Introduction
In 2010, an estimated 1.5 million individuals died
from lung cancer (LC) worldwide, accounting for 19% of all cancer
deaths (1). In 2008, statistics
from the International Agency for Research on Cancer (IARC)
reported that ~520,000 new cases of LC were diagnosed in China
(2,3). There are two major types of LC, i.e.,
non-small-cell lung cancer (NSCLC) and small-cell lung cancer
(SCLC), and the disease is the leading cause of cancer-related
mortality worldwide (4,5). NSCLC accounts for ~80% of LC cases
(6). Despite recent advances in
the treatment of patients with NSCLC, its high mortality rate has
not declined significantly (7),
and the 5-year survival rate does not exceed 15% (8). Thus, effective treatment strategies
for NSCLC patients are urgently required.
MicroRNAs (miRNAs) are a type of endogenous short
and highly conserved non-coding RNA that participate in numerous
developmental processes. miRNAs repress gene expression by
base-pairing with the 3′ untranslated region (UTR) of mRNA
(9). Deregulation of miRNAs is
associated with cancer initiation and progression, indicating their
roles as oncogenes or tumor suppressor genes (10). Numerous miRNAs involved in LC
pathogenesis have emerged, and research into miRNA-targeted therapy
has attracted significant attention (7,11,12).
Recently, miR-584 has attracted the attention of researchers; for
example, in 2015, miR-584 was found to suppress cell invasion and
cell migration in thyroid carcinoma through targeting
Rho-associated coiled-coil containing protein kinase 1 (ROCK1)
(13); in 2016, microRNA-584-3p
was reported to reduce cell migration and cell invasion in glioma
through targeting ROCK1 (14); and
in 2017, miR-584-5p overexpression was found to inhibit cell
proliferation and induce cell apoptosis in gastric cancer through
targeting WW domain-containing E3 ubiquitin protein ligase 1
(15). Interestingly, miR-584-5p
was verified as a potential biomarker for the diagnosis of LC
(16). However, how dose
miR-584-5p affect the progression of NSCLC has not been reported to
date. The aim of the present study was to investigate the action of
miR-584-5p in NSCLC.
Materials and methods
Cell culture
A549 cells were incubated in DMEM (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% antibiotics
(penicillin-streptomycin; Gibco; Thermo Fisher Scientific, Inc.) in
an incubator with 95% humidity and 5% CO2 at 37°C.
Cell transfection
A549 cells (2×105 cells/well) were seeded
in 6-well plates and cultured until reaching 90% confluence, after
which time cell transfection was performed. In brief, 2.5 µl
hsa-miR-584-5p mimics and 5 µl Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) were added to 250
µl DMEM. The mixture was thoroughly mixed and added to the
6-well plates to a concentration of 20 nmol/l.
Prediction of promoter-targeting
miRNA
The binding sites between miR-584-5p and mRNAs were
analyzed by algorithm miRWalk (17) and genome-wide Argonaute-chromosome
interaction profiling data (GSE40536).
Luciferase reporter assay
Cells transfected with miR-584-5p mimics or miR-NC
mimics were seeded into 96-well plates (1×103
cells/well). The cells were cultured in DMEM supplemented with 10%
FBS for 24 h. MMP-14 3′UTR was amplified from cDNA of A549 cells
and inserted into p LightSwitch Prom (Switchgear Genomics, Menlo
Park, CA, USA). Subsequently, pLightMMP-14-WT-3′UTR reporter
plasmid or pLightMMP-14-MUT-3′UTR was transfected into the cells by
FuGENE transfection reagent (Roche Diagnostics, Basel, Switzerland)
in accordance with the manufacturer's protocol. At 24 h after cell
transfection, luciferase activity was examined by LightSwitch
Luciferase Assay (Switchgear Genomics) in accordance with the
manufacturer's protocol.
Patients
A total of 30 pairs of tumor tissues and paired
normal tissues from NSCLC patients used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) were
acquired from Weifang Second People's Hospital after obtaining
written informed consent from all the subjects. The present study
was approved by the Ethical Committee of Weifang Second People's
Hospital (Shandong, China). The patients included 22 men and 8
women, 17 of whom were aged ≥60 years and 13 were aged <60
years. A total of 10 patients had never smoked and 20 patients had
a history of smoking; in addition, 22 patients had lymph node
metastasis and 8 patients had no metastatic lymph nodes; there was
no gender bias in the lymph node metastasis.
