Introduction
Renal interstitial fibrosis (RIF), which represents
a universal pathway for all progressive kidney diseases, has long
been associated with progressive renal function loss and end-stage
renal disease (1,2). RIF is characterized by the excessive
extracellular matrix component deposition in the tubular
interstitium by activated fibroblasts (also referred to as
myofibroblasts) (3,4). Activated fibroblasts often express
α-smooth muscle actin (α-SMA), fibronectin, fibroblast-specific
protein 1 (FSP-1) and collagen I (5,6).
Changes in the expression levels of these proteins are often
accompanied by the epithelial-mesenchymal transition (EMT), in
which endothelial cells and tubular epithelial cells transform into
a more mesenchymal-like phenotype (5,7).
This transition is characterized by the loss of epithelial proteins
including E-cadherin, cytokeratin and zonula occludens-1, and the
upregulation of mesenchymal markers, including α-SMA, fibronectin,
vimentin, FSP-1 and collagen I (8,9).
During EMT in RIF, the EMT of tubular epithelial cells serves a key
function (4,5), and transforming growth factor β1
(TGF-β1) is regarded as a central regulator of the process. TGF-β1
is able to initiate and support the progression of the entire EMT
process (7,10).
Bone morphogenetic protein-7 (BMP-7) is a member of
the TGF-β superfamily of proteins. Previous studies have revealed
that in the mature kidney, BMP-7 exhibits protective and
regenerative potential, and also serves a crucial function in
suppressing the gradual development of RIF in a mouse model of
unilateral urethral obstruction (11–13).
Furthermore, it has been reported that the exogenous administration
of BMP-7 or BMP-7 mimics may present a promising therapeutic option
for serious diseases of the kidney (14,15).
However, BMP-7 is freely soluble in water and has a short
biological half-life span in vivo, which results in the
maintenance of local concentrations being difficult (16). Lentiviral-based gene therapy
systems offer prolonged gene expression (17), and may be ideal for gene therapy
strategies. Therefore, the present study constructed lentiviral
vectors that overexpress BMP-7 and evaluated the potential function
and mechanism of BMP-7 in the progression of RIF. Furthermore, to
the best of our knowledge, the effect of BMP-7 on the migration
induced by TGF-β1 during EMT, a key event in RIF, has not yet been
determined.
Previous studies have demonstrated that BMP-7
attenuates TGF-β-induced EMT in cholangiocarcinoma (18) and pulmonary fibrosis (19). However, the effect and mechanisms
of BMP-7 on EMT during RIF remain yet to be elucidated. In the
present study, it was hypothesized that BMP-7 may inhibit
TGF-β1-induced EMT in renal tubule epithelial cells. To validate
this hypothesis, lentiviral vectors were used to overexpress BMP-7
in human renal proximal tubular epithelial cells (HK-2). Cells were
treated with TGF-β1 for various durations and concentrations of
TGF-β1. Subsequently, the potential effects of BMP-7 on EMT and the
potential underlying mechanisms of BMP-7 in HK-2 cells were
determined.
Materials and methods
Reagents and antibodies
TGF-β1 was obtained from R&D Systems, Inc.
(Minneapolis, MN, USA). Lipofectamine® 3000 Transfection
Reagent (cat. no. L3000015) was purchased from Thermo Fisher
Scientific, Inc. Anti-E-cadherin (cat. no. ab76055), anti-α-SMA
(cat. no. ab5694), anti-FSP-1 (cat. no. ab41532), anti-collagen I
(cat. no. ab34710), anti-vimentin (cat. no. ab92547), anti-Wnt3/3a
(cat. no. ab172612) and anti-BMP-7 (cat. no. ab56023) antibodies
were purchased from Abcam (Cambridge, UK). Anti-phospho-Smad2 (cat.
no. 3108), anti-phospho-Smad3 (cat. no. 9520), anti-Smad2 (cat. no.
3122), anti-Smad3 (cat. no. 9513), anti-glycogen synthase kinase 3β
(GSK-3β; cat. no. 12456), anti-phospho-GSK-3β (cat. no. 5558),
anti-phospho-β-Catenin (Ser33/37/Thr41) (cat. no. 9561),
anti-Non-phospho (Active) β-Catenin (Ser33/37/Thr41) (cat. no.
8814), anti-rabbit horseradish peroxidase (HRP)-linked secondary
(cat. no. 7074) or anti-mouse HRP-linked secondary (cat. no. 7076)
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-β-actin (cat. no. sc-8432) antibody was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Cell culture and treatment
The cell lines HK-2 (cat. no. CRL-2190™) and HEK
293T (cat. no. CRL-11268™) were purchased from the American Type
Culture Collection (Manassas, VA, USA). HK-2 cells were maintained
in Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2 in a humidified incubator. HEK 293T cells
were grown in DMEM media (Gibco; Thermo Fisher Scientific, Inc.)
under the same conditions. For inducing EMT, cells were starved
without serum for 12 h and subsequently treated with different
concentrations of TGF-β1 (0, 2, 5 and 10 ng/ml) at 37°C for 0, 24
and 48 h as described previously (20).
Lentiviral vectors for BMP-7
overexpression
pGCL-green fluorescent protein (GFP)-lentivirus
carrying a full-length human BMP-7 cDNA sequence (LV-BMP-7) and a
non-targeting sequence (LV-Control) were synthesized by Shanghai
GeneChem Co., Ltd. Lentivirus packaging and infection were
performed as described previously (21). One day before transfection,
1.2×107 HEK 293T cells were plated at 90–95% confluence
(in 15-cm dishes). At the time of transfection, the packaging HEK
293T cells were cotransfected with pGC-LV (20 µg), plasmid pHelper
1.0 (15 µg) and plasmid pHelper 2.0 (10 µg) using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.).
