Introduction
Recurrent spontaneous abortions (RSA) are defined as
aborting three or more times within 20 gestational weeks with the
same sexual partner. Epidemiological studies have suggested that
the occurrence rate of RSA is 1–5%. Multiple factors, including
anatomy, genetics, infection, environment, endocrine factors,
thrombus and immune factors are involved in the occurrence and
development of RSA and some of the possible precipitating factors
of RSA remain unknown (1–3). RSA has become a challenge to women of
childbearing age and reproductive medicine workers alike. RSA
affects the physical and mental health of patients and their
families. Therefore, it is important to further investigate the
pathogenesis of RSA and seek effective methods to treat RSA, which
may provide a novel strategy for the clinical treatment of RSA and
be of significance in the field of the RSA research (4,5).
The normal activation of the inflammasome serves an
important role in maintaining the stability of the immune system.
The inflammasome is gaining increasing amounts of attention in the
pathological process of metabolic disorders, autoimmune diseases
and types of cancer. The NACHT, LRR and PYD domains-containing
protein 3 (NLRP3) inflammasome, which is an important component of
innate immunity, serves a role in the immune response and disease
occurrence. The abnormal activation and regulation of the NLRP3
inflammasome is associated with a number of autoimmune diseases
(6,7). The NLRP3 inflammasome is composed of
a variety of protein compounds, which are considered to be innate
immune system sensors. The NLRP3 inflammasome recognizes all types
of danger signals, induces cell activation and releases
inflammatory cytokines causing inflammation (8). The NLRP3 inflammasome hydrolyses the
adjacent two pro-caspase-1, then the enzyme initiates the
production of the active caspase-1. Active caspase-1 mediates the
activation of interleukin (IL)-1β and IL-18 from the precursors of
IL-1β and IL-18. The activation of the NLRP3 inflammasome also
regulates the adaptive immune response. The NLRP3 inflammasome
affects the differentiation and function of active helper T cells
(9). The NLRP3 inflammasome is
closely associated with autoimmune diseases, which indicates that
the NLRP3 inflammasome may be associated with the occurrence of
recurrent miscarriage (10). In
the present study, our aim is to investigate the role and mechanism
of NLRP3 inflammasome in RSA. The significance of activation of the
NLRP3 inflammasome, caspase-1 and IL-1β, IL-18 inflammatory
cytokines was studied, which may explain the occurrence of
recurrent miscarriage and provide novel ideas for the diagnosis and
treatment of inflammation and autoimmune diseases, including
RSA.
Patients and methods
Patients and controls
The case-control study was approved by the Ethics
Committee of Nanjing Medical University (Nanjing, China). A total
of 30 patients with RSA and 30 controls from Wuxi Hospital for
Maternal and Child Health Care, The Affiliated Hospital of Nanjing
Medical University were recruited between July 2014 and June 2017,
and all peripheral blood samples were collected from these people
subsequent to obtaining written informed consent. The age (mean ±
standard deviation) of women with RSA (31.2±3.2 years) was not
significantly different from that of the controls (30.5±3.7 years).
Excluding any definite causes, including abnormalities of the
uterus or cervix, chromosomal abnormality, infection, endocrine and
metabolic diseases, congenital thrombophilia and autoimmune
disease, RSA was diagnosed. Peripheral blood (20 ml) was collected
from two different groups at the late follicular phase (days 9–12)
of the menstrual cycle. A total of 30 fertile women who had at
least one successful pregnancy and no previous abortions, with
regular menstrual cycles, were used as the control group. None of
the women in the present study were taking oral contraceptives.
Experimental animals of RSA
A total of 30 10-week-old mice [female CBA/J
(h-2k) mice, male DBA/2J (h-2d) and male
Balb/c (h-2d); 10 mice in each group] weighing 20–30 g
were obtained from the Beijing Institute of Laboratory Animals of
the Chinese Academy of Medical Sciences (Beijing, China). The mice
were housed at 24°C with 12-h light/dark cycle and free access to
food and water with breeding conditions complying with special
pathogen free standards. The experimental protocols for animals
were approved by the Ethics Committee of Nanjing Medical
University. Male Balb/c or DBA/2J mice were bred with female CBA/J
mice and the vaginal plugs in individual mated female mice were
examined daily to determine potential pregnancy. CBA/J mice mated
with Balb/c mice were used as the normal pregnancy group. CBA/J
mice mated with DBA/2J were considered as the spontaneous abortion
group. Each group had 10 mice.
