Introduction
Cyclic nucleotide phosphodiesterases (PDEs) are a
superfamily of enzymes that catalyze cAMP and cGMP intracellular
secondary messengers and regulate a wide array of genes and
proteins. Phosphodiesterase 4 (PDE4) is a member of a family of
enzymes that selectively degrade intracellular cAMP by increasing
levels of cAMP, and PDE4 inhibitors are used in the treatment of
asthma, chronic obstructive pulmonary disease (COPD) and atopic
dermatitis (1,2). Various isoforms of PDE4 have been
shown to interact with other proteins and lipids, allowing specific
isoforms to be targeted to distinct intracellular sites and
signaling complexes within cells. Our previous study reported that
ovalbumin (OVA) sensitization and challenge significantly increased
the activity of PDE4 and mRNA expression of PDE4A, PDE4C and PDE4D
in the lung of a rat model of allergic asthma, and rolipram
significantly inhibited the upregulation of PDE4 (3). PDE4B is mainly expressed in
neutrophils and monocytes and is essential in the
lipopolysaccharide (LPS)-induced secretion of tumor necrosis factor
(TNF-α) (4–6), whereas PDE4D has been shown to be
associated with airway hyper-reactivity. Singh et al
(7) reported that prenatal
exposure to cigarette smoke affected airway reactivity by
modulating levels of cAMP in the lung through changes in the
activity of PDE4D, indicating the importance of altered PDE4
activity.
Ciclamilast
[N-(3,5-dichloropyrid-4-yl)-3-cyclopentyl-oxy-4-methylbenzamid] is
a novel PDE4 inhibitor and is structurally analogous to piclamilast
(RP 73401). In terms of its side effects, our previous study
reported that ciclamilast may be a superior PDE4 inhibitor with
fewer gastrointestinal side effects than piclamilast (8), and oral administration of ciclamilast
significantly improved lung function and reduced the secretion of
cytokines, neutrophil infiltration and goblet cell hyperplasia in
OVA-sensitized and challenged mice (9), however, there is no currently
available data to compare the activity of ciclamilast to
piclamilast in treating allergic lung inflammation.
To compare the activities of ciclamilast and
piclamilast, and elucidate the possible biochemical basis for the
superior activity of ciclamilast, the present study investigated
the effects of ciclamilast on the activity and expression of PDE4
in the lung of an allergic rat model, and compared them with the
effects of piclamilast and dexamethasone. In addition, the present
study performed a systemic pharmacodynamic review of the novel PDE4
inhibitor ciclamilast in asthma.
Materials and methods
Sensitization and treatment
A total of 70 Male Sprague-Dawley rats (weight,
150–170 g; age, 5–6 weeks old), were purchased from the Laboratory
Animal Center of Zhejiang University School of Medicine (Zhejiang,
China), and were initially housed in a light-controlled (12-h
light/dark cycle), humidity-controlled (50–60%) and
temperature-controlled (22–25°C) room with free access to food and
water. The animal experiments were approved by The Zhejiang Medical
Laboratory Animal Administration Committee.
The rats were sensitized subcutaneously with an
injection (1 ml) of a saline suspension containing 0.2% OVA
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 10% aluminum
hydroxide into the footpad, neck, back, groin and abdomen on day 0.
The rats were aerosolized with OVA (1% in saline) for 30 min every
day between days 15 and 21 using a jet nebulizer (PARI GmbH,
Starnberg, Germany) as previously described (3) and sacrificed on day 21. Control mice
were aerosolized with an equal volume of saline. Ciclamilast and
piclamilast were suspended in 1% hydroxyethylcellulose solution.
Ciclamilast (1, 3 and 10 mg/kg/day, i.g.), piclamilast (10
mg/kg/day, i.g.), and dexamethasone (0.3 mg/kg/day, i.p.) were
administered 30 min prior to OVA challenge between days 15 and 21.
Negative (Sham) control rats were gavaged with vehicle (1%
hydroxyethylcellulose solution). Ciclamilast and piclamilast were
provided by Beijing Joinn Drug Research Center (Beijing, China) and
the administered dosages were lower than the maximum tolerated dose
of piclamilast and ciclamilast (up to 10 mg/kg in beagles).
Preparation of bronchoalveolar lavage
fluid (BALF)
Following the final OVA challenge, the rats were
anaesthetized with urethane (2 g/kg, i.p.) prior to being
sacrificed. Their lungs were lavaged with D-Hanks' solution via a
tracheal catheter. This process was repeated three times with a
total volume of 5 ml D-Hanks', and the BALF recovery rate was
>90%. Total cells counts were determined with a hemocytometer.
Slides for differential cell counts were prepared by a centrifuge
at 450 × g at 4°C for 10 min. The slides were stained with Wright's
stain, and the total numbers of eosinophils/neutrophils,
monocytes/macrophages and lymphocyte were recorded on each slide
using an Olympus CH-20 light microscope (Olympus Corporation,
Tokyo, Japan; magnification, ×400), followed by calculation of the
percent and absolute number.
Histological examination
The lungs were immersed in 4% neutral formalin for 7
days and then used to prepare paraffin blocks. The slides were
prepared at a thickness of 8-µM and then stained with alcian
blue-periodic acid-Schiff for measuring goblet cell hyperplasia and
with hematoxylin and eosin (H&E) for examining cell
infiltration and tissue lesions using an Olympus CH-20 light
microscope (Olympus Corporation; magnification, ×400). For
semi-quantified assessment of goblet cell hyperplasia, which
reflects the extent of mucus production in the airway epithelium,
the numbers of goblet cells in at least three different fields of
each lung section were recorded. The mean scores were obtained from
six rats. For semi-quantified assessment of pulmonary histological
changes, eosinophil influx, edema and epithelial lesions were
divided into five grades (10): 0,
normal; 1, rare; 2, mild; 3, moderate; 4, severe. The score was
recorded from at least three different fields of each lung section.
The mean scores were obtained from six animals. Histopathological
assessment was performed in a blinded-manner on randomized
sections.
Assay for PDE activity
The lung lobes were harvested, and 10% homogenates
were prepared using a homogenizer (JiangShu HaiMeng QiLin Medical
Instrument Factory, Haimeng, China) in ice-cold hypotonic
homogenization buffer (30 mmol/l HEPES, pH 7.4, 1 mmol/l EDTA, 1
mmol/l β-mercaptoethanol, 2 mmol/l PMSF and 10 g/l pepstatin A,
0.1% Triton X-100) and centrifuged (Eppendorf Centrifuge 5804R,
Eppendorf, Hamburg, Germany) at 1, 2000 × g for 30 min at 4°C. The
supernatants were collected and stored at −80°C until assaying the
activity of PDE. Assays of cAMP-PDE and cGMP-PDE activity have been
described in detail in our previous study (3).
