Introduction
Intermittent fasting (ImF) or intermittent caloric
restriction refers to the dietary restriction that reduces food
intake during a defined period (1). Previous studies have revealed
positive effects of dietary restriction. For example, ImF improves
the tolerance of neurons against brain ischemic damage in mice and
rats (2,3). Furthermore, dietary restriction
ameliorates age-related decline of presynaptic proteins (i.e.,
synaptophysin) in the rat hippocampus (4), and caloric restriction increases
hippocampal-dependent spatial learning memory in mice (5), suggesting that ImF improves spatial
learning memory.
Neurogenesis in adults is characterized by
proliferation and differentiation of a small number of neural
progenitor cells (NPCs) into mature neurons (granule cells) or
glial cells (astrocytes or oligodendrocytes), which occurs in the
subgranular zone (SgZ) of the hippocampal dentate gyrus (6,7). The
processes of the adult neurogenesis include proliferation,
differentiation, morphogenesis and maturation of adult-born
neurons, and integration of newborn neurons into the neural
circuitry of the hippocampus (8).
It has been reported that dietary restriction regimen can enhance
cell proliferation and differentiation of NPCs through upregulation
of neurotrophic factors, such as brain-derived neurotrophic factor
and neurotrophin-3 in the hippocampus (9,10).
Superoxide dismutases (SODs) such as Cu/Zn SOD
(SOD1) and Mn SOD (SOD2) are one of major antioxidant enzymes
involves in removing superoxide anion radicals (11), and catalase (CAT) is a common
enzyme as a H2O2 scavenger (12). A previous study has reported that
predominant expression of SOD1 is shown in early neural precursors
and migrating neuroblasts in the SgZ (13). In addition, expression level of CAT
mRNA is maintained during all three stages of neural
differentiation (undifferentiated cells, embryoid bodies, and
post-plating) in embryonic stem cells as an in vitro model
for neural differentiation (14).
In this regard, antioxidant enzymes play important roles in the
process of neurogenesis.
Despite many researches on beneficial effects of
ImF, studies on cell proliferation and the differentiation of
neuroblasts including their dendrite remodeling according to the
period of ImF in the hippocampal dentate gyrus remains to be
elucidated. Thus, in the present study, we investigated effects of
ImF for 1 to 3 months on expressions of Ki67 (a cell proliferation
marker) and doublecortin (DCX, a neuroblast differentiation
marker), and the complexity of neuroblast dendrites in the dentate
gyrus of the gerbil hippocampus. In addition, we examined changes
in expressions of endogenous antioxidant enzymes such as SOD1,
SOD2, and CAT in the dentate gyrus to study their related
mechanisms of ImF in the cell proliferation and neuroblast
differentiation.
Materials and methods
Experimental animals
Male gerbils were obtained at six months of age
(body weight, 65±4.6 g) from the Experimental Animal Center,
Kangwon University, Chuncheon, Republic of Korea, and maintained at
a constant temperature (23±0.4°C) and humidity (50±0.6%) with a
12-h light/dark cycle. The process of handling and caring animals
conformed to the guidelines being in compliance with current
international laws and policies (NIH Guide for the Care and Use of
Laboratory Animals, The National Academies Press, 8th edition,
2011). The protocol of this experiment was approved by the
Institutional Animal Care and Use Committee (IACUC) at Kangwon
National University (approval no. KW-180124-1; Gangwon, Republic of
Korea). No animals died during this experiment.
ImF and experimental groups
Animals were fed commercially available rodent
normal diet or ImF (provided food on alternate days) was applied
for 1, 2, and 3 months according to published methods (1,2,15).
Food intake of ImF group was controlled daily (10 g per day), and
body weight of normal diet and ImF groups was monitored every
month. Animals with normal diet or ImF were randomly assigned to
following groups: i) Control group (n=7), which was allowed free
access to water and food; ii) 1-month (1-M) ImF group (n=7); iii)
2-M ImF group (n=7); and iv) 3-M ImF group (n=7). To investigate
effects of ImF on neuroblasts and antioxidant enzymes, animals in
each group were sacrificed at the designated times.
Preparation of histological
sections
As described previously (16), animals were anesthetized with 60
mg/kg pentobarbital sodium (JW Pharm. Co., Ltd., Republic of Korea)
(17,18) at 1, 2, and 3 months after ImF, and
perfused transcardially with 0.1 M phosphate buffered saline (PBS,
pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer
(PB, pH 7.4). Their brains were removed, and tissues containing
hippocampi were cut, cryoprotected and serially sectioned into
25-µm frontal sections in a cryostat (Leica, Wetzlar, Germany).
