Introduction
As a type of mesenchymal stromal cell,
adipose-derived stem cells (ADSCs) are characterized by their
ability to self-renew and differentiate into multiple cell lineages
(1). ADSCs are obtained from white
or brown adipose tissue (2).
Advantages of ADSCs are that they are abundant, collection results
in minimal morbidity, they may differentiate into multiple cell
lineages, and can be transplanted safely and effectively (3). It is generally accepted that adipose
tissue contains multipotent progenitor cells (4). In addition, it comprises a useful and
clinically important cell population called the stromal vascular
fraction (SVF). SVF may be used directly or cultured as ADSCs
(2,5,6).
ADSCs are abundantly sourced and readily attained. SVF/ADSCs may be
collected for clinical use by harvesting adipose tissue, digesting
type I collagen enzymes, and removing red blood cells. ADSCs have
been extensively applied in various clinical fields, including
treatment for immune disorders, tissue degeneration and ischemic
conditions, as well as soft tissue, craniofacial tissue and
cardiovascular tissue regeneration (2,7).
Considering their clinical characteristics and high demand,
maintaining ADSC viability during transit or prior to clinical
application is a wide concern, and the present study focused on
methods for preserving ADSCs.
The primary methods for in vitro storage
include 4°C non-frozen preservation, −80°C cryopreservation and
−196°C programmed cryopreservation in liquid nitrogen. Gonda et
al (8) demonstrated that
adipocytes may be optimally preserved for viability by cooling to
sub-zero temperatures in liquid nitrogen for 6 months with a set
preservation protocol. Following long-term cryopreservation, the
proliferation and multiple differentiation capacities of human
ADSCs are not lost (9). Matsumoto
et al (10) determined oil
volume ratio, glycerol-3-phosphate dehydrogenase activity and
cell-surface marker expression via scanning electron microscopy to
assess the viability of ADSCs at room temperature (RT) and at 4°C.
Furthermore, it was reported that adipose tissue should be stored
promptly, as storage overnight at 4°C results in no evident loss or
alteration in the biological properties or yield of ADSCs (10). However, it has also been indicated
that low temperatures may irreversibly damage the ADSC membrane
(11,12). In this regard, an appropriate
protection medium should be adopted when freezing cells, such as
dimethyl sulfoxide (DMSO). To mitigate this damage, Bunnell et
al (13) stored the cells in a
specialized container. This allowed the temperature to be lowered
at a rate of 1°C/min until −80°C was reached, and the cells were
stored overnight. The following day, the cells were transferred to
liquid nitrogen (13). Thus far,
the majority of studies have been concerned with the long-term
preservation of stem cells. For stem cells intended for clinical
application, the current methods of preservation are inadequate, as
human ADSC transplantation requires higher standards of security,
survival and proliferation ability. In addition to the temperature,
the preservation medium is also of critical significance. Several
reports have concluded that an environment that mimics that of the
native cell, containing 0.9% NaCl, 5% glucose and albumin or human
serum (HS), is the most appropriate for cell survival (14). Other preservation media have also
been studied, such as DMSO; however, the clinical use of DMSO
causes diverse problems, including leukoencephalopathy, nausea,
vomiting and potential renal function decrease (15). Liu et al (16) determined that 10–12.5% of human
platelet-rich plasma (hPRP) resulted in the best outcome in bone
formation tests, and involved injecting a mixture of ADSCs, hPRP
and injectable tissue engineering bone into a nude mouse. As
demonstrated by Shafaei et al (17), HS creates a better
micro-environment for cell survival than fetal bovine serum
(FBS).
For the clinical application of ADSCs, short-term
preservation is more crucial than other time frames. Thus,
identifying a safer temperature, more suitable medium and
appropriate time frame is of crucial importance. In the present
study, the effect of different temperatures [4°C and RT (~26°C)]
and three types of preservation media (10% PRP, 10% HS and 0.9%
normal saline) on ADSCs was tested to determine optimal time and
storage conditions. Survival, proliferation and differentiation
abilities of ADSCs were investigated under these various
conditions.
Materials and methods
Ethics statement
The protocol of the present study was approved by
the Ethics Committee of The First Affiliated Hospital of Jinan
University (Guangzhou, China). Informed consent was obtained from
all subjects prior to the current study. The fat tissue used in the
present study came from a 65-year-old woman and the blood used in
the present study came from a 26-year-old woman.
Preparation and activation of PRP
PRP was prepared following the method reported by
Jalowiec et al (18). In
brief, blood samples from a 26-year-old man were collected in
anti-agglutination tubes and centrifuged twice at 200 × g for 10
min and 400 × g for 15 min at RT to collect adequate platelets. The
supernatant platelet-poor plasma was transferred to another tube
and used as a diluent when necessary. Factoring in the PRP platelet
concentration, which was counted in a Malassez counting chamber
(EMD Millipore, Billerica, MA, USA), a minimum value of
800×109/l, and a maximum value of
1,200×109/l, was expected for the final PRP platelet
concentration (the platelet concentration of PRP is ~5 times that
of whole blood) (19). A
concentration of 862×109/l PRP was attained. The product
was activated with 10% calcium gluconate and subsequently stored at
4°C overnight. Prior to application, the product was centrifuged at
3,000 × g for 15 min (RT).
Preparation of HS
HS was prepared following the method described by
Freymann et al (20). Blood
samples from the same person were collected in dry blood collecting
tubes. Once the blood had coagulated, the serum was isolated from
the entire blood clot by centrifugation at 2,000 × g for 10 min at
RT. The PRP and HS were stored at −20°C and thawed prior to
application.