ELISA
To assess the levels of caspase-1 activity, and
IL-1β and IL-18 expression in the peripheral blood monocytes (PBMC)
of patients with RSA and spleen tissues of model mice, ELISA
analysis was performed using respective ELISA kits (eBioscience;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for IL-1β
(KHC0019) and IL-18, (MAN0014360) and Beyotime Institute of
Biotechnology, Haimen, China for caspase-1 activity (P70380)
according to the manufacturer's protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the PBMCs of patients or
the spleen tissue of mice with TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and was reverse transcribed at 50°C for 60
min using a PrimeScript RT reagent kit (Takara Bio, Inc., Otsu,
Japan), according to the manufacturer's protocol. Subsequently,
cDNA was subjected to qPCR using a Lightcycler480 and SYBR-Green
system (Roche Diagnostics, Basel, Switzerland) following the
manufacturer's protocol. The primer sequences used in the present
study were as follows: i) forward, NLRP3:
5′-CGAGACCTCTGGGAAAAAGCT-3′ and reverse,
5′-GCATACCATAGAGGAATGTGATGTAC-3′; ii) GAPDH forward,
5′-CTCTGGAAAGCTGTGGCGTGATG-3′ and reverse,
5′-ATGCCAGTGAGCTTCCCGTTCAG-3′. GAPDH was used as a housekeeping
gene for normalization. The cycling parameters were as follows:
Denaturation at 95°C for 10 min, amplification at 95°C for 15 sec,
58°C for 1 min and 72°C for 1 min for 40 cycles. NLRP3 expression
was calculated following normalization to GAPDH expression levels
by the comparative 2−∆∆Cq quantification cycle method
(11).
Inhibition of caspase-1
In the present study, a highly specific,
cell-permeable and competitive inhibitor of caspase-1 was used as
previously described (12,13). Briefly, the mated model mice
received the first intraperitoneal injection of 0.5 mg
Ac-Tyr-Val-Ala-Asp-2,6-dimethylbenzoyloxymethyl ketone
(Ac-YVAD-CHO; Bachem AG, Bubendorf, Switzerland) dissolved in 0.1
ml sterile phosphate-buffered saline (pH 7.4) on day 0. This was
repeated every three days. Mice receiving treatment with PBS were
used as a control.
Detection of Th17 [cluster of
differentiation (CD)4+ IL-17A+] and
regulatory T cells [Treg; CD4+ CD25+ forkhead
box protein P3 (FOXP3+)] in RSA and controls by flow
cytometry
For analysis of Th17 cells, PBMCs (2×106
cells/ml) were suspended in culture medium including RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc.) with L-glutamine,
penicillin (100 U/ml), streptomycin (10 mg/ml) and 10% fetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
stained for the surface marker CD4 by incubation with fluorescein
isothiocyanate-conjugated anti-human CD4 (1:30; cat. no.
11-0041-82; eBioscience; Thermo Fisher Scientific, Inc.) at 4°C for
30 min. Subsequently, the cells were fixed in 4% paraformaldehyde
at 4°C for 30 min and then stained with phycoerythrin
(PE)-conjugated anti-human IL-17A (1:50; cat. no. 25-7177-82;
eBioscience; Thermo Fisher Scientific, Inc.) for Th17 cell
detection, following fixation and permeabilization according to the
manufacturer's protocol, by flow cytometric analysis as in a
previous study (14). Similarly,
for Treg cell staining, cells were incubated with PerCP anti-human
CD4 (1:40; cat. no. 46-0047-42; eBioscience; Thermo Fisher
Scientific, Inc.) and CD25 (1:40; cat. no. 11-0257-42; eBioscience;
Thermo Fisher Scientific, Inc.) at 4°C for 30 min. Following
surface staining, the cells were fixed in 4% paraformaldehyde for
30 min at 4°C and intracellularly stained with PE-anti-human FOXP3
(1:60; cat. no. 12-4777-42; eBioscience; Thermo Fisher Scientific,
Inc.). All data were analyzed by flow cytometry and analyzed by
FlowJo software version 10 (FlowJo LLC, Ashland, OR, USA), as
described in a previous study (14,15).