Western blot analysis
At 48 h after transfection, A549 cells were rinsed
by cold phosphate-buffered saline followed by the addition of
protein lysis buffer. Total protein was extracted through
centrifugation of A549 cells at 14,000 rpm at 4°C for 10 min.
Proteins were first separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and the proteins were
transferred onto nitrocellulose membranes. Subsequently, the
nitrocellulose membranes were incubated with primary antibodies at
4°C overnight followed by treatment by horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at room temperature for 2 h.
Finally, the blots were treated with ECL (Thermo Fisher Scientific,
Inc.).
RT-qPCR analysis
Total RNA from A549 cells and patient tissue samples
was extracted by TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
Reverse transcription and the TaqMan microRNA assay were performed
using the Hairpin-it™ miRNA qPCR Quantitation kit
(Shanghai GenePharma Co., Ltd., Shanghai, China) in accordance with
the manufacturer's protocol. Thereafter, RT-qPCR was performed with
a SYBR Green kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and the reaction was performed with the ABI 7500 Real-time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermocycling conditions were as follows: 95°C for 2 min,
followed by 40 cycles of 95°C for 15 sec, 58°C for 30 sec and 72°C
for 30 sec. Relative expression levels were determined by the
2−ΔΔCq method (18).
The expression of miR-584-5p was normalized to U6 and the
expression of matrix metalloproteinase (MMP)-14 was normalized to
GAPDH.
Wound healing assay
A549 cells (1×105 cells/well) transfected
with miR-584-5p mimics or miR-NC mimics were inoculated into 6-well
plates and cultured in DMEM for 6 h. A wound was created using a
10-µl Eppendorf pipette tip. After washing with serum-free
medium 3 times to remove the detached cells, A549 cells were
cultured in DMEM in an incubator with 5% CO2 for 24 h at
37°C. The distance between the edges of the gap was measured using
Image-Pro Plus v6.0 software.
Transwell assay
Cell invasion was evaluated by Transwell assay
(Corning Incorporated, Corning, NY, USA) with 10 µl Matrigel
(BD Biosciences, San Jose, CA, USA). A549 cells (5×105
cells per 200 µl) transfected with miR-584-5p mimics or
miR-NC mimics were inoculated into the upper chambers, while DMEM
was added into the lower chambers. Following incubation at 37°C for
24 h, the invasive cells were fixed with 4% paraformaldehyde and
stained with crystal violet solution (0.1%). Five random areas from
each chamber were examined under a microscope.
Statistical analysis
Statistical analyses were performed with SPSS v13.0
software (SPSS Inc., Chicago, IL, USA). Data were expressed as mean
± standard deviation. Differences between two groups were analyzed
using two-tailed Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Evaluation of miR-584-5p levels in
tissue samples in vivo and cells in vitro
First, we examined the miR-584-5p level in tissue
samples. As shown in Fig. 1A, the
level of miR-584-5p was significantly lower in tumor samples
compared with that in normal tissues (P<0.05). This result was
consistent with a recent study published in 2017, which indicated
decreased miR-584-5p levels in the arterial plasma as well as tumor
tissues of LC patients and identified miR-584-5p as a biomarker in
the diagnosis of LC (16).
We were eager to know the effects of miR-584-5p
overexpression in tumor progression. Thereafter, we tested the
expression change of miR-584-5p in A549 cells after the
transfection of miR-584-5p mimics. As showed in Fig. 1B, compared with the miR-NC group,
miR-584-5p mimics significantly elevated the expression of
miR-584-5p in vitro, which provide the evidence that the
transfections were successful.
As shown in Table
I, there was no significant difference in miR-584-5p expression
by sex (P=0.896), age (P=0.552) or smoking history (P=0.676).
However, there was a significant difference by lymph node
metastasis, as 22 patients with lymph node metastasis expressed
significantly lower miR-584-5p levels compared with the remaining 8
patients who had no metastatic lymph nodes (P=0.009); and a
significant difference in TNM stage, 13 patients at stage I + II
expressed higher miR-584-5p levels compared with the remaining 17
patients who were at stage III+IV (P=0.038).
 | Table I.Characteristics of non-small cell
liver cancer patients. |
Table I.
