Introduction
Few subjects in biomedicine have attracted the
interest of the scientific and lay communities alike as has
Alzheimer's disease. With the sharp rise in life expectancy during
the 20th century, more and more old people from ~49 to >76 years
suffer from neurodegenerative disorders in the United States
(1–3). Among them, AD appears as the most
common form of mental failure in old age (4). The main symptoms of AD are
progressive memory disorder, cognitive dysfunction, personality
change and language disorder, which seriously affect social,
professional and life functions (5). Amyloid β (Aβ) initiates inflammatory
reaction in the early stage of disease (6–8).
This pathological change can promote the increase of Aβ production
and abnormal accumulation, and form cascade amplification effect,
resulting in the decrease of neurons and abnormality and causing
AD. In addition, Aβ induced neurotoxicity and oxidative stress also
participate in the pathogenesis of AD (9). Therefore, the pathogenesis of AD must
be understood as soon as possible and corresponding measures taken
to prevent and treat it.
Compound porcine cerebroside and ganglioside
injection (CPCGI) is a compound preparation, which is used to treat
brain dysfunction clinically. It is estimated that each ml of CPCGI
contains 0.24 mg monosialotetrahexosyl ganglioside (GM-1), 3.2 mg
of polypeptides and 0.125 mg of hypoxanthine (10,11).
Hypoxanthine is an important substance in human life, which can
improve the metabolism of substance and energy, accelerate the
repair of damaged tissue, and restore normal physiological function
of anoxic tissue (12).
Hypoxanthine, small polypeptide, and amino acid coordination can
promote the metabolism of the body. CPCGI has been widely used in
China. In clinical studies, CPCGI can significantly shorten the
time of fracture healing and promote the curative effect of
fracture healing (13). In
addition, CPCGI has a significant effect in treating hypoxic
ischemic encephalopathy, where it helps to improve the recovery of
consciousness and muscle strength, and there is no obvious adverse
reaction during the treatment (11,14).
A previous study demonstrated that CPCGI could also activate
mitochondrial autophagy to improve cerebral ischemia reperfusion
injury (10). A clinical study
demonstrated that CPCGI can promote the metabolism of brain tissue,
participate in the growth, differentiation and regeneration of
neurons in brain tissue, and improve the function of cerebral blood
circulation and brain metabolism (15).
However, the effect of CPCGI on AD has not been
studied so far. Therefore, the present study investigated the
effect of CPCGI on the progress of AD in vivo and in
vitro and explored its molecular mechanism.
Materials and methods
Establishment of AD rat model
A total of 40 Wistar rats were selected (age, 10–11
weeks; weight, 240–260 g) from the laboratory animal room of
Liaoning University of Traditional Chinese Medicine. The rats fed
and drank freely at room temperature (20-22°C) with 40–50%
humidity, and were maintained under a 12-h light/dark cycle. The
present study was performed according to the principles and
procedures of the National Institutes of Health's Guide for the
Care and Use of Laboratory Animals (16). This study was approved by the
Animal Care and Use Committee of the Second Hospital of Hebei
Medical University.
The rat model of AD was established using Aβ1–42
(Sigma-Aldrich; Merck KGaA) as in previous studies (17–19).
The Wistar male rats were randomly divided into 4 groups with 10
rats in each group: Sham, model, model + vehicle (saline) and model
+ CPCGI (1 ml/kg/d). Rats in the sham group were treated with
saline by a gradual intracerebroventricular (icv) injection (1
µl/min) into the lateral ventricle. Rats in the model group were
treated with Aβ1–42 (400 pmol/3 µl/rat) by gradual
intracerebroventricular (icv) injection (1 µl/min) into the lateral
ventricle (17). The AD model rats
received CPCGI treatment (1 ml/kg/d; intraperitoneal injection) for
15 consecutive days starting 1 h after AD induction. Rats in the
model + vehicle group received an equal amount of saline. At the
end of the experiment, the rats were anaesthetized with
pentobarbital (40 mg/kg, intraperitoneal injection) before being
sacrificed through cervical dislocation (rats without a heartbeat
that were not breathing were confirmed as dead). Subsequently, the
hippocampal tissues of rats from the different groups were
collected. No rats died during the experiment. Tests were
terminated when the rats lost more than 15% of their body weight
and every effort was made to alleviate their suffering.
Sucrose preference test
On day 12 after CPCGI treatment, to assess anhedonic
behavior of rats, the sucrose preference test was performed as in a
previous study (17). Briefly,
rats were acclimated to the two-bottle choice paradigm (two
identical bottles were placed on the cages) for three days. In
order to avoid withdrawal symptoms in rats, each rat was given two
bottles, one containing a 2% sucrose solution and the other
containing tap water. The two bottles were changed every 12 h to
avoid a ‘side’ bias. The amount of the sucrose solution or tap
water consumed was detected by weighing the bottles immediately
before and after the test. The sucrose preference ratio was
calculated as following: Sucrose preference value (%)=sucrose
intake (g) ×100%/[sucrose intake (g) + water intake (g)].
