Introduction
Diabetic nephropathy (DN) is among the major
diabetic microvascular complications and the leading cause of
end-stage renal disease (ESRD). According to a report by the
International Diabetes Federation in 2017, ~425 million adults have
been recorded as having diabetes worldwide (1). A high percentage of patients with
diabetes mellitus (up to 30%) develop DN (2). Various factors are involved in the
development of DN. Nitric oxide (NO) is a gaseous molecule that is
also known as endothelium-derived relaxing factor and has proven
functional in the development of DN (3). In particular, NO may be involved in
the progressive alteration of glomerular hemodynamics in diabetic
subjects (4,5). The production of NO is catalyzed by
the enzyme NO-synthase. A total of three NO-synthase (NOS) enzymes,
namely endothelial NOS (eNOS), neuronal NOS and inducible NO
comprise the major isoforms of this enzyme family (6). Several studies have demonstrated that
eNOS depletion can accelerate nephropathy progression in diabetic
mice (7,8). Therefore, pharmacological improvement
of impaired eNOS function or NO production in the glomerular
endothelium may mitigate the progression of DN.
Piperazine ferulate (PF) is a derivative of ferulic
acid, which has been used in China for the treatment of different
types of glomerular disease, including nephritis and nephritic
syndrome. A clinical study performed in children with
immunoglobulin (Ig)A nephropathy reported that PF tablets combined
with eucalyptol-limonene-pinene enteric soft capsules were safe and
effective for the treatment of this type of nephropathy (9). It has also been reported that PF can
inhibit TGF-β1-induced renal interstitial fibroblast activation
(10) and synthesis of the
extracellular matrix in TGF-β1-induced mesangial cells (11). However, the renoprotective effect
of PF in DN and the underlying mechanism of its action have not yet
been investigated.
In the present study, whether PF exerts a preventive
effect against DN was investigated and the underlying mechanism of
its action was also investigated. The data indicated that PF
delayed the development of DN and the mechanism of action was
associated with normalized expression and activity of eNOS.
Materials and methods
Materials
MTT, streptozotocin (STZ) and dimethyl sulfoxide
(DMSO) were obtained from Beijing Solarbio Biotechnology Co., Ltd.,
(Beijing, China). N-nitro-L-arginine methylester (L-NAME), NO and
nitric oxide synthase (NOS) assay kits were purchased from Beyotime
Institute of Biotechnology (Haimen, China). PF was obtained from
Hunan Qianjin Xiangjiang Pharmaceutical Industry Co., Ltd.
(Zhuzhou, China). A mouse albumin ELISA kit was purchased from
Abcam (Cambridge, UK; cat. no. ab108791). Polyclonal rabbit
anti-mouse antibodies specific for eNOS (cat. no. 9572) and
p-eNOS1177 (cat. no. 9571) were obtained from Cell
Signaling Technology Inc., (Danvers, MA, USA). A β-actin antibody
(cat. no. BM0626) was purchased from Wuhan Boster Biological
Technology, Ltd., (Wuhan, China). Creatinine (cat. no. C011-2) and
blood urea nitrogen (BUN, cat. no. C013-2) assay kits were obtained
from Jiancheng Technology Co., Ltd (Nanjing, China). The glucose
assay kit (cat. no. 361500) was purchased from Shanghai Rongsheng
Biotechnology Co., Ltd. (Shanghai, China). All other chemicals were
of analytical grade.
Animals
A total of 75 C57BL/6J male mice (aged 8 weeks,
19–22 g) were obtained from the Experimental Animal Center of
Silaikejingda (Changsha, China). The mice were maintained in a 12 h
light/dark cycle at 22±2°C and 50–60% humidity, with free access to
a standard diet and tap water. The animals underwent an
acclimatization period of 7 days prior to the experimental conduct.
The animal protocol was approved by the Ethics Committee of Animal
Experiments of the Central South University and was performed in
accordance with the Guide for the Care and Use of Laboratory
Animals.
Experimental design
Initially, animals were randomly divided into two
groups: A control group (n=15) and a model group (n=60). The model
group mice were intraperitoneally injected for 5 consecutive days
with STZ (65 mg/kg body weight). This chemical was dissolved in
citrate buffer (0.1 M, pH 4.0) and administered to the animals
following overnight fasting as described previously (12). The control group mice were injected
with citrate buffer solution (0.1 M, pH 4.0). The blood glucose
concentration in mice was monitored for 48 h following the
injection and was measured every 3 days thereafter. A blood glucose
level of >16.7 mmol/l indicated successful induction of
diabetes. Following one week of animal maintenance, the diabetic
mice were randomly divided into four groups: A model control group
(n=15), a low PF dosage group (PF-L; n=15), a medium PF dosage
group (PF-M; n=15) and a high PF dosage group (PF-H; n=15). The
normal control and model control groups were provided with 0.5%
sodium carboxymethylcellulose (CMC-Na), whereas the experimental
mice received PF suspended in 0.5% CMC-Na at a dosage of 100, 200
and 400 mg/kg body weight once a day. Following treatment for 12
weeks, the 24-h urine was collected from each animal using
metabolic cages. At the termination of the study, all mice were
anaesthetized using sodium pentobarbital (50 mg/kg body weight),
then the blood was collected. Finally, mice were sacrificed by
exsanguination and the kidney tissue was quickly removed. Animal
death was confirmed by comprehensively examining the vital signs of
animal, including lack of breathing, lack of a heartbeat, reflexes
and the presence of rigor mortis.
