Introduction
Endothelial progenitor cells (EPCs) play a pivotal
role in neoangiogenesis and mediate recovery and repair of damaged
endothelium (1–4). A meta-analysis of cell-based
therapies in patients with refractory angina found improvements in
cardiovascular outcome (5).
Different surface markers can be used to subcategorize the
heterogeneous EPC family, two of the most commonly employed being
CD34 and CD133 (6–9). Recently, intramyocardial
transplantation of CD133+ EPCs was shown to improve
heart function after severe myocardial infarction (10,11).
We identified a novel CD34−CD133+ EPC
subpopulation, which gives rise to more mature
CD34+CD133+ EPCs, and more potently mediates
homing and vascular reparation than the latter EPC subset (6,12–14).
Subsequently, numbers of CD34−CD133+ EPCs
were found to be decreased in patients with vascular disease and
diabetes in contrast to numbers of CD34+ EPCs (15), which might indicate a clinical
relevance of the former EPC subset.
Hematopoietic precursors have been found to have the
potential of transdifferentiation into non-hematopoietic cells, i.
e. hepatocytes (16), neurons
(17), and cardiomyocytes
(18). Interestingly, following
heart transplantation a proportion of cardiomyocytes from donor
organs originated from non-cardiac sources (19), which may include the bone marrow
(20). It has previously been
shown that EPCs can be transdifferentiated in vitro into
functionally active cardiomyocytes when co-cultivated with rat
cardiomyocytes (21), a process
depending on E-cadherin (22).
In our present study we sought to analyze
CD34−CD133+ and
CD34+CD133+ EPC subpopulations for their
differentiation potential into non-endothelial cell-types, which
might have important implications for regenerative therapeutic
purposes.
Materials and methods
Purification of EPC subpopulations
from human peripheral blood
The local ethics committee (Ethik-Kommission der
Ärztekammer des Saarlandes) approved all investigations (compliance
no. 122/09), which were carried out as specified by institutional
guidelines. All volunteers provided written informed consent.
Peripheral blood mononuclear cells (PBMNCs) were obtained from
peripheral blood sampled from healthy volunteers (n=7, age 32±1.7
years, 5 male, 2 female) followed by separation into
CD34−CD133+ and
CD34+CD133+ EPCs as previously described
(6,9,23,24).
A detailed description of the two EPC separation methods used
(MACS® Cell separation and EasySep® Cell
separation) can be found in the supplementary material.
MACS® cell separation
This separating system is based on microbeads, which
are superparamagnetic particles. Magnetically labelled cells are
separated over a column placed in a specific separator. Cells are
then retained on the column, while unlabeled cells pass through.
These cells are then collected as the unlabeled fraction and the
retained cells are eluted from the MACS Column after removal from
the magnet. For magnetically labelling cells were initially
suspended in 300 µl MACS buffer (PBS, pH 7.2 with 0.5% BSA and 2 mM
EDTA) per 108 cells. Then 100 µl FcR block reagent was added in
order to avoid non-specific and Fc-receptor mediated binding of the
antibody, as well as 100 µl CD34 MicroBeads. The cell suspension
was then mixed well and incubated for 30 min at 4°C. After ten
minutes washing in 15 ml MACS buffer, the cell pellet was suspended
in 500 µl MACS buffer per 108 cells. Two columns were
then prepared by being flushed with buffer. Then, cell suspension
was added to one of the columns, which had been previously placed
in the MACS separator. The CD34+ cells were retained by
the magnet in the column. The column was then rinsed with buffer 3
times and then placed on the second prepared column. To increase
purity three rinse steps were repeated once more, and finally
CD34+ cells get transferred in a new tube. In order to
these cells once again magnetically, it was necessary to remove the
microbeads from the cells. For this, 20 µl MACS multisort release
reagent were added to the cell suspension and incubated for 10 min.
Using a pre-rinsed MS column the remaining magnetically labelled
cells were removed. After another washing, the cell pellet was
taken up in 50 µl MACS buffer per 107 cells. Afterwards
CD34+ and CD34− cells were labelled with
CD133-microBeads as described above.