The titer of the recombinant lentiviral vector was 3×108
TU/ml. The recombinant lentivirus was stored at −80°C. For
lentiviral infection, add recombinant lentivirus (4 µl) at 3×10
8 TU/ml to each well (2×105 cells per well in
6-well plates). Polybrene (Shanghai GeneChem Co., Ltd.) was added
to a final concentration of 5 µg/ml. Following incubation at 37°C
for 24 h, the cell culture medium was then replaced with DMEM and
10% (vol/vol) FBS. Cells were incubated at 37°C for another 48 h
and then the stable overexpression of BMP-7 in HK-2 cells was
confirmed using reverse transcription-quantitative (RT-q)PCR and
western blotting.
RT-qPCR
Total RNA from HK-2 cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and cDNA was synthesized using a M-MLV kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's protocol.
Specific primers for α-SMA, collagen I, FSP-1, vimentin,
E-cadherin, BMP-7 and GAPDH are listed in Table I. qPCR was performed using a
SYBR-Green Real-Time PCR assay kit (Takara Bio, Inc.) on a CFX96
Touch Sequence Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The thermocycling conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 35
amplification cycles at 95°C for 15 sec and 60°C for 1 min. GAPDH
was used as the endogenous control for normalization, and the
expression was analyzed using the 2−ΔΔCq method
(22).
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primers |
|---|
| α-SMA |
|
Forward |
5′-CCGAGATCTCACCGACTACC-3′ |
|
Reverse |
5′-TCCAGAGCGACATAGCACAG-3′ |
| FSP-1 |
|
Forward |
5′-ACCTCTCTGTTCAGCACTTCC-3′ |
|
Reverse |
5′-GAACTTGTCACCCTCGTTGC-3′ |
| Collagen I |
|
Forward |
5′-ACATGCCGAGACTTGAGACTCA-3′ |
|
Reverse |
5′-GCATCCATAGTACATCCTTGGTTAGG-3′ |
| E-cadherin |
|
Forward |
5′-CACACTGATGGTGAGGGTACAAGG-3′ |
|
Reverse |
5′-GGGCTTCAGGAACACATACATGG-3′ |
| Vimentin |
|
Forward |
5′-GTTTCCCCTAAACCGCTAGG-3′ |
|
Reverse |
5′-AGCGAGAGTGGCAGAGGA-3′ |
| BMP-7 |
|
Forward |
5′-TGGCAGCATCCAATGAACAAGATCC-3′ |
|
Reverse |
5′-TTCCTTTCGCACAGACACCAATGTG-3′ |
| GAPDH |
|
Forward |
5′-ACAAGATGGTGAAGGTCGGTG-3′ |
|
Reverse |
5′-AGAAGGCAGCCCTGGTAACC-3′ |
Western blotting
Western blotting was performed as described
previously (23,24). Total protein was isolated from HK-2
cells using NE-PER™ Nuclear and Cytoplasmic Extraction reagents
(Thermo Fisher Scientific, Inc.; cat. no. 78833), and the protein
concentration was examined using a Pierce bicinchoninic acid assay
(Pierce; Thermo Fisher Scientific, Inc.; cat. no. 23227). Equal
quantities of protein (20 µg) were loaded on a 10–12% SDS-gel and
resolved using SDS-PAGE. Resolved proteins were transferred to a
0.22 µm polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). Subsequently, membranes were blocked using 5%
non-fat milk for 1 h at room temperature and incubated overnight at
4°C with anti-E-cadherin (1:1,000), anti-α-SMA (1:1,000),
anti-FSP-1 (1:1,000), anti-collagen I (1:1,000), anti-vimentin
(1:1,000), anti-Wnt3/3a (1:500), anti-BMP-7 (1:1,000),
anti-phospho-Smad2 (1:500), anti-phospho-Smad3 (1:500),
anti-phospho-GSK-3β (1:1,000), anti-phospho-β-Catenin (1:1,000),
anti-Smad2 (1:1,000), anti-Smad3 (1:1,000), anti-GSK-3β (1,000),
anti-Non-phospho (Active) β-Catenin (1:1,000) and anti-β-actin
(1:2,000). Subsequent to incubation with the primary antibodies,
membranes were washed with 0.1% tris-buffered saline with 0.5%
Tween-20 for 3 times, and the membranes were incubated at room
temperature for 1 h with the anti-rabbit or anti-mouse HRP-linked
secondary antibodies (1:2,000). Signals were visualized using an
enhanced chemiluminescence detection system (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Densitometry analysis was performed using Quantity One version 4.62
(Bio-Rad Laboratories, Inc.).
Cell Counting Kit-8 and cell
morphology assays
A total of 3×103 HK-2 cells were plated
in 96-well plates and, after a 24 h incubation period, cells were
treated with different concentrations of TGF-β1 (0, 2, 5 and 10
ng/ml) at 37°C for 72 h. Subsequently, 10 µl CCK8 solution (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was added to each
well and incubated at 37°C for a further 2 h, and cell viability
was determined by measuring the absorbance at 450 nm on a
spectrophotometer. Cell morphological changes were observed by
phase-contrast light microscopy (magnification ×100; Olympus
Corporation).
Migration assay
HK-2 cells transfected with LV-BMP-7 and control
untransfected cells were pre-treated with 10 ng/ml TGF-β1 at 37°C
for 48 h. Cells were suspended in 200 µl serum-free medium. Cell
suspensions containing 10 ng/ml TGF-β1 were added to the upper
chamber of a Transwell insert (8 µm pores; Corning, Inc.) at a
density of 1×105 cells/ml (200 µl per chamber). The
lower chamber contained culture medium (600 µl per chamber)
supplemented with 10% FBS which was used as a chemoattractant.
