Antibodies against iNOS (cat. no. sc-651), COX-2 (cat. No.
sc-17454), Inhibitor kappa B alpha (Iκ-Bα) (cat. no. sc-371), NF-κB
(cat. no. sc-372), β-actin (cat. no. sc-47778), and horseradish
peroxidase-conjugated mouse anti-rabbit IgG (cat. no. sc-2357) and
goat anti-mouse IgG (cat. no. sc-2005) were purchased from Santa
Cruz Biotechnology. Antibodies against pERK (cat. no. #9101), ERK
(cat. no. #9102), pJNK (cat. no. #9251), JNK (cat. no. #9252), pP38
(cat. no. #9211), P38 (cat. no. #9212), and Inhibitory kappa B
alpha (Iκ-Bα) (cat. no. #2859) were purchased from Cell Signaling
Technology, Inc. Easy-blue™ Total RNA extraction kit (cat. no.
17061) was purchased from iNtRON Biotechnology. Enzyme-linked
immunosorbent assay (ELISA) kits for anti-mouse IL-1β (cat. no.
MAB401), IL-6 (cat. no. MAB406) and TNF-α (cat. no. MAB425)
antibodies, and mouse IL-1β (cat. no. BAF401), IL-6 (cat. no.
BAF406) and TNF-α (cat. no. BAF425) biotinylated antibodies were
purchased from R&D Systems. Nuclear extraction kit (cat. no.
2900) was purchased from EMD Millipore. Trans AM NF-κB activation
assay kit (cat. no. 40096) was purchased from Active Motif.
Cell culture
Mouse inner medullary collecting duct (mIMCD-3)
cells, the mouse renal epithelial cell line (cat. no. CRL2123),
were purchased from the American Type Culture Collection (ATCC).
mIMCD-3 cells were routinely cultured in DMEM/F-12 supplemented
with 10% FBS and 1% penicillin/streptomycin, and were maintained in
a humidified chamber containing 5% CO2 at 37°C and were
used in passages 3–5.
Cell viability assay
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay was performed to detect the cytotoxicity of BBR on
mIMCD-3 cells. The cells were seeded in each well of 24-well plates
(5×104 cells/well) and incubated with BBR at a
concentration of 0.05, 0.1, 0.5, 1 or 10 µM for 24 h. Thereafter,
the medium was changed, and cells were incubated with MTT solution
(0.5 mg/ml) for 30 min at 37°C. The medium was removed and the
purple colored precipitate of formazan was dissolved in 200 µl of
dimethyl sulfoxide. Aliquots of the dissolved precipitate were
taken in a 96-well plate, in duplicates, and estimated at 540 nm
using a micro-ELISA plate reader.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNAs were isolated with Easy-Blue™, and the
purity of the samples was confirmed by RNA calculator (Gene Quant
Pro, Biochrom). The total RNA extraction kit was used according to
the manufacturer's instructions and reverse transcription of RNA
(10 µg) to cDNA was performed using the ABI cDNA synthesis kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) (conditions:
37°C for 1 h, followed by 95°C for 5 min). TaqMan quantitative
RT-PCR with an ABI StepOne Plus detection system was performed
according to the manufacturer's instructions (Applied Biosystems;
Thermo Fisher Scientific, Inc.). All qPCR data were normalized to
the expression levels of the housekeeping gene hypoxanthine guanine
phosphoribosyltransferase (HPRT). The thermocycling conditions used
for qPCR were as follows: 50°C for 2 min and 95°C for 10 min,
followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. The
commercial forward, reverse, and probe oligonucleotide primers for
multiplex real-time TaqMan PCR were purchased from ABI (cat. no.
4304437; Applied Biosystems; Thermo Fisher Scientific, Inc.). The
2−ΔΔCq method was used to determine the relative mRNA
expression level (21).
Flow cytometry analysis
mIMCD-3 cells treated with BBR (0.1, 0.5 or 1 µM)
and LPS (5 µg/ml) for 24 h were harvested with cell scraper and
washed with PBS by centrifugation for 5 min. Cells were then
incubated with the blocking solution for 30 min at RT.
Subsequently, cells were incubated with a PE-conjugated anti-mouse
TLR4/MD-2 complex antibody (1:250; cat. no. 117605; BioLegend) for
30 min at 4°C. Data were acquired by BD FACS calibur cell analyser
and analysed in CellQuest Pro software (BD Biosciences; Thermo
Fisher Scientific, Inc.).
Western blotting
The cells were washed with PBS and lysed with lysis
buffer (1% cocktail of protease inhibitor and 1% phosphatase
inhibitor in 1X RIPA Buffer). Protein concentration was determined
by bicinchoninic acid assay. Subsequently, Total cell proteins (20
µg) were then separated by SDS-PAGE on a 10% gel and transferred to
a PVDF membrane (cat. no. 10600023; GE Healthcare Life Sciences).
The membrane was blocked with 5% skim milk in phosphate-buffered
saline (PBS) with Tween-20 (PBST) for 2 h at room temperature (RT)
and washed PBST for 10 min, three times. Then, incubated with
primary antibody (1:1,000) at 4°C overnight: iNOS, COX-2, Ik-Ba,
b-actin, pERK, ERK, pJNK, JNK, pP38 and P38. After washing three
times with PBST, each blot was incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG or donkey
anti-goat IgG secondary antibody for 1 h at RT. The proteins were
visualized using an enhanced chemiluminescence detection system
(cat. no. RPN2232; Amersham; GE Healthcare). Captures protein bands
and quantitative analysis were performed using Quantity
One® software version 4.6.6 (Bio-Rad Laboratories,
Inc.).
