Introduction
Dental pulp inflammation is a pathological process
characterized by various bacterial virulence factors that often
elicits a dental emergency, and may develop into periapical disease
or pulp necrosis (1).
Lipopolysaccharide (LPS) is commonly released from gram-negative
bacteria. When LPS enters the dental pulp, it can evoke an
inflammatory response; LPS is also closely associated with pulpitis
and periapical periodontitis (2).
Previous studies have found that LPS can stimulate Toll-like
receptor (TLR)4 in the cell membranes of human dental pulp cells
(hDPCs), and activate the NF-κB, ERK1/2 and p38 pathways, thereby
producing inflammation-related cytokines, including interleukin
(IL)-6 and IL-8 (3,4). Although there are a number of
mechanisms associated with the development of dental pulp
infection, the specific molecular mechanism is still unclear
(5,6). Recent studies have suggested that
epigenetic alterations are crucial regulators in the occurrence and
development of dental pulp infection (7–9).
DNA methylation that occurs at
cytosine-phosphate-guanine (CpG) dinucleotide sites is the most
common epigenetic modification event in the genome (10). The DNA methylation process involves
placing a methyl group onto the 5-position of cytosines situated in
CpG dinucleotides and turning the cytosine into 5-methylcytosine
(5mC), which is catalyzed by members of the DNA methyltransferase
(DNMT) family (11). DNMT1 can
methylate hemimethylated CpGs and is a well-known maintenance
methyltransferase that can preserve methylation patterns during DNA
replication (12). DNMT3a and
DNMT3b are de novo methyltransferases that can methylate
unmethylated and hemimethylated DNA, and establish DNA methylation
patterns in embryo development (13). The roles and functions of DNA
methylation patterns have attracted extensive attention, but there
has been particular emphasis on their roles in the pathological
processes of cancer (14). Only
recently have studies begun to shed light on the contribution of
DNMTs to the initiation and progression of inflammatory diseases
(15). A study on inflamed
peripheral blood mononuclear cells (PBMCs) showed that DNMT1
expression decreased after treatment with LPS; DNMT1 modulated the
methylation level of gene promoters, thus mediating the
transcription of pro-inflammatory cytokines, including IL-6, IL-8
and tumor necrosis factor-α (TNF-α) (16). In macrophages, DNMT1 contributes to
the hypermethylation of suppressor of cytokine signaling 1, a
negative regulator of cytokine signal transduction, thereby
enhancing the secretion of pro-inflammatory cytokines induced by
LPS indirectly (17). DNA
methylation could also affect inflammatory reactions by modulating
the activation levels of crucial proteins of the NF-κB and/or MAPK
pathways (18,19). In addition, DNA methylation
epigenetically regulates the transcription of TLRs and signal
transduction molecules, including TNF receptor-associated factor 6
(TRAF6) and myeloid differentiation primary response 88 (MyD88).
This suggests that DNA methylation is engaged in signaling pathways
related to inflammation (20,21).
These studies provide evidence indicating that DNA methylation can
epigenetically regulate inflammatory reactions via several
different mechanisms. However, whether DNA methylation is involved
in the modification of dental pulp immunity remains unclear.
Preliminary experiments by our lab showed that in
LPS-treated hDPCs, 5-aza-2′-deoxycytidine (5-Aza-CdR), a DNA
methyltransferase inhibitor, increased the production of several
inflammation-related cytokines (unpublished data). The present
study aimed to investigate the effect of DNMT1 on the LPS-induced
inflammatory response in hDPCs, thereby exploring the role of DNA
methylation in dental pulp inflammation. The results demonstrated
that DNMT1 knockdown promoted the expression of
pro-inflammatory cytokines and the phosphorylation of IKKα/β and
p38 in LPS-treated hDPCs. Moreover, DNMT1 depletion
decreased the 5mC level in the IL-6 and TRAF6
promoters. These data suggested that DNMT1 may be involved in
inhibiting the LPS-induced inflammatory response in hDPCs.
Materials and methods
Isolation and culture of hDPCs
Healthy permanent premolars and third molars were
collected from donors aged 18 to 25 for orthodontic reasons from
the Department of Oral and Maxillofacial Surgery, Guanghua School
of Stomatology, Sun Yat-sen University for approximately one year
between March 2018 and April 2019. Only healthy teeth without
carious disease or hyperemic pulp tissue were selected. A total of
128 teeth from 58 donors (29 males and 29 females) were obtained
for dental pulp tissue isolation and cell culture. hDPCs were
isolated and cultivated using an enzymatic method as described by
Gronthos et al (22). After
extraction, the teeth were washed with 70% ethanol and PBS (pH 7.4)
and then split open to expose the pulp chamber. The dental pulp
tissue was gently isolated with forceps and minced into small
pieces, which were then digested in 3 mg/ml collagenase type I
(Gibco; Thermo Fisher Scientific, Inc.) for 20 min at 37°C.
Subsequently, the minced pulp tissue was cultured in DMEM
containing 20% FBS, 100 mg/ml streptomycin and 100 U/ml penicillin
(all purchased from Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. The medium was changed every 3 days. When
the cells reached 80% confluence, they were detached using
trypsin/EDTA (Gibco; Thermo Fisher Scientific, Inc.) and
subcultured at a ratio of 1:2. Generally, 2–3 teeth from one donor
were used for each primary culture. For each primary culture,
~106 cells at the zero passage were obtained. All
experiments were performed with cells from passages two or three.
To avoid inter-individual variation, the experiments were performed
at least three times for each sample and each experiment, and
average data were generated. For each parameter, experiments were
replicated three times each using donor cells from three samples,
and average data for the three different cell types were
obtained.
Treatment with LPS
hDPCs were stimulated for the indicated times (0, 3,
6, 12 and 24 h) with 1 µg/ml purified Escherichia coli
(E. coli) LPS (Sigma-Aldrich; Merck KGaA) at 37°C with 5%
CO2 (4,23). The blank controls were cells
without LPS stimulation.
DNMT1 small interfering RNA (siRNA)
transfection
siRNA was used in hDPCs to knockdown DNMT1. A
total of 3 siRNA sequences (Invitrogen; Thermo Fisher Scientific,
Inc.) were designed to target the human DNMT1 gene. Before
transfection, hDPCs were seeded in 6-well plates in 2 ml of α-MEM
at 4×105 cells/well containing 10% FBS for 24 h. After
attachment overnight, hDPCs were then transfected with siRNA (50
nM) against DNMT1 or a nontargeting siRNA control using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. After
incubation for 24 h, the media was changed, and DMEM supplemented
with 10% FBS was added with or without 1 µg/ml E. coli LPS.