Cells were incubated at 37°C for 24 h. Cells on the upper surfaces
of the chambers were gently scraped off with cotton swabs and the
cells which had migrated to the lower surface of the chamber were
fixed with 100% methanol at room temperature for 20 min, stained
with 0.1% crystal violet at room temperature for 5 min, and the
number of cells in 10 random fields of view were counted per well
using a light microscope (magnification ×100; Olympus
Corporation).
Statistical analysis
GraphPad Prism version 8 (GraphPad Software Inc., La
Jolla, CA, USA) was used for all statistical analyses. Data are
presented as the mean ± standard error of the mean. A one-way or
two-way analysis of variance or an unpaired Student's t-test were
used to compare differences between groups and the LSD test served
as the post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Transfection of LV-BMP-7 increases
BMP-7 mRNA and protein expressions levels in HK-2 cells
To assess the potential effect of BMP-7 on EMT in
RIF, lentiviral vectors encoding the BMP-7 gene were used to infect
HK-2 cells. Infection efficiencies were visualized by fluorescence
microscopy (magnification, ×100). To evaluate the efficiency of
transfection, RT-qPCR and western blotting were performed.
Subsequent to viral infection, >90% of the cells were
GFP-positive, suggesting a high infection efficiency (Fig. 1). Additionally, as presented in
Fig. 2, BMP-7 mRNA (Fig. 2A) and protein (Fig. 2B) expression levels in the HK-2
infected cells were significantly increased compared with the
LV-Control and normal control cells (all P<0.001).
 | Figure 1.Lentiviral infection efficiency of
LV-Control and LV-BMP-7. HK-2 cells were transfected with
LV-Control or LV-BMP-7 for 72 h, and the infection efficiency was
observed by a fluorescent microscope. Left, bright field; right,
GFP. GFP expression (right panels) revealed that >90% of the
cells had been successfully infected with lentiviruses.
Magnification, ×100. Scale bar, 100 µm. BMP-7, bone morphogenetic
protein-7; LV-Control, pGCL-GFP-lentivirus carrying a non-targeting
sequence; LV-BMP-7, pGCL-GFP-lentivirus carrying full-length human
BMP-7 cDNA sequence; HK-2, human renal proximal tubular epithelial
cells; GFP, green fluorescent protein. |
BMP-7 overexpression alters expression
of EMT-associated genes in HK-2 cells
The expression of EMT-associated biomarkers in
infected and control HK-2 cells was determined. BMP-7
overexpression significantly increased the mRNA expression levels
of E-cadherin, and significantly reduced the mRNA expression levels
of α-SMA, collagen I, FSP-1 and vimentin compared with the
LV-Control cells (P<0.01; Fig.
3A). Furthermore, western blotting exhibited similar changes in
the protein expression of these proteins (Fig. 3B).
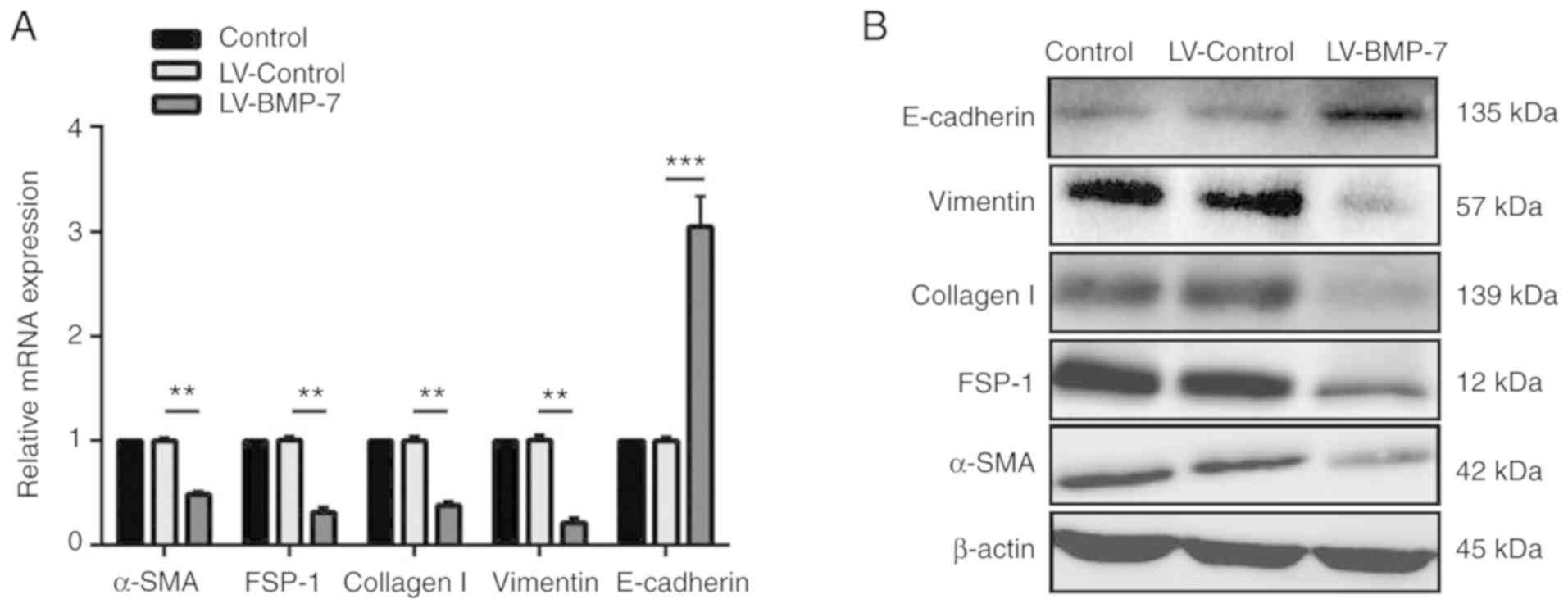 | Figure 3.BMP-7 overexpression alters the
expression of EMT-associated genes in HK-2 cells. (A) BMP-7
overexpression significantly reduced the mRNA expression of α-SMA,
FSP-1, collagen I and vimentin, and significantly increased
E-cadherin expression levels. Data are normalized to GAPDH.