Cresyl violet (CV) histochemistry
CV histochemical staining was performed to
investigate cellular distribution and morphology. In brief,
according to the method of our previous study (16), One % of CV acetate (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was dissolved in distilled water
(DW), and glacial acetic acid was added to this solution. Sections
of each group were mounted on gelatin-coated microscopy slides,
stained with CV solution and dehydrated with serial ethanol.
Finally, the stained sections were covered with Canada balsam
(Kanto, Tokyo, Japan).
Immunohistochemistry
In brief, according to our published method
(19), sheep anti-SOD1 (1:1,200;
Calbiochem, San Diego, CA, USA), sheep anti-SOD2 (1:1,200;
Calbiochem), rabbit anti-CAT (1:250; Abcam, Cambridge, MA, USA),
rabbit anti-Ki-67 (1:250; Abcam), and rabbit anti-DCX (1:5,500;
Abcam), were used as primary antibodies. Sections of each group
were sequentially treated with 0.3% H2O2 for
40 min and 10% normal goat serum for 40 min. The treated sections
were incubated with each primary antibody overnight at 5°C. The
reacted sections were exposed to biotinylated goat anti-rabbit,
rabbit anti-sheep, or goat anti-mouse IgG (1:300; Vector
Laboratories, Inc., Burlingame, CA, USA) and streptavidin
peroxidase complex (1:300; Vector Laboratories, Inc.). Finally, the
reacted sections were visualized by visualizing with 3,
3′-diaminobenzidine tetrahydrochloride (in 0.05 M Tris-HCl buffer,
pH 7.2).
Data analysis
First, we quantitatively analyzed SOD1, SOD2, and
CAT immunoreactivities according to our published method (20). In brief, we selected six sections
like the above-mentioned method. Images of SOD1, SOD2, and CAT
immunoreactive structures were captured from the dentate gyrus
through an AxioM1 light microscope (Carl Zeiss, Göttingen, Germany)
equipped with a camera (Axiocam, Carl Zeiss, Germany) connected to
a PC monitor. The taken images were calibrated into an array of
512×512 pixels under ×10 primary magnification. Each
immunoreactivity was measured by a 0–255 gray scale system and
evaluated by optical density (OD), which was obtained after
transformation of the mean gray level using a formula, OD=log
(255/mean gray level). A ratio of the OD was calibrated as %
(relative OD, ROD) using Adobe Photoshop version 8.0 and analyzed
using Image J 1.46 software (National Institutes of Health,
Bethesda, MD, USA). A ratio of the ROD was calibrated as %, with
the control group designated as 100%.
Second, we analyzed numbers of Ki67 and DCX positive
cells according to our published method (19). In brief, we selected six sections
from each gerbil with 140-µm interval according to antero-posterior
(AP) −1.4 to −2.2 mm of the gerbil brain atlas. We took images of
the cells from the dentate gyrus through an AxioM1 light microscope
(Carl Zeiss,) equipped with a camera (Axiocam; Carl Zeiss)
connected to a PC monitor. Cell counts were carried out by
averaging the total number of Ki67 and DCX positive cells from all
sections taken from each animal by using an image analyzing system
(software: Optimus 6.5, CyberMetrics, Scottsdale, AZ, USA).
Statistical analysis
Data are expressed as the means ± standard error of
the mean. Differences of the mean number of immunoreactive
structures among the groups was statistically analyzed with one-way
analysis of variance followed by post hoc Tukey's test using
GraphPad Instat (Instat Statistics; GraphPad Software Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Body weight
In the control group, body weight was slightly
increased for 3 months (Fig. 1).
In the ImF groups, body weight was also gradually increased over
time. However, body weight between the control and ImF groups was
not significantly different (Fig.
1).
Antioxidants immunoreactivities
SOD1 immunoreactivity
In the control group, SOD1 immunoreactivity was
found in cells in the granule cells layer (GCL) and polymorphic
layer (PL) of the dentate gyrus (Fig.