Tail suspension test
Following the final CPCGI treatment, the tail
suspension test was performed as previously described (17). In brief, every rat was individually
suspended by the tail using a clamp, 3–4 cm from the end, in a gray
wooden enclosure (60×30×20 cm). A square platform was placed under
the rat's forepaws and lightly touching them to avoid hemodynamic
stress and limb pain. The immobility time was recorded (in seconds)
during the 5-min test period.
PC12 AD cell model establishment and
treatment
Rat adrenal pheochromocytoma (PC12) cells were
purchased from American Type Culture Collection (ATCC, cat. no.
CRL-1721). CPCGI was obtained from Jilin Buchang Pharmaceutical
Co., Ltd. DMEM high-sugar medium, horse serum, and fetal bovine
serum were purchased from Gibco (Thermo Fisher Scientific,
Inc.).
PC12 cells were cultured in DMEM containing 10%
fetal bovine serum and 5% horse serum, and cultured at 37°C with
95% humidity and 5% CO2 (20). PC12 cells were divided into
control, model, model + vehicle (PBS) and model + CPCGI. In the
model + vehicle and model + CPCGI groups, PC12 cells were
pre-treated with PBS and CPCGI separately for 1 h before
stimulation with 50 µM Aβ25–35 (Sigma-Aldrich; Merck KGaA) for 24
h. PC12 cells in the model group were stimulate with 50 µM Aβ25–35
for 24 h (21). Cells in the
control group were not subjected to any treatment. Subsequently,
PC12 cells in each group were subjected to the following
experiments.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from tissues (100 mg) was collected by
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
and reverse transcribed into cDNA using PrimeScript RT Reagent Kit
(Takara Bio, Inc.) according to the manufacturer's protocols. The
temperature protocol for the reverse transcription reaction was
25°C for 5 min, 42°C for 60 min and 80°C for 2 min. qPCR was
performed to analyze gene expression using the cDNA the SYBR RT-PCR
kit (Takara Bio, Inc.) according to the manufacturer's protocols.
The thermocycling conditions were as follows: 95°C for 5 min,
followed by 38 cycles of denaturation at 95°C for 15 sec and
annealing/elongation at 60°C for 30 sec. Primer sequences were:
GAPDH, forward 5′-CTTTGGTATCGTGGAAGGACTC-3′; reverse
5′-GTAGAGGCAGGGATGATGTTCT-3′; Aβ42, forward
5′-ATGGCGAGCAAAGTCTCGATC-3′; reverse 5′-CGCAATCACCACGCCGCCCAC-3′.
GAPDH was used as the internal control, and the gene expression was
quantified by the 2−ΔΔCq method (22).
Western blot analysis
Protein expression was detected using western
blotting. Radioimmunoprecipitation assay buffer (Auragene
Bioscience) was used to extract the proteins from hippocampus or
PC12 cells. A bicinchoninic acid protein quantitative kit (Thermo
Fisher Scientific, Inc.) was used to detect protein concentrations
in line with the manufacturer's instructions. 10% SDS-PAGE gel
electrophoresis was used to isolate proteins (30 µg/lane), and then
the proteins were transferred onto PVDF membranes (EMD Millipore).
5% skimmed milk was used to block the membrane at room temperature
for 1 h, and then the membranes were incubated with primary
antibodies: β-Amyloid (for Aβ42 and Aβ40 detection; cat no. 8243;
1:1,000; Cell Signaling Technology, Inc.), Bcl-2 (cat no. ab196495;
1:1,000; Abcam), Bax (cat no. ab32503; 1:1,000; Abcam), cleaved
caspase3 (cat no. ab49822; 1:1,000; Abcam), pro-caspase3 (cat no.
ab183179; 1:1,000; Abcam), phosphorylated (p)-p38 (cat no. ab4822;
1:1,000; Abcam), p38 (cat no. ab170099; 1:1,000; Abcam), p65 (cat
no. ab16502; 1:1,000; Abcam), p-p65 (cat no. ab86299; 1:1,000;
Abcam), and β-actin (cat no. ab179467; 1:1,000; Abcam) at room
temperature for 3 h. Subsequently, the PVDF membranes were
hybridized with horseradish peroxidase-conjugated anti-rabbit IgG
secondary antibody (cat. no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) for 1 h. Finally, ECL reagent (Applygen
Technologies, Inc.) was used to visualize protein bands. The band
density was semi-quantified with Gel-Pro Analyzer densitometry
software (version 6.3, Media Cybernetics, Inc.).
Enzyme linked immunosorbent assay
(ELISA)
The expression of TNF-α (cat. no. PT516) and IL-1β
(cat. no. PI303) in the hippocampus of rats from different groups
were detected using ELISA kits (Beyotime Institute of
Biotechnology) according to the manufacturers instructions.