ELISA
ELISAs for IL-1β, IL-6, and TNF-α were carried out
in duplicates in 96-well plates coated with anti-mouse IL-1β, IL-6,
and TNF-α monoclonal antibodies in PBS (pH 7.4) at 4°C overnight.
The plates were washed with PBST and blocked with PBS containing
10% FBS for 2 h at RT. After two times washes, standards and
samples were added and incubated for 2 h at RT, and then the wells
were washed and biotinylated anti-mouse IL-1β, IL-6, or TNF-α were
added and incubated for 1 h at RT. The wells were washed,
avidin-peroxidase was added, and plates were incubated for 30 min
at RT. The wells washing again and followed by the addition of TMB
substrate. Color development was measured at 450 nm using an
automated microplate ELISA reader. Standard samples were run on
each assay plate by using serial dilutions of recombinant IL-1β,
IL-6, and TNF-α.
Immunocytochemistry
mIMCD-3 cells were plated in a chamber slide and
incubated with LPS (5 µg/ml) for 30 min at 37°C. The cells were
treated with BBR (1 µM) for 1 h before LPS treatment. The cells
were fixed in 4% paraformaldehyde for 15 min at RT and washed 3
times with PBS. The cells were treated with 0.1% TritonX-100 for 15
min at RT. After washing, non-specific binding sites were blocked
with serum (3% BSA) for 1 h at RT, and incubated with NF-κB
antibody (1:250) at 4°C overnight. The cells were then washed and
incubated with AlexaFluor®568 goat anti-rabbit IgG
(1:2,000; cat. no. A-11011) for NF-κB antibody for 2 h at RT in a
darkened room. For nuclear staining, the cells were incubated with
DAPI (cat. no. H-1200; Vector Laboratories) at 5 µg/ml for 5 min at
RT. The slide was finally washed and mounted for microscopic
examination. Stained sections were visualized using a confocal
laser microscope (Olympus).
Nuclear extraction
mIMCD-3 cells were plated in 100-mm dishes
(1×107 cells/dish). Then, LPS (5 µg/ml) treated for 0,
15, 30 and 60 min with or without pretreatment of BBR (1 µM) for 1
h. Nuclear extraction assay performed according to the
manufacturer's instructions. Briefly, cells were harvested with
cell scraper and washed with PBS by centrifugation at 250 × g for 5
min. Add 1X Cytoplasmic Lysis Buffer (containing DTT and Protease
inhibitor cocktail) to the pellet and incubation on ice for 15 min.
Centrifuge at 250 × g for 5 min, discard the supernatant and add 1X
Cytoplasmic Lysis Buffer. Five times drawing and ejecting by
27-gauge syringe, then centrifuge at 8,000 × g for 20 min. Discard
supernatant, and add Nuclear Extraction Buffer (containing DTT and
Protease inhibitor cocktail). Five times drawing and ejecting by
27-gauge syringe, then gently agitate on rotator at 4°C for 60 min.
Centrifuge at 16,000 × g for 5 min, then supernatant was used for
experiments.
NF-κB p65 activation assay
NF-κB activation was determined in the nuclear
extracts of mIMCD-3 cells, following the manufacturer's
instructions. Briefly, 15 µg nuclear extract was added to a
biotinylated oligonucleotide containing the NF-κB consensus site
attached to the streptavidin-coated 96-well plates with agitation
on rocking platform at 100 rpm for 1 h. Plates were washed with
wash buffer to remove all the unbound reagents. NF-κB p65 primary
antibody (1:1,000) was added for 1 h at room temperature without
agitation, followed by a goat anti-rabbit secondary antibody
conjugated with horseradish peroxidase (1:1,000) at room
temperature without agitation. Subsequent to an incubation for 1 h
at room temperature, 100 µl developing solution was added to all
wells for 3 min at room temperature protected from direct light.
The blue color development in the sample wells was monitored until
it turned medium to dark blue. Subsequently, 100 µl stop solution
was added and the blue color turned yellow. Finally, the absorbance
value was ascertained using a spectrophotometer at a wavelength of
450 nm. As a positive control for NF-κB p65 activation, nuclear
extracts from Jurkat cells, provided with the kit, were used.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean (SEM). Statistical significance of intergroup
differences was evaluated using two-way ANOVA, with time and dose
as variables, followed by a Duncan's post-hoc test. All statistical
analyses were performed using SPSS. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were carried out in triplicates.
Results
Effects of BBR on production of iNOS
and COX-2
Before studying the biological activity of BBR, we
performed the MTT assay to evaluate the cytotoxicity of BBR in
mIMCD-3 cells. As shown in Fig.
1A, up to a dose of 1 µM, BBR did not affect cell viability.
Thus, we chose BBR concentrations of 0.1, 0.5 and 1 µM for further
experiments.
 | Figure 1.Cytotoxicity of BBR and inhibitory
activity of BBR on iNOS and COX-2 production in mIMCD-3 cells. (A)
mIMCD-3 cells were incubated with BBR (0.05, 0.1, 0.5, 1 or 10 µM)
for 24 h. Subsequently, an MTT assay was performed to evaluate
cytotoxicity of BBR. mIMCD-3 cells were incubated with BBR (0.1,
0.5 or 1 µM) for 1 h. Subsequently, cells were exposed to LPS (5
µg/ml) for 3 h (iNOS) and 1 h (COX-2). mRNA levels of (B) iNOS and
(D) COX-2 were evaluated by reverse transcription-PCR. Protein
levels of (C) iNOS and (E) COX-2 were measured by western blotting.