**P<0.01 and ***P<0.001 vs. LV-Control group. (B) Western
blotting revealed that the protein expression of α-SMA, FSP-1,
collagen I and vimentin was substantially decreased in the BMP-7
overexpressing cells, whereas the expression of E-cadherin was
increased. BMP-7, bone morphogenetic protein-7; LV-Control,
pGCL-green fluorescent protein-lentivirus carrying a non-targeting
sequence; LV-BMP-7, pGCL-green fluorescent protein-lentivirus
carrying full-length human BMP-7 cDNA sequence; HK-2, human renal
proximal tubular epithelial cells; α-SMA, α-smooth muscle actin;
FSP-1, fibroblast-specific protein 1; EMT,
epithelial-to-mesenchymal transition. |
BMP-7 overexpression reverses the
effects of TGF-β1
TGF-β1 has been demonstrated to be a strong promoter
of EMT in renal tubular epithelial cells (25). Additionally, TGF-β1-induced EMT
results in reduced cell proliferation (26). The effect of TGF-β1 on cell
viability in HK-2 cells overexpressing BMP-7 was assessed. A CCK-8
assay was performed to assess cell viability in the HK-2 cells.
Cell viability of HK-2 cells was not significantly affected by 5
ng/ml TGF-β1 (Fig. 4A). After 72
h, 10 ng/ml TGF-β1 significantly inhibited the viability rate of
HK-2 cells (P=0.0003 vs. cells treated with 2 or 5 ng/ml TGF-β1;
Fig. 4A), however, in the BMP-7
overexpressing cells, the viability rate was partially restored
(P=0.0022 vs. cells treated with LV-Control+10 ng/ml TGF-β1;
Fig. 4A).
To further investigate the potential function of
BMP-7 in regulating the migratory capacity of HK-2 cells, Transwell
assays were performed. As presented in Fig. 4B, 10 ng/ml TGF-β1 significantly
increased the migratory capacity of HK-2 cells (P=0.0008 vs. cells
infected with the LV-Control or LV-BMP-7) and overexpression of
BMP-7 significantly reversed this effect (P=0.0262 vs. cells
treated with LV-Control+10 ng/ml TGF-β1). These results demonstrate
that BMP-7 overexpression reversed the suppression of viability and
the increase in the migration induced by TGF-β1 in HK-2 cells.
BMP-7 overexpression inhibits
TGF-β1-induced EMT in HK-2 cells
To investigate whether BMP-7 overexpression resulted
in the suppression of TGF-β1-induced EMT, HK-2 cells were treated
with 10 ng/ml TGF-β1 for 48 h and the morphological changes were
observed in HK-2 cells. As presented in Fig. 5A, HK-2 cells, which traditionally
exhibit a cobblestone-like morphology, exhibited a spindle-like
morphology when treated with TGF-β1, and also exhibited reduced
cell-cell adhesion. However, in cells overexpressing BMP-7,
treatment with TGF-β1 did not result in morphological changes.
Western blotting was performed to assess the expression levels of
EMT-associated markers. As presented in Fig. 5B, 10 ng/ml TGF-β1 reduced BMP-7
expression in a time-dependent manner in the HK-2 cells. This
reduction was accompanied by increased expression of the
mesenchymal markers α-SMA, collagen I, FSP-1 and vimentin, and
decreased the expression of E-cadherin, an epithelial marker.
However, overexpressing BMP-7 in cells resulted in the upregulation
of E-cadherin and downregulation of the mesenchymal markers α-SMA,
collagen I, FSP-1 and vimentin compared with untransfected cells
treated with TGF-β1.
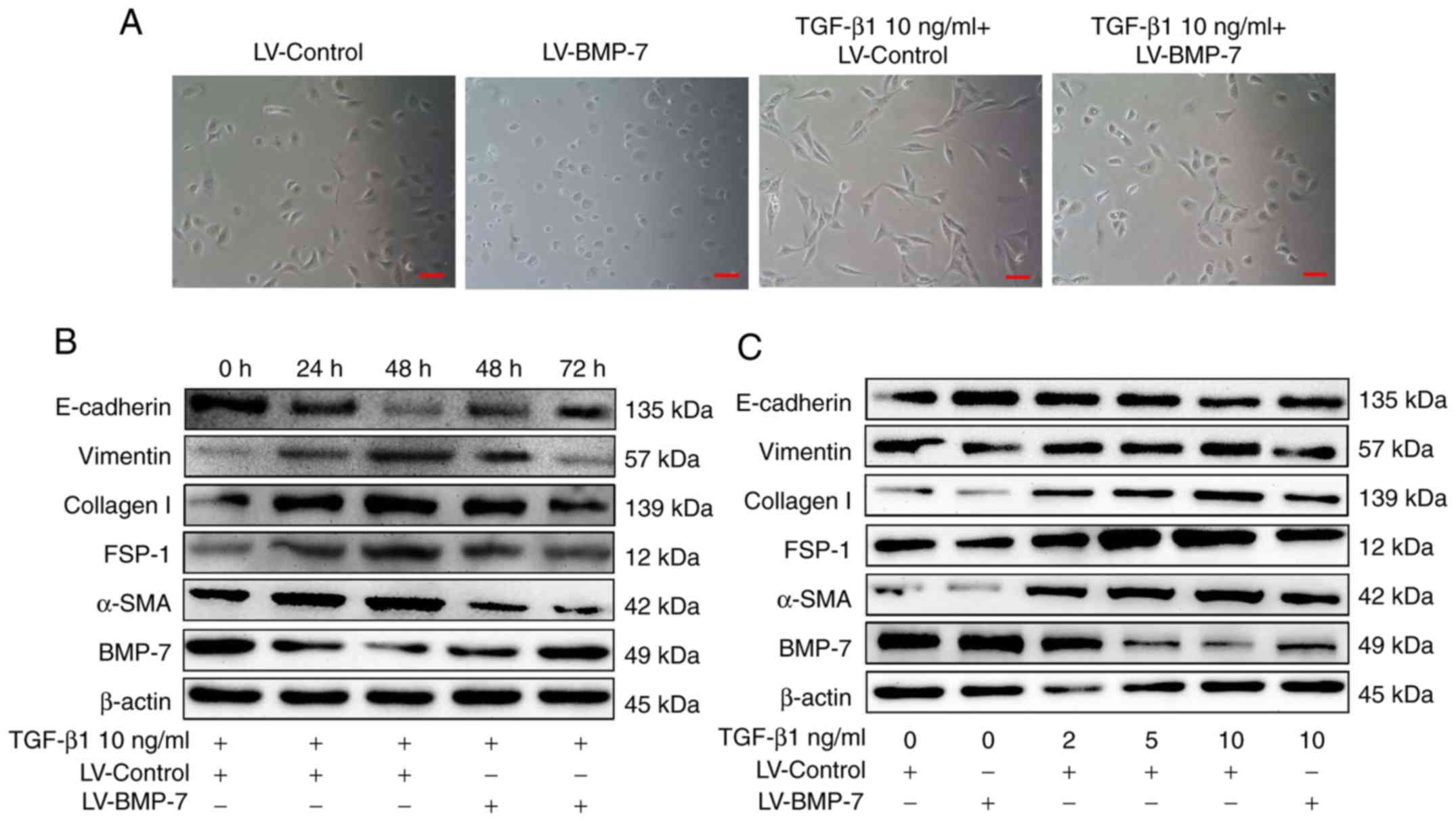 | Figure 5.BMP-7 overexpression notably