Cell isolation and culture
Isolation and culture of ADSCs was partially
performed using the standard protocols of Bura et al
(21) and Guo et al
(22). Permission and a signed
consent to participate from the Institutional Review Board of
Medical Science (Jinan University, Guangzhou, China) was obtained
prior to the collection of 100 ml abdominal subcutaneous fat from a
65-year-old patient in November 2016. The adipose tissue was
digested with 0.2% collagenase I (Biochrom GmbH, Berlin, Germany)
for 30 min at 37°C, centrifuged for 10 min at 300 × g (RT) and
filtered with a 100 µm mesh filter (neoLab Migge GmbH, Heidelberg,
Germany). NaCl (0.3%) was used to remove the red blood cells prior
to transfer of cells into culture medium including Dulbecco's
Modified Eagle's Medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc.), 1% 100 U/ml penicillin and 100
µg/ml streptomycin, and culture in 5% CO2 at 37°C. The
culture medium was changed at 3 day intervals. Primary cells were
cultured for ~10 days and defined as ‘passage 0’. ADSCs of passage
2 which were cultured at culture medium and passaged twice, then
prepared for subsequent experimentation.
Cell phenotype identification
To characterize the phenotype of ADSCs, the surface
markers were determined, using flow cytometry as previously
described (10). Briefly, ADSCs
were incubated with Thy-1 cell surface antigen (CD90, catalog
number: 561970), protein tyrosine phosphatase, receptor type C
(CD45, catalog number: 555482), CD34 molecule (CD34, catalog
number: 550761), integrin subunit β1 (CD29, catalog number:
557332), endoglin (CD105, catalog number: 561441), CD44 antigen
(CD44, catalog number: 555478) and major histocompatibility
complex, class II DR (HLA-DR, catalog number: 555812) antibodies
(BD Biosciences, Franklin Lakes, NJ, USA) at 4°C for 30 min in the
dark. Excess antibody was removed, cells were washed twice with PBS
and 300 µl fixative solution was added. Flow cytometric analyses
were performed with FlowJo version 7.6.1 (FlowJo LLC, Ashland, OR,
USA).
In vitro osteogenic
differentiation
ADSCs were seeded onto 24-well plates at a density
of 1×104 cells/well. Following culture for 24 h in 10%
FBS medium (Gibco; Thermo Fisher Scientific, Inc.) 37°C, cells were
cultured in osteogenic differentiation medium at 37°C, composed of
standard medium supplemented with FBS, penicillin-streptomycin,
glutamine, ascorbate, β-glycerophosphate and dexamethasone (Cyagen
Biosciences, Inc., Santa Clara, CA USA). During osteogenic
induction, the medium was changed every three days. After 14 days
of in vitro osteogenic induction, cells were washed twice
with PBS and subsequently fixed with 10% neutral formaldehyde for
30 min at RT. Fixed cells were stained 4 min at 37°C with 0.1%
alizarin red dye to visualize calcium deposition (23,24).
The stained plates were air-dried for 2 min and observed under a
light microscope (magnification, ×100).
In vitro adipogenic
differentiation
ADSCs were seeded onto 24-well plates at a density
of 1×104 cells/well following culture for 24 h at 37°C
in DMEM with 10% FBS. Cells were subsequently placed in adipogenic
differentiation medium (Cyagen Biosciences, Inc.). During
adipogenic differentiation, the medium was changed every three
days. Following culture for 14 days, cells were washed with PBS and
fixed with 10% neutral formaldehyde for 30 min at RT. Fixed cells
were stained with 0.5% oil red O dye for 30 min at 37°C (25,26).
Cells were washed again in PBS and observed under a light
microscope (magnification, ×100).
Experimental groups
ADSCs were placed in six 15 ml tubes at
5×105 cells/tube and were numbered 1–6. The following
media were added to the tubes: i) 0.5 ml 0.9% NaCl, ii) 0.5 ml 10%
HS, iii) 0.5 ml 10% PRP, iv) 0.5 ml 0.9% NaCl, v) 0.5 ml 10% HS and
vi) 0.5 ml 10% PRP. Tubes 1–3 were stored at 4°C, while tubes 4–6
were stored at RT. Following a 2, 4 and 6 h culture, cell survival,
apoptosis, proliferation and differentiation abilities were
determined.
Cell proliferation assay
Cell proliferation was assessed with a Cell Counting
Kit-8 (CCK8) assay (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). The optical density (OD) values obtained indirectly report
on cell proliferation. ADSCs from each group were seeded onto
96-well plates at a density of 3×103 cells/well (5
parallel wells were seeded for each group). The cells were cultured
at 37°C for 24 h, rinsed with PBS and mixed with 10 µl CCK8 in 100
µl DMEM at 37°C until the medium turned orange. The absorbance of
each well was detected by a microplate reader at a wavelength of
450 nm. The OD value was recorded, and the difference was analyzed
using statistical methods (22).
Cell survival assay
Cell suspension (500 µl) from each group was placed
into a flow tube. Propidium iodide (PI; 5 µl) was added to the flow
tube with 5 µl Annexin V-FITC stain and mixed for 10 min in the
dark at RT. The values of the side scatter, which represented the
entire cell population, and the FL3 channel, which represented
living cells, were recorded according to the detection of Annexin
V-FITC/PI double staining cell apoptosis kit (KeyGEN) by flow
cytometry. Data were analyzed with FlowJo version 7.6.1 (FlowJo
LLC, Ashland, OR, USA).
Semi-quantification of in vitro
osteogenic differentiation
ADSCs from each group were seeded onto 96-well
plates at a density of 3×103 cells/well (5 parallel
wells were seeded for each group) and maintained in osteogenic
differentiation medium for 14 days. Cultivation procedures and
alizarin red S dye staining was performed according to the protocol
described above. In brief, the 96-well plates were decolored using
100 µl 10% hexadecylpyridinium chloride for 30 min at RT, and the
OD value was detected with a microplate reader at a wavelength of
562 nm to perform a semi-quantitative analysis of osteogenic
differentiation, as described previously (27,28).