Results are presented as the mean ± SEM of at least three
independent experiments. *P<0.05 vs. control; †P<0.05 vs. LPS
alone. BBR, berberine; iNOS, inducible nitric oxide synthase;
COX-2, cyclooxygenase-2; mIMCD-3, mouse IMCD-3; LPS,
lipopolysaccharide. |
To determine the pro-inflammatory effects of BBR
against LPS in mIMCD-3 cells, production of pro-inflammatory
molecules such as iNOS and COX-2 after LPS stimulation in mIMCD-3
cells was investigated. In accordance with our previous report
(2), production of iNOS and COX-2
was elevated in LPS-exposed mIMCD-3 cells. However, the elevation
of iNOS and COX-2 was significantly inhibited by BBR in these cells
(Fig. 1B-E).
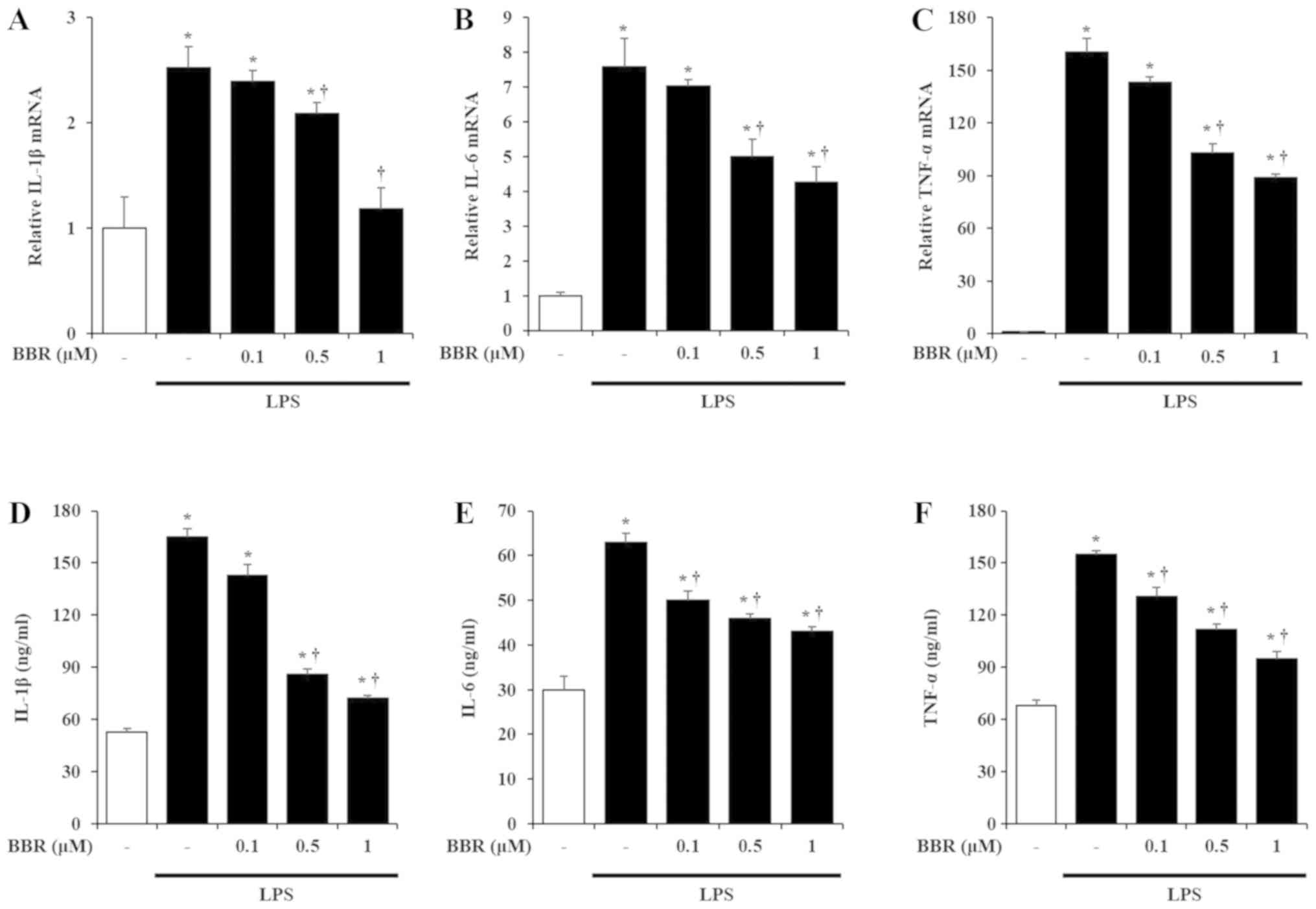
Effects of BBR on production of
pro-inflammatory cytokines
IL-1β, IL-6, and TNF-α are the representative
pro-inflammatory cytokines known to be induced by LPS treatment in
mIMCD-3 cells (2). Thus, to
measure the effects of BBR on the production of these
pro-inflammatory cytokines in LPS-exposed mIMCD-3 cells, we
investigated the mRNA and protein expression of IL-1β, IL-6, and
TNF-α after LPS stimulation. As reported earlier (2), LPS treatment increased the mRNA and
protein levels of IL-1β, IL-6, and TNF-α in mIMCD-3 cells. However,
BBR treatment inhibited the induction of IL-1β, IL-6, and TNF-α
mRNA and protein levels in a dose-dependent manner (Fig. 2).