abrogates TGF-β1-induced epithelial-mesenchymal transition in HK-2
cells. (A) Representative images of morphological changes in HK-2
cells after 48 h. Scale bar, 100 µm. TGF-β1 treatment substantially
increased expression of α-SMA, collagen I, FSP-1 and vimentin and
decreased expression of E-cadherin in a (B) time- and (C)
dose-dependent manner. BMP-7 overexpression notably suppressed the
TGF-β1-induced decrease in E-cadherin expression and increase in
α-SMA, collagen I, FSP-1 and vimentin expression. BMP-7, bone
morphogenetic protein-7; HK-2, human renal proximal tubular
epithelial cells; α-SMA, smooth muscle actin; FSP-1,
fibroblast-specific protein 1; TGF-β1, transforming growth factor
β1; LV-Control, pGCL-green fluorescent protein-lentivirus carrying
a non-targeting sequence; LV-BMP-7, pGCL-green fluorescent
protein-lentivirus carrying full-length human BMP-7 cDNA
sequence. |
To determine the effect of different concentrations
of TGF-β1 on the expression of EMT markers, HK-2 cells were
incubated with 0, 2, 5 and 10 ng/ml TGF-β1 for 48 h, and the
expression of EMT markers were determined by western blotting.
TGF-β1 substantially decreased the protein expression of the
epithelial marker E-cadherin and increased the expression of the
mesenchymal markers α-SMA, collagen I, FSP-1 and vimentin in a
dose-dependent manner, with peak expression observed with 10 ng/ml
TGF-β1 (Fig. 5C). Next, the effect
of TGF-β1 on the BMP-7 overexpressing cells was determined with
regards to the expression of EMT markers. Treatment with 10 ng/ml
TGF-β1 did not result in a change in the expression of epithelial
and mesenchymal markers in the overexpressing cells, with the cells
possessing an epithelial expression profile (Fig. 5C). These data suggest that TGF-β1
induced EMT in a time- and dose-dependent manner in HK-2 cells, and
that the overexpression of BMP-7 reversed the effects of
TGF-β1.
BMP-7 overexpression antagonizes the
TGF-β1-induced EMT of HK-2 cells by inhibiting Wnt3/β-catenin and
TGF-β1/Smad2/3 signaling
To elucidate the mechanism by which BMP-7 treatment
inhibited TGF-β1-induced EMT in HK-2 cells, the Wnt3/β-catenin and
TGF-β1/Smad2/3 signaling pathways were assessed. As presented in
Fig. 6A, TGF-β1 activated the
Wnt3/β-catenin and TGF-β1/Smad2/3 signaling pathways in a
time-dependent manner. However, in the BMP-7-overexpressing HK-2
cells, the activation of the two signaling pathways were notably
reduced.
To determine the effect of different concentrations
of TGF-β1 on the activation of the Wnt3/β-catenin and
TGF-β1/Smad2/3 signaling pathways, HK-2 cells were incubated with
various concentrations of TGF-β1 for 48 h, and the protein
expression of members of the Wnt3/β-catenin and TGF-β1/Smad2/3
signaling pathways were determined. The expression of Wnt3/3a,
active β-catenin, phospho-Smad2 and phospho-Smad3 were increased
substantially, in addition to the expression of phospho-β-catenin
and phospho-GSK-3β being decreased, whereas this effect was
reversed in the BMP-7-overexpressing cells after 72 h of treatment
(Fig. 6B). Altogether, these
results demonstrate that BMP-7 overexpression notably attenuated
the TGF-β1-induced activation of Wnt3/β-catenin and TGF-β1/Smad2/3
signaling pathways in HK-2 cells.