Analysis of PDE4B and PDE4D mRNAs
Total RNA was isolated from each tissue using
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), preparation of first-strand cDNA from rat was performed
using First-strand cDNA Synthesis kit (Shanghai Sangon Biological
Engineering Technology and Service, Shanghai, China). Reverse
transcription was performed under the following conditions:
Denaturation (70°C for 5 min), annealing (42°C for 60 min) and
elongation (70°C for 10 min). The sequences of the polymerase chain
reaction (PCR) primers used for PDE4B and PDE4D were as reported
previously (9) and presented in
Table I. The PCR primer sets were
able to detect all known variants derived from the appropriate
PDE4B and PDE4D genes. PCR amplification was performed in a
standard PCR buffer [10 mM Tris-HCl, pH 9.0, 100 mM KCl, 80 mM
(NH4)2SO4 and 0.1% NP-40]
containing 0.2 mM of each dNTP, 1.5 mM of MgCl2, 500 nM
of each primer and 1 unit of Taq DNA polymerase in a total volume
of 25 µl for 30 cycles, with the following cycle parameters:
Denaturing, 94°C for 45 sec; annealing, 58°C for 70 sec; and
extension, 72°C for 2 min. Following PCR amplification, 8 µl each
reaction mixture was resolved by electrophoresis on a 1.5% agarose
gel containing ethidium bromide, and the PCR product bands were
quantified by using a UVP Gel Documentation system (UVP LLC,
Upland, CA, USA). The levels of PDE4 mRNAs were calculated relative
to β-actin. It was confirmed that, under these conditions, the PCR
product accumulation did not reach plateau levels.
 | Table I.Primer sequences of PDE4s in
rats. |
Table I.
Primer sequences of PDE4s in
rats.
| Gene | Primer
sequences | Amplification
product length, base pairs | Annealing
temperature, °C |
|---|
| β-actin | Forward:
5′-AACCCTAAGGCCAACCGTGAAAAG-3′ | 343 | 57 |
|
| Reverse:
5′-GCTCGAAGTCTAGGGCAACATA-3′ |
|
|
| PDE4B | Forward:
5′-AGGATTCTGAAGGACCGG-3′ | 154 | 56 |
|
| Reverse:
5′-AGATTATGTGTCGATCAG-3′ |
|
|
| PDE4D | Forward:
5′-GGCTTCATAGACTACATTG-3′ | 418 | 56 |
|
| Reverse:
5′-TTACACTGTTACGTGTCAGG-3′ |
|
|
PDE4B and PDE4D immunohistochemical
(IHC) staining
The right lower lung lobes were collected intact,
and the fresh lung tissues were snap-frozen in liquid nitrogen
prior to being stored in frozen blocks at −80°C. When cut into
sections, the lung tissues were embedded in the OCT compound in
cryomolds, and serial 8-µm-thick sections were prepared on a
cryostat (Leica CM1900; Leica Microsystems, Inc., Buffalo Grove,
IL, USA) and then mounted on glass slides pretreated with 1%
polylysine. The slides were then dried at room temperature for 5
min and fixed in ice-cold acetone for 10 min prior to being stored
at −80°C until required. In double-step immunohistochemical
staining, the slides were first incubated in 3%
H2O2 in methanol for 10–15 min at room
temperature to block any endogenous peroxidase activity and then
incubated with PDE4B-specific antibody (cat. no. PD4-201AP;
1:2,000; FabGennix International, Inc., Frisco, TX, USA) and
PDE4D-specific antibody (cat. no. PD4-401AP; 1:2,000; FabGennix
International, Inc.) at 4°C overnight. Following three rinses in
PBS, the sections were then incubated with ready-to-use
peroxidase-conjugated secondary antibody rabbit anti-rat
immunoglobulin G (IgG; cat. no. E046801; 1:500; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 30 min at room
temperature. Following three rinses with PBS, the sections were
incubated with diaminobenzidine tetrahydrochloride (cat. no.
K346811; Dako; Agilent Technologies, Inc.) solution at room
temperature for 2–8 min. The samples were then counterstained with
Mayer's hematoxylin at room temperature for 1 min, and the slides
were dehydrated and mounted. In each case, a serial H&E-stained
section was examined for orientation and the histological diagnosis
was confirmed using an Olympus CH-20 light microscope (Olympus
Corporation; magnification, ×400).
Western blot analysis of PDE4B and
PDE4D
To determine the protein levels of PDE4B and PDE4D,
whole protein was extracted from the lung. The proteins were
extracted by radioimmunoprecipitation assay buffer (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) containing 1× PhosSTOP
(Roche Diagnostics, Basel, Switzerland), 1% protease inhibitor
cocktail (Roche Diagnostics) and 2% phenylmethylsulfonyl fluoride
(Wuhan Boster Biological Technology, Ltd.) The protein
concentration was determined by the Bio-Rad Protein Assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Subsequently, 100
µg total protein was loaded into each lane and proteins were
separated by 10% SDS-PAGE gel and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA) using an
electrophoresis system (Bio-Rad Laboratories, Inc.). The membranes
were blocked at room temperature for 1 h with blocking solution,
which was composed of 5% milk prepared in TBS/Tween 20, and
subsequently incubated overnight at 4°C with the PDE4B-specific
antibody (cat. no. PD4-201AP; 1:1,500; FabGennix International,
Inc.) and PDE4D-specific antibody (cat. no. PD4-401AP; 1:500;
FabGennix International, Inc.). The membranes were washed with TBST
and incubated for 1 h at room temperature with the secondary
antibody, horseradish peroxidase-linked anti-rabbit IgG (cat. no.
E046801; 1:500; Dako; Agilent Technologies, Inc.), which was
prepared in blocking solution (1:2,000). Following washing, the
membrane was processed with a chemiluminescent kit (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and subsequently exposed
to X-ray film (XK-1; Kodak, Rochester, NY, USA). Detection of
β-actin (cat. no. sc-47778; 1:4,000; Santa Cruz Biotechnology,
Inc.) served as a loading control. Quantification was performed
using Labwork software (version 4.0; UVP, LLC).