Semi-quantification of in vitro
adipogenic differentiation
ADSCs from each group were seeded onto 96-well
plates at a density of 3×103 cells/well (5 parallel
wells were seeded for each group) and cultured in adipogenic
differentiation medium, as described above, prior to observation
under a light microscope. Cells in the 96-well plates were
subsequently decolored with 100 µl 100% isopropanol for 60 min, and
the OD value was detected using a microplate reader at a wavelength
of 510 nm to semi-quantitatively analyze adipogenic differentiation
(26).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from each group was isolated using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. RNA concentration was determined using
a spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). RNAs with 260/280 ratios between 1.7 and 2.1 were used. cDNA
was synthesized according to the protocol accompanying the
PrimeScript® RT Master Mix kit (Takara Biotechnology
Co., Ltd., Beijing, China) using 500 ng total RNA, and the reaction
conditions were 25°C for start, 37°C for 15 mins, 85°C for 5 sec
and then resting at 4°C. The primers for each gene were designed
using the PrimerBank (29). RT-PCR
was performed using 2 µl cDNA. PCR conditions were: 95°C for 30 sec
for denaturation, 95°C for 5 sec (45 cycles) for amplification and
at 60°C for 20 sec. The RNA analysis employed SYBR Green (Roche
Diagnostics, Indianapolis, IN, USA). Data were quantified using the
2−∆∆Cq method (30) by
normalizing the expression of the target genes to the housekeeping
gene, GAPDH. The values were described as the expression of the
target genes, including runt related transcription factor 2
(RUNX2), SRY-box 9 (SOX9) and sp7 transcription factor (osterix)
for osteogenic differentiation, as well as fatty acid-binding
protein 4 (FABP4), peroxisome-proliferator-activated receptor
(PPAR)γ and CCAAT/enhancer-binding protein (CEBP)α for adipogenic
differentiation. All primer sequences are listed in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward
(5′→3′) | Reverse
(3′→5′) |
|---|
| RUNX2 |
TGGCAGTCACATGGCAGATT |
CTTGGGTGGGTGGAGGATTC |
| SOX9 |
GAGGAAGTCGGTGAAGAACGG |
CCCTCTCGCTTCAGGTCAG |
| Osterix |
GTAGGACTGTAGGACCGGA |
GCCATAGTGAACTTCCTCCTCA |
| FABP4 |
TGGGCCAGGAATTTGACGAA |
GCGAACTTCAGTCCAGGTCA |
| PPARγ |
GCAAACCCCTATTCCATGCT |
CCACGGAGCTGATCCCAAAG |
| CEBPα |
GACTAGGAGATTCCGGTGCC |
GCATTGGAGCGGTGAGTTTG |
| GAPDH |
GCTAAGGCTGTGGGGAAAGT |
TCAGCAGCAGCCTTCACTAC |
Western blot analysis of osteocalcin
and PPARγ
Following osteogenic and adipogenic differentiation,
ADSCs from each group were lysed using radioimmunoprecipitation
assay buffer (Dalian Meilun Biotechnology Co., Ltd., Dalian,
China). Equal quantities of total protein (25 µg) from each group
were separated by 12% SDS-PAGE (Beyotime Institute of
Biotechnology, Shanghai, China). The proteins were subsequently
transferred onto a polyvinylidene fluoride membrane. Membranes were
blocked with 5% non-fat milk for 1 h at RT prior to incubation with
mouse monoclonal anti-PPARγ (diluted with 5% BSA to 1:10,000, cat.
no. 41928; Abcam, Cambridge, UK) and anti-osteocalcin antibodies
(1:10,000, cat. no. 13421; Abcam), followed by incubation with
goat-anti-mouse horseradish peroxidase-conjugated secondary
antibody (Genetech Co., Ltd., Shanghai, China). The levels of
osteocalcin and PPARγ were normalized to those of GAPDH (1:10,000,
cat. no. 9482; Abcam). ImageJ version 2.0 software (National
Institutes of Health, Bethesda, MD, USA) was used for
densitometry.
Statistical analysis
All values were reported as the mean ± standard
error of the mean (n=5/group). Data were analyzed using Student's
t-test or one-way analysis of variance followed by the standard
Tukey test for post-hoc analysis using SPSS version 13.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell identification
ADSCs from P2 were spindle-shaped (Fig. 1A), and the differentiation
identification results are presented in Fig. 1B and C. Cells differentiated in
osteogenic or adipogenic medium were stained with alizarin red
(Fig. 1B) or oil red O (Fig. 1C), which detected calcium salt or
lipid droplets, respectively. The phenotype identification results
(Fig. 2A) revealed that ADSCs were
positive for CD44 (Fig. 2B), CD105
(Fig. 2C), CD29 (Fig. 2D), and CD90 (Fig. 2E), expressed low levels of CD45
(Fig. 2F), and were negative for
CD34 (Fig. 2G) and HLA-DR
(Fig. 2H).
 | Figure 2.ADSC phenotype identification. (A)
Phenotypes were identified by flow cytometry. (B) ADSCs were
positive for CD44, (C) CD105, (D) CD29 and (E) CD90. (F) Low levels
of CD45 were expressed, and ADSCs were negative for (G) CD34 and
(H) HLA-DR. ADSC, adipose-derived stem cells; CD90, Thy-1 cell
surface antigen; CD45, protein tyrosine phosphatase, receptor type
C; CD34, CD34 molecule; CD29, integrin subunit β 1; CD105,
endoglin; CD44, CD44 antigen; HLA-DR, major histocompatibility
complex, class II DR. |
Cell proliferation is dependent on
preservation temperature, duration and medium
The results of the CCK8 cell proliferation assay
revealed that the proliferative ability of each group declined with
time (Fig. 3). Cells in the same
media when stored at 4°C had increased proliferation compared with
the RT group (Fig. 3A). In
addition, the data demonstrated that the 10% PRP and 10% HS groups
had higher OD values compared with the 0.9% NaCl group (Fig. 3B); however, the difference between
10% PRP and 10% HS was not statistically significant. In addition,
the storage of cells at 4°C in 10% PRP or 10% HS facilitated cell
proliferation for up to 4 h. There was an obvious decline between 4
and 6 h, whereas the difference between 2 and 4 h was relatively
similar (Fig. 3C). Therefore,
cells should be used earlier than 4 h and stored at 4°C to maintain
proliferative ability.