Characteristics of non-small cell
liver cancer patients.
| Characteristics | Cases (n) | miR-584-5p (mean ±
SD) | P-value |
|---|
| Gender |
|
| 0.896 |
| Male | 22 | 0.830±0.063 |
|
|
Female | 8 | 0.815±0.071 |
|
| Age (years) |
|
| 0.552 |
|
≥60 | 17 | 0.852±0.077 |
|
|
<60 | 13 | 0.792±0.056 |
|
| Smoking
history |
|
| 0.676 |
| No | 10 | 0.796±0.107 |
|
|
Yes | 20 | 0.841±0.054 |
|
| Lymph node
metastasis |
|
| 0.009 |
| No | 8 | 1.033±0.047 |
|
|
Yes | 22 | 0.781±0.058 |
|
| TNM stage |
|
| 0.038 |
|
I+II | 13 | 0.942±0.080 |
|
|
III+IV | 17 | 0.737±0.054 |
|
Identification of the mRNA target of
miR-584-5p
miR-584-5p could target multiple mRNAs, including
WWP1 (15), ROCK1 (19), and KLRG1 (20). The binding site between miR-584-5p
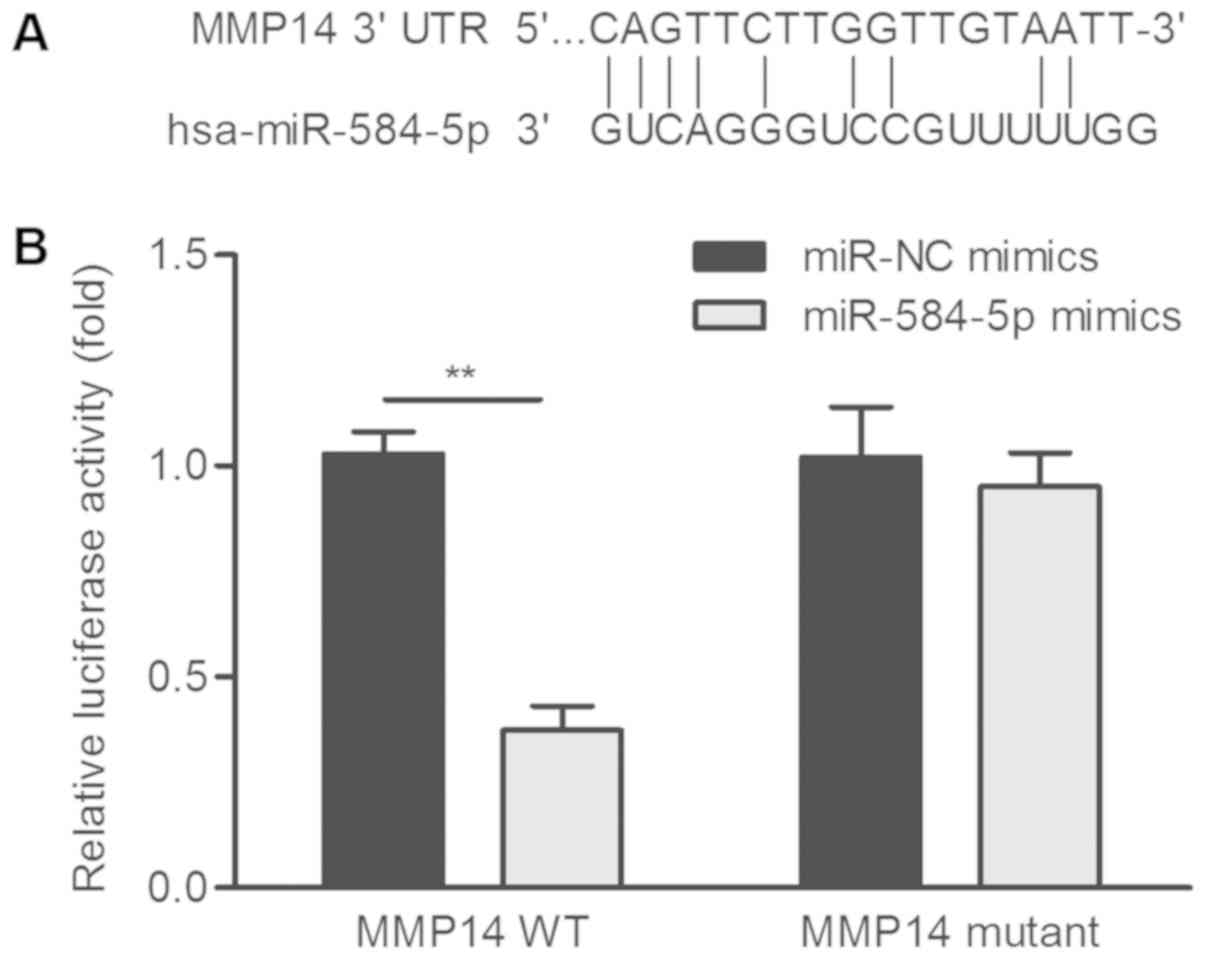
and MMP-14 is shown in Fig. 2A. As
is well known, MMP-14 plays a key role in tumor invasion and
metastasis (21–23). Overexpression of MMP-14 has been
correlated with poor prognosis in NSCLC patients (23), and the MMP-14 protein may be
helpful in predicting the prognosis of NSCLC (24,25).