2A). In the 1-M ImF group, SOD1 immunoreactivity in the cells
was not significantly different compared to that in the control
group (Fig. 2B). However, in the
2-M ImF group, SOD1 immunoreactivity in the cells was significantly
increased by 56.7 and 43.8% compared to that in the control and 1-M
ImF groups, respectively (Fig.
2C). In the 3-M ImF group, SOD1 immunoreactivity was reduced to
the level of the control group (Fig.
2D). SOD1 immunoreactivity in the dentate gyrus in the control
and ImF groups was shown in Fig.
2E.
 | Figure 2.SOD1, SOD2 and CAT
immunohistochemistry. Immunoreactivities for (A-E) SOD1, (F-J) SOD2
and (K-O) CAT of the control and ImF groups. In the control group,
the SOD1, SOD2 and CAT immunoreactivities were observed in cells in
the GCL and PL. SOD1 immunoreactivity was significantly increased
in the GCL (asterisk in C) in the 2-M ImF group. SOD2
immunoreactivity was significantly increased in the GCL (asterisk
in G) at 1 month of ImF. CAT immunoreactivity was significantly
increased in the GCL (asterisk in L) in the 1-M ImF group.
Magnification, ×10; Scale bar=100 µm. (E, J and O) ROD of (E) SOD1,
(J) SOD2 and (O) CAT immunoreactive structures. Bars indicate the
mean ± standard error of the mean (n=7 per group). *P<0.05 vs.
the control group; †P<0.05 vs. the 1-M ImF group.
GCL, granule cell layer; ML, molecular layer; ROD, relative optical
density; SOD, superoxide dismutase; CAT, catalase; PL, polymorphic
layer; ImF, intermittent fasting. |
SOD2 immunoreactivity
In the control group, weak SOD2 immunoreactivity was
shown in cells in the GCL and PL of the dentate gyrus (Fig. 2F). One month of ImF, SOD2
immunoreactivity was significantly increased by 103.8% compared to
that in the control group (Fig.
2G). Two and three months of ImF, SOD2 immunoreactivity in the
cells was decreased by 60.9 and 95.1%, respectively, compared to
the 1-M ImF group, showing that SOD2 immunoreactivity after three
months of ImF 3 was similar to that in the control group (Fig. 2H and I). SOD2 immunoreactivity in
the dentate gyrus in the control and ImF groups was shown in
Fig. 2J.
CAT immunoreactivity
In the control group, very weak CAT immunoreactivity
was observed in cells in the GCL and PL of the dentate gyrus
(Fig. 2K). In the 1-M ImF and 2-M
ImF groups, CAT immunoreactivity in the cells was significantly
increased by 183.9 and 123.0%, respectively, compared to that in
the control group (Fig. 2L and M).
In the 3-M ImF group, CAT immunoreactivity in the cells was
markedly decreased by 108.3% compared to that in the 2-M ImF group,
showing that there was no significant difference from the control
group (Fig. 2N). CAT
immunoreactivity in the dentate gyrus in the control and ImF groups
was shown in Fig. 2O.
Cell proliferation
In the control group, a few Ki67 positive cells were
mainly located in the subgranular zone (SgZ) of the dentate gyrus
(Fig. 3A). In the 1-M ImF group,
many Ki67 positive cells were observed in the SgZ, and the number
of Ki67 positive cells was significantly increased by 250.0%
compared to that in the control group (Fig. 3B). In the 2-M ImF group, Ki67
positive cells were scattered in the SgZ, showing that the Ki67
positive cells were significantly increased in number by 128.6%
compared to those in the control group (Fig. 3C). At 3 months of ImF, the number
of Ki67 positive cells was significantly increased by 185.7%
compared to that in the control group (Fig. 3D). However, Ki67 positive cells in
the 2-M ImF and 3-M ImF groups were significantly decreased in
number compared to that in the 1-M ImF group (Fig. 3E).