EasySep® cell
separation
This separating system is based on Tetrameric
Antibody Complexes (TAC) recognizing specific cell surface antigens
and dextran-coated magnetic particles for selection or depletion of
cells. Labelled cells are linked to magnetic particles and are
separated using a special magnet. For positive cell isolation,
cells of interest are labelled and remain in the tube in the
magnet, while unlabeled cells are poured off. For negative cell
isolation, unwanted cells are labelled for depletion while cells of
interest are poured off into a new tube, and are untouched. The
subpopulations were isolated using the EasySep® system
(StemCell Technologies) in two steps. Mononuclear cells were at
first separated into CD34-positive and CD34-negative cells. For
this, 2×108 cells were resuspended in 1 ml of a special
buffer (PBS with 2% FBS and 1 mM EDTA) and incubated at room
temperature for 15 min with 100 µl EasySep®
CD34-positive selection cocktail. Then, 50 µl magnetic
nanoparticles were added and incubated for 10 min. Then, buffer was
added and the tube was placed in the EasySep® magnet.
After 15 min, the supernatant was poured off and the tube was
filled again with buffer. This procedure was repeated a total of
four times. The remaining cells in the tube (CD34+) population were
suspended in 500 µl of PBS. The supernatant (CD34-) was further
separated into CD133-positive and-negative cell populations. They
were incubated for 15 min with 100 µl human FcR-blocking Reagent
and 100 µl PE-conjugated antibody at room temperature, then for 15
min with PE EasySep® Selection cocktail and for another
10 min with 50 µl EasySep® Magnetic Nanoparticles. The
procedure of the first separation was repeated and the supernatant
consisted of CD34−CD133− cells and in the
tube remained CD34−CD133+ cells.
EPC culture
Growth capacity was tested using the commercially
available cell media endothelial basal medium (EBM), DMEM, RPMI
(Roswell Park Memorial Institute), and StemSpan® (H3000,
StemCell Technologies).
Migration and adhesion assays
We measured the migratory and adhesive capacities of
EPC-subsets using modified Boyden chambers, fibronectin-(Sigma)
coated plates, and a parallel-plate, laminar-flow chamber
(Immunetics, Cambridge, MA, USA) as previously described (6,25).
Differentiation assays
Differentiation capacity of EPC-subpopulations
(CD34+CD133+,
CD34−CD133+,
CD34−CD133−) into fibroblasts, hepatocytes,
cardiomyocytes, endothelial cells, and neuroblasts was analyzed by
stimulation with the indicated growth factors followed by specific
staining. The indicated cell-types (1×105) were cultured
in 100 µl DMEM in 96-well plates, and then stimulated with
fibroblast growth factor (FGF, 1 ng/ml) for fibroblasts, hepatocyte
growth factor (HGF, 30 ng/ml) for hepatocytes, Cardiogenol C (100
nM) for cardiomyocytes, beta-nerve growth factor (β-NGF, 2 ng/ml)
for neuroblasts, and vascular endothelial growth factor (VEGF, 4
ng/ml) for endothelial cells. Differentiation was assessed by cell
morphology and immunohistochemistry. For the latter, the following
monoclonal antibodies were used: Anti-FGF-2/basic-FGF for
fibroblasts (clone bFM-2, Millipore, Billerica, USA),
anti-α-Fetoprotein (AFP) for hepatocytes (clone AFP-11, R&D
Systems, Minneapolis, USA), anti-α-sarcomeric actin (clone 5C5,
Sigma Aldrich, Steinheim) for cardiomyocytes, anti-CD31 PE (clone
WM59, BD, Heidelberg) for endothelial cells, and anti-neuron
specific enolase (NSE, clone EPR3377, Abcam, Cambridge, UK) for
neuronal cells. Specificity of the antibodies was previously tested
in positive control experiments. For this purpose, murine
fibroblasts (LMTK, LGC Standards GmbH), human neuroblastoma cells
(SH-SY5Y (ATCC® CRL-2266™), LGC Standards GmbH), rat
cardiomyoblasts (H9c2, LGC Standards GmbH), human endothelial cells
(HUVECs), and human liver-carcinoma cells (HuH7) were used.
Authentification of SH-SY5Y (ATCC® CRL-2266™) was
performed using short tandem repeat (STR)-profiling with a
commercially available PCR amplification kit (Applied Biosystems).
Culture media were employed according to manufacturer's instruction
(LMTK and H9c2: DMEM, 10% FCS, 1% PenStrep; SHSY5Y: DMEM, 10% FCS,
1% PenStrep, 1% MEM; HUVEC: ECGM; HuH7: RPMI, 10% FCS, 1% PenStrep,
5 µl/ml Ciprobay, 1% L-glutamine).
Statistical analysis
The SigmaStat program was used or all statistical
analyses in the present study. Mean ± standard error of the mean
(SEM) of the data was calculated for statistical analysis.