Statistical analysis
Data are presented as the mean ± standard deviation
from three independent experiments. Comparisons between patients
with RSA and controls were analyzed via the Student's t test.
Correlation analysis was performed via the Pearson correlation
test. All analyses were performed using GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of the NLRP3 inflammasome
in RSA patients
Embryos carry one-half of the tissue antigens
derived from the parents, therefore the embryo is half allogeneic
grafts to the mother. However, throughout the pregnancy, the embryo
survives attacks from the immune system of the mother, which is
attributed to tolerance towards the embryo by the mother's immune
system (16–18). Once the immune tolerance is
overcome, however, it will lead to abortion (19,20).
Therefore, how the embryo escapes from attack by the immune system
in pregnant women is complex.
Inflammasomes have been widely implicated in the
development and progression of various chronic diseases (21–23).
The NOD-like receptor (NLR) family was the first researched
inflammasome. Once the NLR family is activated, NLRP3 recruits the
adapter apoptosis-related speck-like protein containing a caspase
recruitment domain, which in turn recruits procaspase-1.
Procaspase-1 autocatalyzes its own cleavage and activation,
resulting in maturation of the precursor forms of IL-1β and IL-18
into active proinflammatory cytokines and the initiation of
pyroptotic cell death (24).
Abortion is closely associated with the immune
response in pregnant women. To investigate whether the NLRP3
inflammasome is involved in RSA, the expression of NLRP3 mRNA
expression levels in PBMCs of patients with RSA and controls was
analyzed. It was demonstrated that the NLRP3 mRNA expression levels
were significantly increased in RSA patients compared with the
healthy controls (P<0.05; Fig.
1A). The same results were obtained in the decidual tissue
(data not shown). It was then demonstrated that caspase-1 activity
was significantly upregulated in patients with RSA compared with
the healthy controls (P<0.05; Fig.
1B). Furthermore, expression levels of IL-1β (Fig. 1C) and IL-18 (Fig. 1D) were significantly increased in
the patients with RSA (P<0.05). These results suggested that the
NLRP3 inflammasome was activated in patients with RSA. Further
studies were performed to investigate the mechanism of activation
of the NLRP3 inflammasome in RSA patients.
Expression of the NLRP3 inflammasome
in the RSA mouse model
CBA/J mice mated with Balb/c mice were used as the
control group with normal pregnancy. CBA/J mice mated with DBA/2J
served as the spontaneous abortion group. The NLRP3 mRNA expression
levels in the splenic tissue in the RSA and control mouse models
were analyzed. The results demonstrated that the NLRP3 mRNA level
was also significantly upregulated in the RSA model (P<0.05;
Fig. 2A). Following this, the
caspase-1 activity and expression levels of IL-1β and IL-18 in the
RSA and control mice model were examined. The results demonstrated
that the caspase-1 activity was significantly increased (P<0.05;
Fig. 2B) as was IL-1β (P<0.05;
Fig. 2C) and IL-18 expression
(P<0.05; Fig. 2D). These
results suggested that the NLRP3 inflammasome was activated in the
RSA mouse model.
Inhibition of NLRP3 inflammasome in
the RSA mouse model could rescue the abortion-prone mice
The above results suggested that the NLRP3
inflammasome was activated in the patients with RSA and the mouse
model. The caspase-1 inhibitor YVAD was used to pretreat the
abortion-prone mice. On day 14 of pregnancy, the abortion-prone
mice treated with YVAD had a low embryo resorption rate compared
with the untreated abortion-prone mice. These results proved that
the inhibition of the NLRP3 inflammasome in the RSA mouse model was
able to protect the abortion-prone mice (Table I).
 | Table I.Treatment with YVAD was able to
protect the abortion-prone mice in the RSA mouse model. |
Table I.