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS (version 12; SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis, and one-way analysis of variance followed by
Dunnett's test was used to determine significant differences
between the treatment groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
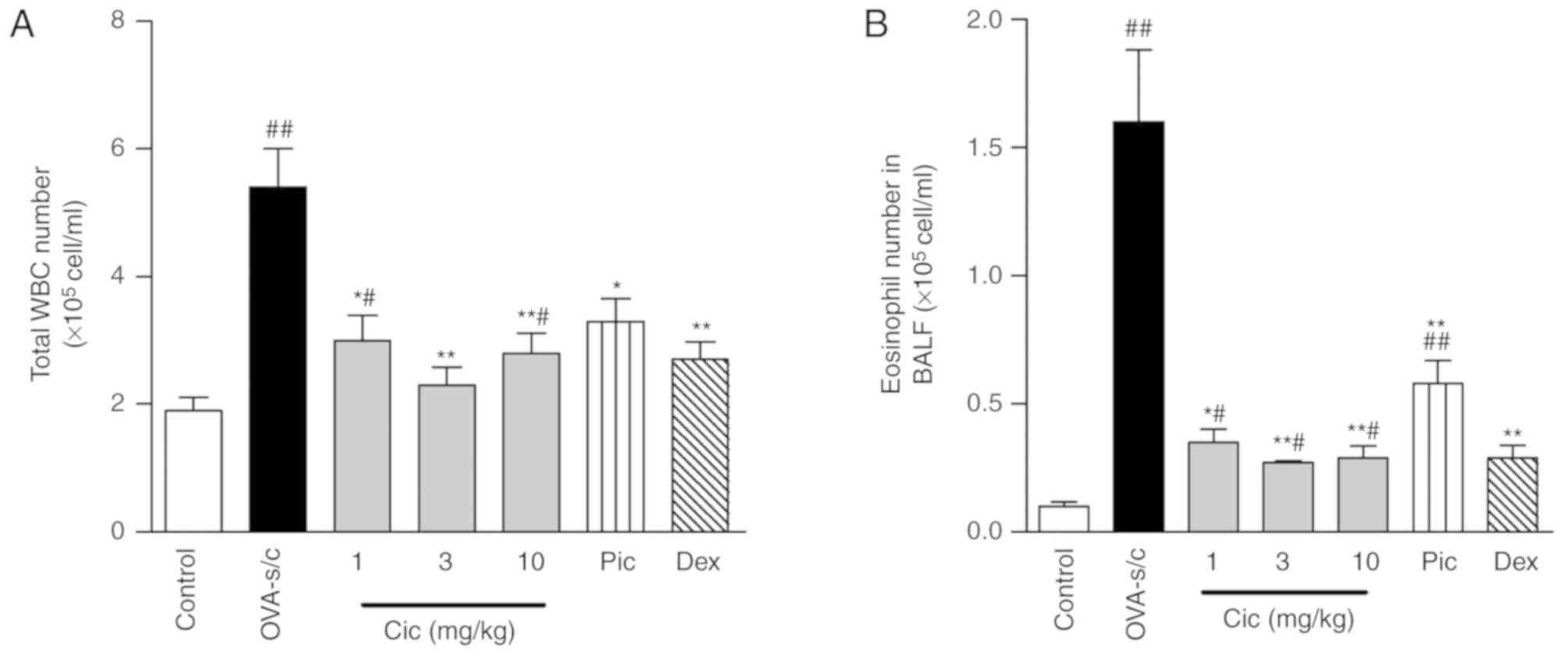
Ciclamilast decreases total cell
number and eosinophil infiltration in the BALF
The total cell number and eosinophil number in the
BALF were significantly increased in the model group. Daily
administration of different doses of ciclamilast between day 15
(the first day of challenge with aerosol ovalbumin) and 21 (the
final day of challenge) significantly reduced airway infiltration
of the cells, which was indicated by the significant reduction in
the number of total cells and eosinophils. In particular, at a dose
of 3 mg/kg, there was significant suppression. Piclamilast at a
dose of 10 mg/kg significantly affected the total cell number in
the BALF, but only marginally decreased the number of eosinophils.
Dexamethasone at a dose of 0.3 mg/kg significantly decreased the
total cell number and eosinophil number and had a similar potency
to ciclamilast at 10 mg/kg (Fig. 1A
and B).
Ciclamilast suppresses eosinophil
infiltration and mucus production in the lung
Marked eosinophilic infiltration was observed around
the airways and blood vessels in the model group compared with the
control group (Fig. 2A and B).
Dexamethasone significantly inhibited the infiltration of
inflammatory cells compared with that in the model (Fig. 2C). Treatments with 10 mg/kg
ciclamilast markedly attenuated the OVA-induced inflammatory
alterations and the epithelial lesions compared with the untreated
OVA model (Fig. 2D). Treatment
with piclamilast (10 mg/kg) did not attenuate the OVA-induced
epithelial lesions; however, it was effective in preventing
eosinophil infiltration (Fig. 2E).
In contrast, ciclamilast at a dose of 1 or 3 mg/kg was able to
inhibit the inflammatory alterations and the epithelial lesions in
the wall of small and large bronchioles (Fig. 2F). The number of goblet cells
containing acid (purple) and neutral (magenta) mucins were
increased in the OVA model group compared with control group
(Fig. 3A and B), and dexamethasone
significantly inhibited mucus secretion compared with the OVA model
(Fig. 3C). The effect of
ciclamilast was similar with dexamethasone (Fig. 3D). However, treatment with
piclamilast (10 mg/kg) did not affect goblet cell hyperplasia
(Fig. 3E). Compared with
ciclamilast, dexamethasone exhibited an increased effectiveness on
OVA-induced mucus secretion (Fig.
3F).