SOD activity and MDA content
measurement
To determine SOD activity (cat. no. S0101) and MDA
content (cat. no. S0131) in the hippocampus of rats, commercial
colorimetric detection kits (Beyotime Institute of Biotechnology)
were performed following the manufacturer's instructions.
Reactive oxygen species (ROS)
production detection
A Reactive Oxygen Species Assay kit (cat. no. S0033,
Beyotime Institute of Biotechnology) was used to determine the
production of ROS in the hippocampus of rats according to the
manufacturer's instructions. The ROS level was expressed as the
percentage of the dichlorofluorescein (DCF) fluorescence level in
control group whose DCF level was set to 100%.
Cell viability analysis
Cell viability was determined by the conventional
MTT assay. Following treatment, PC12 cells were seeded in 96-well
plates (104 cells/well) and incubated at 37°C for 24 h.
Then MTT solution (10 µl) was added to each well and the cells were
incubated for further 4 h at 37°C. Subsequently, 100 µl DMSO
(Nanjing KeyGen Biotech Co., Ltd.) was used to dissolve the
formazan crystals. Finally, the absorbance was measured at the
wavelength of 490 nm by using a micro-plate reader (Synergy2,
BioTek Instruments, Inc.).
Apoptosis assay
Flow cytometry (BD Accuri Flow Cytometer, BD
Biosciences) was performed to analyze cell apoptosis. PC12 cells
were treated with or without CPCGI for 48 h. Then, the cell
apoptosis was determined by using the Annexin V-fluorescein
isothiocyanate/propidium iodide apoptosis detection kit [cat. no.
70-AP101-100; Multisciences (Lianke) Biotech Co., Ltd.] in
accordance with the manufacturer's protocols. FlowJo 7.6 software
(FlowJo LLC) was used to analyze the cell apoptosis rate.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS, Inc.). All experiments were performed three times. Data are
presented as mean ± standard deviation. The differences between
groups were analyzed by one-way analysis of variance with Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
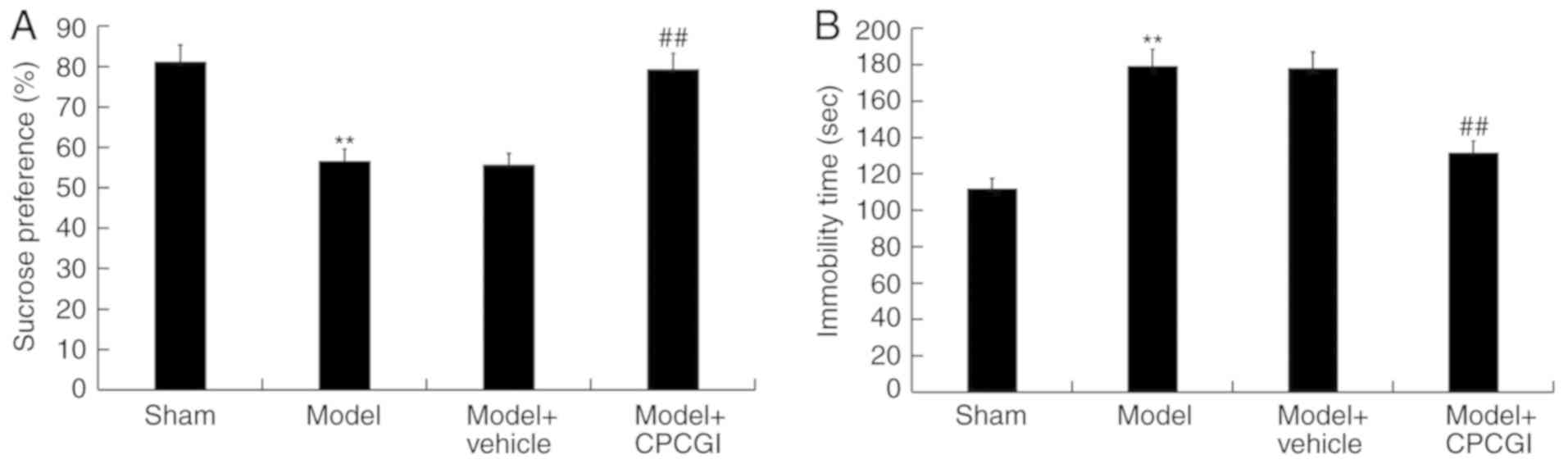
CPCGI ameliorates AD-associated
symptoms of rats induced by Aβ1–42
To study the therapeutic effect of CPCGI on AD, the
rat model of AD was constructed and then treated with CPCGI. It was
found that the reduced the percentage of sucrose preference of rats
induced by Aβ1–42 was significantly increased by CPCGI treatment
(Fig. 1A). The enhanced immobility
time of rats caused by Aβ1–42 administration was significantly
reduced by CPCGI treatment (Fig.
1B).