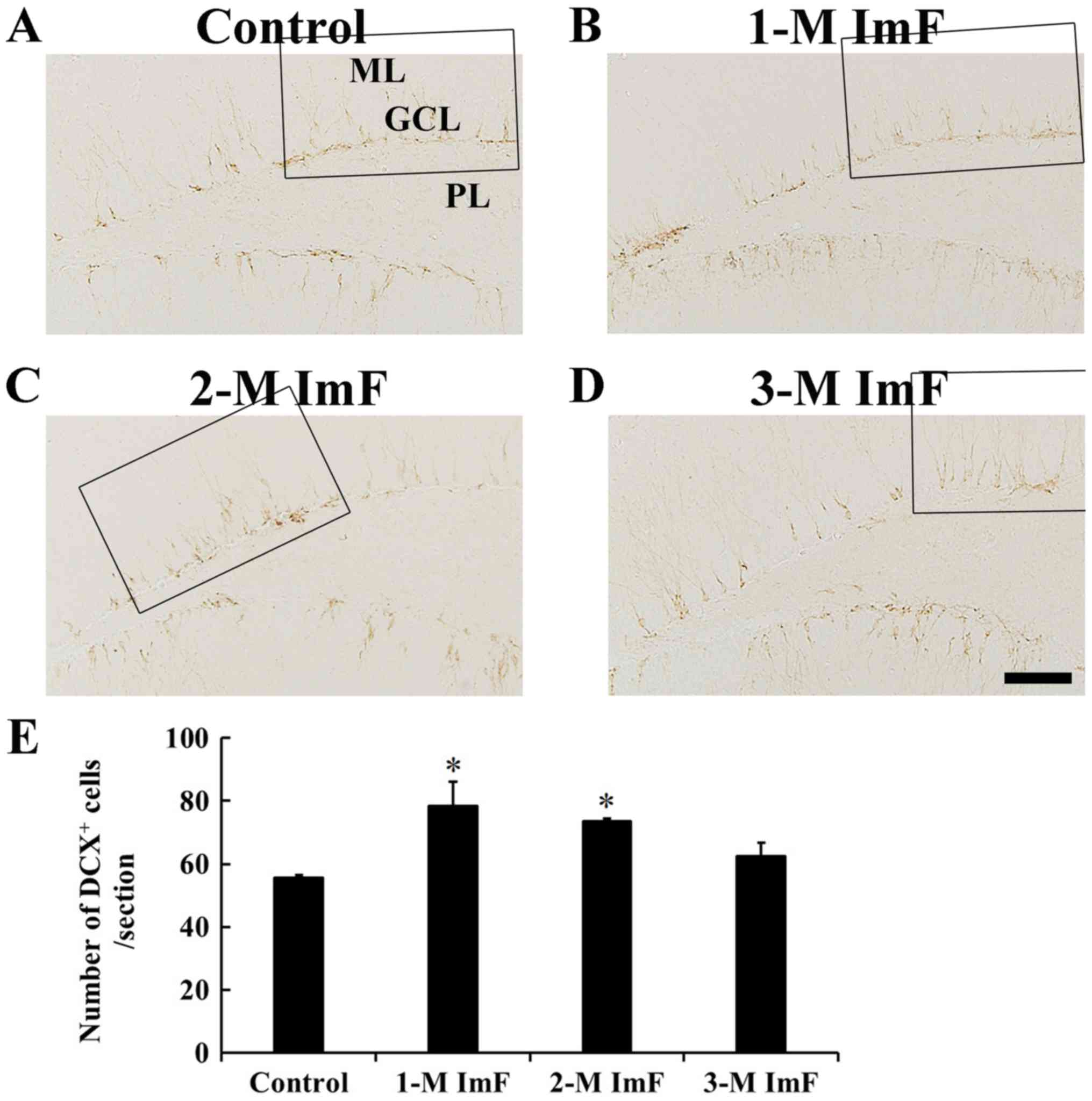
Neuroblast differentiation
In the control group, DCX positive cells were mainly
observed in the SgZ, and their processes projected to the GCL
(Fig. 4A). In the 1-M ImF group,
DCX positive cells were also found in the SgZ, and their number was
significantly increased by 41.1% compared to that in the control
group (Fig. 4B). In the 2-M ImF
group, DCX positive cells were also significantly increased in
number by 32.4% compared to that in the control group (Fig. 4C). At 3 months of ImF, the number
of DCX positive cells was slightly decreased compared to that in
the 2-M ImF group (Fig. 4D). The
number of DCX positive cells in the control and ImF groups was
shown in Fig. 4E.
In this study, according to our previous studies
(17,21), we categorized DCX positive cells
into types a, b and c based on the morphology and complexity of the
dendrites: Dendrites of type a cells were absent or shorter than
soma size, type b cells had one primary dendrite with one branch,
and type c had dendrites with highly arborized branches that
extended into the upper two-thirds of the molecular layer (Fig. 5A). In the control group, the
proportion of dendritic types of DCX positive cells was relatively
uniform (Fig. 5B and C). However,
in the 1-M ImF and 2-M ImF groups, the ratio of the type c
dendrites was increased slightly (Fig.