Treatment with YVAD was able to
protect the abortion-prone mice in the RSA mouse model.
|
| No. of mice | Surviving
fetuses | Resorbed
fetuses | Resorption rate,
% |
|---|
| Control | 10 | 69 | 12 | 17.39 |
| RSA | 10 | 51 | 22 | 43.14 |
| Vehicle | 10 | 47 | 20 | 42.55 |
| YVAD | 10 | 45 | 12 | 26.67 |
Activated NLRP3 inflammasome is
involved in the pathogenesis of RSA through regulation of the Th17
and Treg imbalance
Th17 cells serve an important role in the
inflammatory reaction through the release of the pro-inflammatory
cytokine IL-17. Therefore, Th17 cells may be proinflammatory
factors in embryonic immune rejection. However, Treg cells
(primarily CD4+ CD25+ Treg) induce immune
tolerance, which is a unique immune regulatory function in the T
cell subgroup (25). Treg and Th17
cells are important in immune rejection; a number of studies have
demonstrated that an imbalance of Th17 and Tregs may lead to the
occurrence of RSA (23,25). Whether the NLRP3 inflammasome is
associated with Treg and Th17 cells was investigated in the present
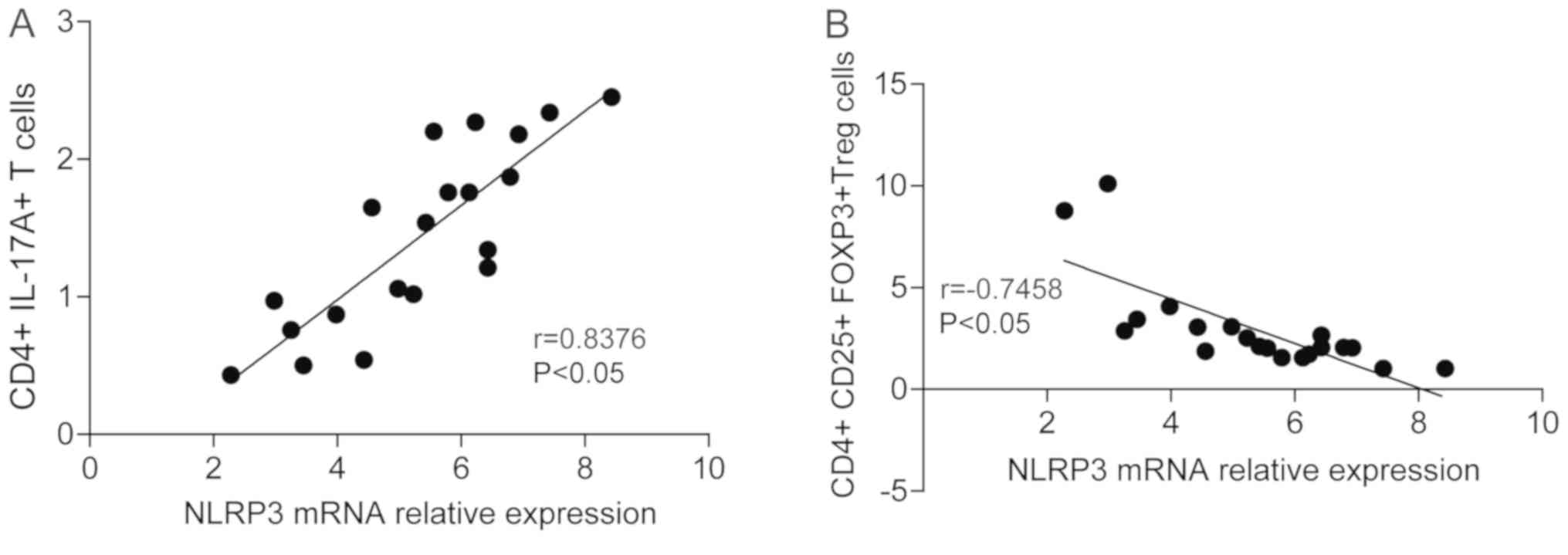
study. The correlation between NLRP3 and IL-17, and NLRP3 and
CD4+ CD25+ Treg cells was analyzed. The
results demonstrated that the NLRP3 expression was positively
correlated with the CD4+ IL-17A+ levels
(r=0.76; Fig. 3A), while NLRP3
expression was negatively correlated with CD4+
CD25+ Treg cells (r=−0.81; Fig. 3B). Similar results were observed
the RSA mouse model (Fig. 4). The
CD4+ IL-17A+ and CD4+
CD25+ Treg levels in the RSA mice were investigated with
the caspase-1 inhibitor YVAD. The results demonstrated that
treatment with YVAD increased the CD4+ CD25+
Treg levels while the CD4+ IL-17A+ levels
were downregulated (Fig. 5). These
results suggested that the activated NLRP3 inflammasome was
involved in the pathogenesis of RSA by regulating the Th17 and Treg
imbalance.