Following this, the Kolmogorov-Smirnov test was used to analyze for
normal distribution and compared with the one-way ANOVA test, which
was also used for comparisons of categorical variables, and the
Bonferroni post hoc test was used. The null hypothesis was rejected
at P<0.05 as it was considered to indicate a statistically
significant difference.
Results
Growth capacity of PBMNCs in different
media
First, growth capacity of PBMNCs was analyzed in
different media in order to determine optimized culture conditions
for subsequent expansion. After four days in culture with DMEM or
RPMI, PBMNCs did not differentiate into any endothelial-specific
cell-types such as EPCs as defined by double-positivity in the
DiLDL/Lectin staining (Fig. 1A).
However, PBMNCs expanded significantly into EPCs when using
endothelial-specific media such as EBM or, to a lower extent, in
stem cell-specific media such as StemSpan®. In contrast
to EBM, RPMI, and StemSpan®, cells expanded
significantly superior (DAPI-positivity, Fig. 1B) and had a higher proliferation
rate as assessed by Ki67-staining when cultured in DMEM (Fig. S1A and B). Therefore, DMEM
supplemented with specific growth factors was used for subsequent
differentiation experiments.
Identification and characterization of
EPCs
Formation of vascular tubes was measured by matrigel
assay (BD Biosciences) as instructed by the manufacturer. After
four days in culture, the growth morphology of EPC colonies was
characterized by the formation of spindles and clusters. Closed
network units were quantified by counting in four adjacent wells.
CD34−CD133+ EPCs formed significantly more
CFUs per well than CD34+CD133+ cells
(Fig. S2A). EPCs were then
defined by binding of FITC-labeled Ulex europaeus-lectin and
the uptake of DiI-Ac-LDL (Fig.
S2B). Double-positivity of EPCs (yellow-merged images) was
analyzed by immunofluorescence staining and characterized most of
the EPCs. These results confirm our data from previous studies
(6,9).
Cell separation of EPC
subpopulations
Next we compared MACS® Cell separation
vs. EasySep® Cell separation, by analyzing the purity of
obtained cells in terms of the distinct EPC-subpopulations
(CD34+CD133+ and
CD34−CD133+). Both methods show a nearly
equal purity for cell separation of CD34+ cells
(Fig. 2). In contrast to this,
purity of CD133+ cells was significantly higher using
EasySep® (P<0.001; Fig.
2, right panel). Among these cells,
CD34−CD133+ EPCs were reliably gained to a
higher extent using the EasySep® Cell separation method
when compared with the MACS® Cell separation method
(P<0.001; Fig. 2).
Further characterization of EPC
subpopulations
FACS analysis of the endothelial markers CD31
(PECAM) and VEGFR-2 revealed them to be present on both
CD34−CD133+ and
CD34+CD133+ cells, but to a significantly
higher extent (P<0.001) on CD34+CD133+
cells (Fig. S3). The monocytic
surface marker CD14 was significantly higher expressed on
CD34+CD133+ cells (P=0.002; Fig. S4), whereas the lymphocytic markers
In contrast, CD3 expression was significantly higher on
CD34−CD133+ cells (P<0.001; Fig. S4).
Proliferation capacity of EPC
subpopulations
Staining of EPC subpopulations with the
proliferation marker Ki67 was significantly higher in cell cultures
originating from CD34−CD133+ EPCs compared to
cell cultures originating from CD34+CD133+
(P<0.005; Fig. S5).
Apoptosis and Necrosis in EPC
subpopulations
The apoptosis marker Annexin-V was detectable to
nearly the same extent in both subpopulations (P=0.469; Fig. S6A). In contrast, the necrosis
marker Propidium-Iodide was detectable in a significantly higher
amount (P=0.005) on CD34+CD133+ cells
compared to CD34−CD133+ cells (Fig. S6B and C). Representative FACS
plots of CD34−CD133+ and
CD34+CD133+ cells as well as of apoptosis
(lower right section) and necrosis (upper right section) in
CD34+CD133+ cells are presented in Fig. S6C and D, respectively.
Migration capacity of EPC subsets
Assays of SDF-1-triggered migration of EPC subsets
revealed than CD34−CD133+ EPCs display a
higher migratory capacity than CD34+CD133+
EPCs (P<0.001, Fig. 3A;
Fig. S7, top panel).
Adhesion capacity of EPC subsets
Then, adhesion assays of EPC subsets were performed
under static and dynamic conditions. Under static conditions,
CD34−CD133+ EPCs cells display higher levels
of SDF-1-triggered static adhesion as compared with
CD34+CD133+ EPCs (P<0.027; Fig. S7, bottom panel). These findings
could be verified under dynamic adhesion conditions using a flow
chamber (Fig. 3B).