All siRNA sequences are listed in Table I. siRNA #1 with the best
interference effect was selected as the DNMT1 target sequence for
the subsequent experiments.
 | Table I.Sequences used for DNMT1 siRNA. |
Table I.
Sequences used for DNMT1 siRNA.
| DNMT1 siRNA | Sequence
(5′-3′) |
|---|
| #1 siRNA | Sense:
GGGACUGUGUCUCUGUUAUTT dTdT |
|
| Antisense: dTdT
AUAACAGAGACACAGUCCCTT |
| #2 siRNA | Sense:
GCACCUCAUUUGCCGAAUATT dTdT |
|
| Antisense: dTdT
UAUUCGGCAAAUGAGGUGCTT |
| #3 siRNA | Sense:
GAGGCCUAUAAUGCAAAGATT dTdT |
|
| Antisense: dTdT
UCUUUGCAUUAUAGGCCUCTT |
Reverse transcription quantitative
(RT-q)PCR
Cells were lysed using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocols, and RNA was extracted and reverse
transcribed into cDNA with a RevertAid First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc.). PCR was performed
using the complementary DNA as a template. SYBR-Green I (Roche
Diagnostics) RT-qPCR results were detected by a
LightCycler® 480 thermal cycler. Thermal cycling
conditions consisted of initial denaturation at 95°C for 5 min,
followed by 45 cycles of 95°C for 10 sec, 65°C for 20 sec and 72°C
for 30 sec. The relative results were normalized to the
GAPDH mRNA levels (24).
The primer sequences were designed using Primer Express Software
v3.0.1 (Thermo Fisher Scientific, Inc.) and are listed in Table II.
 | Table II.Primers used for the analysis of mRNA
levels by reverse transcription-quantitative PCR. |
Table II.
Primers used for the analysis of mRNA
levels by reverse transcription-quantitative PCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| DNMT1 | F:
GGCTGAGATGAGGCAAAAAG |
|
| R:
ACCAACTCGGTACAGGATGC |
| DNMT3 A | F:
AGGGAAGACTCGATCCTCGTC |
|
| R:
GTGTGTAGCTTAGCAGACTGG |
| DNMT3 B | F:
GCCTCAATGTTACCCTGGAA |
|
| R:
CAGCAGATGGTGCAGTAGGA |
| IL-6 | F:
TGCAATAACCACCCCTGACC |
|
| R:
AGCTGCGCAGAATGAGATGA |
| IL-8 | F:
GGTGCAGTTTTGCCAAGGAG |
|
| R:
TTCCTTGGGGTCCAGACAGA |
| GAPDH | F:
TCTCCTCTGACTTCAACAGCGACA |
|
| R:
CCCTGTTGCTGTAGCCAAATTCGT |
Western blot analysis
Protein was extracted from hDPCs using RIPA buffer
(Beyotime Institute of Biotechnology), and the concentrations were
detected using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Proteins (30 µg) were separated using
electrophoresis on 8% SDS-polyacrylamide gels and transferred to
PVDF membranes (EMD Millipore). Next, the membranes were blocked
with TBS-Tween 20 (20 mmol Tris-HCl, 150 mmol NaCl, 0.05% Tween-20)
containing 5% BSA (Biofroxx; neoFroxx GmbH) for 1 h at room
temperature. Then, the membranes were incubated with primary
antibodies against DNMT1 (1:2,000; Abcam), IκB kinase αβ (IKKαβ),
phosphorylated (p)-IKKαβ, p65, p-p65, IκBα, p-IκBα (1:1,000; NF-κB
Pathway Sampler kit, 9936, Cell Signaling Technology, Inc.), p38,
ERK, JNK (1:1,000; MAPK Family Antibody Sampler kit, 9926, Cell
Signaling Technology, Inc.), p-p38, p-ERK, p-JNK (1:1,000;
phospho-MAPK Family Antibody Sampler kit, 9910, Cell Signaling
Technology, Inc.) and GAPDH (1:1,000; Cell Signaling Technology,
Inc.) overnight at 4°C. After rinsing, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies
(1:2,000; AQ160P and AP307P, EMD Millipore) at room temperature for
1 h. An enhanced chemiluminescence system (EMD Millipore) was used
to visualize the antibody binding. The relative protein expression
levels were normalized to that of the GAPDH gene, and the protein
band densities were determined by ImageJ v1.47 software (National
Institutes of Health). ReBlot Plus (EMD Millipore) was used to
strip and re-probe with the p-antibodies for IκBα, p38, ERK and JNK
to distinguish different target proteins when they share similar
molecular weights with the total IκBα, p38, ERK and JNK,
respectively, on the same membrane.
ELISA
Human IL-6 ELISA kits (D6050, R&D Systems, Inc.)
and Human IL-8 ELISA kits (D8000C, R&D Systems, Inc.) were used
to analyze the culture supernatant protein concentrations of IL-6
and IL-8 collected after LPS stimulation for 6 h according to the
manufacturer's protocols. A microplate reader (Tecan Safire
microplate reader; Tecan Group, Ltd.) was used to evaluate the
optical density (OD) values. Based on the standard solution
concentration and corresponding OD value, sample concentrations
were calculated.
Methylated DNA immunoprecipitation
(MeDIP) and RT-qPCR
DNA was extracted from hDPCs and fragmented to
200–500-bp fragments with a Bioruptor Waterbath Sonicator (8
cycles, 15 sec on/15 sec off, at the highest output level while
cooling the tube to 1°C in a waterbath). Then, the DNA fragments
were diluted to 700 µl with TE buffer (Invitrogen; Thermo Fisher
Scientific, Inc.) with 60 µl Protein G Magnetic Beads (S1430S; New
England BioLabs, Inc.) and denatured for 10 min at 94°C. Following
denaturation, DNA was immunoprecipitated at 4°C overnight with an
anti-5mC antibody (1:40, C02010031; Diagenode SA). Then it was
incubated for 2 h with anti-IgG Magnetic Beads (S1430S; New England
BioLabs, Inc.) at 4°C with agitation. The beads were trapped on a
magnetic rack, the supernatant discarded, and washed three times
with 1 ml 1XIP buffer [2 mM EDTA, 20 mM Tris (pH=8.0), 1% Triton
X-100, 0.1% SDS, 150 mM NaCl] for 10 min at 4°C with agitation.