Discussion
The present study demonstrated that BMP-7
overexpression suppressed TGF-β1-induced EMT in HK-2 cells. BMP-7
overexpressing exhibited a notably higher expression of E-cadherin
and treatment with TGF-β1 did not affect E-cadherin expression.
Expression of the mesenchymal markers was substantially lower in
the BMP-7 overexpressing cells compared with the control cells
treated with TGF-β1. Additionally, changes in the morphology
induced by TGF-β1 were not observed in the BMP-7 overexpressing
cells. Furthermore, the mechanism by which BMP-7 overexpression
prevented TGF-β1-induced EMT was associated with inhibition of the
Wnt3/β-catenin and TGF-β1/Smad2/3 signaling pathways.
RIF is considered as the end outcome common to all
end-stage renal diseases. Previous studies have demonstrated that
tubular epithelial cells undergoing EMT is a crucial event in the
progression of RIF (5,27). Therefore, blocking EMT may be an
effective treatment method for preventing the progression of RIF.
Studies have revealed that TGF-β1 is crucially involved in the
pathogenesis of RIF and is associated with progressive kidney
diseases (6,25). TGF-β1, a primary inducer of EMT,
has been demonstrated to be necessary and sufficient for initiating
and supporting the entire EMT process (4,28).
Therefore, inhibiting TGF-β1-mediated signaling may be a promising
therapeutic option for treating patients with RIF. Previously,
BMP-7 has been demonstrated to antagonize TGF-β1-mediated fibrosis
through the suppression of EMT in fibroses of a number of different
organs, including the lung and liver (29–32).
However, the effects of BMP-7 on EMT in RIF have not yet been
determined previously.
A universal feature of EMT is the loss of expression
of epithelial markers and gain of expression of mesenchymal markers
(33,34). Considering that TGF-β1 is a
critical mediator which contributes to EMT in RIF, the present
study specifically examined whether BMP-7 overexpression reversed
TGF-β1-induced EMT. Lentiviral vectors were used to overexpress
BMP-7 in HK-2 cells to examine its effects on cell morphology, cell
viability, migration and changes in the expression of EMT markers
(α-SMA, collagen I, FSP-1, vimentin and E-cadherin), and the effect
of TGF-β1 stimulation in BMP-7 expressing cells. Treatment with 10
ng/ml TGF-β1 for 48 h resulted in untransfected HK-2 cells
exhibiting a mesenchymal phenotype. Furthermore, TGF-β1 treatment
induced the inhibition of cell viability and resulted in increased
migration in HK-2 cells. These alterations were accompanied with a
notably increased expression of mesenchymal markers (α-SMA,
collagen I, FSP-1 and vimentin), and a decreased expression of
E-cadherin. These results are in agreement with previous studies
(35–37). Interestingly, BMP-7 overexpression
prevented this transformation from an epithelial to mesenchymal
phenotype when treated with TGF-β1. Therefore, the results of the
present study revealed that BMP-7 overexpression may notably impede
EMT, suggesting that BMP-7 exhibits a protective potentially
tumor-suppressive effect in RIF.
The Wnt3/β-catenin signaling pathway is a highly
conserved, extremely complex pathway which is thought to be
associated with the pathogenesis of RIF (38,39).
Previously, studies have revealed that Wnt3/β-catenin signaling is
associated with proteinuria, renal dysfunction and renal fibrosis
in a variety of chronic kidney diseases (40,41).
An increased mesenchymal-like phenotype, consisting of reduced
E-cadherin expression, and increased β-catenin and N-cadherin
expression, have been demonstrated to serve a key function in EMT
progression in renal fibrosis (14,34).
It was hypothesized that the BMP-7 mediated attenuation of
TGF-β1-induced EMT may be through inhibition of the Wnt3/β-catenin
signaling pathway. BMP-7 overexpression substantially attenuated
the activation of the Wnt3/β-catenin signaling pathway, which was
induced by TGF-β1. These results are consistent with previous
studies demonstrating that blocking the Wnt3/β-catenin signaling
attenuates EMT in RIF (39,42,43).
Numerous studies have demonstrated that the
TGF-β1/Smad2/3 signaling pathway is an important molecular pathway
which regulates EMT in RIF (6,44,45).
The results of the present study demonstrated that HK-2 cells
overexpressing BMP-7 overexpression displayed the notably reduced
phosphorylation of Smad2/3 when treated with TGF-β1. This result
suggested that BMP-7 overexpression inhibited TGF-β1-induced EMT
through inhibiting the Smad2/3 signaling pathway. However, the
precise mechanisms underlying the inhibition of Wnt3/β-catenin and
TGF-β1/Smad2/3 signaling by BMP-7 requires further study.
In summary, the present study identified a novel
mechanism by which BMP-7 overexpression significantly attenuated
TGF-β1-induced EMT, by suppressing Wnt3/β-catenin and
TGF-β1/Smad2/3 signaling. The results highlight a potentially novel
mechanism for preventing EMT in patients with RIF.
Acknowledgements
The authors would like to thank Professor Yang Jiang
(Department of Hematology, The Second Hospital of Shandong
University, Shandong University, Shandong, China) for statistical
consultation.
Funding
The present study was supported by the Key Research
and Development Plan of Shandong Province (grant nos. 2018GSF118084
and 2017GSF18113), the Youth Foundation of the Second Hospital of
Shandong University (grant no. 2018YT28) and the National Natural
Science Foundation of China (grant nos. 81570653 and 81773790).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS, GL and CP designed the research. YS, SLv, FW,
XL, JC, SLi and XW conducted the experiments. WC and GG analyzed
the data. YS and CP wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berchtold L, Ponte B, Moll S, Hadaya K,
Seyde O, Bachtler M, Vallee JP, Martin PY, Pasch A and de Seigneux
S: Phosphocalcic markers and calcification propensity for
assessment of interstitial fibrosis and vascular lesions in kidney
allograft recipients. PLoS One. 11:e01679292016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng GH, Wang YJ, Wen X, Han XR, Shen M,
Wang S, Zhuang J, Zhang ZF, Wang L, Hu B, et al: Silencing of
SOCS-1 and SOCS-3 suppresses renal interstitial fibrosis by
alleviating renal tubular damage in a rat model of hydronephrosis.