 | Figure 3.Representative histological images of
lung tissue mucus secretions in lung tissue harvested from OVA-s/c
rats (original magnification, ×400). Lung tissues were fixed,
sectioned at 5-µm thickness, and stained with periodic acid-Schiff
for mucus production (magenta-staining epithelial cells are
positive for mucus). (A) Control group; (B) model group; (C) Dex
0.3 mg/kg;(D) Cic 10 mg/kg; (E) Pic 10 mg/kg. (F) Using
semi-quantitative analyses, the number of goblet cell was recorded
in airway epithelia, and the summarized data show the OVA-induced
changes of mucus production and effects of Cic, Pic and Dex. Data
are shown as the mean ± standard error of the mean. There were six
rats per group. #P<0.05 and ##P<0.01
vs. control rats; *P<0.05 and **P<0.01 vs. OVA-s/c rats. OVA,
ovalbumin; OVA-s/c, OVA-sensitized and challenged; Cic,
ciclamilast; Pic, piclamilast; Dex, dexamethasone. |
Ciclamilast decreases the
allergen-induced upregulation of PDE activity in the lungs of
allergic rats
To determine the effect of ciclamilast on the
allergen-induced increase of PDE activity, the activities of
cAMP-PDE and cGMP-PDE in the lung were examined. The activity of
cAMP-PDE in the lung of the model rat was significantly elevated,
resulting in an almost 1.5-fold higher activity than that of the
normal rats (Fig. 4A). The
activity of cGMP-PDE was also increased, however, the change was
not significant (Fig. 4B). Our
previous study showed that the increase in total cAMP-PDE activity
by OVA sensitization and challenge may be caused primarily by the
upregulation of PDE4 (3). In the
present study, treatment with ciclamilast (1, 3 and 10 mg/kg)
significantly reduced the activity of cAMP-PDE in a dose-dependent
manner (ID50=0.2 mg/kg; Fig. 4A). Piclamilast 10 mg/kg and
dexamethasone 0.3 mg/kg fully inhibited the increase in cAMP-PDE
activity induced by OVA sensitization and OVA challenge
(P<0.01). Of note, ciclamilast, piclamilast and dexamethasone
also significantly decreased the activity of cGMP-PDE (Fig. 4B). At the same dosage of 10 mg/kg,
ciclamilast was more effective than piclamilast at altering
cAMP-PDE activity, but was weaker than piclamilast at altering
cGMP-PDE activity. These results suggested that ciclamilast may
have higher selectivity towards PDE4 compared with piclamilast. In
addition, dexamethasone appeared to be as effective as either PDE4
inhibitor, inhibiting the activity of both cAMP-PDE and
cGMP-PDE.
Ciclamilast downregulates the
allergen-induced mRNA expression of PDE4B and PDE4D in lung
tissues
Previous comparative studies have suggested that the
mRNA expression of PDE4A, PDE4B, PDE4C and PDE4D in several
toxicologically relevant tissues and blood leukocytes are
significantly higher in rats than in humans, and the higher
expression levels of PDE4 are correlated with a higher level of
enzyme activity in rat leukocytes (11). Our previous study confirmed that
the mRNA expression levels of PDE4A, PDE 4C and PDE 4D were
upregulated in the lung from the rat model of allergic lung
inflammation, whereas there was no effect on the mRNA expression of
PDE4B (3). To clarify the effect
of ciclamilast on PDE subtype, the present study determined the
subtype expression in lung tissues. The results showed that the
model rats had significantly higher mRNA levels of PDE4D (~2-fold)
than the control rats, but not of PDE4B (Fig. 5A and B). These results are
consistent with our earlier study. Treatment with ciclamilast at 1,
3 and 10 mg/kg, respectively, inhibited PDE4D-specific mRNA
expression by 61.8, 52.6 and 57.9% (P<0.01, some data not shown)
compared with that in the model group. Of note, ciclamilast at 10
mg/kg also inhibited PDE4B-specific mRNA expression by 27%
(P<0.05). However, piclamilast at 10 mg/kg inhibited
PDE4D-specific mRNA expression by 62% (P<0.01) but had no effect
on PDE4B. Dexamethasone also inhibited the mRNA expression of PDE4B
and PDE4D by 23 and 34%, respectively; however, the difference in
the expression levels PDE4B was not significant.
Negative feedback regulation of the
allergen-induced protein expression of PDE4B and PDE4D by
ciclamilast
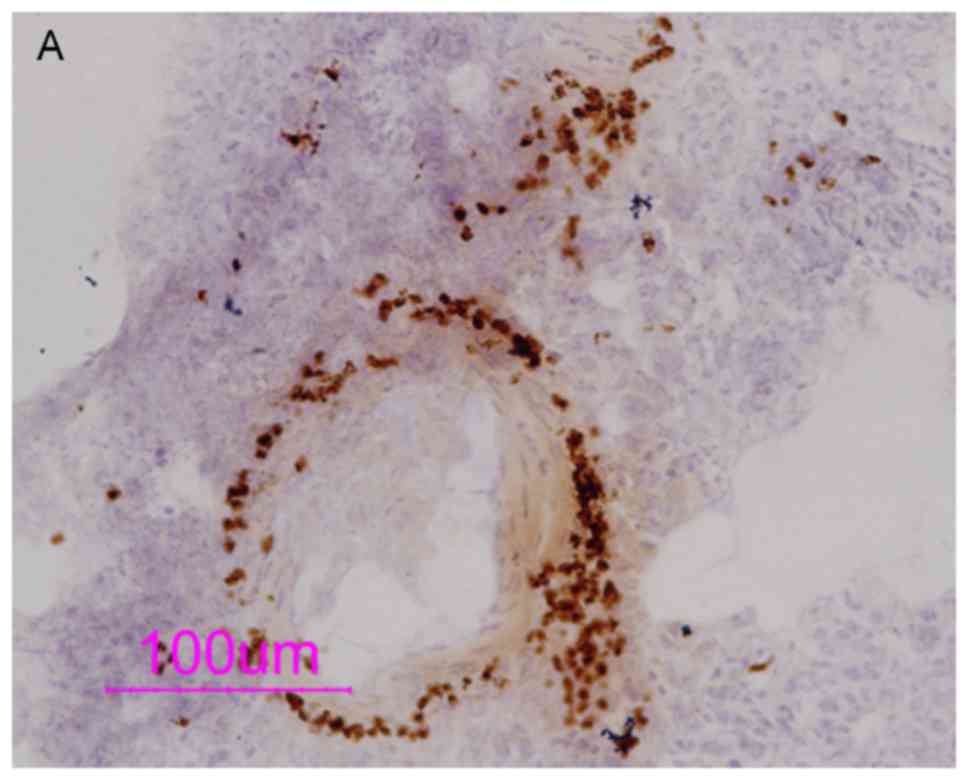
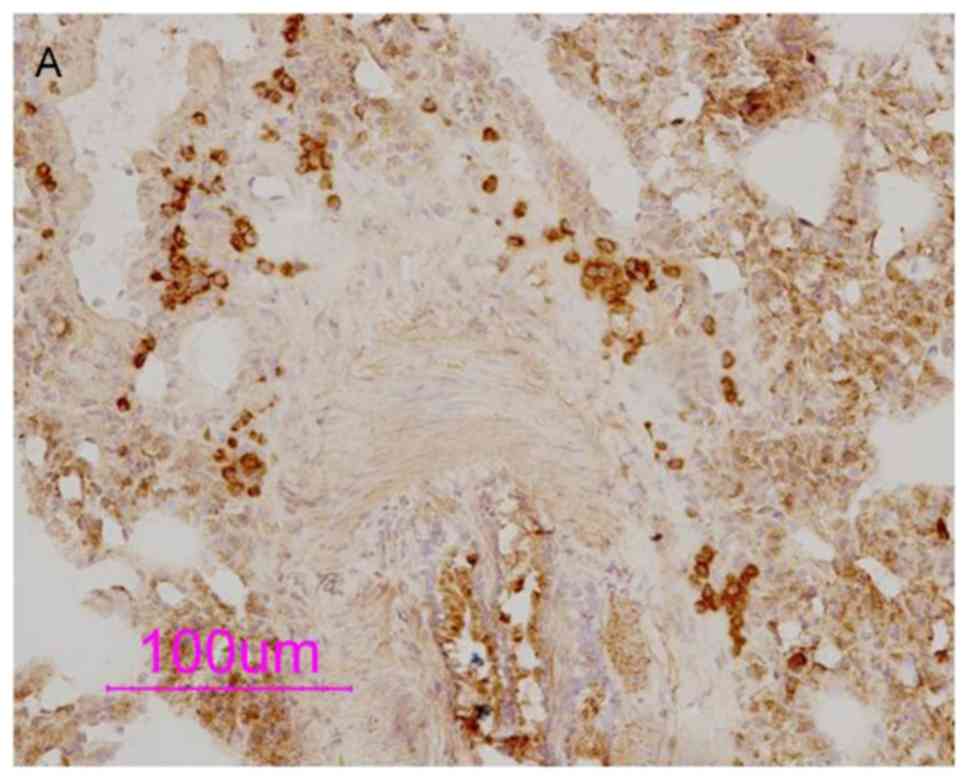
To determine the distribution of the PDE4 subtype,
IHC staining was used to detect the expression of PDE4 isoforms.