Ex vivo differentiation capacity of
EPC subpopulations
Compared to CD34+CD133+ cells,
the CD34−CD133+ progenitor subset showed a
significantly higher differentiation capacity into unrelated
cell-types such as fibroblasts, hepatocytes, cardiomyocytes, and
neurocytes. Prior to these experiments, positive controls for the
specific staining of each cell type were performed (Fig. S8).
Fibroblast cell-type
Differentiation of CD34−CD133+
EPCs (30%) into a fibroblast cell-type could be detected after two
days in culture in contrast to CD34+CD133+
cells (2%) (P<0.001, Fig. 4A;
Fig. S9). After four days, 71% of
CD34−CD133+ cells were positive for the
fibroblast-marker FGF in comparison to only 20% of the
CD34+CD133+ cells (Fig. S9). After eight days in culture,
CD34−CD133+ cells were significantly more
positive than CD34+CD133+ cells, but after
twelve days both subpopulations were nearly completely
differentiated into fibroblast-like cells (95% vs. 97%; Fig. 4A).
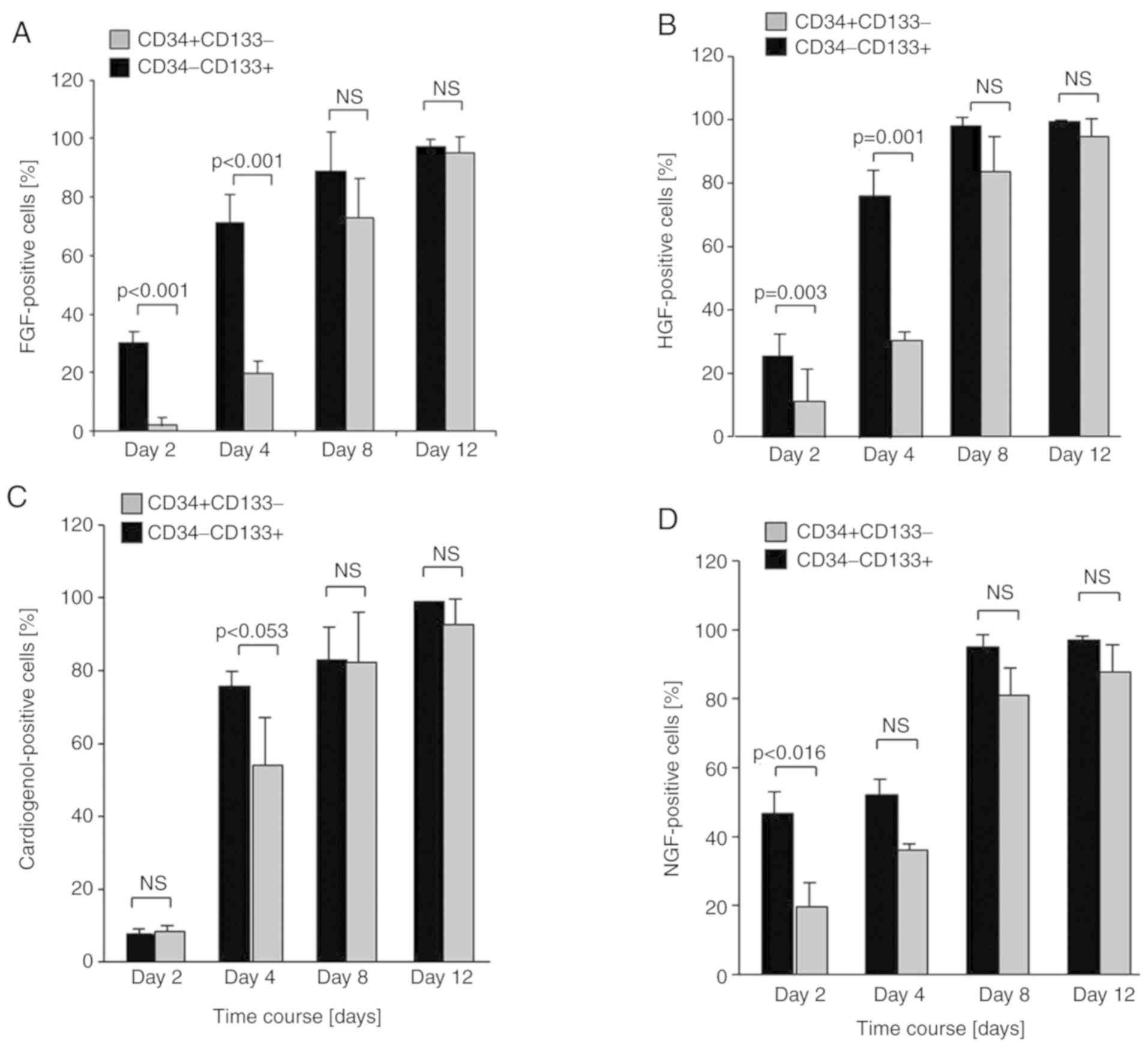 | Figure 4.Ex vivo differentiation
capacity of CD34−CD133+ (black panel) and
CD34+CD133+ (grey panel) EPCs into different
cell-types. (A) Fibroblast cell-type: After 2 days of culture, 30%
of CD34−CD133+ cells stained positive for the
fibroblast-marker FGF compared to CD34+CD133+
cells with 2%. After 4 days, 71% of
CD34−CD133+ cells were FGF-positive in
comparison to only 20% of the CD34+CD133+
cells. After 8 days in culture, CD34−CD133+
cells were significantly more FGF-positive than
CD34+CD133+ cells, but after 12 days both
subpopulations were nearly completely differentiated into
fibroblast-like cells (95% compared with 97%). (B) Hepatocyte
cell-type: After two days of culture, 26% of
CD34−CD133+ differentiated compared to
CD34+CD133+ with 10%. After 4 days, the
disparity between both cell types increased (77% compared with 26%;
P=0.001). In the following differentiation period into HGF-positive
cells up to 12 days, no significant difference could be detected
between the two EPC subsets (P=0.204 and P=0.442). (C)
Cardiomyocyte cell-type: Differentiation took place after 4 days
and showed a trend towards a difference between
CD34−CD133+ and
CD34+CD133+ cells, but to no significance
(P=0.053). In the subsequent 8 days differentiation into