Beads were then resuspended in 400 µl of Elution Buffer (50 mM
Tris-HCl, pH=8.0; 10 mM EDTA, Ph=8.0; 1% SDS) with 10 µl of
Proteinase K (Qiagen GmbH). IP with non-specific human IgG was
measured as a negative control. After IP, the DNA samples were
eluted using phenol-chloroform and precipitated using ethanol.
After resuspending the precipitated samples in 10 µl Tris buffer,
RT-qPCR was performed using 1 µl harvested DNA fragments. The
primers designed for MeDIP-PCR are shown in Table III.
 | Table III.Primers used for methylated DNA
immunoprecipitation PCR. |
Table III.
Primers used for methylated DNA
immunoprecipitation PCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| IL6 | F:
TGGCAGCACAAGGCAAACC |
|
| R:
GCTTCAGCCCACTTAGAGGAGG |
| IL8 | F:
TAGGAAGTGTGATGACTCAGGTT |
|
| R:
GTCAGAGGAAATTCCACGATT |
| TRAF6 | F:
GCTTACTGTAGCCTTGACTGCC |
|
| R:
GTGGTGCATATCTGTAGTCTCGG |
| MYD88 | F:
TTCGCTCACCGACACAGATG |
|
| R:
GGTCACTGCGGCTGCTCTT |
Statistical analysis
All experiments were carried out at least three
times. The data were analyzed by the SPSS 20.0 software package
(IBM Corp.) and are shown as the mean ± SD. Student's t-test was
used to measure the differences between two groups. To evaluate the
differences in multiple sets of data, one-way ANOVA or
repeated-measures ANOVA with a post hoc Dunnett's test was
performed. P<0.05 was considered statistically significant.
Results
DNMT1 expression in LPS-inflamed
hDPCs
To detect the effect of LPS on the inflammatory
reaction in hDPCs, hDPCs were stimulated with LPS at a
concentration of 1 µg/ml for the indicated times. As illustrated in
Fig. 1A and B, compared with the
control group, IL-6 and IL-8 mRNA and protein
expression was significantly increased by LPS. IL-6 and IL-8
expression was upregulated and peaked after 3 h, which was followed
by a gradual decrease. The levels of DNMT1 mRNA were
significantly reduced within 24 h after treatment with LPS. DNMT1
protein expression also decreased, with the most significant change
at 3 h (Fig. 1C and D). Moreover,
the mRNA expression of DNMT3a and DNMT3b did not
change significantly before or after LPS treatment (Fig. 1).
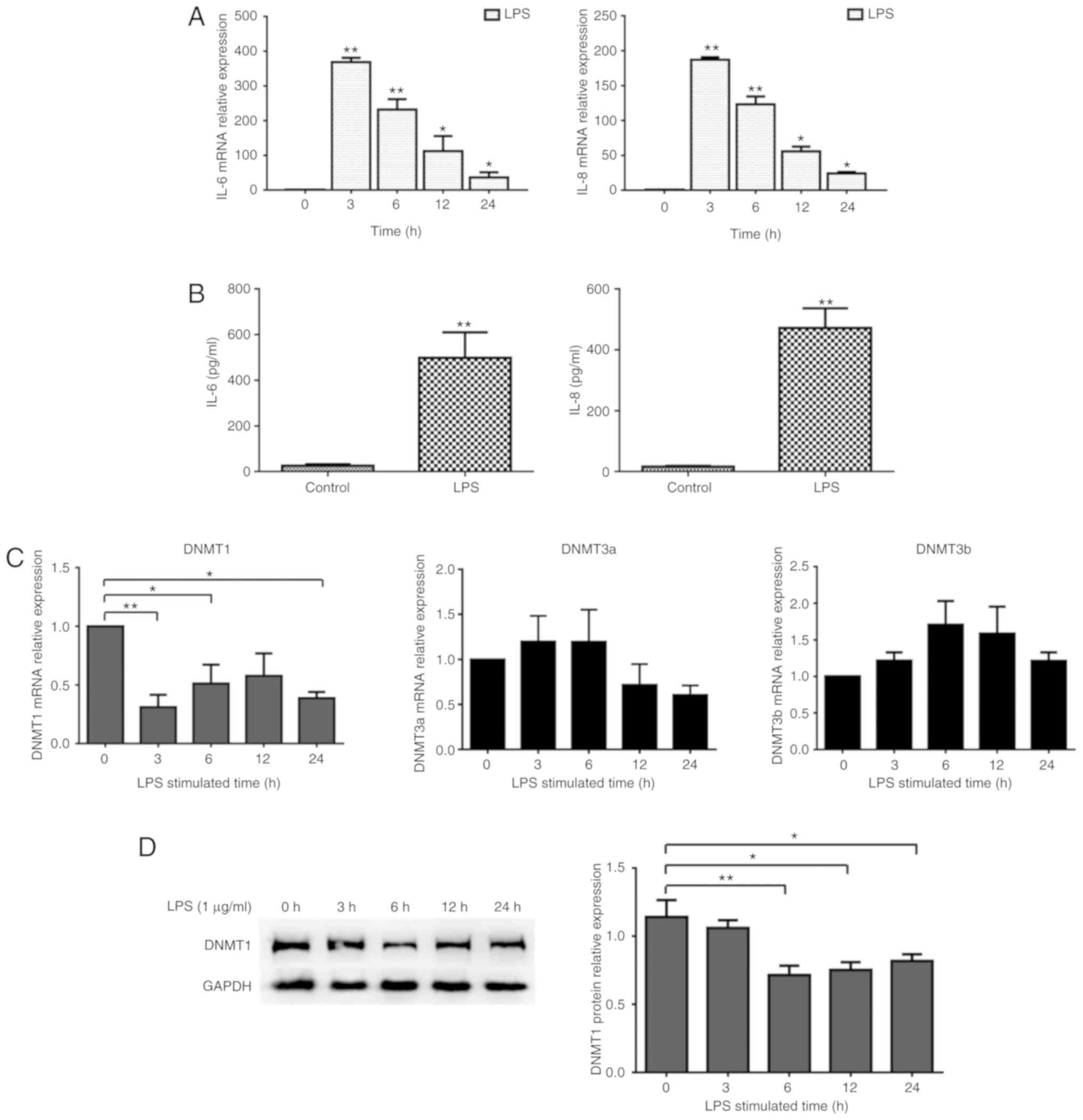 | Figure 1.DNMT expression in hDPC inflammatory
responses. (A) mRNA expression of IL-6 and IL-8 in
hDPCs was measured by RT-qPCR after stimulation with LPS for 0, 3,
6, 12 and 24 h; *P<0.05; **P<0.01. (B) Protein expression
levels of IL-6 and IL-8 from the supernatant were detected using
ELISA; **P<0.01. (C) mRNA expression of DNMT1, DNMT3a and
DNMT3b was examined using RT-qPCR in hDPCs after the
stimulation with LPS for 3, 6, 12 and 24 h. (D) Protein levels of
DNMT1 were determined by western blotting after relative
quantitative analysis; GAPDH served as the control. Results were
presented as the mean ± SD of three independent experiments.