Effects of BBR on activation of NF-κB
and MAPKs
Since BBR was effective in inhibiting
pro-inflammatory mediators in LPS-treated mIMCD-3 cells, we sought
to investigate the mechanistic details of the beneficial activity
of BBR. Firstly, to rule out the possibility that BBR directly
regulates LPS or TLR4, we examined the effect of BBR on TLR4/MD2
complex expression. The methods of Limulus amebocyte lysate (LAL)
assay and RT-qPCR are described in Data S1. As shown in Fig. 3A and Fig. S1, BBR did not directly affect LPS
and TLR4 expression in mIMCD-3 cells, which means BBR regulates the
down-streams upon TLR4-LPS interaction. Thus, we ought to examine
the activation of NF-κB and MAPKs, because activation of both
signaling pathways produce pro-einflammatory mediators (22). As shown in Fig. 3B-E, LPS stimulation triggered Iκ-Bα
degradation, NF-κB p65 translocation from cytosol to nucleus,
elevation of NF-κB p65 binding activity, and MAPK phosphorylation.
However, treatment with BBR inhibited Iκ-Bα degradation, NF-κB p65
translocation and NF-κB p65 binding activity elevation but not MAPK
phosphorylation.
 | Figure 3.Inhibitory effects of BBR on the
NF-κB and MAPK pathways in mIMCD-3 cells. (A) mIMCD-3 cells were
pre-treated with BBR (1 µM) 1 h before LPS (5 µg/ml) treatment. The
expression of TLR4/MD-2 complex was detected after 24 h LPS
treatment by flow cytometry. mIMCD-3 cells were incubated with BBR
(1 µM) for 1 h. Thereafter, mIMCD-3 cells were harvested after LPS
(5 µg/ml) stimulation for 0, 15, 30 or 60 min. Western blotting was
performed to demonstrate that BBR could inhibit (B) Iκ-Bα
degradation and (E) MAPK phosphorylation in mIMCD-3 cells. (C)
Immunofluorescence staining was performed to evaluate BBR could
inhibit NF-κB translocation from cytosol to nucleus in mIMCD-3
cells. NF-κB p65 was stained with AlexaFluor 568 (red) and Nucleus
was stained with DAPI (blue) in mIMCD-3 cells. (D) Binding activity
of NF-κB p65 from mIMCD-3 cells was measured using the Trans AM
NF-κB p65 assay kit. *P<0.05 vs. saline + LPS 0 min; †P<0.05.
Figure shows a representative image from three independent
experiments. Scale bar, 10 µm. BBR, berberine; mIMCD-3, mouse
IMCD-3; LPS, lipopolysaccharide; Iκ-Bα, inhibitory κ-Bα; NF-κB,
nuclear factor-κB; TLR4, toll-like receptor 4; NS, not
significant. |
Effects of NF-κB on production of
inflammatory mediators
To evaluate whether NF-κB deactivation could
contribute to amelioration of inflammatory responses in LPS-treated
mIMCD-3 cells, we used three well known NF-κB inhibitors (NAC, Bay
11-7082, and Parthenolide). As is known, three inhibitors could
block NF-κB p65 translocation from nucleus to cytosol against LPS
treatment in mIMCD-3 cells (Fig.
S2). Next, we hypothesized that NF-κB inhibitors might
downregulate the production of pro-inflammatory mediators, similar
to BBR. As expected, NF-κB inhibitors treatment reduced the mRNA
levels of iNOS, COX-2, IL-1β, IL-6, and TNF-α after LPS treatment
in mIMCD-3 cells to a similar degree as in the cells treated with
BBR (Fig. 4).
 | Figure 4.Reduction of pro-inflammatory
molecules and cytokine expression by inhibition of the NF-κB
pathway in mIMCD-3 cells. mIMCD-3 cells were incubated with 10 mM
NAC, 5 5 µM Bay, and 10 µM Par, which is an NF-κB inhibitor/or BBR
(1 µM) for 1 h. Thereafter, cells were exposed to LPS (5 µg/ml) for
3 h (iNOS), 1 h (COX-2, IL-1β and TNF-α), and 24 h (IL-6). mRNA
levels of (A) iNOS, (B) COX-2, (C) IL-1β, (D) IL-6 and (E) TNF-α
were evaluated by reverse transcription-PCR. Results are expressed
as the mean ± SEM of at least three independent experiments.
*P<0.05 vs. control; †P<0.05 vs. LPS alone. NF-κB, nuclear
factor-κB; mIMCD-3, mouse IMCD-3; NAC, N-acetylcysteine; Bay,
Bay11-7082; Par, Parthenolide; BBR, berberine; LPS,
lipopolysaccharide; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase-2; IL, interleukin; TNF-α, tumor necrosis
factor-α. |
Discussion
In this study, we revealed that BBR inhibited
LPS-mediated inflammatory responses in mIMCD-3 cells. LPS
stimulation induced the expression of pro-inflammatory mediators
(iNOS and COX-2) and pro-inflammatory cytokines (IL-1β, IL-6, and
TNF-α) in mIMCD-3 cells. However, BBR treatment could inhibit these
inflammatory mediators and cytokines dramatically. In addition, the
mechanism underlying the inhibitory effects of BBR upon LPS
stimulation involved the inhibition of NF-κB activation. Our
results show that BBR suppresses LPS-mediated inflammation via
NF-κB deactivation in mIMCD-3 cells.