 | Figure 3.Comparison of ADSC proliferative
ability when preserved in different conditions. ADSCs from
different groups were observed following the addition of 10 µl cell
counting kit 8 reagent, and the OD value was measured. (A) ADSC
suspended in 0.9% NaCl were preserved at RT and 4°C for 2 h. (B)
ADSCs suspended in three types of medium for 2 h at 4°C. (C) ADSCs
suspended in 0.9% NaCl were preserved at 4°C for 2, 4 and 6 h. (D)
Following the addition of 5 µl Annexin V-FITC/PI staining, ADSCs
were analyzed and the SS and FL3 values were recorded, according to
the detection of Annexin V-fluorescein isothiocyanate/PI. The
survival rate of ADSCs suspended in 0.9% NaCl at 4°C for 2, 4 and 6
h is presented. (E) Survival rate ADSCs preserved at RT and 4°C in
0.9% NaCl for 6 h. (F) Survival rate of ADSCs suspended in three
types of medium for 4 h at 4°C. *P<0.05. ADSCs, adipose-derived
stem cells; OD, optical density; PI, propidium iodide; RT,
temperature; HS, human serum; PRP, platelet-rich plasma. |
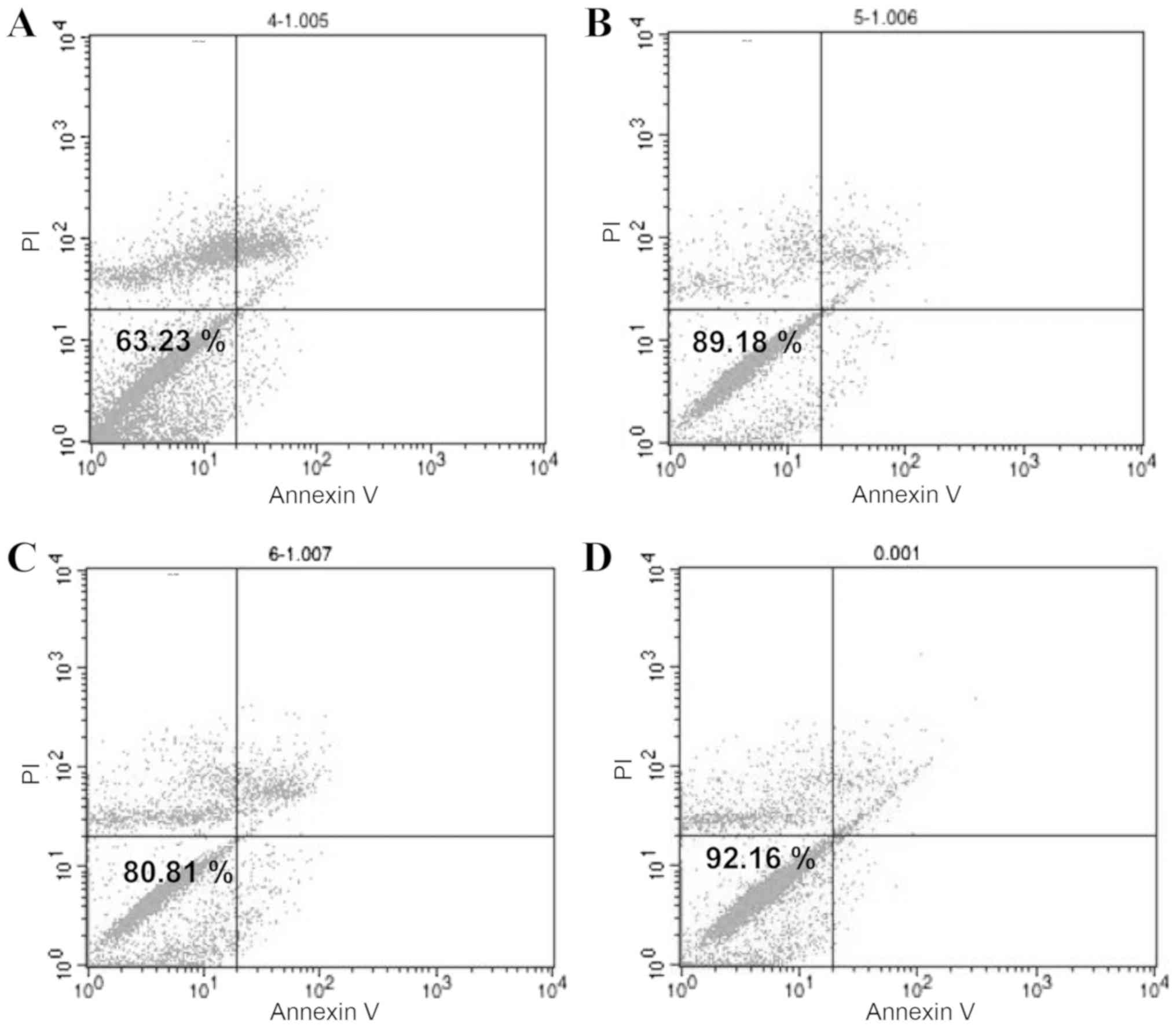
Cell survival is dependent on
preservation temperature, duration and medium
The survival rates of all groups declined with time
(Fig. 3D). When comparing the two
temperatures, 4°C improved ADSC preservation when stored in 0.9%
NaCl, as evidenced by the higher survival rate (Fig. 3E). Furthermore, the data
demonstrated that groups stored in 10% PRP and 10% HS had higher
survival rates, compared with the 0.9% NaCl group (Fig. 3F). There was a common decline among
ADSCs in different media at different times; however, ADSCs
maintained a stable survival rate under 2 h. The survival rate of
ADSCs stored at 4°C in 0.9% NaCl was 63.23% (Fig. 4A). ADSCs stored at 4°C in 10% HS
had the best survival rate of 89.18% (Fig. 4B). ADSCs stored at 4°C in 10% PRP
had the next highest survival rate of 80.81% (Fig. 4C), whereas the survival rate of
ADSCs without treatment was 92.16% (Fig. 4D). In conclusion, to maintain a
survival rate above 80%, ADSCs should be used within 2 h and stored
for no longer than 4 h. These results demonstrated that 10% HS was
the optimum medium.