*P<0.05, **P<0.01. DNMT, DNA methyltransferase; hDPCs, human
dental pulp cells; RT-qPCR, reverse transcription-quantitative PCR;
IL, interleukin; LPS, lipopolysaccharide. |
Effects of DNMT1 on inflammatory
cytokine expression in LPS-induced hDPCs
Our preliminary study found that 5-Aza-CdR, a DNMT
inhibitor, can increase the secretion of inflammatory cytokines in
LPS-stimulated hDPCs, and among the upregulated cytokines, IL-6 and
IL-8 experienced the greatest increase (unpublished data). To
investigate the effect of DNMT1-dependent methylation on
inflammatory cytokine production in hDPCs stimulated with LPS, the
IL-6 and IL-8 expression levels after DNMT1 knockdown in
hDPCs transfected with siRNAs were measured. As shown in Fig. 2A and B, after DNMT1 siRNA
(#1, #2 and #3) interference, DNMT1 mRNA expression levels
were significantly reduced when compared to the negative control
group. These data were further confirmed by western blotting, which
showed a reduction in the protein expression. Among the
DNMT1 siRNAs, the siRNA #1 group showed the best
interference effect at ~72% (Fig.
2B). Therefore, siRNA #1 was selected as the DNMT1
target sequence for the subsequent experiments.
IL-6 and IL-8 gene expression levels
were then measured in cells stimulated by LPS after DNMT1
depletion (Fig. 2C). The results
showed that IL-6 and IL-8 mRNA expression within 24 h
after LPS stimulation was notably higher in the DNMT1
knockdown group compared with the control group. In LPS-inflamed
hDPCs, the protein levels of IL-6 and IL-8 were also significantly
increased after DNMT1 knockdown (Fig. 2D).
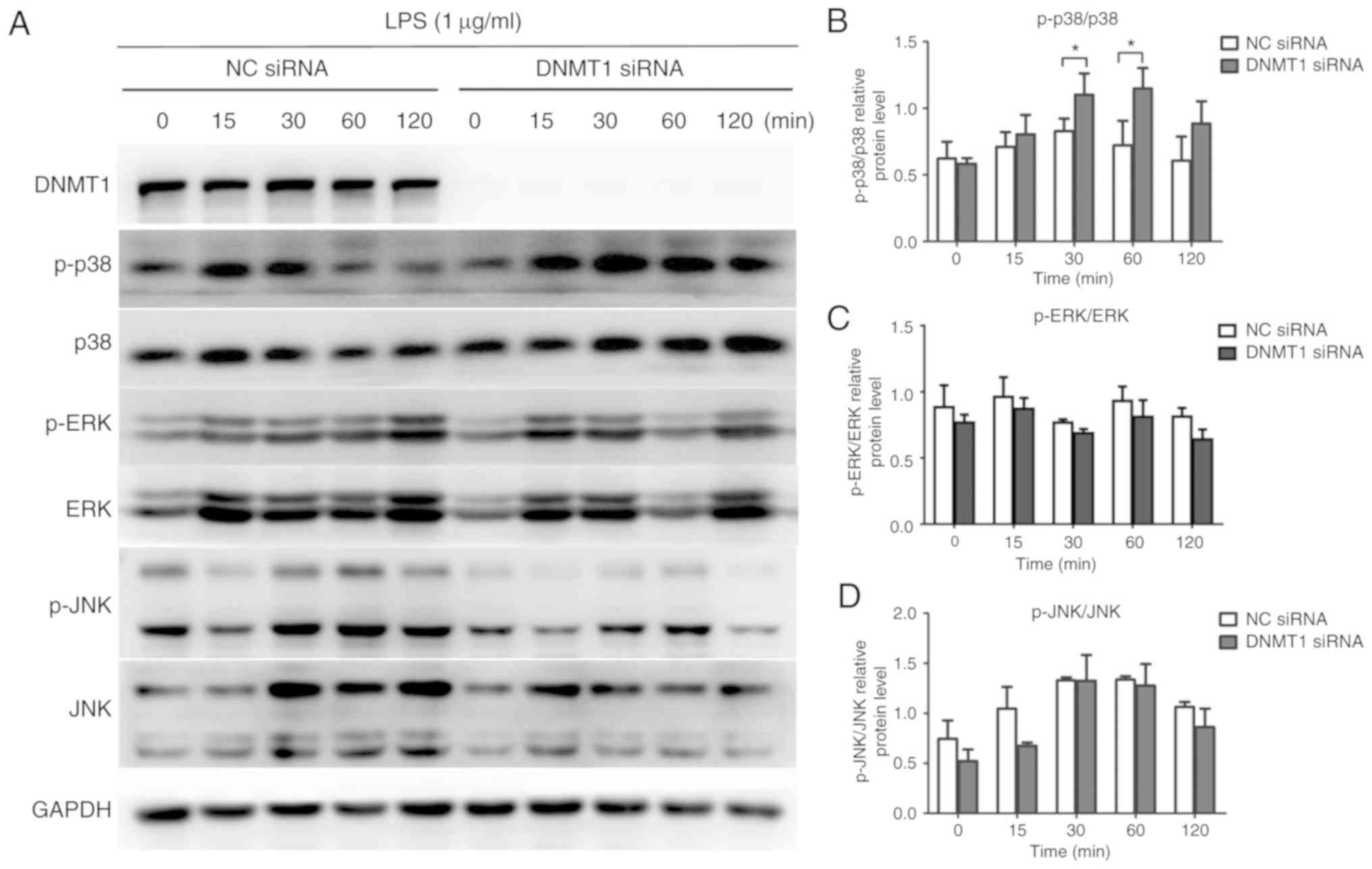
Effects of DNMT1 on the NF-κB
signaling pathway in LPS-induced hDPCs
One of the most important signaling pathways that
influences inflammatory cytokine production in inflammation induced
by LPS is the NF-κB signaling pathway (25). By means of western blotting, the
phosphorylation levels of three crucial proteins of the NF-κB
signaling pathway were examined (IKKα/β, p65 and IκBα) to determine
whether DNMT1 is engaged in NF-κB pathway activation. As
illustrated in Fig. 3A and B,
DNMT1 knockdown significantly increased the phosphorylation
of IKKα/β at 15 and 30 min after LPS treatment in hDPCs. The p65
and IκBα phosphorylation levels also increased at several time
points, but there was no significant difference.