Since BBR is so popular with variety biochemical and
pharmacological effects and cheap, there are already many
ready-to-use drugs associated with BBR. Today, BBR has been
developed to dietary supplement, and BBR was approved Unique
ingredient identifier (UNII) by FDA (UNII: 0I8Y3P32UF). Based on
this information, we can easily examine the beneficial potential of
BBR in both non-clinical and clinical experiments. In non-clinical
experiments, Pietra et al (13) reported that BBR reduce fibrosis and
inflammatory cytokine in human dermal fibroblasts in vitro.
It was also reported that BBR could protect ulcerative colitis and
neuropathic pain in mice (23,24).
In addition, many researches have focused on the beneficial effects
of BBR in renal diseases. It has been reported that BBR could
ameliorate cisplatin-induced nephrotoxicity in mice, that might be
through the reduction of histopathological damage, renal functional
markers, oxidative stress, cell death signals, and NF-κB activation
(25). In addition, BBR have shown
to protective and beneficial activities in various renal diseases
such as diabetic nephropathy and renal dysfunction (26). Based on many researches about
beneficial effects of BBR on renal injury, BBR has been applied to
clinical trials in human renal diseases, ands has been reported to
alleviate the renal injury in type 2 diabetes mellitus (27–29).
In addition to previous reports, our results suggest that BBR could
be applied in urinary tract infection (UTI), such as cystitis and
pyelonephritis. The most common cause of UTI is Escherichia
coli (30), and infection
spreads from the bladder to the kidneys and collecting systems
(31). Therefore, we suggest that
BBR inhibits LPS-infected renal inflammation, especially in
UTI.
Collecting duct system of the kidney is a renal
tubular segment and plays a role in the reabsorption of filtered
water (~15%). A key feature of the collecting duct is water
reabsorption through hormonal regulation in an autocrine/paracrine
manner (32–40). For instance, vasopressin, also
called as antidiuretic hormone (ADH), affects osmotic water
permeability and increases water reabsorption in IMCD cells
(35). Hence, most reports of IMCD
have been in the context of regulation of water homeostasis such as
osmotic water, urea, and electrolyte permeability (35,41–44).
However, because many renal diseases are accompanied by
inflammatory mediator production as well as water imbalance
(45), it is very important to
identify the underlying patho-physiology of inflammatory responses
in renal diseases. Thus, regulation of inflammatory mediators could
be key in the treatment of renal diseases. Through this study, we
propose BBR as a beneficial agent in managing renal
inflammation.
Toll-like receptor 4 (TLR4), a transmembrane
receptor, belongs to the super-family of pattern recognition
receptors (PRRs). Generally, a well-known function of TLR4 is
recognition of exogenous molecules from pathogens-associated
molecular pattern molecules (PAMPs) and/or damage-associated
molecular pattern molecules (DAMPs), which is released upon
cellular or tissue damage. TLR4 existed in many cells such as
endothelial cells, myocytes, thyroid cells, endometrial cells,
mesangial cells, and adipocytes (46–51).
We and other researchers identified the expression of TLR4 in IMCD
cells (2,5,8).
When TLR4 ligand such as LPS binds to TLR4, pro-inflammatory
mediators such as iNOS, COX-2, IL-1β, IL-6, and TNF-α are
up-regulated (5). As these
inflammatory mediators released from collecting duct cells could
trigger renal injury and inflammation, inhibition of inflammatory
mediators in collecting duct cells might be important (5). In this study, BBR treatment inhibited
TLR4-mediated elevation of inflammatory mediators such as iNOS,
COX-2, IL-1β, IL-6, and TNF-α in LPS-treated mIMCD-3 cells, which
suggests that BBR attenuated local inflammation in collecting duct
cells (Figs. 1 and 2).
In this study, we examined whether activation of
NF-κB could regulates the inflammatory mediators. NF-κB belongs to
a family of dimeric transcription factors and participates in
numerous biological procedures, including immune responses,
inflammation, cellular differentiation, proliferation, and survival
(52–55). NF-κB activation, followed by LPS
treatment, results in induction of cytokines (IL-1, IL-6, and
TNF-α) and inflammatory enzymes (iNOS and COX-2) (56–58).
Thus, negative regulation of NF-κB might be responsible for
inhibition of inflammatory mediators. Our findings showed that BBR
treatment inhibited the degradation of Iκ-Bα and translocation of
NF-κB into the nucleus after LPS treatment, suggesting that BBR
inhibited the activation of NF-κB (Fig. 3). In addition, to examine whether
NF-κB activation after LPS leads to production of inflammatory
mediators in mIMCD-3 cells, we investigated the production of
inflammatory mediators after NF-κB inhibition by 3 well known
inhibitor such as NAC (59,60),
Bay 11-7082 (61,62), and Parthenolide (62). As expected, the inhibitors showed
the inhibitory effects on NF-κB activation in our mIMCD-3 models
(Fig. S2). The inhibition of
NF-κB by NAC, Bay 11-7082, and Parthenolide significantly inhibited
the production of inflammatory mediators (Fig. 4), which suggest that BBR has
anti-inflammatory effects mainly through NF-κB deactivation in
LPS-exposed mIMCD-3 cells. Some previous researches have reported
that BBR inhibits inflammatory mediators and NF-κB signaling in
various different disease models (19,63–67).