ADSC differentiation capacity
decreases when stored at RT
The experimental results revealed that the
osteogenic (Fig. 5A) and
adipogenic (Fig. 5B)
differentiation of cells stored at 4°C was more successful compared
with the cells stored at RT. Thus, compared with RT, 4°C is a more
suitable environment for the preservation of ADSCs (P<0.05).
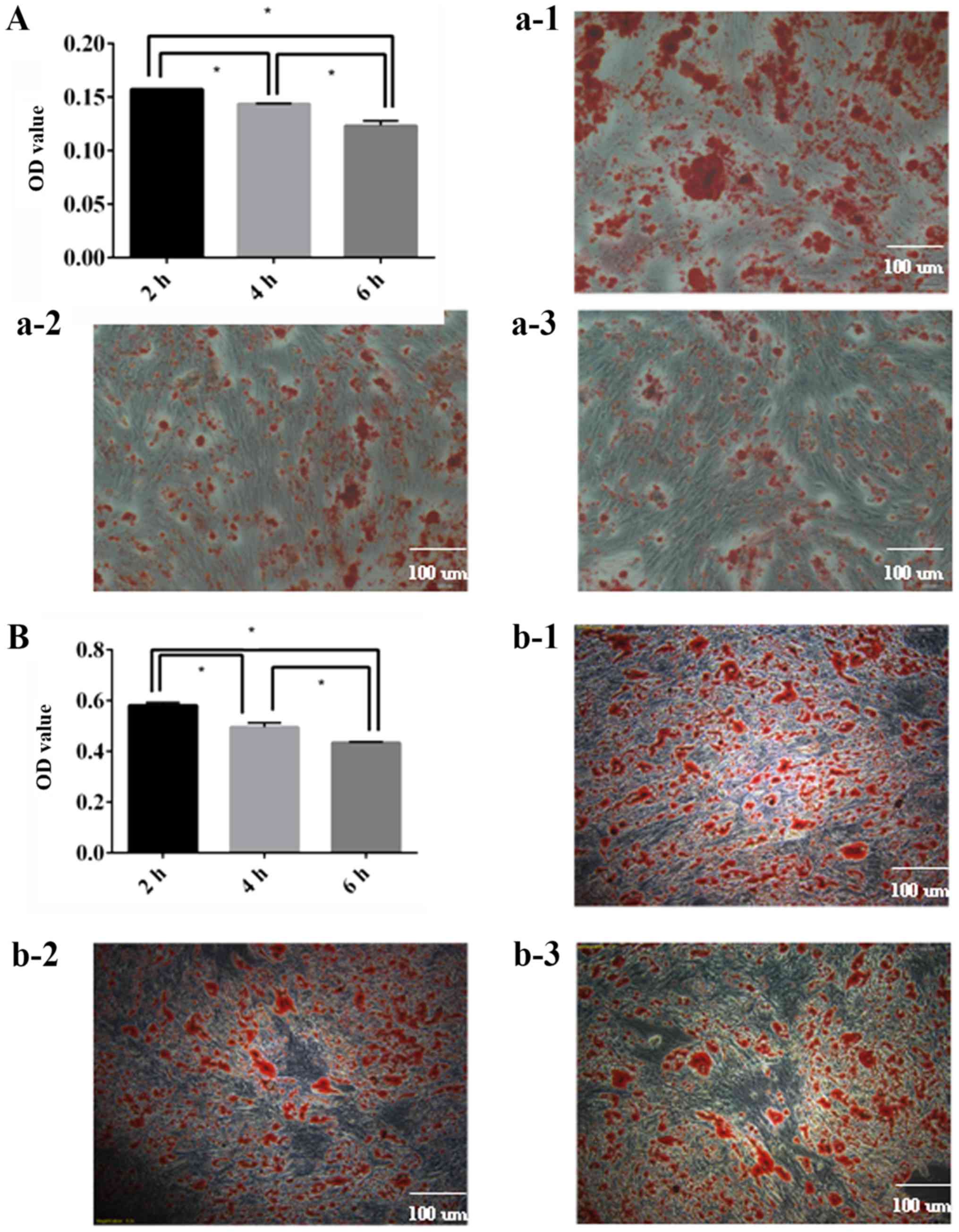
ADSC differentiation capacity
decreases over time
It was demonstrated that the osteogenic (Fig. 6A) and adipogenic (Fig. 6B) differentiation capacity of ADSCs
decreased over time, and there were statistically significant
differences between each group. It was concluded that ADSCs should
be utilized as soon as possible.
ADSC differentiation capacity
decreases when stored in 0.9% NaCl
It was determined that 10% PRP and 10% HS were more
optimal media for osteogenic and adipogenic differentiation,
compared with 0.9% NaCl medium (P<0.05; Fig. 7A). However, there was no
statistically significant difference between the 10% PRP and 10% HS
groups for adipogenic differentiation (Fig. 7B). To maintain a higher
differentiation rate, 10% HS or 10% PRP should be used as the
suspension media for the short-term preservation of ADSCs.