Effects of DNMT1 on the MAPK signaling
pathway in LPS-induced hDPCs
Another vital signaling transduction pathway
involved in inflammation in the LPS-related inflammatory response
is the MAPK signaling pathway (26). The phosphorylation levels of three
key proteins in the MAPK signaling pathway were assessed (p38,
ERK1/2 and JNK) to determine whether DNMT1 plays an important role
in MAPK signaling pathway activation. As illustrated in Fig. 4A and B, after DNMT1
knockdown in LPS-inflamed hDPCs, the p38 phosphorylation level was
increased, while both p-ERK and p-JNK levels were not significantly
altered.
Effects of DNMT1 on the dynamic
methylation levels of the IL-6, IL-8, TRAF6 and MyD88 gene
promoters in LPS-induced hDPC inflammation
DNA methylation can regulate the occurrence and
progression of inflammatory responses by modulating the methylation
levels of inflammation-related cytokines and signaling molecule
promoters (27). TRAF6 and MyD88
are key intracellular signal transducers of LPS-induced signaling
pathways (28). To identify
whether the methylation of IL-6, IL-8, TRAF6 and
MyD88 was regulated through DNMT1, the levels of 5mC present
at their gene promoters were examined by means of MeDIP-PCR. The
results illustrated that the levels of 5mC at the IL-6 and
TRAF6 promoters decreased notably in LPS-stimulated hDPCs
after DNMT1 knockdown. However, no significant change was
observed in the 5mC levels of the IL-8 and MyD88
promoters (Fig. 5). These
experimental results indicated that DNMT1 can modulate the
methylation of IL-6 and TRAF6 in hDPCs stimulated by
LPS.
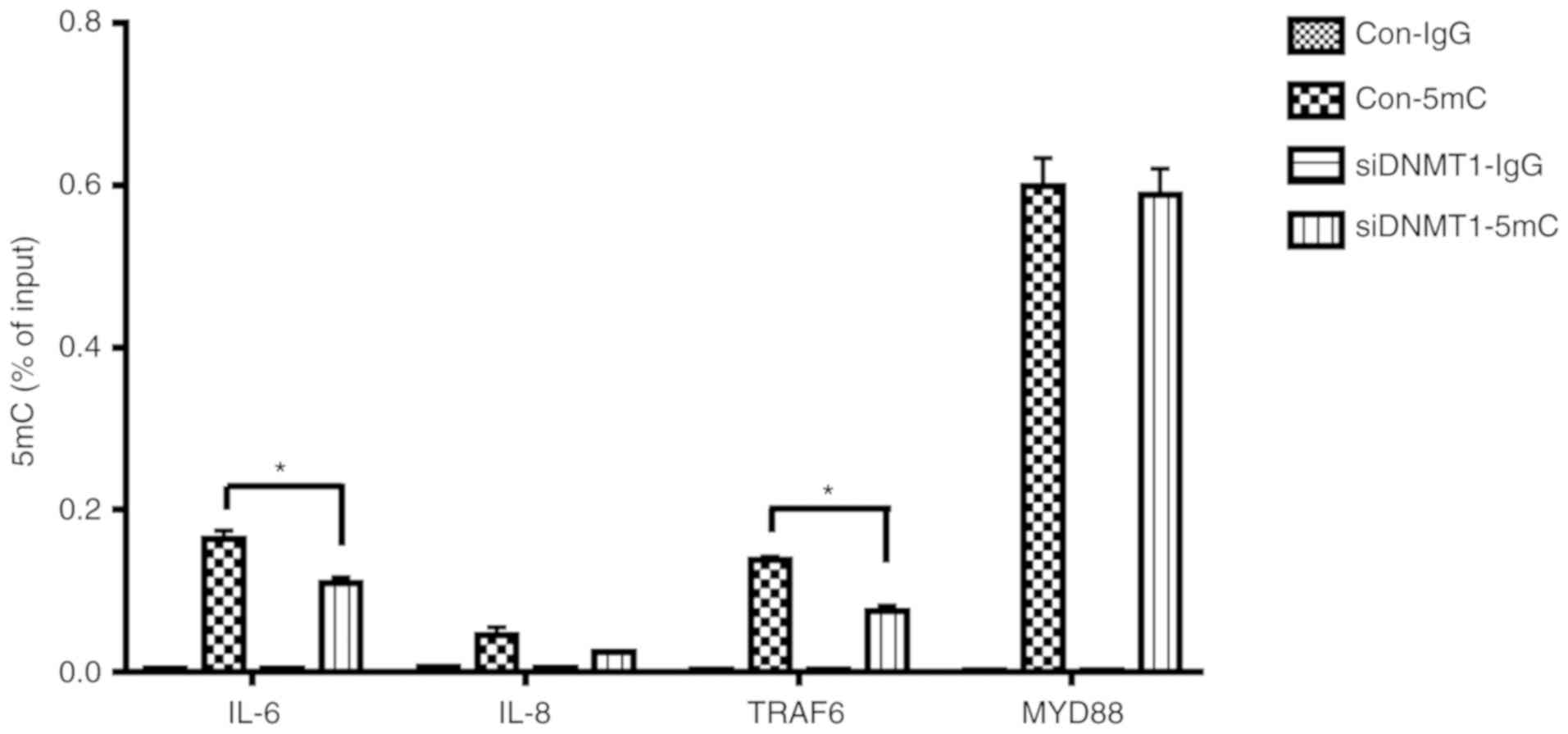 | Figure 5.Effects of DNMT1 knockdown on
the 5mC levels of the IL-6, IL-8, MyD88 and TRAF6
gene promoters in LPS-induced human dental pulp cells. IL-6,
IL-8, MyD88 and TRAF6 gene promoters' dynamic
methylation levels were assessed using methylated DNA
immunoprecipitation PCR after DNMT1 knockdown, and after 3 h of
stimulation with LPS (control groups). The results were repeated at
least three times and presented as the mean ± SD of three
independent experiments; *P<0.05. DNMT, DNA methyltransferase;
IL, interleukin; LPS, lipopolysaccharide; 5mC, 5-methylcytosine;
TRAF6, TNF receptor-associated factor 6; MyD88, myeloid
differentiation primary response 88; si, small interfering
(RNA). |
Discussion
As a major component of the outer membrane of
gram-negative bacteria, LPS serves as the primary pathogenic factor
leading to dental pulp inflammation (29). When healthy dental pulp cells are
exposed to LPS, pro-inflammatory chemokines and cytokines,
including IL-6 and IL-8, are released, thus triggering subsequent
inflammatory events (2). DNA
methylation is a major epigenetic regulator that can influence the
transcriptional expression of pro-inflammatory cytokines in the
initiation and development of the inflammatory response (15–17).