However, there is no report that BBR could have anti-inflammatory
and protective effects in LPS-exposed inner medullary collecting
duct (IMCD) cells. In this study, we firstly reported that BBR has
protective effects upon LPS-induced inflammation by downregulation
of NF-κB pathway in mouse IMCD-3 cells.
In summary, we firstly revealed that the
anti-inflammatory effects of BBR via deactivation of NF-κB in
LPS-stimulated mIMCD-3 cells. Our results suggest that BBR might be
a potential and beneficial drug in renal injury. However, the
current data are not sufficient to explain the beneficial effects
of BBR in in vivo renal injury systems. Therefore, further
studies in this area would be needed.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by The
National Research Foundation of Korea grant funded by the Korea
government (grant no. NRF-2017R1A5A2015805).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DGK, JWC and IJJ designed the study, conducted the
data analysis and prepared the manuscript. MJK assisted with the
experiments. HSL, HJS and SHH analyzed the data and prepared the
manuscript. HJS revised the manuscript for intellectual and
scientific content. GSB and SJP designed the study and prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tchapyjnikov D, Li Y, Pisitkun T, Hoffert
JD, Yu MJ and Knepper MA: Proteomic profiling of nuclei from native
renal inner medullary collecting duct cells using LC-MS/MS. Physiol
Genomics. 40:167–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim DG, Bae GS, Jo IJ, Choi SB, Kim MJ,
Jeong JH, Kang DG, Lee HS, Song HJ and Park SJ: Guggulsterone
attenuated lipopolysaccharide-induced inflammatory responses in
mouse inner medullary collecting Duct-3 cells. Inflammation.
39:87–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Welch AK, Jeanette Lynch I, Gumz ML, Cain
BD and Wingo CS: Aldosterone alters the chromatin structure of the
murine endothelin-1 gene. Life Sci. 159:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohaupt MG, Schwöbel J, Elzie JL, Kannan
GS and Kone BC: Cytokines activate inducible nitric oxide synthase
gene transcription in inner medullary collecting duct cells. Am J
Physiol. 268:F770–F777. 1995.PubMed/NCBI
|
|
5
|
Chassin C, Vimont S, Cluzeaud F, Bens M,
Goujon JM, Fernandez B, Hertig A, Rondeau E, Arlet G, Hornef MW and
Vandewalle A: TLR4 facilitates translocation of bacteria across
renal collecting duct cells. J Am Soc Nephrol. 19:2364–2374. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chassin C, Goujon JM, Darche S, du Merle
L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, Le Bouguénec C,
Buzoni-Gatel D and Vandewalle A: Renal collecting duct epithelial
cells react to pyelonephritis-associated escherichia coli by
activating distinct TLR4-dependent and -independent inflammatory
pathways. J Immunol. 177:4773–4784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi JY, Nam SA, Jin DC, Kim J and Cha JH:
Expression and cellular localization of inducible nitric oxide
synthase in lipopolysaccharide-treated rat kidneys. J Histochem
Cytochem. 60:301–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Küper C, Beck FX and Neuhofer W: Toll-like
receptor 4 activates NF-κB and MAP kinase pathways to regulate
expression of proinflammatory COX-2 in renal medullary collecting
duct cells. Am J Physiol Renal Physiol. 302:F38–F46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun SF, Zhao TT, Zhang HJ, Huang XR, Zhang
WK, Zhang L, Yan MH, Dong X, Wang H, Wen YM, et al: Renoprotective
effect of berberine on type 2 diabetic nephropathy in rats. Clin
Exp Pharmacol Physiol. 42:662–670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Linn YC, Lu J, Lim LC, Sun H, Sun J, Zhou
Y and Ng HS: Berberine-induced haemolysis revisited: Safety of
Rhizoma coptidis and cortex phellodendri in chronic haematological
diseases. Phytother Res. 26:682–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong Y, Chen YT, Yang YX, Zhou XJ, Dai SJ,
Tong JF, Shou D and Li C: Metabolomics study of type 2 diabetes
mellitus and the antidiabetic effect of berberine in zucker
diabetic fatty rats using Uplc-ESI-Hdms. Phytother Res. 30:823–828.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Peng Y, Liu Y, Yang J, Ding N and
Tan W: Berberine, a natural compound, suppresses Hedgehog signaling
pathway activity and cancer growth. BMC Cancer. 15:5952015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pietra D, Borghini A and Bianucci AM: In
vitro studies of antifibrotic and cytoprotective effects elicited
by proto-berberine alkaloids in human dermal fibroblasts. Pharmacol
Rep. 67:1081–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng L, Kang S, Yin Z, Jia R, Song X, Li
L, Li Z, Zou Y, Liang X, Li L, et al: Antibacterial activity and
mechanism of berberine against Streptococcus agalactiae. Int J Clin
Exp Pathol. 8:5217–5223. 2015.PubMed/NCBI
|
|
15
|
Kwon OJ, Kim MY, Shin SH, Lee AR, Lee JY,
Seo BI, Shin MR, Choi HG, Kim JA, Min BS, et al: Antioxidant and
anti-inflammatory effects of Rhei rhizoma and coptidis rhizoma
mixture on reflux esophagitis in rats. Evid Based Complement Altern
Med. 2016:20521802016. View Article : Google Scholar
|
|
16
|
Wang B, Xu X, He X, Wang Z and Yang M:
Berberine improved aldo-induced podocyte injury via inhibiting
oxidative stress and endoplasmic reticulum stress pathways both in
vivo and in vitro. Cell Physiol Biochem. 39:217–228. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adil M, Kandhare AD, Dalvi G, Ghosh P,