Discussion
RSA occurs in 1–5% of women during their
reproductive years (26). The
exact mechanism of RSA is remains unclear. Certain studies have
suggested that the onset of RSA has been associated with maternal
immune system attack on the fetus by allo-rejection (27–29).
A previous study observed that Th2-anti-inflammatory predominance
facilitated the survival of the fetus in the maternal uterus
(30). However, a previous study
demonstrated that the lack of Th2 cytokine secretion of genetically
deficient mice did not always cause abortion, suggesting that
multiple factors were involved in the pathogenesis of RSA (31). Previous studies have demonstrated
that Treg (31) and Th17 (32) are involved in abortion. The
imbalance of Treg and Th17 leads to abortion through the secretion
of inflammatory cytokines. The focus of the present study was the
mechanism underlying the imbalance of Treg and Th17 cells. A
previous study demonstrated that the NLRP3 inflammasome serves an
important role in inflammatory and autoimmune diseases (33). In the present study, it was
demonstrated that the NLRP3 inflammasome was activated in patients
with RSA and the mouse model. Further experiments demonstrated that
the inhibition of the NLRP3 inflammasome in the RSA mouse model was
able to rescue abortion-prone mice. Finally, the results
demonstrated that the activated NLRP3 inflammasome is involved in
the pathogenesis of RSA by regulating the Th17 and Treg balance
disturbance.
Once danger signals meet the immune system, the
NLRP3 inflammasome is activated. Following this, the NLRP3
inflammasome increases the secretion of active caspase-1, and IL-1β
and IL-18, which are inflammatory cytokines (34). In the present study it was
demonstrated that the mRNA expression levels of NLRP3 were
increased in addition to the active caspase-1, IL-1β and IL-18 in
the women with RSA compared with the controls. These results
suggested that the NLRP3 inflammasome may be involved in
abortion.
The cytokines induced by the NLRP3 inflammasome have
individual characteristics. IL-1, which is considered to be a
powerful inflammatory mediator, may induce acute systemic or local
inflammation and contributes to a number of chronic diseases. IL-1
is associated with a number of autoimmune diseases, including
atherosclerosis, osteoarthritis, metabolic syndrome and type 2
diabetes (35). The secretion of
IL-1β consists of a number of stages. First, an activated antigen
triggers IL-1β expression in inflammatory cells. Second, the IL-1β
precursor protein, which has no biological activity in the cytosol,
is translated from the IL-1β mRNA. Finally, the cell secretes
activated IL-1β. The inflammasome, including activated caspase-1
and pro-IL-18, was discovered by Cerretti et al (36), Thornberry et al (37), Miura et al (38) and Martinon et al (39).
Although inflammasomes exist in different forms, the
constituent parts of the inflammasomes are similar, including the
NLR gene family, adaptor proteins and procaspase-1 molecules
(40). The NLRP3 inflammasome is
the most important inflammasome in sterile inflammation. Diverse
stimulation, including pore-forming toxins, bacteria, viruses and
endogenous molecules, may activate the NLRP3 inflammasome (41,42).
Pathogen-associated and damage-associated molecular
patterns may activate the NLRP3 inflammasome. Following this, the
NLRP3 inflammasome induces the secretion of important
pro-inflammatory cytokines, including IL-1β and IL-18 (43,44).