Expression of osteogenic and
adipogenic-associated genes is altered by ADSC preservation
temperature, medium and duration
The differential expression of RUNX2, SOX9, osterix,
FABP4, PPARγ, and CEBPα for different temperatures and over time
are presented in Fig. 8. Similar
trends were observed; the expression of genes associated with
osteogenic and adipogenic differentiation was lower for cells
stored at RT than for those stored at 4°C. Furthermore, the longer
the cells were stored, the lower the osteogenic differentiation
capacity. In terms of the preservation media, excluding osterix,
there were no significant differences between the 10% PRP group and
the 0.9% NaCl group. The expression of other genes was higher in
the 10% PRP and 10% HS groups compared with the 0.9% NaCl group
(Fig. 8).
 | Figure 8.Expression of osteogenic and
adipogenic-associated genes under different preservation
conditions. Reverse transcription-quantitative polymerase chain
reaction was performed to determine the gene expression levels of
(A) osterix, (B) RUNX2, (C) SOX9, (D) FABP4, (E) PPARγ, and (F)
CEBPα among the different groups. *P<0.05. HS, human serum; PRP,
platelet-rich plasma; RT, room temperature; osterix, sp7
transcription factor; RUNX2, runt-related transcription factor 2;
SOX9, SRY-box 9; FABP4, fatty acid-binding protein 4; PPARγ,
peroxisome-proliferator-activated receptor γ; CEBPα, CCAAT/enhancer
binding protein α. |
Osteocalcin and PPARγ protein
expression is altered by ADSC preservation temperature, medium and
duration
The protein expression of osteocalcin, which is
involved in osteogenic differentiation, and PPARγ, which is
involved in adipogenic differentiation, was detected by western
blotting. ADSCs stored at 4°C exhibited an increased expression of
osteocalcin and PPARγ compared with those stored at RT (Fig. 9A and B). With increasing time, the
expression of osteocalcin and PPARγ decreased (Fig. 9C and D). It was also observed that
the secretion of osteocalcin and PPARγ was higher in the 10% PRP
and 10% HS groups, compared with the 0.9% NaCl group (Fig. 9E and F).
Discussion
ADSCs are characterized by low immunogenicity and
the adaptation of ADSCs to their environment provides a promising
direction for future clinical applications (31). Martinez-Gonzalez et al
(32), demonstrated that ADSCs
protect the alveoli structure in patients with asthma by reducing
the inflammation generated by neutrophil granulocytes, reducing IgE
secretion and inhibiting lymphocyte infiltration. Additionally, Won
et al (33) revealed that
local injection of ADSCs promoted hair growth.
Several procedures are involved in the clinical
preparation of ADSCs, including isolation, cultivation, passage and
preservation. Especially in the preservation period, if, for
instance, a patient has an accident, such as a sudden increased
blood pressure or other cases that might affect the success of the
surgery, a viable environment should be found to preserve the
ADSCs.
PPARγ directly activates genes involved in lipid
synthesis (34), and osteocalcin
is a marker of osteogenic differentiation (35). The expression of RUNX2, SOX9 and
osterix was detected to determine the osteogenic differentiation
capacity of ADSCs stored under various conditions. FABP4, PPARγ,
and CEBPα expression was also detected to determine adipogenic
differentiation capacity (34,36–39).
According to the results of the present study, it
was concluded that, compared with RT, 4°C was suitable as an
appropriate temperature for ADSC storage. ADSCs preserved at 4°C do
not require cryoprotectant or a procedure for cell thawing, which
avoids cryoprotectant toxicity and/or irreversible damage to the
cell membrane (11). The results
of the present study suggest that, in addition to temperature, the
preservation medium also served an important role in the cell
microenvironment. PRP and HS are both derived from patients here,
and NaCl is also present in the body, which makes it a safer
choice.
PRP is obtained from the patient's whole blood by
concentrating the blood to a high platelet concentration (40,41).
PRP contains numerous growth factors, including platelet-derived
growth factor, transforming growth factor-β, vascular endothelial
growth factor and epidermal growth factor, which are delivered when
PRP is activated (42–44). The results of the present study
demonstrated that PRP improved osteogenic and adipogenic
differentiation, which can be used to the fullest advantage, if
necessary. PRP has been safely used in numerous fields, including
oral and maxillofacial surgery, soft tissue ulcers, as well as
stubborn acne and scar treatment (41,43,45,46).
The appropriate concentration of PRP for optimum cell proliferation
and osteogenic differentiation was determined to be 10 and 12.5% by
Liu et al (16).
HS is also obtained from the whole blood of
patients, is safe for autologous use, and is abundantly available.
Josh et al (47) used HS
instead of FBS in the DMEM to demonstrate that HS is a viable
alternative to FBS. As with adipose tissue, HS can be easily
collected. As with PRP, HS also contains numerous growth factors,
nutritive substances and other active factors, such as
immunoglobulins, which may have a comprehensive effect on stem cell
culture (48). For example, serum
could provide nutrients needed for cell metabolism (49). Kobayashi et al (49) revealed that HS may be more optimal
than FBS for human bone marrow growth.
In summary, 10% HS and 10% PRP improved ADSC
preservation. Compared with 10% PRP, 10% HS may be more optimal.
This may be since HS has characteristics that approximate more
closely to the normal ADSC environment, and therefore is more
suitable for ADSC activity. Activated PRP releases growth factors
that rapidly stimulate ADSC growth, and this may be perceived as a
potential cause of the results obtained in the cell proliferation
assay where PRP appears to have a higher OD value (Fig. 4B). Over time, ADSCs in the 10% PRP
group appeared to be too undernourished to maintain normal growth.
Based on these results, 10% HS may be the best choice for the
preservation of ADSCs. In addition, from the same quantity of whole
blood, a larger quantity of HS is obtained compared with PRP, and
the process of retrieving HS is more convenient than that of PRP,
which reduces the risk of infection (20). In conclusion, the storage of ADSCs
at 4°C in 10% HS was recommended. Furthermore, ADSCs should be used
in ≤4 h.
The survival rate and stability of ADSCs declined
over time. Optimal preservation of ADSCs allows their function to
be fully exerted upon their clinical use. ADSCs should be used in
<2 h, and no later than 4 h. If storage is required, 10% HS at
4°C should be used. No differences were obtained between
individuals in each experiment (data not shown).
Several problems remain when extensively using
ADSCs. Standardization is required in order to apply ADSCs more
safely and rationally, and equipment involving the separation and
culture of ADSCs in clinical use should be optimized.