cardiomyocyte-like cells was equal in both subpopulations ending up
in 93 and 99% (ns; P=0.193), respectively. (D) Neural cell-type:
After 2 days in culture, CD34−CD133+ cells
were significantly more positive for NGF-staining in contrast to
CD34+CD133+ cells (P=0.016). The difference
between the EPC subsets was not traceable anymore in the following
culture period up to day 12, when up to 95–97% of the cells were
neurocyte-like in both subpopulations. EPC, endothelial progenitor
cell; FGF, fibroblast growth factor; HGF, hepatocyte growth factor;
NGF, nerve growth factor; ns, not significant. |
Hepatocyte cell-type
Differentiation capacity into hepatic-like cells was
significantly increased in CD34−CD133+ EPCs
in contrast to CD34+CD133+ cells after two
days being in culture (P=0.003, Fig.
4B; Fig. S9). After four
days, the disparity between both cell types increased (77% vs. 26%,
P=0.001, Fig. 4B; Fig. S9). In the following
differentiation period into HGF-positive cells, no significant
difference could be determined (P=0.204 and P=0.442).
Cardiomyocyte cell-type
Differentiation into a cardiomyocyte cell-type could
be detected after four days and showed a non-significant difference
between CD34−CD133+ and
CD34+CD133+ cells (P=0.053, Fig. 4C; Fig. S9). In the subsequent eight days,
differentiation into cardiomyocyte-like cells was equal in both
subpopulations ending up in 93 and 99% (P=0.193; Fig. 4C).
Neural cell-type
After two days in culture with β-NGF,
CD34−CD133+ cells were significantly more
positive for NGF-staining in contrast to
CD34+CD133+ cells (P=0.016, Fig. 4D; Fig. S9). The difference was not
traceable anymore in the following four to twelve days, when
finally 95–97% of the cells were neurocyte-like in both
subpopulations (Fig. 4D).
Endothelial cell-type
Growth into endothelial cells was significantly
higher for CD34−CD133+ cells after four days
of culture (P=0.080; Fig. S10),
and highly significant after eight days (P<0.001; Fig. S10). After twelve days, both
subpopulations, CD34−CD133+ and
CD34+CD133+ cells, were positive for VEGF in
97–99%. Additionally, endothelial staining using DiLDL/Lectin
revealed a higher amount of double-positivity in
CD34−CD133+ EPCs compared to
CD34+CD133+ cells (Fig. S2).