The NLRP3 inflammasome is involved in a variety of biological
activities, including inflammation and tissue repair. Recent
studies demonstrated that numerous sensor proteins of endogenous
and exogenous origins have been involved in NLRP3 inflammasome
activation. In metabolic syndrome and inflammatory bowel disease
(45), NLRP3 inflammasome
activation exerts important roles. Furthermore, mutations in
components of inflammasome complexes have been associated with a
propensity for the development of a number of immune-mediated
diseases in humans (46).
A number of studies have suggested that Treg and
Th17 cells serve important roles in the pathogenesis of RSA
(6,7,25,47–49).
Treg cells may induce tolerance and maintain tolerance to
alloantigens. Th17 cells produce the signature cytokine IL-17 to
mediate inflammation, autoimmunity and immunological rejection of
foreign tissue (50,51). The results of the present study
demonstrated that the activated NLRP3 inflammasome is involved in
the pathogenesis of RSA by regulating Th17 and Treg balance
disturbance.
In conclusion, the authors of the present study
suggested that the NLRP3 inflammasome was activated in patients
with RSA by regulating Th17 and Treg balance disturbance. The NLRP3
inflammasome may be used as a therapeutic target for RSA in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by: The National
Natural Scientific Foundation of China (grant nos. 30872999 and
81372480); a grant from Revitalize and Defend the Key Talent's
Subsidy Project in Science and Education of Department of Public
Health of Jiangsu Province (grant no. RC2011033); a grant for the
Key Talents of Young Medical Science in the Jiangsu Province (grant
no. QNRC2016169); a grant for Jiangsu Natural Science Youth Fund
(grant no. BK20170209); The China Postdoctoral Research Foundation
(grant no. 180942); The Jiangsu Province Postdoctoral Research
Foundation (grant no. 1701012A); The Wuxi Science and Technological
Developmental Project (grant no. CSE31N1427); and The Project of
Wuxi Health and Family Planning Commission (grant no.
YGZXM1401).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
The experiments were conceived and designed by ML
and DC, and the experiments were performed by LY and NL. FM and JX
contributed reagents, materials and analytical tools, and analyzed
the data. ML and DC wrote the paper.
Ethics approval and consent to
participate
The case-control study was approved by the Ethics
Committee of Nanjing Medical University. Informed consent was
obtained from all participants. The experimental protocols for
animals were approved by the Ethics Committee of Nanjing Medical
University.
Patients consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Larsen EC, Christiansen OB, Kolte AM and
Macklon N: New insights into mechanisms behind miscarriage. BMC
Med. 11:1542013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwak-Kim J, Agcaoili MS, Aleta L, Liao A,
Ota K, Dambaeva S, Beaman K, Kim JW and Gilman-Sachs A: Management
of women with recurrent pregnancy losses and antiphospholipid
antibody syndrome. Am J Reprod Immunol. 69:596–607. 2013.PubMed/NCBI
|
|
3
|
Shetty S, Patil R and Ghosh K: Role of
microparticles in recurrent miscarriages and other adverse
pregnancies: A review. Eur J Obstet Gynecol Reprod Biol.
169:123–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hovdenak N and Haram K: Influence of
mineral and vitamin supplements on pregnancy outcome. Eur J Obstet
Gynecol Reprod Biol. 164:127–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martínez-Zamora MÁ, Cervera R and Balasch
J: Recurrent miscarriage, antiphospholipid antibodies and the risk
of thromboembolic disease. Clin Rev Allergy Immunol. 43:265–274.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saini V, Arora S, Yadav A and
Bhattacharjee J: Cytokines in recurrent pregnancy loss. Clin Chim
Acta. 412:702–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Mourik MS, Macklon NS and Heijnen CJ:
Embryonic implantation: Cytokines, adhesion molecules, and immune
cells in establishing an implantation environment. J Leukoc Biol.