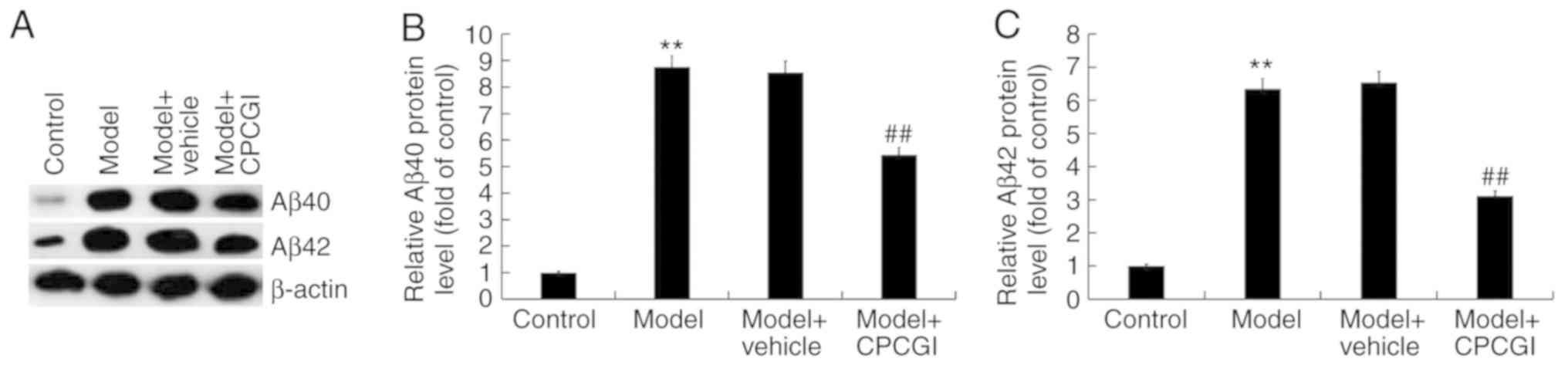
CPCGI treatment inhibited inflammatory
response and oxidative stress in AD model rats induced by
Aβ1–42
Results suggested that compared with sham group, the
protein expression of Aβ40 and Aβ42 in the hippocampus of AD model
rats was markedly increased, while CPCGI significantly decreased
the expression of Aβ40 and Aβ42 (Fig.
2A). Compared with the sham group, the mRNA expression of Aβ40
and Aβ42 in the hippocampus of AD model rats was significantly
increased, while CPCGI significantly decreased the mRNA expression
of Aβ40 and Aβ42 (Fig. 2B and C).
Compared with the rats from the sham group, the production of TNF-α
and IL-1β in the hippocampus of AD model rats was significantly
increased, and these increases were inhibited by CPCGI treatment
(Fig. 2D and E). In addition, the
content of MDA in the hippocampus of Aβ1–42 induced rats was
significantly increased (Fig. 2F),
the activity of SOD was significantly decreased (Fig. 2G), and the ROS level significantly
enhanced (Fig. 2H). CPCGI
treatment significantly reduced the content of MDA, increased the
activity of SOD, and decreased ROS level in the hippocampus of AD
model rats (Fig. 2F-H).
 | Figure 2.Effect of CPCGI on AD rat model. (A)
Western blot assay was used to detect the protein expression of
Aβ40 and Aβ42 in the hippocampus of AD rats treated with or without
CPCGI, and Aβ40/β-actin and Aβ42/β-actin was calculated and
presented. (B and C) RT-qPCR was used to detect the mRNA expression
of Aβ40 and Aβ42 in the hippocampus of AD rats treated with or
without CPCGI. (D and E) ELISA was used to detect the levels of
inflammatory cytokines TNF-α and IL-1β in the hippocampus of AD
rats treated with or without CPCGI. The effect of CPCGI on (F) MDA
secretion, (G) SOD activity and (H) ROS production. Experiments
were repeated three times. Data are given as the mean ± standard
deviation. **P<0.01 vs. the sham group; #P<0.05
vs. the model group; ##P<0.01 vs. the model group.
CPCGI, compound porcine cerebroside and ganglioside injection; AD,
Alzheimer's disease; Aβ, amyloid-β; RT-qPCR, reverse
transcription-quantitative PCR; TNF, tumor necrosis factor; IL,
interleukin; MDA, malondialdehyde; SOD, superoxide dismutase; ROS,
reactive oxygen species. |
CPCGI reduced the protein expression
of Aβ40 and Aβ42 in Aβ25–35 induced PC12 cells
In order to detect the protein expression of Aβ40
and Aβ42, western blot analysis was used. The results demonstrated
that the protein expression of Aβ40 and Aβ42 in Aβ25–35 induced
PC12 cells increased significantly compared with the control group,
and that CPCGI could decrease the protein expression of Aβ40 and
Aβ42 in Aβ25–35 induced PC12 cells (Fig. 3A-C).