Venkata S, Raygude KS and Bodhankar SL: Ameliorative effect of
berberine against gentamicin-induced nephrotoxicity in rats via
attenuation of oxidative stress, inflammation, apoptosis and
mitochondrial dysfunction. Ren Fail. 38:996–1006. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Zhang Y, Zhu Z, Liu H, Guo H,
Xiong C, Xie K, Zhang X and Su S: Protective effect of berberine on
doxorubicin-induced acute hepatorenal toxicity in rats. Mol Med
Rep. 13:3953–3960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan
T, Xu S, Peng J, Xie X and Huang H: Berberine attenuates
lipopolysaccharide-induced extracelluar matrix accumulation and
inflammation in rat mesangial cells: Involvement of NF-κB signaling
pathway. Mol Cell Endocrinol. 331:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yokozawa T, Ishida A, Kashiwada Y, Cho EJ,
Kim HY and Ikeshiro Y: Coptidis Rhizoma: Protective effects against
peroxynitrite-induced oxidative damage and elucidation of its
active components. J Pharm Pharmacol. 56:547–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pålsson-McDermott EM and O'Neill LA:
Signal transduction by the lipopolysaccharide receptor, Toll-like
receptor-4. Immunology. 113:153–162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu L, Gu P and Shen H: Protective effects
of berberine hydrochloride on DSS-induced ulcerative colitis in
rats. Int Immunopharmacol. 68:242–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rezaee R, Monemi A, SadeghiBonjar MA and
Hashemzaei M: Berberine alleviates paclitaxel-induced neuropathy. J
Pharmacopuncture. 22:90–94. 2019.PubMed/NCBI
|
|
25
|
Domitrović R, Cvijanović O, Pernjak-Pugel
E, Skoda M, Mikelić L and Crnčević-Orlić Ž: Berberine exerts
nephroprotective effect against cisplatin-induced kidney damage
through inhibition of oxidative/nitrosative stress, inflammation,
autophagy and apoptosis. Food Chem Toxicol. 62:397–406. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu L, Han J, Yuan R, Xue L and Pang W:
Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB
pathway. Biol Res. 51:92018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li ZY, Liu B, Zhuang XJ, Shen YD, Tian HR,
Ji Y, Li LX and Liu F: Effects of berberine on the serum cystatin C
levels and urine albumin/creatine ratio in patients with type 2
diabetes mellitus. Zhonghua Yi Xue Za Zhi. 98:3756–3761. 2018.(In
Chinese). PubMed/NCBI
|
|
28
|
Hu Y, Ehli EA, Kittelsrud J, Ronan PJ,
Munger K, Downey T, Bohlen K, Callahan L, Munson V, Jahnke M, et
al: Lipid-lowering effect of berberine in human subjects and rats.
Phytomedicine. 19:861–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin J, Xing H and Ye J: Efficacy of
berberine in patients with type 2 diabetes mellitus. Metabolism.
57:712–717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flores-Mireles AL, Walker JN, Caparon M
and Hultgren SJ: Urinary tract infections: Epidemiology, mechanisms
of infection and treatment options. Nat Rev Microbiol. 13:269–284.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stone SC, Mallon WK, Childs JM and
Docherty SD: Emphysematous pyelonephritis: Clues to rapid diagnosis
in the Emergency Department. J Emerg Med. 28:315–319. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JE, Jung HJ, Lee YJ and Kwon TH:
Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney
collecting duct cells. Am J Physiol Renal Physiol. 308:F749–F764.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao M, Cao R, Du S, Jia X, Zheng S, Huang
S, Han Q, Liu J, Zhang X, Miao Y, et al: Disruption of
prostaglandin E2 receptor EP4 impairs urinary concentration via
decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad
Sci USA. 112:8397–8402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kishore BK, Nelson RD, Miller RL, Carlson
NG and Kohan DE: P2Y(2) receptors and water transport in the
kidney. Purinergic Signal. 5:491–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li YX, Huang Y, Liu S, Mao Y, Yuan CY,
Yang X and Yao LJ: Glycogen synthase kinase-3 modulates
hyperosmotic-induced urea transporter A1 relocation in the inner
medullary collecting duct cells. Nephron. 133:71–79. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hyndman KA, Dugas C, Arguello AM,
Goodchild TT, Buckley KM, Burch M, Yanagisawa M and Pollock JS:
High salt induces autocrine actions of ET-1 on inner medullary
collecting duct NO production via upregulated ETB receptor
expression. Am J Physiol Regul Integr Comp Physiol. 311:R263–R271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pandit MM, Gao Y, van Hoek A and Kohan DE:
Osmolar regulation of endothelin-1 production by the inner
medullary collecting duct. Life Sci. 159:135–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fuson AL, Komlosi P, Unlap TM, Bell PD and
Peti-Peterdi J: Immunolocalization of a microsomal prostaglandin E
synthase in rabbit kidney. Am J Physiol Renal Physiol.
285:F558–F564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gueutin V, Vallet M, Jayat M, Peti-Peterdi
J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D and Chambrey
R: Renal β-intercalated cells maintain body fluid and electrolyte
balance. J Clin Invest. 123:4219–4231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huo TL, Grenader A, Blandina P and Healy
DP: Prostaglandin E2 production in rat IMCD cells. II. Possible
role for locally formed dopamine. Am J Physiol. 261:F655–F662.