Dual luciferase assay in A549 cells revealed that
miR-584-5p mimics resulted in decreased MMP-14 promoter activity
(P<0.01; Fig. 2B).
Evaluation of the effects of
miR-584-5p on cell migration and cell invasion
In the wound healing assay, overexpression of
miR-584-5p inhibited the migration of A549 cells compared with that
in the miR-NC mimics group (P<0.01, Fig. 3A and B).
In the Transwell assay, miR-584-5p mimics reduced
A549 cell invasion compared with that in the miR-NC mimics group
(P<0.01; Fig. 3C and D).
These results are consistent with those of a
previous study on the tumor suppressive role of miR-584-5p in human
neuroblastoma (26).
Evaluation of the effects of
miR-584-5p on MMP-14 level
Afterwards, we examined the changes in the
expression of MMP-14 at the protein and mRNA level in A549 cells.
The expression of MMP-14 was found to be reduced by miR-584-5p
compared with that in the miR-NC mimics group (P<0.01; Fig. 4A-C).
Evaluation of the effects of
miR-584-5p on MMP4 and Slug level
Afterwards, we examined the changes in the
expression of MMP-4 and Slug at the protein and mRNA levels in A549
cells. The expression levels of MMP-4 and Slug were reduced by
miR-584-5p compared with that in the miR-NC mimics group
(P<0.01; Fig. 5A-C).
Discussion
In breast cancer, miR-584 downregulation is a
prerequisite for transforming growth factor β-induced cell
migration and motility, its downstream target being phosphatase and
actin regulator 1 (27); in renal
cell carcinoma, miR-584 downregulation is indispensable for
decreasing cancer cell invasion by targeting ROCK1 (28). In conclusion, these results
demonstrated that miR-584 potentially acts as a tumor suppressor.
In accordance with the aforementioned reports, the present study
also demonstrated that miR-584-5p was decreased in the tumor
tissues of NSCLC patients.
Due to the elevation of MMP-14 and its role in
predicting poor overall survival in glioma (29,30),
we suggested that miR-584-5p downregulation may induce a decrease
in the MMP-14 level in NSCLC. We demonstrated that, compared with
the miR-NC group, miR-584-5p mimics significantly elevated the
expression of miR-584-5p in vitro, which provide the
evidence that the transfections were successful; and compared with
the miR-NC mimics group, miR-584-5p mimics significantly inhibited
MMP-14 expression by targeting the promoter, as shown by a
luciferase reporter gene assay, and that miR-584-5p inhibited the
expression of MMP-14 at the protein and mRNA level.
LC is characterized by a high propensity for tumor
invasion and migration, which is the main cause of LC-related death
(31,32). We next investigated the effects of
miR-584-5p on the migration and invasion of the NSCLC cell line
A549. Consistent with previous reports demonstrating the tumor
suppressive role of miR-584-5p in breast cancer and renal cell
carcinoma (27,28), our results also revealed that
miR-584-5p acted as a tumor suppressive factor in NSCLC, as
evidenced by miR-584-5p overexpression decreasing the migration and
invasion of NSCLC cells in vitro.
Changes in proteins involved in tumor invasion and
metastasis, such as MMP4 (33) and
Slug (34) were also detected by
western blot. Results demonstrated that the expression levels of
MMP-4 and Slug were reduced by miR-584-5p compared with that in the
miR-NC mimics group.
In summary, the findings of the present study
demonstrated the tumor suppressive role of miR-584-5p in NSCLC, and
indicated that miR-584-5p/MMP-14 axis may be a novel target for the
treatment of NSCLC patients.