CPCGI enhanced the viability of
Aβ25–35 induced PC12 cells
MTT assay was used to investigate the effect of
CPCGI on the viability of PC12 cells. The results demonstrated that
compared with the control group, the viability of PC12 cells was
significantly decreased in Aβ25–35 treated PC12 cells. CPCGI
treatment could significantly increase the viability of Aβ25–35
induced PC12 cells (Fig. 4A).
CPCGI reduced apoptosis of Aβ25–35
induced PC12 cells
Flow cytometry was used to analyze the effect of
CPCGI on apoptosis in PC12 cells. Compared with the control group,
the apoptosis of PC12 cells in AD cell model group increased
significantly. The apoptosis of Aβ25–35 induced PC12 cells
(Fig. 4B and C) was significantly
reduced by CPCGI treatment. In addition, the protein expression of
Bcl2 (Fig. 4D and E) and
pro-caspase3 decreased (Fig. 4D and
H), while the protein level of Bax (Fig. 4D and F) and cleaved-caspase3
(Fig. 4D and G) increased
significantly in Aβ25–35 induced PC12 cells, and these changes were
inhibited by CPCGI treatment (Fig.
4D-H).
CPCGI reduced p-p38 and p-p65
expression in Aβ25–35 induced PC12 cells
To explore the mechanism by which CPCGI affected AD,
the p38 mitogen-activated protein kinase (MAPK)/NF-κB pathway was
analyzed. In addition, the protein expression of p38, p65, p-p38
and p-p65 in PC12 cells was detected using western blot analysis.
The results demonstrated that the protein expression of p-p38 and
p-p65 and the ratio of p-p38/p38 and p-p65/p65 in Aβ25–35 induced
PC12 cells were significantly higher than that in the control
group. CPCGI treatment significantly reduced the protein expression
of p-p38 and p-p65 and the ratio of p-p38/p38 and p-p65/p65 in
Aβ25–35 induced PC12 cells (Fig.
5A-C), indicating the inhibitory effect of CPCGI on
p38MAPK/NF-κB pathway in Aβ25–35 induced PC12 cells.
Discussion
Alzheimer's disease is a degenerative disease of the
central nervous system characterized by progressive memory
impairment and mental retardation (1,5).
With the aging of the world population, the incidence of
Alzheimer's disease is increasing, therefore, it is urgent to find
new and effective methods for treating AD. In recent years, a great
deal of research has been performed on the etiology and
pathogenesis of Alzheimer's disease (23,24).
Aβ (6–8), inflammatory factors (25) and oxidative stress markers MDA and
SOD (26,27) have been found to serve a leading
role in the occurrence and development of AD. Previous studies have
found that folic acid could improve learning and memory in rats
with AD by directly or indirectly inhibiting or clearing the
deposition of Aβ in the hippocampus and olfactory region (28–30).
In addition, zuo-gui pills, a classic TCM formulation (31,32),
can effectively increase the activity of SOD and decrease the
content of MDA in brain tissue of AD model rats (33). In the study of CPCGI, a previous
study found that CPCGI has a neuroprotective effect on middle
cerebral artery occlusion injured rats by inhibiting apoptosis and
improving synaptic and mitochondrial function (10). In a study of the protective effect
of CPCGI on cerebral ischemia-reperfusion injury in rats, the
results suggested that one of the neuroprotective mechanisms of
CPCGI may be related to activation of mitochondrial autophagy and
improvement of mitochondrial function (15).
Previous studies have demonstrated that CPCGI has
neuroprotective effect (10,11,15),
but its neuroprotective mechanism for AD is still unclear,
therefore, the present study investigated the effect of CPCGI on
AD. In the present study, the rat model of AD was established using
Aβ1–42 and then treated with CPCGI. Depression is prevalent in
patients with AD (34,35). Previous studies have revealed that
intracerebroventricular or hippocampal injection of Aβ1–42 results
in depressive-like symptoms in rats (36–39).
Therefore, the present study determined the effect of CPCGI on the
percentage of sucrose preference and the immobility time of rats
with AD, and found that CPCGI significantly enhanced the percentage
of sucrose preference of AD rats and reduced the immobility time of
rats treated with Aβ1-42. Each ml of CPCGI contains 0.24 mg
Monosialotetrahexosyl ganglioside (GM-1), 3.2 mg of polypeptides,
and 0.125 mg of hypoxanthine (10,11).
As GM-1 (40,41) and polypeptides (42) all have been reported to have
neuroprotective effects, it was hypothesized that CPCGI may serve a
protective role in AD mainly through GM-1 and polypeptides,
however, this need further research.