1991.PubMed/NCBI
|
|
41
|
Chen M, Cai H, Klein JD, Laur O and Chen
G: Dexamethasone increases aquaporin-2 protein expression in ex
vivo inner medullary collecting duct suspensions. Front Physiol.
6:3102015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ranieri M, Tamma G, Di Mise A, Russo A,
Centrone M, Svelto M, Calamita G and Valenti G: Negative feedback
from CaSR signaling to aquaporin-2 sensitizes vasopressin to
extracellular Ca2. J Cell Sci. 128:2350–2360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng P, Lin Y, Wang F, Luo R, Zhang T, Hu
S, Feng P, Liang X, Li C and Wang W: 4-PBA improves lithium-induced
nephrogenic diabetes insipidus by attenuating ER stress. Am J
Physiol Renal Physiol. 311:F763–F776. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hyndman KA, Boesen EI, Elmarakby AA,
Brands MW, Huang P, Kohan DE, Pollock DM and Pollock JS: Renal
collecting duct NOS1 maintains fluid-electrolyte homeostasis and
blood pressure. Hypertension. 62:91–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Anders HJ and Muruve DA: The inflammasomes
in kidney disease. J Am Soc Nephrol. 22:1007–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hijiya N, Miyake K, Akashi S, Matsuura K,
Higuchi Y and Yamamoto S: Possible involvement of toll-like
receptor 4 in endothelial cell activation of larger vessels in
response to lipopolysaccharide. Pathobiology. 70:18–25. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tsukumo DM, Carvalho-Filho MA, Carvalheira
JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassalo J, Curi
R, Velloso LA and Saad MJ: Loss-of-function mutation in toll-like
receptor 4 prevents diet-induced obesity and insulin resistance.
Diabetes. 56:1986–1998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nicola JP, Vélez ML, Lucero AM, Fozzatti
L, Pellizas CG and Masini-Repiso AM: Functional toll-like receptor
4 conferring lipopolysaccharide responsiveness is expressed in
thyroid cells. Endocrinology. 150:500–5008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hirata T, Osuga Y, Hirota Y, Koga K,
Yoshino O, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O and
Taketani Y: Evidence for the presence of toll-like receptor 4
system in the human endometrium. J Clin Endocrinol Metab.
90:548–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wolf G, Bohlender J, Bondeva T, Roger T,
Thaiss F and Wenzel UO: Angiotensin II upregulates toll-like
receptor 4 on mesangial cells. J Am Soc Nephrol. 17:1585–1593.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vitseva OI, Tanriverdi K, Tchkonia TT,
Kirkland JL, McDonnell ME, Apovian CM, Freedman J and Gokce N:
Inducible toll-like receptor and NF-kappaB regulatory pathway
expression in human adipose tissue. Obesity (Silver Spring).
16:932–937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: Remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Caamaño J and Hunter CA: NF-kappaB family
of transcription factors: Central regulators of innate and adaptive
immune functions. Clin Microbiol Rev. 15:414–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
May MJ and Ghosh S: Signal transduction
through NF-kappa B. Immunol Today. 19:80–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Oka S, Kamata H, Kamata K, Yagisawa H and
Hirata H: N-acetylcysteine suppresses TNF-induced NF-kappaB
activation through inhibition of IkappaB kinases. FEBS Lett.
472:196–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gupta SC, Sundaram C, Reuter S and
Aggarwal BB: Inhibiting NF-κB activation by small molecules as a
therapeutic strategy. Biochim Biophys Acta. 1799:775–787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Strickson S, Campbell DG, Emmerich CH,
Knebel A, Plater L, Ritorto MS, Shpiro N and Cohen P: The
anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent
signalling network by targeting the ubiquitin system. Biochem J.
451:427–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ghashghaeinia M, Toulany M, Saki M,
Bobbala D, Fehrenbacher B, Rupec R, Rodemann HP, Ghoreschi K,
Röcken M, Schaller M, et al: The NFĸB pathway inhibitors bay
11-7082 and parthenolide induce programmed cell death in anucleated
erythrocytes. Cell Physiol Biochem. 27:45–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao C, Wang Y, Yuan X, Sun G, Shen B, Xu
F, Fan G, Jin M, Li X and Liu G: Berberine inhibits
lipopolysaccharide-induced expression of inflammatory cytokines by
suppressing TLR4-mediated NF-ĸB and MAPK signaling pathways in
rumen epithelial cells of Holstein calves. J Dairy Res. 86:171–176.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang H, Shan Y, Wu Y, Xu C, Yu X, Zhao J,
Yan J and Shang W: Berberine suppresses LPS-induced inflammation
through modulating Sirt1/NF-κB signaling pathway in RAW264.7 cells.
Int Immunopharmacol. 52:93–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gao MY, Chen L, Yang L, Yu X, Kou JP and
Yu BY: Berberine inhibits LPS-induced TF procoagulant activity and
expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells.
Pharmacol Rep. 66:480–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang X, Feng S, Ding N, He Y, Li C, Li M,
Ding X, Ding H, Li J, Wu J and Li Y: Anti-inflammatory effects of
berberine hydrochloride in an LPS-induced murine model of mastitis.
Evid Based Complement Alternat Med. 2018:51643142018.PubMed/NCBI
|
|
67
|
Lee IA, Hyun YJ and Kim DH: Berberine
ameliorates TNBS-induced colitis by inhibiting lipid peroxidation,
enterobacterial growth and NF-κB activation. Eur J Pharmacol.
648:162–170. 2010. View Article : Google Scholar : PubMed/NCBI
|