However, there are several limitations in the
present study as listed: 1. We mainly focus on the effects of
miR-584-5p on cell migration and cell invasion, there was no data
about the effect of miR-584-5p on LC cell proliferation which we
will complement in our further study; 2. MMP-14 plays a key role in
tumor invasion and metastasis, we mainly checked the expression
changes of MMP-14 in the present study. stretching, MMP4, Slug are
involved in tumor invasion and metastasis, which will be detected
in the future work; 3. there was no data about the role of
miR-584-5p in vivo, and the effects of miR-584-5p on
clinical cases related to the clinical staging of LC, which will be
complemented in our further study; 4. It would be necessary to use
either primary cell lines from a few patients or use aggressive and
non-aggressive NSCLC cell lines in the future study.
Acknowledgements
The authors would like to thank Weifang Second
People's Hospital for the support during the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TG, CZ and ZW performed the experiments. TG, CZ and
XZ analyzed the data. XZ designed the project and prepared the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Weifang Second People's Hospital (Shandong, China).
Written informed consent was obtained from all the subjects.
Patient consent for publication
Written informed consent was obtained from all the
subjects.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regioal mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang
Y, Zhao H, Wu J, Zhang Y, Zhao L, et al: National survey of the
medical treatment status for non-small cell lung cancer (NSCLC) in
China. Lung Cancer. 77:371–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Gu J, Roth JA, Hildebrandt MA,
Lippman SM, Ye Y, Minna JD and Wu X: Pathway-based serum microRNA
profiling and survival in patients with advanced stage non-small
cell lung cancer. Cancer Res. 73:4801–4809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oak CH, Wilson D, Lee HJ, Lim HJ and Park
EK: Potential molecular approaches for the early diagnosis of lung
cancer (review). Mol Med Rep. 6:931–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Q, Xu E, Dai J, Liu B, Han Z, Wu J,
Zhang S, Peng B, Zhang Y and Jiang Y: A novel long noncoding RNA
AK001796 acts as an oncogene and is involved in cell growth
inhibition by resveratrol in lung cancer. Toxicol Appl Pharmacol.
285:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kesanakurti D, Maddirela DR, Chittivelu S,
Rao JS and Chetty C: Suppression of tumor cell invasiveness and in
vivo tumor growth by microRNA-874 in non-small cell lung cancer.
Biochem Biophys Res Commun. 434:627–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho S, Park TI, Lee EB and Son SA: Poor
prognostic factors in surgically resected stage I non-small cell
lung cancer: Histopathologic and immunohistochemical analysis.
Korean J Thorac Cardiovasc Surg. 45:101–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esquela-Kerscher A, Trang P, Wiggins JF,
Patrawala L, Cheng A, Ford L, Weidhaas JB, Brown D, Bader AG and
Slack FJ: The let-7 microRNA reduces tumor growth in mouse models
of lung cancer. Cell Cycle. 7:759–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang J, Wu Y, Li DS, Wang ZY, Shen Q, Sun
TQ, Guan Q and Wang YJ: miR-584 suppresses invasion and cell
migration of thyroid carcinoma by regulating the target oncogene
ROCK1. Oncol Res Treat. 38:436–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue H, Guo X, Han X, Yan S, Zhang J, Xu S,
Li T, Guo X, Zhang P, Gao X, et al: MicroRNA-584-3p, a novel tumor
suppressor and prognostic marker, reduces the migration and
invasion of human glioma cells by targeting hypoxia-induced ROCK1.
Oncotarget. 7:4785–4805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Li Z, Wei S, Wang W, Chen Z, Zhang
L, Chen L, Li B, Sun G, Xu J, et al: Overexpression of miR-584-5p
inhibits proliferation and induces apoptosis by targeting WW
domain-containing E3 ubiquitin protein ligase 1 in gastric cancer.
J Exp Clin Cancer Res. 36:592017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Wen W, Shan X, Zhu W, Xu J, Guo R,
Cheng W, Wang F, Qi LW, Chen Y, et al: A six-microRNA panel in
plasma was identified as a potential biomarker for lung
adenocarcinoma diagnosis. Oncotarget. 8:6513–6525. 2017.PubMed/NCBI
|
|
17
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roberto GM, Delsin LEA, Vieira GM, Silva
MO, Hakime RG, Gava NF, Engel EE, Scrideli CA, Tone LG and
Brassesco MS: ROCK1-predictedmicroRNAs dysregulation contributes to
tumor progression in ewing sarcoma. Pathol Oncol Res.