The protein expression of Aβ40 and Aβ42,
inflammatory factors TNF-α and IL-1β, the MDA secretion and SOD
activity associated with oxidative stress markers, and ROS
production, which are involved in the occurrence and development of
AD, were identified in the hippocampus of AD model rats. The
findings indicated that CPCGI administration significantly
decreased the protein expression of Aβ40 and Aβ42, and that the
production of TNF-α and IL-1β in the hippocampus of AD model rats
was also reduced by CPCGI treatment. CPCGI treatment significantly
reduced the MDA content, increased SOD activity and reduced ROS
level in the hippocampus of AD model rats. However, as the
detection of injected Aβ may be controversial because of the
detected expression could be due to the injected Aβ, the expression
of injected Aβ in rats or PC12 cells were not measured in the
present study.
An in vitro model of AD was established by
subjecting PC12 cells to Aβ25-35, and it was found that the
viability of AD model cells significantly decreased and the
apoptosis of cells increased. The results of the current study were
consistent with previous studies (43,44):
CPCGI treatment significantly enhanced the viability of PC12 cells
induced by Aβ25–35 and reduced cell apoptosis.
The MAPK/NF-κB pathway, serves important roles in
the regulation of cell growth and inflammatory response, and has
been revealed to be activated during the development of AD
(45,46). Thus, to explore the mechanism by
which CPCGI affected AD, the p38MAPK/NF-κB pathway was analyzed. As
expected, it was observed that the activated p38MAPK/NF-κB pathway
caused by Aβ25–35 induction was inhibited by CPCGI treatment.
In conclusion, the results of the present study
suggested that CPCGI served a protective role in AD development by
reducing Aβ accumulation, inhibiting inflammatory response and
oxidative stress, and preventing neuronal apoptosis by inhibiting
MAPK/NF-κB signaling pathway activation. However, the present study
is a preliminary study of the role of CPCGI in AD, and has some
limitations. For example, the pathological changes of AD rats were
not analyzed using microscopy. The p38MAPK/NF-κB pathway was not
analyzed in vivo. A group of p38 specific inhibitor or NF-κB
specific inhibitor combination therapies was not conducted in the
present study nor did it investigate the effect of CPCG1 on normal
rats and PC12 cells. In order to make the role of CPCGI in AD more
convincing, more research is needed. For instance, whether CPCGI
serves a role in AD by directly affecting p38MAPK and NF-κB
signaling in vivo and in vitro should be
investigated. In addition, the effect of CPCGI on normal rats and
normal PC12 cells should be studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW performed study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. JZ performed data collection and statistical
analysis.
Ethics approval and consent to
participate
The present study was performed according to the
principles and procedures of the National Institutes of Health's
Guide for the Care and Use of Laboratory Animals. This study was
approved by the Animal Care and Use Committee of the Second
Hospital of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Selkoe DJ: Alzheimer's disease: Genes,
proteins, and therapy. Physiol Rev. 81:741–766. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dubois B, Feldman HH, Jacova C, Dekosky
ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D,
Gauthier S, Jicha G, et al: Research criteria for the diagnosis of
Alzheimer's disease: Revising the NINCDS-ADRDA criteria. Lancet
Neurol. 6:734–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang J, Lemaire HG, Unterbeck A, Salbaum
JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K and
Müller-Hill B: The precursor of Alzheimer's disease amyloid A4
protein resembles a cell-surface receptor. Nature. 325:733–736.
1987. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glenner GG and Wong CW: Alzheimer's
disease: Initial report of the purification and characterization of
a novel cerebrovascular amyloid protein. Biochem Biophys Res
Commun. 120:885–890. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lane CA, Hardy J and Schott JM:
Alzheimer's disease. Eur J Neurol. 25:59–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frost PS, Barros-Aragão F, da Silva RT,
Venancio A, Matias I, Lyra E Silva NM, Kincheski GC,
Pimentel-Coelho PM, De Felice FG, Gomes FCA, et al: Neonatal
infection leads to increased susceptibility to Aβ oligomer-induced
brain inflammation, synapse loss and cognitive impairment in mice.
Cell Death Dis. 10:3232019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blennow K, Leon MJD and Zetterberg H:
Alzheimer's disease. Lancet. 368:387–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ballard C, Gauthier S, Corbett A, Brayne
C, Aarsland D and Jones E: Alzheimer's disease. Lancet.
377:1019–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teng E, Taylor K, Bilousova T, Weiland D,
Pham T, Zuo X, Yang F, Chen PP, Glabe CG, Takacs A, et al: Dietary
DHA supplementation in an APP/PS1 transgenic rat model of AD
reduces behavioral and Aβ pathology and modulates Aβ
oligomerization. Neurobiol Dis. 552–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang M, Zhang Y, Feng L, Zheng J, Fan S,
Liu J, Yang N, Liu Y and Zuo P: Compound porcine cerebroside and
ganglioside injection attenuates cerebral ischemia-reperfusion
injury in rats by targeting multiple cellular processes.