However, very little research has sought to define the function of
DNA demethylation in the development of the LPS-inflamed dental
pulp.
DNA methylation plays a pivotal role in a wide range
of inflammatory diseases, and aberrant DNA methylation is often
observed in some inflammation-related conditions (30). DNMT1 expression is increased in the
rectal epithelium during ulcerative colitis progression in patients
and may be a relatively early event in ulcerative
colitis-associated tumorigenesis; consequently, this factor may be
useful for predicting the risk of colorectal neoplasia in
ulcerative colitis (31). In
periodontitis, treating human oral keratinocytes with LPS
downregulated DNMT1 expression (32). In Sjögren's syndrome, the global
DNA methylation level in patient salivary gland epithelial cells
was reduced, with a 7-fold decrease in DNMT1 but no significant
difference in DNMT3a/b expression (33). To determine the relationship
between DNMTs and LPS-inflamed dental pulp, the expression of three
DNMTs in LPS-treated hDPCs were examined. After LPS stimulation,
both mRNA and protein expression levels of DNMT1 decreased and
reached their lowest level 3 h after stimulation. In addition, the
mRNA expression levels of DNMT3a and DNMT3b
fluctuated but did not differ significantly. These results
suggested that DNMT1-dependent methylation may be involved in the
inflammatory progression of dental pulp.
Researchers previously demonstrated that DNA
methylation can function as a key epigenetic regulator in the
pathogenesis of inflammation-related diseases (34,35).
LPS stimulation can induce pro-inflammatory cytokine expression,
and the methylation status of their gene promoters is involved in
regulating the inflammatory response. In bovine endometrial cells,
treatment with LPS can increase IL-6 and IL-8 mRNA
expression and decrease the methylation levels of specific CpG
sites at the IL-6 promoter (at −366 and −660) and the
IL-8 promoter (at −120 and −48) (35). Treating PBMCs with LPS induces the
expression of pro-inflammatory cytokines, including IL-6, TNF-α and
IL-1β, while also demethylating the IL-6 gene at the −302
and −264 CpG sites, as well as the TNF-α gene at the −371
CpG site (36). However, in human
intestinal epithelial cells, the 5 CpG sites located near the
IL-8 transcription start site (−83, −7, +73, +119 and +191)
were unmethylated on the lower and upper strands in both LPS
treated and untreated groups (37). In our previous research, SEQUENOM
MassARRAY was used to measure the methylation levels of the
IL-6 and IL-8 promoters in hDPCs after LPS
stimulation. The results showed that the methylation level at the
−276 CpG site in the IL-6 promoter decreased after LPS
stimulation. However, there was no difference in the methylation
level of the IL-8 promoter (unpublished data). In the
present study, to investigate the function of DNMT1 in inflammatory
cytokine production by hDPCs after LPS stimulation, DNMT1 knockdown
in hDPCs was established through siRNA transfection. The expression
of DNMT1 was significantly decreased following depletion of DNMT1,
which is consistent with our previous research (20,38).
Next, the LPS-stimulated cytokine expression after knocking down
DNMT1 was examined. DNMT1 silencing prominently
enhanced the production of the cytokines IL-6 and IL-8, thereby
indicating that DNMT1 may be a regulator that negatively targets
cytokine accumulation in hDPCs inflamed by LPS.
It is commonly known that the MAPK and NF-κB
signaling pathways play critical roles in mediating inflammatory
reactions and are likely regulated by DNA methylation (19,39).
In aged mouse macrophages, phosphorylation of IκBα in the NF-κB
signaling pathway was increased after treatment with the
demethylation agent 5-Aza-CdR (40). 5-Aza-CdR also increased IκBα and
IKKα/β phosphorylation levels to promote the activation of NF-κB
signaling in gastric cancer cells (41). A study on lung tissue inflammation
revealed that 5-Aza-CdR can markedly decrease p38, JNK and ERK
phosphorylation levels, thereby inhibiting MAPK signaling pathway
activation under LPS stimulation (19). The levels of DNA methylation were
affected in 27 gene promoters of the MAPK pathway in PBMCs and
plasma samples from children who were constantly exposed to air
pollutants (42). To explore
whether DNA methylation influences the signaling pathways in
LPS-treated hDPCs, the phosphorylation levels of several important
signaling molecules in the MAPK and NF-κB signaling pathways were
examined. The data from the present study showed that compared to
LPS exposure alone, DNMT1 depletion upregulated the
phosphorylation levels of IKKα/β in the NF-κB signaling pathway and
the phosphorylation level of p38 in the MAPK signaling pathway.
Therefore, DNMT1 suppressed both the MAPK and NF-κB signaling
pathways in LPS-stimulated hDPCs, further confirming that DNMT1
acts as a negative regulator in inflamed hDPCs.
Previous studies have proposed that DNA methylation
not only affects the methylation level of inflammatory cytokine
promoters, but also changes the methylation status of intracellular
signal transducers of signaling pathways (43). TRAF6 and MyD88, key intracellular
signal transducers of the MAPK and NF-κB signaling pathways, can be
regulated by DNA methylation (20,21).