The results showed abundant expression of PDE4B and PDE4D in
inflammatory cells around the blood vessels and airway (Figs. 6 and 7). OVA sensitization and challenge caused
an increase in the protein expression of PDE4B and PDE4D in
eosinophils in contrast to the H&E-stained slides (P<0.05).
PDE4B was mainly distributed in the cytoplasm, whereas PDE4D was
mainly distributed in the cell membrane. The different distribution
of PDE4 subtypes indicates different functions of the PDE4
subtypes.
The protein expression of PDE4B in the eosinophils
of the OVA-sensitization and -challenged group was significantly
enhanced, suggesting that the combination of OVA sensitization and
OVA challenge is likely to upregulate the expression of PDE4B.
Ciclamilast increased the expression of the PDE4B (P<0.05),
whereas piclamilast did not. This result suggests that ciclamilast
exhibits higher selectivity towards PDE4B than piclamilast.
PDE4D is mainly expressed in airway smooth muscle,
vascular smooth muscle, airway epithelial cells and the membrane of
inflammatory cells. In the bronchial epithelial cells, tracheal
smooth muscle cells and vascular smooth muscle cells of the model
group, OVA-sensitization without OVA challenge group, and
OVA-challenge without OVA sensitization group, the expression of
PDE4D was significantly enhanced, indicating that challenge
following OVA sensitization, or challenge or sensitization alone
increased the expression of PDE4D, and ciclamilast inhibited this
increased expression. Although piclamilast also inhibited the
increased expression of PDE4D in the OVA-induced bronchial
epithelial cells, it did not inhibit expression in the OVA-induced
tracheal smooth muscle or vascular smooth muscle cells, in which
the expression of PDE4D was increased. This result indicated that
piclamilast had higher selectivity towards PDE4D than ciclamilast.
The expression of PDE4B/4D was decreased by treatment with
dexamethasone (P<0.05), mainly due to decreased numbers of
eosinophils.
Discussion
Asthma is characterized by airway inflammation,
tissue injury and remodeling, bronchoconstriction and mucus
hypersecretion, involving multiple tissues including leukocytes
from the circulating blood, airway sensory neurons, smooth muscle
cells and epithelial cells. Numerous preclinical in vivo
studies have shown that PDE4 inhibitors suppress characteristic
features of these diseases, including cell recruitment, activation
of inflammatory cells and physiological changes in lung function
(12). Therefore, if PDE4 is
involved in the pathogenesis of asthma, lung PDE4 may be as
important as, if not more important than, leukocyte PDE4. Various
PDE4 subtypes are known to be expressed in different tissues and
have distinct biological roles. For example, 4B, but not 4D, is the
predominant PDE4 subtype in monocytes and neutrophils (4,5),
involved in the regulation of TNF production (13). As a novel anti-inflammatory
therapy, PDE4 inhibitors have been developed for asthma and chronic
obstructive pulmonary disease COPD since the 1980s. Since 2010, the
first PDE4 inhibitor, roflumilast (Daxas®) has been
marketed in the European Union and Canada for the treatment of
severe COPD. In the majority of cases, problems in the clinical
development of PDE4 inhibitors are due to a lack of efficacy or a
low tolerated dose (14).
Therefore, the development of newer PDE4 inhibitors to overcome
this limitation has been encouraged (14,15).
In terms of piclamilast (RP 73401), various studies
have investigated its anti-inflammatory and bronchodilator
properties since 1994 (16–23).
Recent evidence shows that piclamilast prolonged the survival of
pre-term rat pups with neonatal hyperoxic lung injury by inhibiting
inflammation and reducing alveolar fibrin deposition (24), and further investigation found that
piclamilast attenuated and partially reversed pulmonary
hypertension and right ventricular hypertrophy, but did not affect
advanced alveolar development in neonatal rats with
hyperoxia-induced lung injury or affect normal lung and heart
development (25). Piclamilast
also attenuated the respiratory burst of sputum cells from patients
with mild asthma and COPD in vitro (22).
There have been various reports on the potency of
piclamilast, rolipram and roflumilast, including our previous
studies and those of others (Table
II). There is conflicting evidence regarding the potency of
piclamilast and rolipram, including effects on LPS-induced
circulating TNF-α (26). It has
been demonstrated that RP 73401 and rolipram exert the same potency
on the inhibition of antigen- and mediator-induced bronchospasms in
guinea pigs (27), and relaxation
of the tracheae pre-contracted with histamines (28). However, Bundschuh et al
(29) reported that roflumilast
exhibited effects that were 8- and 25-fold higher than those of
piclamilast and rolipram, respectively, in suppressing
allergen-induced early airway reactions. Conflicting evidence has
also been found for TNFα release, for example, Souness et al
(26) demonstrated that RP 73401
(IC50: 6.9±3.3 nM, n=5) exhibited 71-fold higher potency
than (±)-rolipram (IC50: 490±260 nM, n=4) at inhibiting
LPS-induced TNFα release from human peripheral blood monocytes, and
Wollin et al (30) reported
that rolipram exhibited more potent inhibition of OVA-induced TNF-α
release than piclamilast (9,31,32).