Acknowledgements
Not applicable
Funding
The present study was supported by the National
Nature and Science Foundation, P.R. China (grant nos. 81272100,
81372065 and 81871563), the major project of Guangzhou Municipal
Science and Technology Bureau (grant nos. 201300000091 and
201508020253), and the Natural Science Foundation of Guangdong,
P.R. China (grant no. S2013010015264).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL designed the study; YW and ML performed the
experiments and wrote the manuscript; XL, SL, JY, LF, WS and JS
analyzed the data.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Ethics Committee of The First Affiliated Hospital of Jinan
University (Guangzhou, China). Informed consent was obtained from
all subjects prior to study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gimble JM, Katz AJ and Bunnell BA:
Adipose-derived stem cell for regenerative medicine. Circ Res.
100:1249–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bora P and Majumdar AS: Adipose
tissue-derived stromal vascular fraction in regenerative medicine:
A brief review on biology and translation. Stem Cell Res Ther.
8:1452017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gimble JM: Adipose tissue-derived
therapeutics. Expert Opin Biol Ther. 3:705–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baer PC and Geiger H: Adipose-derived
mesenchymal stromal/stem cell: Tissue localization,
characterization, and heterogeneity. Stem Cells Int 2012.
8126932012.
|
|
5
|
Qiu X, Fandel TM, Ferretti L, Albersen M,
Orabi H, Zhang H, Lin G, Lin CS, Schroeder T and Lue TF: Both
immediate and delayed intracavernous injection of autologous
adipose-derived stromal vascular fraction enhances recovery of
erectile function in a rat model of cavernous nerve injury. Eur
Urol. 62:720–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin G, Garcia M, Ning H, Banie L, Guo YL,
Lue TF and Lin CS: Defining stem and progenitor cell within adipose
tissue. Stem Cells Dev. 17:1053–1063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tobita M, Orbay H and Mizuno H:
Adipose-derived stem cell: Current findings and future
perspectives. Discov Med. 11:160–170. 2011.PubMed/NCBI
|
|
8
|
Gonda K, Shigeura T, Sato T, Matsumoto D,
Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase S, et al: Preserved
proliferative capacity and multipotency of human adipose-derived
stem cell after long-term cryopreservation. Plast Reconstr Surg.
121:401–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalez-Fernandez ML, Perez-Castrillo S,
Ordas-Fernandez P, Lopez-Gonzalez ME, Colaco B and Villar-Suarez V:
Study on viability and chondrogenic differentiation of
cryopreserved adipose tissue-derived mesenchymal stromal cell for
future use in regenerative medicine. Cryobiology. 71:256–263. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsumoto D, Shigeura T, Sato K, Inoue K,
Suga H, Kato H, Aoi N, Murase S, Gonda K and Yoshimura K:
Influences of preservation at various temperatures on liposuction
aspirates. Plast Reconstr Surg. 120:1510–1517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saragusty J and Arav A: Current progress
in oocyte and embryo cryopreservation by slow freezing and
vitrification. Reproduction. 141:1–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saragusty J, Gacitua H, Rozenboim I and
Arav A: Protective effects of iodixanol during bovine sperm
cryopreservation. Theriogenology. 71:1425–1432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bunnell BA, Flaat M, Gagliardi C, Patel B
and Ripoll C: Adipose-derived stem cell: Isolation, expansion and
differentiation. Methods. 45:115–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang F, Ren H, Shao X, Zhuang C, Chen Y
and Qi N: Preservation media, durations and cell concentrations of
short-term storage affect key features of human adipose-derived
mesenchymal stem cells for therapeutic application. PeerJ.
5:e33012017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higman MA, Port JD, Beauchamp NJ Jr and
Chen AR: Reversible leukoencephalopathy associated with re-infusion
of DMSO preserved stem cell. Bone Marrow Transplant. 26:797–800.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Zhou Y, Feng H, Ma GE and Ni Y:
Injectable tissue-engineered bone composed of human adipose-derived
stromal cell and platelet-rich plasma. Biomaterials. 29:3338–3345.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shafaei H, Esmaeili A, Mardani M, Razavi
S, Hashemibeni B, Nasr-Esfahani MH, Shiran MB and Esfandiari E:
Effects of human placental serum on proliferation and morphology of
human adipose tissue-derived stem cell. Bone Marrow Transplant.
46:1464–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jalowiec JM, D'Este M, Bara JJ, Denom J,
Menzel U, Alini M, Verrier S and Herrmann M: An in vitro
investigation of platelet-rich plasma-gel as a cell and growth
factor delivery vehicle for tissue engineering. Tissue Eng Part C
Methods. 22:49–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weibrich G, Kleis WK, Hafner G and Hitzler
WE: Growth factor levels in platelet-rich plasma and correlations
with donor age, sex, and platelet count. J Craniomaxillofac Surg.
30:97–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Freymann U, Degrassi L, Kruger JP,
Metzlaff S, Endres M and Petersen W: Effect of serum and
platelet-rich plasma on human early or advanced degenerative
meniscus cells. Connect Tissue Res. 58:509–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bura A, Planat-Benard V, Bourin P,
Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA,
Fleury S, Gadelorge M, et al: Phase I trial: The use of autologous
cultured adipose-derived stroma/stem cell to treat patients with
non-revascularizable critical limb ischemia. Cytotherapy.
16:245–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo X, Li S, Ji Q, Lian R and Chen J:
Enhanced viability and neural differential potential in poor
post-thaw hADSCs by agarose multi-well dishes and spheroid culture.
Hum Cell. 28:175–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu H, Guo F, Zhou X, Gong L, Zhang Y, Zhai
W, Chen L, Cen L, Yin S, Chang J and Cui L: The stimulation of
osteogenic differentiation of human adipose-derived stem cell by
ionic products from akermanite dissolution via activation of the
ERK pathway. Biomaterials. 32:7023–7033. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Ye Y, Tian H, Yang S, Jin X, Tong
W and Zhang Y: In vitro osteogenesis of human adipose-derived stem
cell by coculture with human umbilical vein endothelial cell.