5B, D and E). In the 3-M ImF group, the ratio of the type b
dendrites was significantly decreased, and the ratio of the type c
dendrites was significantly increased compared to that in the
control group (Fig. 5B and F).
Discussion
In the present study, we investigated effects of ImF
on endogenous antioxidant enzymes such as SOD1, SOD2 and CAT as
well as cell proliferation, neuroblast differentiation and their
dendrite arborization according to the period of ImF in the gerbil
dentate gyrus using immunohistochemistry.
Our results showed that body weight was gradually
increased over a period of 3 months in the control and ImF groups
in adult gerbils (6-month old), but there was no significant
difference between the control and ImF groups. This finding is
supported by a previous study which revealed that increased body
weight of control and 5-M ImF groups were not significantly
different in adult C57BL/6 mice (9-weeks old) (22). On the other hand, body weight in
Wistar rats of control and 1-M ImF groups was changed according to
age; however, no significant difference was shown between the
groups (23). Namely, body weight
was progressively increased in young rats (4-month old), whereas
slightly decreased in aged rats (24-month old) (23). Furthermore, Li et al
(1) reported that, for 11 months
of ImF, significant body weight change in adult CD-1 mice (7-week
old) appeared after 37 weeks (around 9 months) of ImF. Based on
these previous studies and our results, it is postulated that
weight change after alternate day ImF regimen might be affected by
age and fasting period.
In this study, 1 and/or 2 months of ImF regimen
significantly increased SOD1, SOD2 and CAT immunoreactivities in
the granule cell and polymorphic layer of the gerbil dentate gyrus.
In Walsh's et al (24)
review article, they reported that the production of mitochondrial
reactive oxidative stress was reduced in the brain as well as in
the heart, kidneys, liver, and skeletal muscle: In particular, SOD
and CAT activities in the brain were increased after ImF in 20–30%
of studies.
Newly generated neurons in the SgZ of the dentate
gyrus go through multi-stages of morphological development: The
growth of dendrites and axons of immature neurons occurs about 1
week after cell birth, and basic structural and physiological
characteristics of adult-born neurons become similar to those of
mature neurons at around 2 months in the dentate gyrus (8). In the present study, we observed that
ImF significantly increased numbers of Ki67 positive (newly
generated) cells for 3 months of ImF as well as numbers of DCX
positive cells (neuroblasts) for 2 months of ImF in the SgZ of the
dentate gyrus. In addition, at 3 months of ImF, the proportion of
DCX positive neuroblasts with tertiary dendrites was significantly
increased. Previous investigators have reported similar results
that show that the number of newly generated progenitor cells
(BrdU+), which differentiate to mature neurons
(BrdU+/MAP2+), in the dentate gyrus are
significantly increased after 3 months of ImF (9,10).
Furthermore, fasting mimicking diet (alternating fasting designed
as low-calorie consumption only for 4 days of diet) also increases
numbers of new born progenitor cells (BrdU+) and newly
generated immature neurons (BrdU+/DCX+) in
aged C57Bl/6 mice (23-month old) as well as increased DCX positive
neuroblasts in number and dendrites maturation in adult CD-1 mice
(6-month old) (25). Taken
together with our present findings and the previous studies,
continued ImF regimen is likely to increase cell proliferation,
neuroblast differentiation and complexity of neuroblast dendrites
in the gerbil hippocampal dentate gyrus.
Previous studies have revealed that antioxidant
enzymes are closely linked to increased neurogenesis. Fishman et
al (26) have demonstrated
that SOD1 and SOD2 knockout mice show a significant reduction in
numbers of newly born neurons in the SgZ of the dentate gyrus. In
addition, mitochondrial CAT overexpressed transgenic mice show an
increase of basal neurogenesis in the dentate gyrus (27) as well as a significant improvement
in dendritic arborization of granular cells against proton
irradiation (28). Furthermore,
transgenic mice with mitochondrial CAT overexpression show a
tendency to increase dendritic complexity and synaptic integrity of
subiculum neurons (29).