85:4–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piccinni MP, Scaletti C, Maggi E and
Romagnani S: Role of hormone-controlled Th1- and Th2-type cytokines
in successful pregnancy. J Neuroimmunol. 109:30–33. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaouat G, Lédée-Bataille N, Zourbas S,
Ostojic S, Dubanchet S, Martal J and Frydman R: Cytokines,
implantation and early abortion: Re-examining the Th1/Th2 paradigm
leads to question the single pathway, single therapy concept. Am J
Reprod Immunol. 50:177–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Ippolito S, Tersigni C, Marana R, Di
Nicuolo F, Gaglione R, Rossi ED, Castellani R, Scambia G and Di
Simone N: Inflammosome in the human endometrium: Further step in
the evaluation of the ‘maternal side’. Fertil Steril.
105:111–118.e1-e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time PCR and the 2(-Delta
Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paszkowski AS, Rau B, Mayer JM, Möller P
and Beger HG: Therapeutic application of caspase
1/interleukin-1beta-converting enzyme inhibitor decreases the death
rate in severe acute experimental pancreatitis. Ann Surg.
235:68–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Westermann D, Van Linthout S, Dhayat S,
Dhayat N, Escher F, Bücker-Gärtner C, Spillmann F, Noutsias M, Riad
A, Schultheiss HP and Tschöpe C: Cardioprotective and
anti-inflammatory effects of interleukin converting enzyme
inhibition in experimental diabetic cardiomyopathy. Diabetes.
56:1834–1841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sereshki N, Gharagozloo M, Ostadi V,
Ghahiri A, Roghaei MA, Mehrabian F, Andalib AA, Hassanzadeh A,
Hosseini H and Rezaei A: Variations in T-helper 17 and regulatory T
cells during the menstrual cycle in peripheral blood of women with
recurrent spontaneous abortion. Int J Fertil Steril. 8:59–66.
2014.PubMed/NCBI
|
|
15
|
Wang WJ, Hao CF, Yi-Lin, Yin GJ, Bao SH,
Qiu LH and Lin QD: Increased prevalence of T helper 17 (Th17) cells
in peripheral blood and decidua in unexplained recurrent
spontaneous abortion patients. J Reprod Immunol. 84:164–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Potdar N, Gelbaya T and Nardo LG:
Endometrial injury to overcome recurrent embryo implantation
failure: A systematic review and meta-analysis. Reprod Biomed
Online. 25:561–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denny KJ, Woodruff TM, Taylor SM and
Callaway LK: Complement in pregnancy: A delicate balance. Am J
Reprod Immunol. 69:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kokcu A, Yavuz E, Celik H and Bildircin D:
A panoramic view to relationships between reproductive failure and
immunological factors. Arch Gynecol Obstet. 286:1283–1289. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakashima A, Shima T, Inada K, Ito M and
Saito S: The balance of the immune system between T cells and NK
cells in miscarriage. Am J Reprod Immunol. 67:304–310. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin LP, Fan DX and Li DJ: Regulation of
costimulatory signal in maternal-fetal immune tolerance. Am J
Reprod Immunol. 66:76–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rock KL, Kataoka H and Lai JJ: Uric acid
as a danger signal in gout and its comorbidities. Nat Rev
Rheumatol. 9:13–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grebe A and Latz E: Cholesterol crystals
and inflammation. Curr Rheum Rep. 15:3132013. View Article : Google Scholar
|
|
23
|
Jourdan T, Godlewski G, Cinar R, Bertola
A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, et
al: Activation of the Nlrp3 inflammasome in infiltrating
macrophages by endocannabinoids mediates beta cell loss in type 2
diabetes. Nat Med. 19:1132–1140. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirwan AM, Lenighan YM, O'Reilly ME,
McGillicuddy FC and Roche HM: Nutritional modulation of metabolic
inflammation. Biochem Soc Trans. 45:979–985. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peck A and Mellins ED: Plasticity of
T-cell phenotype and function: The T helper type 17 example.
Immunology. 129:147–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin QD and Qiu LH: Pathogenesis,
diagnosis, and treatment of recurrent spontaneous abortion with
immune type. Front Med China. 4:275–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Druckmann R and Druckmann MA: Progesterone
and the immunology of pregnancy. J Steroid Biochem Mol Biol.