Neuropsychiatr Dis Treat. 13:927–935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M, Feng L, Fan S, Zheng JI, LI DM,
Yang N, Zuo P and Liu Y: Effect of Compound porcine cerebroside and
ganglioside injection on cerebral ischemia-reperfusion injury in
rats. Chin J Rehabil Theory Prac. 281–285. 2016.
|
|
12
|
Kwak DH, Kim SM, Lee DH, Kim JS, Kim SM,
Lee SU, Jung KY, Seo BB and Choo YK: Differential expression
patterns of gangliosides in the ischemic cerebral cortex produced
by middle cerebral artery occlusion. Mol Cells. 20:354–360.
2005.PubMed/NCBI
|
|
13
|
Zamfir AD: Neurological analyses: Focus on
gangliosides and mass spectrometry. Adv Exp Med Biol. 806:153–204.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang XF, Wang JM, Chen Q, Wei YY and Chen
HW: Meta-analysis on effect of compound Danshen injection in
treating neonatal hypoxic-ischemic encephalopathy. Zhongguo Zhong
Yao Za Zhi. 40:141–148. 2015.(In Chinese). PubMed/NCBI
|
|
15
|
Wang H, Jiang HQ, Li J, et al: Clinical
study of Compound Porcine Cerebroside and Ganglioside Injection on
Alzheimer disease. Chinese Community doctors. 12:127–128. 2010.(In
Chinese).
|
|
16
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39(199): 208–211. 1996.
|
|
17
|
Amani M, Shokouhi G and Salari AA:
Minocycline prevents the development of depression-like behavior
and hippocampal inflammation in a rat model of Alzheimer's disease.
Psychopharmacology (Berl). 236:1281–1292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcez ML, Mina F, Bellettini-Santos T,
Carneiro FG, Luz AP, Schiavo GL, Andrighetti MS, Scheid MG, Bolfe
RP and Budni J: Minocycline reduces inflammatory parameters in the
brain structures and serum and reverses memory impairment caused by
the administration of amyloid β (1–42) in mice. Prog
Neuropsychopharmacology Biol Psychiatry. 77:23–31. 2017. View Article : Google Scholar
|
|
19
|
Cioanca O, Hritcu L, Mihasan M, Trifan A
and Hancianu M: Inhalation of coriander volatile oil increased
anxiolytic-antidepressant-like behaviors and decreased oxidative
status in beta-amyloid (1–42) rat model of Alzheimer's disease.
Physiol Behav. 131:68–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharifi AM and Mousavi SH: Studying the
effects of lead on DNA fragmentation and proapoptotic bax and
antiapoptotic Bcl-2 protein expression in PC12 cells. Toxicol Mech
Methods. 18:55–79. 2008. View Article : Google Scholar
|
|
21
|
Lee S, Youn K, Kim DH, Ahn MR, Yoon E, Kim
OY and Jun M: Anti-neuroinflammatory property of phlorotannins from
Ecklonia cava on Aβ25-35-induced damage in PC12
cells. Mar Drugs. 17:E72018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balin BJ and Hudson AP: Etiology and
pathogenesis of late-onset Alzheimer's disease. Curr Allergy Asthma
Rep. 14:4172014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Area-Gomez E and Schon EA: On the
pathogenesis of Alzheimer's disease: The MAM hypothesis. FASEB J.
31:864–867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bolós M, Perea JR and Avila J: Alzheimer's
disease as an inflammatory disease. Biomol Concepts. 8:37–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nazıroğlu M, Muhamad S and Pecze L:
Nanoparticles as potential clinical therapeutic agents in
Alzheimer's disease: Focus on selenium nanoparticles. Expert Rev
Clin Pharmacol. 10:773–782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dastan Z, Pouramir M, Ghasemi-Kasman M,
Ghasemzadeh Z, Dadgar M, Gol M, Ashrafpour M, Pourghasem M,
Moghadamnia AA and Khafri S: Arbutin reduces cognitive deficit and
oxidative stress in animal model of Alzheimer's disease. Int J
Neurosci. 129:1145–1153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Faux NG, Ellis KA, Porter L, Fowler CJ,
Laws SM, Martins RN, Pertile KK, Rembach A, Rowe CC, Rumble RL, et
al: Homocysteine, vitamin B12, and folic acid levels in Alzheimer's
disease, mild cognitive impairment, and healthy elderly: Baseline
characteristics in subjects of the Australian imaging biomarker
lifestyle study. J Alzheimers Dis. 27:909–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Connelly PJ, Prentice NP, Cousland G and
Bonham J: A randomised double-blind placebo-controlled trial of
folic acid supplementation of cholinesterase inhibitors in
Alzheimer's disease. Int J Geriatr Psychiatry. 23:155–160. 2010.