21–Dec;2017.(Epub ahead of print). View Article : Google Scholar
|
|
20
|
Cipolla GA, Park JK, de Oliveira LA,
Lobo-Alves SC, de Almeida RC, Farias TD, Lemos Dde S, Malheiros D,
Lavker RM and Petzl-Erler ML: A 3′UTR polymorphism marks
differential KLRG1 mRNA levels through disruption of a miR-584-5p
binding site and associates with pemphigus foliaceus
susceptibility. Biochim Biophys Acta 1859. 1306–1313. 2016.
|
|
21
|
Chun TH, Sabeh F, Ota I, Murphy H,
McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED and Weiss SJ:
MT1-MMP-dependent neovessel formation within the confines of the
three-dimensional extracellular matrix. J Cell Biol. 167:757–767.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sabeh F, Ota I, Holmbeck K,
Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S,
Inada M, Krane S, et al: Tumor cell traffic through the
extracellular matrix is controlled by the membrane-anchored
collagenase MT1-MMP. J Cell Biol. 167:769–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tolde O, Rösel D, Mierke CT, Panková D,
Folk P, Vesely P and Brábek J: Neoplastic progression of the human
breast cancer cell line G3S1 is associated with elevation of
cytoskeletal dynamics and upregulation of MT1-MMP. Int J Oncol.
36:833–839. 2010.PubMed/NCBI
|
|
24
|
Wang YZ, Wu KP, Wu AB, Yang ZC, Li JM, Mo
YL, Xu M, Wu B and Yang ZX: MMP-14 overexpression correlates with
poor prognosis in non-small cell lung cancer. Tumor Biol.
35:9815–9821. 2014. View Article : Google Scholar
|
|
25
|
Zhou H, Wu A, Fu W, Lv Z and Zhang Z:
Significance of semaphorin-3A and MMP-14 protein expression in
non-small cell lung cancer. Oncol Lett. 7:1395–1400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiang X, Mei H, Qu H, Zhao X, Li D, Song
H, Jiao W, Pu J, Huang K, Zheng L and Tong Q: miRNA-584-5p exerts
tumor suppressive functions in human neuroblastoma through
repressing transcription of matrix metalloproteinase 14. Biochim
Biophys Acta 1852. 1743–1754. 2015.
|
|
27
|
Fils-Aimé N, Dai M, Guo J, El-Mousawi M,
Kahramangil B, Neel JC and Lebrun JJ: MicroRNA-584 and the protein
phosphatase and actin regulator 1 (PHACTR1), a new signaling route
through which transforming growth factor-β mediates the migration
and actin dynamics of breast cancer cells. J Biol Chem.
288:11807–11823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueno K, Hirata H, Shahryari V, Chen Y,
Zaman MS, Singh K, Tabatabai ZL, Hinoda Y and Dahiya R: Tumour
suppressor microRNA-584 directly targets oncogene Rock-1 and
decreases invasion ability in human clear cell renal cell
carcinoma. Br J Cancer. 104:308–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Yuan J, Tu Y, Mao X, He S, Fu G,
Zong J and Zhang Y: Co-expression of MMP-14 and MMP-19 predicts
poor survival in human glioma. Clin Transl Oncol. 15:139–145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie H, Xue YX, Liu LB, Wang P, Liu YH and
Ying HQ: Expressions of matrix metalloproteinase-7 and matrix
metalloproteinase-14 associated with the activation of
extracellular signal-regulated kinase1/2 in human brain gliomas of
different pathological grades. Med Oncol. 28 Suppl 1:S433–S438.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kraljevic Pavelic S, Sedic M, Bosnjak H,
Spaventi S and Pavelic K: Metastasis: New perspectives on an old
problem. Mol Cancer. 10:222011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sohn EJ, Jung DB, Lee H, Han I, Lee J, Lee
H and Kim SH: CNOT2 promotes proliferation and angiogenesis via
VEGF signaling in MDA-MB-231 breast cancer cells. Cancer Lett.
412:88–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao S and Zhao X, Zhang X, Luo M, Zuo X,
Huang S, Wang Y, Gu S and Zhao X: Notch1 signaling regulates the
epithelial-mesenchymal transition and invasion of breast cancer in
a Slug-dependent manner. Mol Cancer. 14:282015. View Article : Google Scholar : PubMed/NCBI
|