Our previous studies in OVA-induced allergic mice or rats also
support the hypothesis that rolipram has higher selectivity than
piclamilast. Other reports also support these results (28,30).
The reasons for the contradictory results may be due to the sources
of the drug.
 | Table II.Pharmacological characteristics of
ciclamilast and piclamilast on the ovalbumin-induced allergic
model. |
Table II.
Pharmacological characteristics of
ciclamilast and piclamilast on the ovalbumin-induced allergic
model.
| Author, year |
IC50/ED50 | Ciclamilast | Piclamilast | Rolipram | Roflumilast,
mg/kg | (Refs.) |
|---|
| Deng et al,
2006; Sun et al, 2006 | RL PD50
(95% CI) MCh (µg/kg) in mice | 0.59
(0.51–0.69) | 0.82
(0.63–1.07) | – | – | (8,31) |
| Deng et al,
2006; Sun et al, 2006 | Cdyn
PD20 (95% CI) MCh (µg/kg) in mice | 1.24
(0.88–1.76) | 0.73
(0.5–0.94) | – | – | (8,31) |
| Deng et al,
2006; Sun et al, 2006 | cAMP-PDE activity
mice | 1.8 mg/kg | 3.52 mg/kg | – | – | (8,31) |
| Tang et al,
2006 | cAMP-PDE activity
in SD rats | 0.2 mg/kg | 0.98 mg/kg | – | – | (9) |
| Holbrook et
al, 1996 | Relaxed tracheae
pre-contracted with carbachol | – | 39±1 µM | 18±5 µM | – | (28) |
| Holbrook et
al, 1996 | Relaxed tracheae
pre-contracted with histamine | – | 0.2±0.1 µM | 0.2±0.1 µM | – | (28) |
| Wollin et
al, 2006 | Inhibition of
airway hyper-responsiveness in BN rats | – | 16×1.5 mg/kg | 3×1.5 mg/kg | 1.5 | (30) |
| Wollin et
al, 2006 | Inhibition of
neutrophil influx in BN rats | – | 28.1 mg/kg | 6.9 mg/kg | 0.9 | (30) |
| Wollin et
al, 2006 | Inhibition of TNFα
release in BN rats | – | 23×0.9 mg/kg | 9×0.9 mg/kg | 0.9 | (30) |
| Ji et al,
2003 | Bronchorelaxant
effect in vitro |
0.84×10−5 M |
1.00×10−5 M | – | – | (32) |
| Chen et al,
2004 | PDE4A activity in
yeast cell GL62 | 1.27
(0.84–1.91) | 66.4
(33.3–132.2) | 3.73
(2.51–5.53) | – | (33) |
In the present study, the anti-inflammatory effect
of ciclamilast, a derivative of piclamilast, was reported.
Ciclamilast exhibited more potent inhibition of cAMP-PDE activity,
airway hyperresponsiveness, tracheae contraction and lung
inflammation than piclamilast. Our previous comparative study
showed that ciclamilast exhibited more potent bronchorelaxant
effects than piclamilast on resting tension in a guinea pig airway
smooth muscle model (32). Our
previous study also showed that the IC50 values of
piclamilast and rolipram on recombinant PDE4A expressed in GL62
yeast cells were 66.4 (33.3–132.2) and 3.73 (2.51–5.53) µmol/l,
respectively (33), suggesting
that rolipram had improved selectivity towards PDE4A than
piclamilast.
By contrast, in lung tissues, PDE4D, but not PDE4B,
is the dominant PDE4 subtype, with a critical role in the control
of airway smooth muscle contraction (7). However, major PDE4 subtypes
regulating the functions of airway sensory and epithelial cells
remain to be elucidated. Individual PDE4 subtypes are non-redundant
overall but are complementary. Based on their upregulation in the
lungs of allergic rats, PDE4A and PDE4C, as with 4B and 4D, may
also be involved in the pathogenesis of asthma in distinct tissues
(3). In the present study, the
effects of ciclamilast on lung PDE4 activity, PDE4 subtype
expression, inflammation and mucus secretion were investigated in
an asthmatic rat model. In this model, antigen sensitization and
repeated challenges induced airway inflammation, airway goblet cell
hyperplasia, and increases in the enzyme activity of cAMP-PDE and
expression of PDE4B and PDE4D. These changes were suppressed by
ciclamilast, piclamilast, roliparm and dexamethasone. Ciclamilast
showed more potent inhibition of the mRNA expression of PDE4B than
piclamilast. However, piclamilast showed more potent inhibition of
PDE4D. According to the PDE activity assay, ciclamilast exhibited
more potent selectivity towards cAMP-PDE, whereas piclamilast
exhibited more potent selectivity towards cGMP-PDE. Furthermore,
there was a correlation between the activity of cAMP-PDE and
eosinophils in the BALF (Spearman's correlation coefficient=0.314,
P<0.01) and lung tissue (Spearman's correlation
coefficient=0.407, P<0.01). These data suggest that ciclamilast
exerts a superior anti-inflammatory effect, which involves cAMP-PDE
activity, mainly via PDE4B.
A previous study suggested that human primary CD4
(+) T cells expressed PDE4A, PDE4B and PDE4D in response to
anti-CD3/CD28 stimulation in a time-dependent manner. The knockdown
of either PDE4B or PDE4D inhibited the release of interleukin-2,
similar to the effect of the panPDE4 inhibitor, RP 73401
(piclamilast) (21).
PDE4D-targeting small interfering RNA alone was as effective as the
panPDE4 inhibitor RP 73401 (piclamilast) (21). This result also supported the
findings of the present study. While rolipram reversed the
permeability of rat pulmonary microvascular endothelial cells, the
mechanism may involve the downregulated mRNA expression of PDE4D
(34). Jin et al (13) reported that mice deficient in PDE4B
and PDE4D did not develop airway hyper-responsiveness, and the
ablation of PDE4D had no impact on airway inflammation. These
finding suggest the essential role of PDE4B in the development of
allergen-induced airway inflammation, highlighting the fact that
PDE4 inhibitors with PDE4B selectivity may have efficacy in asthma
treatment.
In the present study, the inhibitory effects of
dexamethasone on the mRNA expression of PDE4 subtypes in the lung
suggest that glucocorticoids may also directly downregulate PDE4
activity, at least partly by downregulating the expression of PDE4.