TRAF6 hypermethylation has been linked to low TRAF6 gene
expression levels in PBMCs during inflammatory bowel diseases
(21). In addition, MyD88 was
shown to have consistently higher methylation levels in its
promoter region in moderate localized aggressive periodontitis
(LAP) than in severe LAP (44). In
patients with LAP, the methylation level of the MyD88 promoter is
negatively associated with several cyto/chemokines, such as IL-8
and IL-6 (44). In the present
study, to explore whether these signal transduction factors are
regulated by DNA methylation in LPS-treated hDPCs, MeDIP and
RT-qPCR were used to analyze the dynamic 5mC levels of the IL-6,
IL-8, TRAF6 and MyD88 gene promoters in
DNMT1-deficient cells. Notably, the 5mC levels of the
IL-6 and TRAF6 gene promoters decreased, suggesting
that DNMT1 knockdown downregulated 5mC at the IL-6
and TRAF6 gene promoters. Although a modest decrease in the
IL-8 and MyD88 gene promoter 5mC levels was observed,
there were no significant differences. These observations indicated
that DMNT1 can mediate the 5mC level of IL-6 and
TRAF6 in LPS-inflamed hDPCs.
In summary, the present study showed that
stimulating hDPCs with LPS decreased the expression of the DNA
methyltransferase DNMT1. DNMT1 depletion increased
LPS-induced cytokine secretion in hDPCs, and activated NF-κB and
MAPK signaling. Furthermore, silencing DNMT1 was involved in
downregulating methylation levels at the promoters of IL-6
and TRAF6. This study indicated that DNMT1-dependent DNA
methylation plays a role in the inflammatory response of hDPCs
stimulated by LPS, and provides a novel rationale for researchers
to further reveal the molecular mechanisms of inflamed dental
pulp.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
81771058).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QX designed the study and provided scientific
leadership to junior colleagues. LC and MZ performed the
experiments and statistically analyzed the results. LC wrote the
manuscript. QL and DL analyzed data, providing constructive
comments. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was authorized by the
institutional Ethical Review Boards of the Guanghua School of
Stomatology of Sun Yat-sen University, and written informed consent
for this investigation was provided from all patients who
participated in the experiment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bindal P, Ramasamy TS, Kasim NHA,
Gnanasegaran N and Chai WL: Immune responses of human dental pulp
stem cells in lipopolysaccharide-induced microenvironment. Cell
Biol Int. 42:832–840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Renard E, Gaudin A, Bienvenu G, Amiaud J,
Farges JC, Cuturi MC, Moreau A and Alliot-Licht B: Immune cells and
molecular networks in experimentally induced pulpitis. J Dent Res.
95:196–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li JG, Lin JJ, Wang ZL, Cai WK, Wang PN,
Jia Q, Zhang AS, Wu GY, Zhu GX and Ni LX: Melatonin attenuates
inflammation of acute pulpitis subjected to dental pulp injury. Am
J Transl Res. 7:66–78. 2015.PubMed/NCBI
|
|
4
|
Feng Z, Li Q, Meng R, Yi B and Xu Q:
METTL3 regulates alternative splicing of MyD88 upon the
lipopolysaccharide-induced inflammatory response in human dental
pulp cells. J Cell Mol Med. 22:2558–2568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song F, Sun H, Wang Y, Yang H, Huang L, Fu
D, Gan J and Huang C: Pannexin3 inhibits TNF-α-induced inflammatory
response by suppressing NF-κB signaling pathway in human dental
pulp cells. J Cell Mol Med. 21:444–455. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hui T, A P, Zhao Y, Yang J, Ye L and Wang
C: EZH2 regulates dental pulp inflammation by direct effect on
inflammatory factors. Arch Oral Biol. 85:16–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hui T, Wang C, Chen D, Zheng L, Huang D
and Ye L: Epigenetic regulation in dental pulp inflammation. Oral
Dis. 23:22–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bei Y, Tianqian H, Fanyuan Y, Haiyun L,
Xueyang L, Jing Y, Chenglin W and Ling Y: ASH1L suppresses matrix
metalloproteinase through mitogen-activated protein kinase
signaling pathway in pulpitis. J Endod. 43:306.e2–314.e2. 2017.
View Article : Google Scholar
|
|
9
|
Cardoso FP, Viana MB, Sobrinho AP, Diniz
MG, Brito JA, Gomes CC, Moreira PR and Gomez RS: Methylation
pattern of the IFN-gamma gene in human dental pulp. J Endod.
36:642–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith ZD and Meissner A: DNA methylation:
Roles in mammalian development. Nat Rev Genet. 14:204–220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore LD, Le T and Fan G: DNA methylation
and its basic function. Neuropsychopharmacology. 38:23–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loo SK, Ab Hamid SS, Musa M and Wong KK:
DNMT1 is associated with cell cycle and DNA replication gene sets
in diffuse large B-cell lymphoma. Pathol Res Pract. 214:134–143.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Auclair G, Guibert S, Bender A and Weber
M: Ontogeny of CpG island methylation and specificity of DNMT3
methyltransferases during embryonic development in the mouse.
Genome Biol. 15:5452014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kettunen E, Hernandez-Vargas H, Cros MP,
Durand G, Le Calvez-Kelm F, Stuopelyte K, Jarmalaite S, Salmenkivi
K, Anttila S, Wolff H, et al: Asbestos-associated genome-wide DNA
methylation changes in lung cancer. Int J Cancer. 141:2014–2029.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu J, Zhang YN, Zheng X, Zhang P, Ma G
and Tan H: Notch promotes DNMT-mediated hypermethylation of Klotho
leads to COPD-related inflammation. Exp Lung Res. 44:368–377. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen J, Wu S, Guo W, Liang S, Li X and
Yang X: Epigenetic regulation of pro-inflammatory cytokine genes in
lipopolysaccharide-stimulated peripheral blood mononuclear cells
from broilers. Immunobiology. 222:308–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng C, Huang C, Ma TT, Bian EB, He Y,
Zhang L and Li J: SOCS1 hypermethylation mediated by DNMT1 is
associated with lipopolysaccharide-induced inflammatory cytokines
in macrophages. Toxicol Lett. 225:488–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma SC, Hao YJ, Jiao Y, Wang YH, Xu LB, Mao
CY, Yang XL, Yang AN, Tian J, Zhang MH, et al: Homocysteine-induced
oxidative stress through TLR4/NF-κB/DNMT1-mediated LOX-1 DNA
methylation in endothelial cells. Mol Med Rep. 16:9181–9188. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang X, Kong G, Li Y, Zhu W, Xu H, Zhang
X, Li J, Wang L, Zhang Z, Wu Y, et al: Decitabine and 5-azacitidine
both alleviate LPS induced ARDS through
anti-inflammatory/antioxidant activity and protection of glycocalyx
and inhibition of MAPK pathways in mice. Biomed Pharmacother.