J Cell Biochem. 119:2200–2211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mengel M: Deconstructing interstitial
fibrosis and tubular atrophy: A step toward precision medicine in
renal transplantation. Kidney Int. 92:553–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farris AB and Colvin RB: Renal
interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol
Hypertens. 21:289–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Y, Peng C, Lv S, Cheng J, Liu S, Wen
Q, Guan G and Liu G: Adipose-derived stem cells ameliorate renal
interstitial fibrosis through inhibition of EMT and inflammatory
response via TGF-β1 signaling pathway. Int Immunopharmacol.
44:115–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S and Susztak K: Epithelial
plasticity versus EMT in kidney fibrosis. Trends Mol Med. 22:4–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu XY, Chai JJ, Chen YP, Rui HL, Wang YY,
Dong HR, Man YL and Cheng H: Hirsutella sinensis attenuates
aristolochic acid-induced renal tubular epithelial-mesenchymal
transition by inhibiting TGF-β1 and snail expression. PLoS One.
11:e01492422016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li A, Zhang X, Shu M, Wu M, Wang J, Zhang
J, Wang R, Li P and Wang Y: Arctigenin suppresses renal
interstitial fibrosis in a rat model of obstructive nephropathy.
Phytomedicine. 30:28–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutariya B, Jhonsa D and Saraf MN: TGF-β:
The connecting link between nephropathy and fibrosis.
Immunopharmacol Immunotoxicol. 38:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ninichuk V, Gross O, Segerer S, Hoffmann
R, Radomska E, Buchstaller A, Huss R, Akis N, Schlöndorff D and
Anders HJ: Multipotent mesenchymal stem cells reduce interstitial
fibrosis but do not delay progression of chronic kidney disease in
collagen4A3-deficient mice. Kidney Int. 70:121–129. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao J, Wang W, Li Y, Xia J, Peng Y, Zhang
Y and Xia A: Artesunate attenuates unilateral ureteral
obstruction-induced renal fibrosis by regulating the expressions of
bone morphogenetic protein-7 and uterine sensitization-associated
gene-1 in rats. Int Urol Nephrol. 48:619–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Zhao J, Zhang J, Wei J, Zhang J
and Huang Y: Protective effect of BMP-7 against aristolochic
acid-induced renal tubular epithelial cell injury. Toxicol Lett.
198:348–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng XM, Chung AC and Lan HY: Role of the
TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond).
124:243–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohigashi M, Imai N, Toba H, Kobara M and
Nakata T: Pitavastatin exhibits protective effects on podocytes
accompanied by BMP-7 up-regulation and Rho suppression.
Pharmacology. 97:265–276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kabuto Y, Morihara T, Sukenari T, Kida Y,
Oda R, Arai Y, Sawada K, Matsuda K, Kawata M, Tabata Y, et al:
Stimulation of rotator cuff repair by sustained release of bone
morphogenetic protein-7 using a gelatin hydrogel sheet. Tissue Eng
Part A. 21:2025–2033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugiyama O, An DS, Kung SP, Feeley BT,
Gamradt S, Liu NQ, Chen IS and Lieberman JR: Lentivirus-mediated
gene transfer induces long-term transgene expression of BMP-2 in
vitro and new bone formation in vivo. Mol Ther. 11:390–398. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duangkumpha K, Techasen A, Loilome W,
Namwat N, Thanan R, Khuntikeo N and Yongvanit P: BMP-7 blocks the
effects of TGF-β-induced EMT in cholangiocarcinoma. Tumour Biol.
35:9667–9676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang D, Wang Y, Zhu Z, Yang G, An G, Li
X, Niu P, Chen L and Tian L: BMP-7 attenuated silica-induced
pulmonary fibrosis through modulation of the balance between
TGF-β/Smad and BMP-7/Smad signaling pathway. Chem Biol Interact.
243:72–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JH, He L, Zou ZM, Ding ZC, Zhang X,
Wang H, Zhou P, Xie L, Xing S and Yi CZ: A novel inhibitor of
homodimerization targeting MyD88 ameliorates renal interstitial
fibrosis by counteracting TGF-β1-induced EMT in vivo and in vitro.
Kidney Blood Press Res. 43:1677–1687. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng C, Zhao H, Chen W, Song Y, Wang X, Li
J, Qiao Y, Wu D, Ma S, Wang X and Gao C: Identification of SHCBP1
as a novel downstream target gene of SS18-SSX1 and its functional
analysis in progression of synovial sarcoma. Oncotarget.
7:66822–66834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan D, Deng S, Gan W, Li S and Li Y:
Curcumol attenuates epithelial-mesenchymal transition of
nasopharyngeal carcinoma cells via TGF-β1. Mol Med Rep.
17:7513–7520. 2018.PubMed/NCBI
|
|
24
|
Peng C, Song Y, Chen W, Wang X, Liu X,
Wang F, Wu D, Ma S, Wang X and Gao C: FLVCR1 promotes the
proliferation and tumorigenicity of synovial sarcoma through
inhibiting apoptosis and autophagy. Int J Oncol. 2018. View Article : Google Scholar
|
|
25
|
Loboda A, Sobczak M, Jozkowicz A and Dulak
J: TGF-β1/Smads and miR-21 in renal fibrosis and inflammation.