There is evidence suggesting that dexamethasone treatment can
affect the cAMP signaling pathway in human osteosarcoma cells by
decreasing the mRNA levels of the PDE4A and PDE4B subtypes,
particularly the PDE4A4 and PDE4B1 isoforms (35). The dexamethasone-induced elevation
of intracellular cAMP may represent one way by which
glucocorticoids can modulate the immune response. PDE4 inhibitors
may also be useful as an alternative or adjunctive treatment for
glucocorticoid resistance or insensitivity in common inflammatory
diseases, including asthma, rheumatoid arthritis, inflammatory
bowel disease, COPD and acute respiratory distress syndrome, as
they can reverse glucocorticoid resistance by inhibiting its
underlying mechanisms in a variety of in vivo models
(36,37). Furthermore, PDE4 inhibitor has been
shown to be of use during therapy with glucocorticoids in cases of
leukemia (38,39).
In conclusion, the present study showed that
ciclamilast exhibited more potent inhibition of cAMP-PDE activity
and lung inflammation than piclamilast. The main reason may be due
to its improved selectivity towards PDE4B rather than PDE4D, and
improved selectivity towards cAMP-PDE rather than cGMP-PDE. These
results provide overall insights into the mechanism of action of
ciclamilast as an anti-inflammatory agent and a suggest that PDE4
inhibitors with PDE4B selectivity may have efficacy in asthma
treatment.
Acknowledgements
The authors would like to thank the Beijing Joinn
Drug Research Center (Beijing, China) for supplying ciclamilast and
piclamilast. In addition, the authors would like to thank Professor
Cong-lin Zuo from Beijing Joinn Drug Research Center for providing
the toxicological data of ciclamilast.
Funding
The present study was supported by grants (grant
nos. LY18H310002 and LY14H310004) from Zhejiang Provincial Natural
Science Foundation, grants (grant nos. 81570056 and 81170536) from
the Natural Science Foundation of China, a grant (grant no.
2014A01) from the Health Science & Technology Program of
Hangzhou and a grant (grant no. 2017C37132) from Fund project of
Zhejiang provincial science and Technology Department.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYZ, QMX, JQC and HFT designed the study and wrote
the manuscript. XYZ and JCC prepared the ovalbumin model and
harvested the lung samples, analyzed the polymerase chain reaction,
western blotting and histology results. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Zhejiang
Medical Laboratory Animal Administration Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shu SJ, Lei XG, Liang JH, Song YH, Xu Q,
Chen XD, Mao LG and Li ZG: The effects of second messenger cAMP and
its relative components on the contraction of uterine smooth muscle
of rat. Eur Rev Med Pharmacol Sci. 21:1709–1721. 2017.PubMed/NCBI
|
|
2
|
Sakkas LI, Mavropoulos A and Bogdanos DP:
Phosphodiesterase 4 inhibitors in immune-mediated diseases: Mode of
action, clinical applications, current and future perspectives.
Curr Med Chem. 24:3054–3067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang HF, Song YH, Chen JC, Chen JQ and
Wang P: Upregulation of phosphodiesterase-4 in the lung of allergic
rats. Am J Respir Crit Care Med. 71:823–828. 2005. View Article : Google Scholar
|
|
4
|
Ma D, Wu P, Egan RW, Billah MM and Wang P:
Phosphodiesterase 4B gene transcription is activated by
lipopolysaccharide and inhibited by interleukin-10 in human
monocytes. Mol Pharmacol. 55:50–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang P, Wu P, Ohleth KM, Egan RW and
Billah MM: Phosphodiesterase 4B2 is the predominant
phosphodiesterase species and undergoes differential regulation of
gene expression in human monocytes and neutrophils. Mol Pharmacol.
56:170–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin SL and Conti M: Induction of the
cyclic nucleotide phosphodiesterase PDE4B is essential for
LPS-activated TNF-alpha responses. Proc Natl Acad Sci USA.
99:7628–7633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh SP, Barrett EG, Kalra R,
Razani-Boroujerdi S, Langley RJ, Kurup V, Tesfaigzi Y and Sopori
ML: Prenatal cigarette smoke decreases lung cAMP and increases
airway hyperresponsiveness. Am J Respir Crit Care Med. 168:342–347.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng YM, Xie QM, Tang HF, Sun JG, Deng JF,
Chen JQ and Yang SY: Effects of ciclamilast, a new PDE 4 PDE4
inhibitor, on airway hyperresponsiveness, PDE4D expression and
airway inflammation in a murine model of asthma. Eur J Pharmacol.
547:125–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang HF, Chen JQ, Xie QM, Zheng XY, Zhu
YL, Adcock I and Wang X: The role of PDE4 in pulmonary inflammation
and goblet cell hyperplasia in allergic rats. Biochim Biophys Acta
1762. 525–532. 2006.
|
|
10
|
Xie QM, Chen JQ, Shen WH and Bian RL:
Correlative changes of interferon-gamma and interleukin-4 between
cortical layer and pulmonary airway of sensitized rats. Acta
Pharmacol Sin. 23:248–252. 2002.PubMed/NCBI
|
|
11
|
Bian H, Zhang J, Wu P, Varty LA, Jia Y,
Mayhood T, Hey JA and Wang P: Differential type 4 cAMP-specific
phosphodiesterase (PDE4) expression and functional sensitivity to
PDE4 inhibitors among rats, monkeys and humans. Biochem Pharmacol.
68:2229–2236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spina D: PDE4 inhibitors: Current status.
Br J Pharmacol. 155:308–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin SL, Goya S, Nakae S, Wang D, Bruss M,
Hou C, Umetsu D and Conti M: Phosphodiesterase 4B is essential for
T(H)2-cell function and development of airway hyperresponsiveness
in allergic asthma. J Allergy Clin Immunol. 126:1252–1259.e12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giembycz MA and Newton R: Harnessing the
clinical efficacy of phosphodiesterase 4 inhibitors in inflammatory
lung diseases: Dual-selective phosphodiesterase inhibitors and
novel combination therapies. Handb Exp Pharmacol. 415–446. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giembycz MA: Can the anti-inflammatory
potential of PDE4 inhibitors be realized: Guarded optimism or
wishful thinking? Br J Pharmacol. 155:288–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashton MJ, Cook DC, Fenton G, Karlsson JA,
Palfreyman MN, Raeburn D, Ratcliffe AJ, Souness JE, Thurairatnam S
and Vicker N: Selective type IV phosphodiesterase inhibitors as
antiasthmatic agents. The syntheses and biological activities of
3-(cyclopentyloxy)-4-methoxybenzamides and analogues. J Med Chem.
37:1696–1703. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Souness JE, Maslen C, Webber S, Foster M,
Raeburn D, Palfreyman MN, Ashton MJ and Karlsson JA: Suppression of
eosinophil function by RP 73401, a potent and selective inhibitor
of cyclic AMP-specific phosphodiesterase: Comparison with rolipram.