Discussion
To our knowledge, our data is the first to indicate
that CD34−CD133+ and
CD34+CD133+ EPCs can transdifferentiate into
other cell-types such as hepatocytes, fibroblasts, and neuronal
cells under specific selection pressure. Moreover, our data present
novel evidence that the CD34−CD133+ EPC
subpopulations is characterized by a significantly faster
differentiation capacity than CD34+CD133+
EPCs. This might be important to rapidly tailor differentiation of
CD34−CD133+ EPC according to the local need
of the injured tissue. In a recent study (26), co-culturing EPCs with
VEGF-secreting mesenchymal stem cells (MSCs) enhanced endothelial
marker expression (CD31, von Willebrand factor), which points to a
VEGF-mediated role for MSCs in EPC differentiation. Clearly, more
studies are needed to further discriminate the complex network of
regulators of EPC differentiation including paracrine factors as
well as stem and progenitor cells.
In mice with hind limb ischemia, co-administration
of endothelial colony-forming cells (ECFCs) with MSCs significantly
increased vessel density and foot perfusion by a CD105
(endoglin)-dependant mechanism (27). Interestingly, endoglin was found to
play a critical role in integrin-mediated adhesion of mural cells
to endothelial cells in mice (28). We found that
CD34−CD133+ EPCs display significantly higher
adhesion capacity to TNFα-activated HUVECs and to
fibroncetin-coated surfaces in response to SDF-1 than
CD34+CD133+ EPCs. The cell-cell adhesion
activity of CD34−CD133+ EPCs and
CD34+CD133+ EPCs towards pericytes/vascular
smooth muscle cells as well as the role of endoglin in that respect
will have to be analyzed in future studies.
The EPC family consists of diverse members who are
characterized by different functional potential and have been
implicated in several pathologies. In that respect,
CD133+ from the bone marrow (BM) were shown to contain
more VEGFR+ cells, a higher distribution of primitive
progenitors, and a higher proliferation activity than the
CD34+ BM population or the corresponding mobilized
peripheral blood cells (29). Our
findings shed more light onto these data by showing that
CD133+ cells consist of CD34+ and
CD34− subopulations, which are characterized by specific
adhesive, migrative, and transdifferative potentials. In patients
with congestive heart failure, numbers of CD34+,
CD133+, and CD34+CD133+ cells in
peripheral blood are regulated differently (30). In endarterectomized tissue from
patients with chronic thromboembolic pulmonary hypertension, a
putative EPC subpopulation of CD34+CD133+
fetal liver kinase-1+ (flk-1) cells could be identified
(31). In patients 6 months after
coronary stent implantation, subpopulations of
CD34+CD133+, CD34+ human
VEGFR+, and CD34+CD133+ human
VEGFR+ EPCs inversely correlated with plaque volume and
plaque area (32). In patients
with acute ischemic stroke, elevated inflammatory parameter levels
negatively correlated with circulating
CD133+VEGR2+ EPCs (33). These studies as well as our data
underscore the ongoing debate on the exact definition of EPCs,
which is even more complicated by the diversity of markers used and
the regulation of EPCs in health and disease.
Moreover, genetic factors may be of importance in
EPC regulation as a recent study reported increases of
CD34+ and CD34+VEGFR2+ EPCs and
diverse subsets in individuals with at least one specific
polymorphism allele of KLOTHO KL-VS in comparison with individuals
with wild-type alleles (34).
Besides this, obese teenagers were shown to have higher levels of
CD34− EPCs than CD34+ EPCs which correlated
with elevations of systolic blood pressure, hsCRP, HbA1c, and lower
HDL levels (35). In conclusion,
in overweight adolescents CD34− EPCs may serve as
markers for vascular injury and may point to increased
cardiovascular risk.
In summary, immature
CD34−CD133+ EPCs are characterized by a
particularly high capacity of differentiating into various kinds of
non-endothelial cell types under specific selection pressure and
according to the actual need. The significant higher capacity of
proliferation of CD34−CD133+ EPCs may make it
possible to gain higher numbers of EPCs, thus possibly increasing
the potential for therapy with EPCs.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank technician Miss
Claudia Schormann (Clinic of Internal Medicine III, Cardiology,
Angiology and Intensive Care, University of the Saarland) for her
excellent work.
Funding
The present study was supported by The Deutsche
Forschungsgemeinschaft (KFO 196) and from The University of The
Saarland (HOMFOR grant).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CB performed the experiments. KB analysed and
interpreted the data. EF supervised the study. KB and EF designed
the current study and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The local ethics committee (Ethik-Kommission der
Ärztekammer des Saarlandes) approved all investigations (compliance
no. 122/09), which were carried out as specified by institutional
guidelines. All volunteers provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chopra H, Hung MK, Kwong DL, Zhang CF and
Pow EHN: Insights into endothelial progenitor cells: Origin,
classification, potentials, and prospects. Stem Cells Int.