View Article : Google Scholar
|
|
30
|
Chen H, Liu S, Ji L, Wu T, Ji Y, Zhou Y,
Zheng M, Zhang M, Xu W and Huang G: Folic acid supplementation
mitigates Alzheimer's disease by reducing inflammation: A
randomized controlled trial. Mediators Inflamm. 2016:59121462016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kou S, Zheng Q, Wang Y, Zhao H, Zhang Q,
Li M, Qi F, Fang L, Liu L, Ouyang J, et al: Zuo-Gui and You-Gui
pills, two traditional Chinese herbal formulas, downregulated the
expression of NogoA, NgR, and RhoA in rats with experimental
autoimmune encephalomyelitis. J Ethnopharmacol. 158:102–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang YZ, Kou S, Gu LY, Zheng Q, Li M, Qi
F, Zhao H and Wang L: Effects of Zuogui Pill () and Yougui Pill ()
on the expression of brain-derived neurotrophic factor and cyclic
adenosine monophosphate/protein kinase A signaling transduction
pathways of axonal regeneration in model rats with experimental
autoimmune encephalomyelitis. Chin J Integr Med. 20:24–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bracegirdle S: The effects of Zuo-gui
pills on SOD and MDA of AD model mice. Chin Arch Tradit Chin Med.
2583–2585. 2010.(In Chinese).
|
|
34
|
Engedal K, Barca ML, Laks J and Selbaek G:
Depression in Alzheimer's disease: Specificity of depressive
symptoms using three different clinical criteria. Int J Geriatr
Psychiatry. 26:944–951. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benoit M, Berrut G, Doussaint J, Bakchine
S, Bonin-Guillaume S, Frémont P, Gallarda T, Krolak-Salmon P,
Marquet T, Mékiès C, et al: Apathy and depression in mild
Alzheimer's disease: A cross-sectional study using diagnostic
criteria. J Alzheimers Dis. 31:325–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Souza LC, Jesse CR, Del Fabbro L, de Gomes
MG, Goes ATR, Filho CB, Luchese C, Pereira AAM and Boeira SP:
Swimming exercise prevents behavioural disturbances induced by an
intracerebroventricular injection of amyloid-β1-42
peptide through modulation of cytokine/NF-kappaB pathway and
indoleamine-2,3-dioxygenase in mouse brain. Behav Brain Res.
331:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Souza LC, Jesse CR, Antunes MS, Ruff JR,
de Oliveira Espinosa D, Gomes NS, Donato F, Giacomeli R and Boeira
SP: Indoleamine-2,3-dioxygenase mediates neurobehavioral
alterations induced by an intracerebroventricular injection of
amyloid-β1–42 peptide in mice. Brain Behav Immun. 56:363–377. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo J, Chang L, Li C, Li M, Yan P, Guo Z,
Wang C, Zha Q and Wang Q: Sb203580 reverses memory deficits and
depression-like behavior induced by microinjection of
Aβ1-42 into hippocampus of mice. Metab Brain Dis.
32:57–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song X, Liu B, Cui L, Zhou B, Liu W, Xu F,
Hayashi T, Hattori S, Ushiki-Kaku Y, Tashiro SI and Ikejima T:
Silibinin ameliorates anxiety/depression-like behaviors in amyloid
β-treated rats by upregulating BDNF/TrkB pathway and attenuating
autophagy in hippocampus. Physiol Behav. 179:487–493. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dai R, Zhang S, Duan W, Wei R, Chen H, Cai
W, Yang L and Wang Q: Enhanced autophagy contributes to protective
effects of GM1 ganglioside against Aβ1-42-induced neurotoxicity and
cognitive deficits. Neurochem Res. 42:2417–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gong G, Yin L, Yuan L, Sui D, Sun Y, Fu H,
Chen L and Wang X: Ganglioside GM1 protects against high altitude
cerebral edema in rats by suppressing the oxidative stress and
inflammatory response via the PI3K/AKT-Nrf2 pathway. Mol Immunol.
95:91–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jin W, Xu X, Chen X, Qi W, Lu J, Yan X,
Zhao D, Cong D, Li X and Sun L: Protective effect of pig brain
polypeptides against corticosterone-induced oxidative stress,
inflammatory response, and apoptosis in PC12 cells. Biomed
Pharmacother. 115:1088902019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeng Z, Xu J and Zheng W: Artemisinin
protects PC12 cells against β-amyloid-induced apoptosis through
activation of the ERK1/2 signaling pathway. Redox Biol. 12:625–633.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang YL, Wang ZW and Song W: Set up
Alzheimer's disease cell apoptosis model with PC-12 cell induced by
Aβ(25–35). J Nanjing Med Univ. 208–214. 2003.(In Chinese).
|
|
45
|
Lee S, Youn K and Jun M: Major compounds
of red ginseng oil attenuate Aβ25-35-induced neuronal
apoptosis and inflammation by modulating MAPK/NF-κB pathway. Food
Funct. 9:4122–4134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu H, Deng Y, Gao J, Liu Y, Li W, Shi J
and Gong Q: Sodium hydrosulfide attenuates beta-amyloid-induced
cognitive deficits and neuroinflammation via modulation of
MAPK/NF-κB pathway in rats. Curr Alzheimer Res. 12:673–683. 2015.
View Article : Google Scholar : PubMed/NCBI
|