Br J Pharmacol. 115:39–46. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cooper N, Teixeira MM, Warneck J, Miotla
JM, Wills RE, Macari DM, Gristwood RW and Hellewell PG: A
comparison of the inhibitory activity of PDE4 inhibitors on
leukocyte PDE4 activity in vitro and eosinophil trafficking in
vivo. Br J Pharmacol. 126:1863–1871. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Favot L, Keravis T and Lugnier C:
Modulation of VEGF-induced endothelial cell cycle protein
expression through cyclic AMP hydrolysis by PDE2 and PDE4. Thromb
Haemost. 92:634–645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Favot L, Keravis T, Holl V, Le Bec A and
Lugnier C: VEGF-induced HUVEC migration and proliferation are
decreased by PDE2 and PDE4 inhibitors. Thromb Haemost. 90:334–343.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peter D, Jin SL, Conti M, Hatzelmann A and
Zitt C: Differential expression and function of phosphodiesterase 4
(PDE4) subtypes in human primary CD4+ T cells: Predominant role of
PDE4D. J Immunol. 178:4820–4831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beeh KM, Beier J, Lerch C, Schulz AK and
Buhl R: Effects of piclamilast, a selective phosphodiesterase-4
inhibitor, on oxidative burst of sputum cells from mild asthmatics
and stable COPD patients. Lung. 182:369–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aoki M, Kobayashi M, Ishikawa J, Saita Y,
Terai Y, Takayama K, Miyata K and Yamada T: A novel
phosphodiesterase type 4 inhibitor, YM976
(4-(3-chlorophenyl)-1,7-diethylpyrido[2,3-d]pyrimidin-2(1H)-one),
with little emetogenic activity. J Pharmacol Exp Ther. 295:255–260.
2000.PubMed/NCBI
|
|
24
|
de Visser YP, Walther FJ, Laghmani EH, van
Wijngaarden S, Nieuwland K and Wagenaar GT: Phosphodiesterase-4
inhibition attenuates pulmonary inflammation in neonatal lung
injury. Eur Respir J. 31:633–644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Visser YP, Walther FJ, Laghmani el H,
Steendijk P, Middeldorp M, van der Laarse A and Wagenaar GT:
Phosphodiesterase 4 inhibition attenuates persistent heart and lung
injury by neonatal hyperoxia in rats. Am J Physiol Lung Cell Mol
Physiol. 302:L56–L67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Souness JE, Griffin M, Maslen C, Ebsworth
K, Scott LC, Pollock K, Palfreyman MN and Karlsson JA: Evidence
that cyclic AMP phosphodiesterase inhibitors suppress TNF alpha
generation from human monocytes by interacting with a
‘low-affinity’ phosphodiesterase 4 conformer. Br J Pharmacol.
118:649–658. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raeburn D, Underwood SL, Lewis SA, Woodman
VR, Battram CH, Tomkinson A, Sharma S, Jordan R, Souness JE, Webber
SE and Karlsson JA: Anti-inflammatory and bronchodilator properties
of RP 73401, a novel and selective phosphodiesterase type IV
inhibitor. Br J Pharmacol. 113:1423–1431. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holbrook M, Gozzard N, James T, Higgs G
and Hughes B: Inhibition of bronchospasm and ozone-induced airway
hyperresponsiveness in the guinea-pig by CDP840, a novel
phosphodiesterase type 4 inhibitor. Br J Pharmacol. 118:1192–1200.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bundschuh DS, Eltze M, Barsig J, Wollin L,
Hatzelmann A and Beume R: In vivo efficacy in airway disease models
of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol
Exp Ther. 297:280–290. 2001.PubMed/NCBI
|
|
30
|
Wollin L, Bundschuh DS, Wohlsen A, Marx D
and Beume R: Inhibition of airway hyperresponsiveness and pulmonary
inflammation by roflumilast and other PDE4 inhibitors. Pulm
Pharmacol Ther. 19:343–352. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun JG, Deng YM, Wu X, Tang HF, Deng JF,
Chen JQ, Yang SY and Xie QM: Inhibition of phosphodiesterase
activity, airway inflammation and hyperresponsiveness by PDE4
inhibitor and glucocorticoid in a murine model of allergic asthma.
Life Sci. 79:2077–2085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji H, Xie QM and Chen JQ: Comparison of
piclamilast with ciclamilast in bronchodilating and antiallergic
effects. Zhejiang Da Xue Xue Bao Yi Xue Ban. 32:274–278. 2003.(In
Chinese). PubMed/NCBI
|
|
33
|
Chen JC, Chen JQ, Xie QM and Zhu YL:
Selective inhibition of purified human phosphodiesterase 4A
expressed in yeast cell GL62 by ciclamilast, piclamilast, and
rolipram. Acta Pharmacol Sin. 25:1171–1175. 2004.PubMed/NCBI
|
|
34
|
Thompson WJ, Ashikaga T, Kelly JJ, Liu L,
Zhu B, Vemavarapu L and Strada SJ: Regulation of cyclic AMP in rat
pulmonary microvascular endothelial cells by rolipram-sensitive
cyclic AMP phosphodiesterase (PDE4). Biochem Pharmacol. 63:797–807.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahlstrom M, Pekkinen M, Huttunen M and
Lamberg-Allardt C: Dexamethasone down-regulates
cAMP-phosphodiesterase in human osteosarcoma cells. Biochem
Pharmacol. 69:267–275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barnes PJ and Adcock IM: Glucocorticoid
resistance in inflammatory diseases. Lancet. 373:1905–1917. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Milara J, Navarro A, Almudever P, Lluch J,
Morcillo EJ and Cortijo J: Oxidative stress-induced glucocorticoid
resistance is prevented by dual PDE3/PDE4 inhibition in human
alveolar macrophages. Clin Exp Allergy. 41:535–546. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tiwari S, Dong H, Kim EJ, Weintraub L,
Epstein PM and Lerner A: Type 4 cAMP phosphodiesterase (PDE4)
inhibitors augment glucocorticoid-mediated apoptosis in B cell
chronic lymphocytic leukemia (B-CLL) in the absence of exogenous
adenylyl cyclase stimulation. Biochem Pharmacol. 69:473–483. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong H, Zitt C, Auriga C, Hatzelmann A and
Epstein PM: Inhibition of PDE3, PDE4 and PDE7 potentiates
glucocorticoid-induced apoptosis and overcomes glucocorticoid
resistance in CEM T leukemic cells. Biochem Pharmacol. 79:321–329.
2010. View Article : Google Scholar : PubMed/NCBI
|