97:389–396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen RA, Check JH and Dougherty MP:
Evidence that exposure to progesterone alone is a sufficient
stimulus to cause a precipitous rise in the immunomodulatory
protein the progesterone induced blocking factor (PIBF). J Assist
Reprod Genet. 33:221–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin LP, Fan DX, Zhang T, Guo PF and Li DJ:
The costimulatory signal upregulation is associated with Th1 bias
at the maternal-fetal interface in human miscarriage. Am J Reprod
Immunol. 66:270–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raghupathy R, Makhseed M, Azizieh F,
Hassan N, Al-Azemi M and Al-Shamali E: Maternal Th1- and Th2-type
reactivity to placental antigens in normal human pregnancy and
unexplained recurrent spontaneous abortions. Cell Immunol.
196:122–130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zenclussen AC: Regulatory T cells in
pregnancy. Springer Semin Immunopathol. 28:31–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang WJ, Liu FJ, Qu HM, Hao CF, Qu QL,
Xiong-Wang, Bao HC and Wang XR: Regulation of the expression of
Th17 cells and regulatory T cells by IL-27 in patients with
unexplained early recurrent miscarriage. J Reprod Immunol.
99:39–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mangan MS and Latz E: TAK1ng control: TAK1
restrains NLRP3 activation. J Exp Med. 215:1007–1008. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poudel B and Gurung P: An update on cell
intrinsic negative regulators of the NLRP3 inflammasome. J Leukoc
Biol. Jan 26–2018.(Epub ahead of print). View Article : Google Scholar
|
|
35
|
Dinarello CA: A clinical perspective of
IL-1β as the gatekeeper of inflammation. Eur J Immunol.
41:1203–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cerretti DP, Kozlosky CJ, Mosley B, Nelson
N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T,
Cannizzaro LA, et al: Molecular cloning of the interleukin-1beta
converting enzyme. Science. 256:97–100. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thornberry NA, Bull HG, Calaycay JR,
Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner
JR, Aunins J, et al: A novel heterodimeric cysteine protease is
required for interleukin-1beta processing in monocytes. Nature.
356:768–774. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miura M, Zhu H, Rotello R, Hartwieg EA and
Yuan J: Induction of apoptosis in fibroblasts by IL-1
beta-converting enzyme, a mammalian homolog of the C. elegans cell
death gene ced-3. Cell. 75:653–660. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bauernfeind F, Ablasser A, Bartok E, Kim
S, Schmid-Burgk J, Cavlar T and Hornung V: Inflammasomes: Current
understanding and open questions. Cell Mol Life Sci. 68:765–783.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fernandes-Alnemri T, Yu JW, Datta P, Wu J
and Alnemri ES: AIM2 activates the inflammasome and cell death in
response to cytoplasmic DNA. Nature. 458:509–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hornung V, Ablasser A, Charrel-Dennis M,
Bauernfeind F, Horvath G, Caffrey DR, Latz E and Fitzgerald KA:
AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating
inflammasome with ASC. Nature. 458:514–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Davis BK, Wen H and Ting JP: The
inflammasome NLRs in immunity, inflammation, and associated
diseases. Annu Rev Immunol. 29:707–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kanneganti TD: Inflammatory bowel disease
and the NLRP3 inflammasome. N Engl J Med. 377:694–696. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Neudecker V, Haneklaus M, Jensen O,
Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS,
Gerich ME, et al: Myeloid-derived miR-223 regulates intestinal
inflammation via repression of the NLRP3 inflammasome. J Exp Med.
214:1737–1752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alijotas-Reig J, Melnychuk T and Gris JM:
Regulatory T cells, maternal-foetal immune tolerance and recurrent
miscarriage: New therapeutic challenging opportunities. Med Clin
(Barc). 144:265–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saito S, Nakashima A, Myojo-Higuma S and
Shiozaki A: The balance between cytotoxic NK cells and regulatory
NK cells in human pregnancy. J Reprod Immunol. 77:14–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu XY, Zhou YH, Wang MY, Jin LP, Yuan MM
and Li DJ: Blockade of CD86 signaling facilitates a Th2 bias at the
maternal-fetal interface and expands peripheral CD4+CD25+
regulatory T cells to rescue abortion-prone fetuses. Biol Reprod.
72:338–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage distinct
from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|