84:447–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng R, Li D, Feng Z and Xu Q: MyD88
hypermethylation mediated by DNMT1 is associated with LTA-induced
inflammatory response in human odontoblast-like cells. Cell Tissue
Res. 376:413–423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McDermott E, Ryan EJ, Tosetto M, Gibson D,
Burrage J, Keegan D, Byrne K, Crowe E, Sexton G, Malone K, et al:
DNA methylation profiling in inflammatory bowel disease provides
new insights into disease pathogenesis. J Crohns Colitis. 10:77–86.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung JY, Woo SM, Kim WJ, Lee BN, Nör JE,
Min KS, Choi CH, Koh JT, Lee KJ and Hwang YC: Simvastatin inhibits
the expression of inflammatory cytokines and cell adhesion
molecules induced by LPS in human dental pulp cells. Int Endod J.
50:377–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Guo S, Jiang K, Zhang T, Zhiming W,
Yaping Y, Jing Y, Shaukat A and Deng G: miR-488 mediates negative
regulation of the AKT/NF-κB pathway by targeting Rac1 in
LPS-induced inflammation. J Cell Physiol. 2019. View Article : Google Scholar
|
|
26
|
Shang L, Wang T, Tong D, Kang W, Liang Q
and Ge S: Prolyl hydroxylases positively regulated LPS-induced
inflammation in human gingival fibroblasts via TLR4/MyD88-mediated
AKT/NF-κB and MAPK pathways. Cell Prolif. 51:e125162018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matt SM, Lawson MA and Johnson RW: Aging
and peripheral lipopolysaccharide can modulate epigenetic
regulators and decrease IL-1β promoter DNA methylation in
microglia. Neurobiol Aging. 47:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Zhou X, Zhao Y, Xiao J, Lu Y, Shi
Q, Wang Y, Wang H and Liang Q: Polyphyllin I ameliorates
collagen-induced arthritis by suppressing the inflammation response
in macrophages through the NF-κB pathway. Front Immunol.
9:20912018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Love RM and Jenkinson HF: Invasion of
dentinal tubules by oral bacteria. Crit Rev Oral Biol Med.
13:171–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barnicle A, Seoighe C, Greally JM, Golden
A and Egan LJ: Inflammation-associated DNA methylation patterns in
epithelium of ulcerative colitis. Epigenetics. 12:591–606. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujii S, Katake Y and Tanaka H: Increased
expression of DNA methyltransferase-1 in non-neoplastic epithelium
helps predict colorectal neoplasia risk in ulcerative colitis.
Digestion. 82:179–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Camargo Pereira G, Guimarães GN,
Planello AC, Santamaria MP, de Souza AP, Line SR and Marques MR:
Porphyromonas gingivalis LPS stimulation downregulates DNMT1,
DNMT3a, and JMJD3 gene expression levels in human HaCaT
keratinocytes. Clin Oral Investig. 17:1279–1285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thabet Y, Le Dantec C, Ghedira I,
Devauchelle V, Cornec D, Pers JO and Renaudineau Y: Epigenetic
dysregulation in salivary glands from patients with primary
Sjögren's syndrome may be ascribed to infiltrating B cells. J
Autoimmun. 41:175–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hedrich CM and Tsokos GC: Epigenetic
mechanisms in systemic lupus erythematosus and other autoimmune
diseases. Trends Mol Med. 17:714–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Yan X, Nesengani LT, Ding H, Yang
L and Lu W: LPS-induces IL-6 and IL-8 gene expression in bovine
endometrial cells ‘through DNA methylation’. Gene. 677:266–272.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Angrisano T, Pero R, Peluso S, Keller S,
Sacchetti S, Bruni CB, Chiariotti L and Lembo F: LPS-induced IL-8
activation in human intestinal epithelial cells is accompanied by
specific histone H3 acetylation and methylation changes. BMC
Microbiol. 10:1722010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen J, Liu Y, Ren X, Gao K, Li Y, Li S,
Yao J and Yang X: Changes in DNA methylation and chromatin
structure of pro-inflammatory cytokines stimulated by LPS in
broiler peripheral blood mononuclear cells. Poult Sci.
95:1636–1645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mo Z, Li Q, Cai L, Zhan M and Xu Q: The
effect of DNA methylation on the miRNA expression pattern in
lipopolysaccharide-induced inflammatory responses in human dental
pulp cells. Mol Immunol. 111:11–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jangiam W, Tungjai M and Rithidech KN:
Induction of chronic oxidative stress, chronic inflammation and
aberrant patterns of DNA methylation in the liver of
titanium-exposed CBA/CaJ mice. Int J Radiat Biol. 91:389–398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang M, Xiang Y, Wang D, Gao J, Liu D,
Liu Y, Liu S and Zheng D: Dysregulated expression of miR-146a
contributes to age-related dysfunction of macrophages. Aging Cell.
11:29–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim TW, Lee SJ, Oh BM, Lee H, Uhm TG, Min
JK, Park YJ, Yoon SR, Kim BY, Kim JW, et al: Epigenetic
modification of TLR4 promotes activation of NF-κB by regulating
methyl-CpG-binding domain protein 2 and Sp1 in gastric cancer.
Oncotarget. 7:4195–4209. 2016.PubMed/NCBI
|
|
42
|
Carmona JJ, Sofer T, Hutchinson J, Cantone
L, Coull B, Maity A, Vokonas P, Lin X, Schwartz J and Baccarelli
AA: Short-term airborne particulate matter exposure alters the
epigenetic landscape of human genes associated with the
mitogen-activated protein kinase network: A cross-sectional study.
Environ Health. 13:942014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, Feng Z, Li Q, Yi B and Xu Q: DNA
methylcytosine dioxygenase ten-eleven translocation 2 enhances
lipopolysaccharide-induced cytokine expression in human dental pulp
cells by regulating MyD88 hydroxymethylation. Cell Tissue Res.
373:477–485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shaddox LM, Mullersman AF, Huang H, Wallet
SM, Langaee T and Aukhil I: Epigenetic regulation of inflammation
in localized aggressive periodontitis. Clin Epigenetics. 9:942017.
View Article : Google Scholar : PubMed/NCBI
|