2018:98470152018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salybekov AA, Kawaguchi AT, Masuda H,
Vorateera K, Okada C and Asahara T: Regeneration-associated cells
improve recovery from myocardial infarction through enhanced
vasculogenesis, anti-inflammation, and cardiomyogenesis. PLoS One.
13:e02032442018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mudyanadzo TA: Endothelial progenitor
cells and cardiovascular correlates. Cureus.
10:e33422018.PubMed/NCBI
|
|
4
|
Silvestre JS, Mallat Z, Tedgui A and Lévy
BI: Post-ischaemic neovascularization and inflammation. Cardiovasc
Res. 78:242–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones DA, Weeraman D, Colicchia M, Hussain
MA, Veerapen D, Andiapen M, Rathod KS, Baumbach A and Mathur A:
Impact of cell therapy on cardiovascular outcomes in patients with
refractory angina. Circ Res. 124:1786–1795. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedrich EB, Walenta K, Scharlau J,
Nickenig G and Werner N: CD34-/CD133+/VEGFR-2+ endothelial
progenitor cell subpopulation with potent vasoregenerative
capacities. Circ Res. 98:e20–e25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hristov M, Zernecke A, Liehn EA and Weber
C: Regulation of endothelial progenitor cell homing after arterial
injury. Thromb Haemost. 98:274–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walenta K, Friedrich EB, Sehnert F, Werner
N and Nickenig G: In vitro differentiation characteristics of
cultured human mononuclear cells-implications for endothelial
progenitor cell biology. Biochem Biophys Res Commun. 333:476–482.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steinhoff G, Nesteruk J, Wolfien M, Kundt
G; PERFECT Trial Investigators Group, ; Börgermann J, David R,
Garbade J, Große J, Haverich A, et al: Cardiac function improvement
and bone marrow response -: Outcome analysis of the randomized
PERFECT phase III clinical trial of intramyocardial CD133+
application after myocardial infarction. EBioMedicine. 22:208–224.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Correa A, Ottoboni GS, Senegaglia AC,
Capriglione LGA, Miyague NI, Leite LMB, Jamur VR, Rebelatto CLK,
Olandoski M and Brofman PRS: Expanded CD133+ cells from human
umbilical cord blood improved heart function in rats after severe
myocardial infarction. Stem Cells Int. 2018:54124782018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Werner N and Nickenig G: Clinical and
therapeutical implications of EPC biology in atherosclerosis. J
Cell Mol Med. 10:318–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walenta KL, Bettink S, Böhm M and
Friedrich EB: Differential chemokine receptor expression regulates
functional specialization of endothelial progenitor cell
subpopulations. Basic Res Cardiol. 106:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaiser R, Friedrich D, Chavakis E, Böhm M
and Friedrich EB: Effect of hypoxia on integrin-mediated adhesion
of endothelial progenitor cells. J Cell Mol Med. 16:2387–2393.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung C, Fischer N, Fritzenwanger M, Thude
H, Ferrari M, Fabris M, Brehm BR, Barz D and Figulla HR:
Endothelial progenitor cells in adolescents: Impact of overweight,
age, smoking, sport and cytokines in younger age. Clin Res Cardiol.
98:179–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lagasse E, Connors H, Al-Dhalimy M,
Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL and
Grompe M: Purified hematopoietic stem cells can differentiate into
hepatocytes in vivo. Nat Med. 6:1229–1234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brazelton TR, Rossi FM, Keshet GI and Blau
HM: From marrow to brain: Expression of neuronal phenotypes in
adult mice. Science. 290:1775–1779. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Müller P, Pfeiffer P, Koglin J, Schäfers
HJ, Seeland U, Janzen I, Urbschat S and Böhm M: Cardiomyocytes of
noncardiac origin in myocardial biopsies of human transplanted
hearts. Circulation. 106:31–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deb A, Wang S, Skelding KA, Miller D,
Simper D and Caplice NM: Bone marrow-derived c.ardiomyocytes are
present in adult human heart: A study of gender-mismatched bone
marrow transplantation patients. Circulation. 107:1247–1249. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Badorff C, Brandes RP, Popp R, Rupp S,
Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM and Dimmeler S:
Transdifferentiation of blood-derived human adult endothelial
progenitor cells into functionally active cardiomyocytes.