Mediators Inflamm. 2016:83192832016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moore LD, Isayeva T, Siegal GP and
Ponnazhagan S: Silencing of transforming growth factor-β1 in situ
by RNA interference for breast cancer: Implications for
proliferation and migration in vitro and metastasis in vivo. Clin
Cancer Res. 14:4961–4970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei SY, Wang YX, Zhang QF, Zhao SL, Diao
TT, Li JS, Qi WR, He YX, Guo XY, Zhang MZ, et al: Multiple
mechanisms are involved in salt-sensitive hypertension-induced
renal injury and interstitial fibrosis. Sci Rep. 7:459522017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang YY, Jiang H, Pan J, Huang XR, Wang
YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY and Chen JH:
Macrophage-to-myofibroblast transition contributes to interstitial
fibrosis in chronic renal allograft injury. J Am Soc Nephrol.
28:2053–2067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao J, Li Y, Peng Y, Zhang Y, Li H, Li R
and Xia A: Febuxostat prevents renal interstitial fibrosis by the
activation of BMP-7 signaling and inhibition of USAG-1 expression
in rats. Am J Nephrol. 42:369–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang D, An G, Zhu Z, Wang Y, Yang G, Li
X, Niu P, Chen L and Tian L: The protective effects of bone
morphogenetic protein-7 against epithelial injury and matrix
metalloproteases upregulation induced by silica in vitro. Hum Exp
Toxicol. 36:892–900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, An G, Wang Y, Liang D, Zhu Z, Lian
X, Niu P, Guo C and Tian L: Anti-fibrotic effects of bone
morphogenetic protein-7-modified bone marrow mesenchymal stem cells
on silica-induced pulmonary fibrosis. Exp Mol Pathol. 102:70–77.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LP, Dong JZ, Xiong LJ, Shi KQ, Zou
ZL, Zhang SN, Cao ST, Lin Z and Chen YP: BMP-7 attenuates liver
fibrosis via regulation of epidermal growth factor receptor. Int J
Clin Exp Pathol. 7:3537–3547. 2014.PubMed/NCBI
|
|
33
|
Liu J, Zhong Y, Liu G, Zhang X, Xiao B,
Huang S, Liu H and He L: Role of Stat3 signaling in control of EMT
of tubular epithelial cells during renal fibrosis. Cell Physiol
Biochem. 42:2552–2558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Q, Yang W, Wang X, Li X, Qi S, Zhang
Y and Gao MQ: TGF-β1 induces EMT in bovine mammary epithelial cells
through the TGFβ1/Smad signaling pathway. Cell Physiol Biochem.
43:82–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai J, Xiao X, Zhang X, Cui H, Hao J, Han
J and Cao N: Erythropoietin inhibits hypoxia-induced
epithelial-to-mesenchymal transition via upregulation of miR-200b
in HK-2 cells. Cell Physiol Biochem. 42:269–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang S, Liu F, Niu Q, Li Y, Liu C, Zhang
L, Ni D and Pu X: GLIPR-2 overexpression in HK-2 cells promotes
cell EMT and migration through ERK1/2 activation. PLoS One.
8:e585742013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou J, Cheng H, Wang Z, Chen H, Suo C,
Zhang H, Zhang J, Yang Y, Geng L, Gu M and Tan R: Bortezomib
attenuates renal interstitial fibrosis in kidney transplantation
via regulating the EMT induced by TNF-α-Smurf1-Akt-mTOR-P70S6K
pathway. J Cell Mol Med. 23:5390–5402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nlandu-Khodo S, Neelisetty S, Phillips M,
Manolopoulou M, Bhave G, May L, Clark PE, Yang H, Fogo AB, Harris
RC, et al: Blocking TGF-β and β-Catenin epithelial crosstalk
exacerbates CKD. J Am Soc Nephrol. 28:3490–3503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin X, Zha Y, Zeng XZ, Dong R, Wang QH and
Wang DT: Role of the Wnt/β-catenin signaling pathway in inducing
apoptosis and renal fibrosis in 5/6-nephrectomized rats. Mol Med
Rep. 15:3575–3582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xue H, Xiao Z, Zhang J, Wen J, Wang Y,
Chang Z, Zhao J, Gao X, Du J and Chen YG: Disruption of the Dapper3
gene aggravates ureteral obstruction-mediated renal fibrosis by
amplifying Wnt/β-catenin signaling. J Biol Chem. 288:15006–15014.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang MQ, Wang L, Cao AL, Zhao J, Chen X,
Wang YM, Wang H and Peng W: HuangQi decoction improves renal
tubulointerstitial fibrosis in mice by inhibiting the up-regulation
of Wnt/β-catenin signaling pathway. Cell Physiol Biochem.
36:655–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsu YC, Chang PJ, Ho C, Huang YT, Shih YH,
Wang CJ and Lin CL: Protective effects of miR-29a on diabetic
glomerular dysfunction by modulation of DKK1/Wnt/β-catenin
signaling. Sci Rep. 6:305752016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsujimura T, Idei M, Yoshikawa M, Takase O
and Hishikawa K: Roles and regulation of bone morphogenetic
protein-7 in kidney development and diseases. World J Stem Cells.
8:288–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang Y, Tong J, He F, Yu X, Fan L, Hu J,
Tan J and Chen Z: miR-141 regulates TGF-β1-induced
epithelial-mesenchymal transition through repression of HIPK2
expression in renal tubular epithelial cells. Int J Mol Med.
35:311–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peng C, Zhao H, Song Y, Chen W, Wang X,
Liu X, Zhang C, Zhao J, Li J, Cheng G, et al: SHCBP1 promotes
synovial sarcoma cell metastasis via targeting TGF-β1/Smad
signaling pathway and is associated with poor prognosis. J Exp Clin
Cancer Res. 36:1412017. View Article : Google Scholar : PubMed/NCBI
|




