Biochem Biophys Res Commun. 412:143–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Visweswaran M, Schiefer L, Arfuso F,
Dilley RJ, Newsholme P and Dharmarajan A: Wnt antagonist secreted
frizzled-related protein 4 upregulates adipogenic differentiation
in human adipose tissue-derived mesenchymal stem cell. PLoS One.
10:e1180052015. View Article : Google Scholar
|
|
26
|
Li HX, Luo X, Liu RX, Yang YJ and Yang GS:
Roles of Wnt/beta-catenin signaling in adipogenic differentiation
potential of adipose-derived mesenchymal stem cell. Mol Cell
Endocrinol. 291:116–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samuel S, Ahmad RE, Ramasamy TS,
Karunanithi P, Naveen SV, Murali MR, Abbas AA and Kamarul T:
Platelet-rich concentrate in serum free medium enhances osteogenic
differentiation of bone marrow-derived human mesenchymal stromal
cell. PeerJ. 4:e23472016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bunnell BA, Estes BT, Guilak F and Gimble
JM: Differentiation of adipose stem cell. Methods Mol Biol.
456:155–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Spandidos A, Wang H and Seed B:
PrimerBank: A PCR primer database for quantitative gene expression
analysis, 2012 update. Nucleic Acids Res. 40:D1144–D1149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frese L, Dijkman PE and Hoerstrup SP:
Adipose tissue-derived stem cells in regenerative medicine.
Transfus Med Hemother. 43:268–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martínez-González I, Cruz MJ, Moreno R,
Morell F, Muñoz X and Aran JM: Human mesenchymal stem cell resolve
airway inflammation, hyperreactivity, and histopathology in a mouse
model of occupational asthma. Stem Cells Dev. 23:2352–2363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Won CH, Yoo HG, Kwon OS, Sung MY, Kang YJ,
Chung JH, Park BS, Sung JH, Kim WS and Kim KH: Hair growth
promoting effects of adipose tissue-derived stem cell. J Dermatol
Sci. 57:134–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hernández-Bule ML, Martínez-Botas J,
Trillo MÁ, Paíno CL and Úbeda A: Antiadipogenic effects of
subthermal electric stimulation at 448 kHz on differentiating human
mesenchymal stem cells. Mol Med Rep. 13:3895–3903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rumiński S, Ostrowska B, Jaroszewicz J,
Skirecki T, Włodarski K, Święszkowski W and Lewandowska-Szumieł M:
Three-dimensional printed polycaprolactone-based scaffolds provide
an advantageous environment for osteogenic differentiation of human
adipose-derived stem cells. J Tissue Eng Regen Med. 12:e473–e485.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benazzo F, Botta L, Scaffino MF, Caliogna
L, Marullo M, Fusi S and Gastaldi G: Trabecular titanium can induce
in vitro osteogenic differentiation of human adipose derived stem
cells without osteogenic factors. J Biomed Mater Res A.
102:2061–2071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rada T, Reis RL and Gomes ME: Distinct
stem cells subpopulations isolated from human adipose tissue
exhibit different chondrogenic and osteogenic differentiation
potential. Stem Cell Rev. 7:64–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smyth DC, Takenaka S, Yeung C and Richards
CD: Oncostatin M regulates osteogenic differentiation of murine
adipose-derived mesenchymal progenitor cells through a
PKCdelta-dependent mechanism. Cell Tissue Res. 360:309–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Regassa A, Suh M, Datar J, Chen C and Kim
WK: Fatty acids have different adipogenic differentiation
potentials in stromal vascular cells isolated from abdominal fat in
laying hens. Lipids. 52:513–522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nurden AT, Nurden P, Sanchez M, Andia I
and Anitua E: Platelets and wound healing. Front Biosci.
13:3532–3548. 2008.PubMed/NCBI
|
|
41
|
Whitman DH, Berry RL and Green DM:
Platelet gel: An autologous alternative to fibrin glue with
applications in oral and maxillofacial surgery. J Oral Maxillofac
Surg. 55:1294–1299. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alsousou J, Thompson M, Hulley P, Noble A
and Willett K: The biology of platelet-rich plasma and its
application in trauma and orthopaedic surgery: A review of the
literature. J Bone Joint Surg Br. 91:987–996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nikolidakis D and Jansen JA: The biology
of platelet-rich plasma and its application in oral surgery:
Literature review. Tissue Eng Part B Rev. 14:249–258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Anitua E: Plasma rich in growth factors:
Preliminary results of use in the preparation of future sites for
implants. Int J Oral Maxillofac Implants. 14:529–535.
1999.PubMed/NCBI
|
|
45
|
Drago L, Bortolin M, Vassena C, Romanò CL,
Taschieri S and Del Fabbro M: Plasma components and platelet
activation are essential for the antimicrobial properties of
autologous platelet-rich plasma: An in vitro study. PLoS One.
9:e1078132014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Margolis DJ, Kantor J, Santanna J, Strom
BL and Berlin JA: Effectiveness of platelet releasate for the
treatment of diabetic neuropathic foot ulcers. Diabetes Care.
24:483–488. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Josh F, Kobe K, Tobita M, Tanaka R, Suzuki
K, Ono K, Hyakusoku H and Mizuno H: Accelerated and safe
proliferation of human adipose-derived stem cell in medium
supplemented with human serum. J Nippon Med Sch. 79:444–452. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Adkins JN, Varnum SM, Auberry KJ, Moore
RJ, Angell NH, Smith RD, Springer DL and Pounds JG: Toward a human
blood serum proteome: Analysis by multidimensional separation
coupled with mass spectrometry. Mol Cell Proteomics. 1:947–955.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kobayashi T, Watanabe H, Yanagawa T,
Tsutsumi S, Kayakabe M, Shinozaki T, Higuchi H and Takagishi K:
Motility and growth of human bone-marrow mesenchymal stem cell
during ex vivo expansion in autologous serum. J Bone Joint Surg Br.
87:1426–1433. 2005. View Article : Google Scholar : PubMed/NCBI
|