Circulation. 107:1024–1032. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koyanagi M, Urbich C, Chavakis E, Hoffmann
J, Rupp S, Badorff C, Zeiher AM, Starzinski-Powitz A, Haendeler J
and Dimmeler S: Differentiation of circulating endothelial
progenitor cells to a cardiomyogenic phenotype depends on
E-cadherin. FEBS Lett. 579:6060–6066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Friedrich EB, Werner C, Walenta K, Böhm M
and Scheller B: Role of extracellular signal-regulated kinase for
endothelial progenitor cell dysfunction in coronary artery disease.
Basic Res Cardiol. 104:613–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Werner C, Böhm M and Friedrich EB: Role of
integrin-linked kinase for functional capacity of endothelial
progenitor cells in patients with stable coronary artery disease.
Biochem Biophys Res Commun. 377:331–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedrich EB, Tager AM, Liu E, Pettersson
A, Owman C, Munn L, Luster AD and Gerszten RE: Mechanisms of
leukotriene B4-triggered monocyte adhesion. Arterioscler Thromb
Vasc Biol. 23:1761–1767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge Q, Zhang H, Hou J, Wan L, Cheng W, Wang
X, Dong D, Chen C, Xia J, Guo J, et al: VEGF secreted by
mesenchymal stem cells mediates the differentiation of endothelial
progenitor cells into endothelial cells via paracrine mechanisms.
Mol Med Rep. 17:1667–1675. 2018.PubMed/NCBI
|
|
27
|
Rossi E, Smadja D, Goyard C, Cras A,
Dizier B, Bacha N, Lokajczyk A, Guerin CL, Gendron N, Planquette B,
et al: Co-injection of mesenchymal stem cells with endothelial
progenitor cells accelerates muscle recovery in hind limb ischemia
through an endoglin-dependent mechanism. Thromb Haemost.
117:1908–1918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rossi E, Smadja DM, Boscolo E, Langa C,
Arevalo MA, Pericacho M, Gamella-Pozuelo L, Kauskot A, Botella LM,
Gaussem P, et al: Endoglin regulates mural cell adhesion in the
circulatory system. Cell Mol Life Sci. 73:1715–1739. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koutna I, Peterkova M, Simara P, Stejskal
S, Tesarova L and Kozubek M: Proliferation and differentiation
potential of CD133+ and CD34+ populations from the bone marrow and
mobilized peripheral blood. Ann Hematol. 90:127–137. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fritzenwanger M, Lorenz F, Jung C, Fabris
M, Thude H, Barz D and Figulla HR: Differential number of CD34+,
CD133+ and CD34+/CD133+ cells in peripheral blood of patients with
congestive heart failure. Eur J Med Res. 14:113–117.
2009.PubMed/NCBI
|
|
31
|
Yao W, Firth AL, Sacks RS, Ogawa A, Auger
WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA,
et al: Identification of putative endothelial progenitor cells
(CD34+CD133+Flk-1+) in endarterectomized tissue of patients with
chronic thromboembolic pulmonary hypertension. Am J Physiol Lung
Cell Mol Physiol. 296:L870–L878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Otto S, Nitsche K, Jung C, Kryvanos A,
Zhylka A, Heitkamp K, Gutiérrez-Chico JL, Goebel B, Schulze PC,
Figulla HR and Poerner TC: Endothelial progenitor cells and plaque
burden in stented coronary artery segments: An optical coherence
tomography study six months after elective PCI. BMC Cardiovasc
Disord. 17:1032017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Golab-Janowska M, Paczkowska E,
Machalinski B, Kotlega D, Meller A, Safranow K, Wankowicz P and
Nowacki P: Elevated inflammatory parameter levels negatively impact
populations of circulating stem cells (CD133+), early endothelial
progenitor cells (CD133+/VEGFR2+), and fibroblast growth factor in
stroke patients. Curr Neurovasc Res. 16:19–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skrzypkowska M, Słomiński B,
Ryba-Stanisławowska M, Gutknecht P and Siebert J: Circulating CD34+
and CD34+VEGFR2+ progenitor cells are associated with KLOTHO KL-VS
polymorphism. Microvasc Res. 119:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kleinbongard P and Weber AA: Impaired
interaction between platelets and endothelial progenitor cells in
diabetic patients. Basic Res Cardiol. 103:569–571. 2008. View Article : Google Scholar : PubMed/NCBI
|


