Furthermore, Dias et al (30) have extensively reviewed previous
researches and summarized that dietary polyphenols, which known to
be the most abundant antioxidant in foods, exert the improvement of
NPCs proliferation and neuronal differentiation. Taken together,
our present results, which are described in the 3rd paragraph,
suggest that ImF could affect neuronal differentiation and
maturation by increasing SOD1, SOD2, and CAT expressions in the
gerbil dentate gyrus.
To sum up, findings in this study indicate that ImF
regimen enhances cell proliferation, neuroblast differentiation and
dendrites maturation by increasing SOD1, SOD2, and CAT
immunoreactivities in the gerbil hippocampal dentate gyrus.
Acknowledgements
Not applicable.
Funding
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
NRF-2015R1D1A1A01059728), and by Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Science, ICT &Future Planning
(NRF-2017R1A2B4009079).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contribution
BS, MS, HK, TL, CP and YP performed the experiments.
JP, JL, JY, CL and IH analyzed and interpreted the data. JA, MW and
YL made substantial contributions to the conception and design of
the study, and were involved in drafting and revising the
manuscript, and interpreting the data. All Authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The protocol of this experiment was approved by the
Institutional Animal Care and Use Committee (IACUC) at Kangwon
National University (approval number, KW-180124-1; Gangwon,
Republic of Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li L, Wang Z and Zuo Z: Chronic
intermittent fasting improves cognitive functions and brain
structures in mice. PLoS One. 8:e660692013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeong JH, Yu KS, Bak DH, Lee JH, Lee NS,
Jeong YG, Kim DK, Kim JJ and Han SY: Intermittent fasting is
neuroprotective in focal cerebral ischemia by minimizing autophagic
flux disturbance and inhibiting apoptosis. Exp Ther Med.
12:3021–3028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arumugam TV, Phillips TM, Cheng A, Morrell
CH, Mattson MP and Wan R: Age and energy intake interact to modify
cell stress pathways and stroke outcome. Ann Neurol. 67:41–52.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mladenovic Djordjevic A, Perovic M, Tesic
V, Tanic N, Rakic L, Ruzdijic S and Kanazir S: Long-term dietary
restriction modulates the level of presynaptic proteins in the
cortex and hippocampus of the aging rat. Neurochem Int. 56:250–255.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma L, Wang R, Dong W and Zhao Z: Caloric
restriction can improve learning and memory in C57/BL mice probably
via regulation of the AMPK signaling pathway. Exp Gerontol.
102:28–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J, Duan W and Mattson MP: Evidence
that brain-derived neurotrophic factor is required for basal
neurogenesis and mediates, in part, the enhancement of neurogenesis
by dietary restriction in the hippocampus of adult mice. J
Neurochem. 82:1367–1375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng W, Aimone JB and Gage FH: New neurons
and new memories: How does adult hippocampal neurogenesis affect
learning and memory? Nat Rev Neurosci. 11:339–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee J, Duan W, Long JM, Ingram DK and
Mattson MP: Dietary restriction increases the number of newly
generated neural cells and induces BDNF expression, in the dentate
gyrus of rats. J Mol Neurosci. 15:99–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee J, Seroogy KB and Mattson MP: Dietary
restriction enhances neurotrophin expression and neurogenesis in
the hippocampus of adult mice. J Neurochem. 80:539–547. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution
and expression. Free Radic Biol Med. 33:337–349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chelikani P, Fita I and Loewen PC:
Diversity of structures and properties among catalases. Cell Mol
Life Sci. 61:192–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faiz M, Acarin L, Peluffo H, Villapol S,
Castellano B and Gonzalez B: Antioxidant Cu/Zn SOD: Expression in
postnatal brain progenitor cells. Neurosci Lett. 401:71–76. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ostadsharif M, Ghaedi K, Hossein
Nasr-Esfahani M, Mojbafan M, Tanhaie S, Karbalaie K and Baharvand
H: The expression of peroxisomal protein transcripts increased by
retinoic acid during neural differentiation. Differentiation.
81:127–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tajes M, Gutierrez-Cuesta J, Folch J,
Ortuño-Sahagun D, Verdaguer E, Jiménez A, Junyent F, Lau A, Camins
A and Pallàs M: Neuroprotective role of intermittent fasting in
senescence-accelerated mice P8 (SAMP8). Exp Gerontol. 45:702–710.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn JH, Shin BN, Park JH, Kim IH, Cho JH,
Chen B, Lee TK, Tae HJ, Lee JC, Cho JH, et al: Long-term
observation of neuronal degeneration and microgliosis in the gerbil
dentate gyrus after transient cerebral ischemia. J Neurol Sci.
363:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan BC, Park JH, Chen BH, Cho JH, Kim IH,
Ahn JH, Lee JC, Hwang IK, Cho JH, Lee YL, et al: Long-term
administration of scopolamine interferes with nerve cell
proliferation, differentiation and migration in adult mouse
hippocampal dentate gyrus, but it does not induce cell death.
Neural Regen Res. 9:1731–1739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carpenter JW: Exotic animal formulary (ED
4). J Exotic Pet Med. 22:308–309. 2013.
|
|
19
|
Chen BH, Ahn JH, Park JH, Song M, Kim H,
Lee TK, Lee JC, Kim YM, Hwang IK, Kim DW, et al: Rufinamide, an
antiepileptic drug, improves cognition and increases neurogenesis
in the aged gerbil hippocampal dentate gyrus via increasing
expressions of IGF-1, IGF-1R and p-CREB. Chem Biol Interact.
286:71–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee TK, Park JH, Ahn JH, Shin MC, Cho JH,
Bae EJ, Kim YM, Won MH and Lee CH: Pretreated duloxetine protects
hippocampal CA1 pyramidal neurons from ischemia-reperfusion injury
through decreases of glial activation and oxidative stress. J
Neurol Sci. 370:229–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen BH, Yan BC, Park JH, Ahn JH, Lee DH,
Kim IH, Cho JH, Lee JC, Kim SK, Lee B, et al: Aripiprazole, an
atypical antipsychotic drug, improves maturation and complexity of
neuroblast dendrites in the mouse dentate gyrus via increasing
superoxide dismutases. Neurochem Res. 38:1980–1988. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anson RM, Guo Z, de Cabo R, Iyun T, Rios
M, Hagepanos A, Ingram DK, Lane MA and Mattson MP: Intermittent
fasting dissociates beneficial effects of dietary restriction on
glucose metabolism and neuronal resistance to injury from calorie
intake. Proc Natl Acad Sci USA. 100:6216–6220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vasconcelos AR, Kinoshita PF, Yshii LM,
Marques Orellana AM, Böhmer AE, de Sá Lima L, Alves R, Andreotti
DZ, Marcourakis T, Scavone C and Kawamoto EM: Effects of
intermittent fasting on age-related changes on Na,K-ATPase activity
and oxidative status induced by lipopolysaccharide in rat
hippocampus. Neurobiol Aging. 36:1914–1923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walsh ME, Shi Y and Van Remmen H: The
effects of dietary restriction on oxidative stress in rodents. Free
Radic Biol Med. 66:88–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brandhorst S, Choi IY, Wei M, Cheng CW,
Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M,
et al: A periodic diet that mimics fasting promotes multi-system
regeneration, enhanced cognitive performance, and healthspan. Cell
Metab. 22:86–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fishman K, Baure J, Zou Y, Huang TT,
Andres-Mach M, Rola R, Suarez T, Acharya M, Limoli CL, Lamborn KR
and Fike JR: Radiation-induced reductions in neurogenesis are
ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic Biol
Med. 47:1459–1467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao AC, Craver BM, Tseng BP, Tran KK,
Parihar VK, Acharya MM and Limoli CL: Mitochondrial-targeted human
catalase affords neuroprotection from proton irradiation. Radiat
Res. 180:1–6. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parihar VK, Allen BD, Tran KK, Chmielewski
NN, Craver BM, Martirosian V, Morganti JM, Rosi S, Vlkolinsky R,
Acharya MM, et al: Targeted overexpression of mitochondrial
catalase prevents radiation-induced cognitive dysfunction. Antioxid
Redox Signal. 22:78–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chmielewski NN, Caressi C, Giedzinski E,
Parihar VK and Limoli CL: Contrasting the effects of proton
irradiation on dendritic complexity of subiculum neurons in wild
type and MCAT mice. Environ Mol Mutagen. 57:364–371. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dias GP, Cavegn N, Nix A, do Nascimento
Bevilaqua MC, Stangl D, Zainuddin MS, Nardi AE, Gardino PF and
Thuret S: The role of dietary polyphenols on adult hippocampal
neurogenesis: Molecular mechanisms and behavioural effects on
depression and anxiety. Oxid Med Cell Longev 2012. 5419712012.
|