Serum and urine measurements
Urinary albuminuria was determined with the
commercially available ELISA kit. The parameters of blood glucose,
creatinine and BUN were also measured using respective kits
following the manufacturer's protocols.
Cell culture
Primary human glomerular endothelial cells (GEnCs)
were obtained from ScienCell Research Laboratories, Inc., (San
Diego, CA, USA) and were maintained in endothelial cell medium
(ECM, ScienCell Research Laboratories, Inc.) containing 5% fetal
bovine serum (FBS; ScienCell Research Laboratories, Inc.), 1%
penicillin/streptomycin and 1% endothelial cell growth factor
(ScienCell Research Laboratories, Inc.). All experiments were
performed using GEnCs at passages 3–6. The cells were cultured at
37°C in 5% CO2.
Histological evaluation
The kidney tissues of the mice were fixed at 4°C in
4% paraformaldehyde for 24 h and then embedded in paraffin wax. The
paraffin-embedded kidney tissues were cut into 3-µm slices and the
sections were stained using routine hematoxylin and eosin (H&E)
and periodic acid-Schiff (PAS) staining methods. For H&E
staining, sections were stained in hematoxylin for 3 min and eosin
for 1 min at room temperature. For PAS staining, sections were
stained with periodic acid solution for 20 min and Schiff's reagent
at 37°C for 30 min in the dark, then subsequently counterstained
with hematoxylin for 2 min. Following staining, the
histopathological changes in the kidney tissue slices were observed
under light microscopy at ×400 magnification. The H&E-stained
sections were used for glomerular area studies. The surface area of
the glomerular sections derived from each animal was determined by
digital image analysis using ImageJ 1.37c software (National
Institutes of Health, Bethesda, MD, USA). PAS staining was
performed in order to evaluate the degree of glomerulosclerosis
(glomerulosclerosis index). Semiquantitative scoring of
glomerulosclerosis was performed using a five-grade method as
described previously (13).
Briefly, the degree of sclerosis was graded as follows: 0=no
sclerosis; 1=sclerosis involving <25% of the area examined;
2=sclerosis involving 25–50% of the area examined; 3=sclerosis
involving 50 to 75% of the area examined; 4=sclerosis involving
>75% of the area examined. The glomerulosclerosis index was
calculated using the following formula: Glomerulosclerotic
index=1×N1+2×N2+3×N3+4×N4/N0+N1+N2+N3+N4,
where N is the number of glomeruli in each grade of sclerosis.
Electron microscopy analysis
Cortical kidney tissues were fixed with 2.5%
glutaraldehyde at room temperature for 24 h and then post-fixed in
1% osmium tetroxide at 4°C for 2 h in the dark. The tissue samples
were dehydrated in graded ethanol and treated with propylene oxide
for 3 h at room temperature. Subsequently, the sections were
embedded in EMbed 812 resin (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and polymerized at 60°C for 48 h. The ultrathin
sections (60–80 nm) were stained with 1% uranyl acetate for 10 min,
followed by 2% lead citrate buffer for 2 min at 37°C. The structure
of the glomerular endothelial cell layer was captured via
transmission electron microscopy (Hitachi High-Tech7700).
Assessment of cellular viability
Cellular viability was measured by MTT assay.
Briefly, GEnCs were cultured in a 96-well plate and treated with
different concentrations of PF for 24 h. MTT was added to each
well, and GEnCs were cultured at 37°C for 3 h. The medium
containing MTT was discarded and the formazan was dissolved in 150
µl dimethyl sulfoxide. The absorbance was measured at 490 nm using
a microplate reader. Cellular viability was expressed as the
relative percentage of the absorbance value of PF-treated cells
compared with that of the control cells.
Evaluation of intracellular eNOS
level
GEnCs were seeded in 6-well plates at a density of
1×104 per cm2. When the confluence reached at
50–60%, the medium was replaced with low sugar Dulbecco's modified
Eagle's medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) containing 10% FBS. Then, the cells randomly divided into 5
groups: Control group (normal glucose, 5.5 mM glucose), PF group
(100 µM PF), high glucose group (30 mM D-glucose, HG), PF+HG group
(100 µM PF + 30 mM D-glucose) and mannitol group (24.5 mM
D-mannitol plus 5.5 mM glucose). GEnCs were pre-incubated with PF
(100 µM) for 4 h, then subjected to HG for 24 h. D-mannitol was
used as an osmotic control for the HG. Following treatment, the
cells were harvested and the mRNA and protein of eNOS were
detected.
Assessment of NO expression levels and
NOS activity
Total NO production and NOS activity were measured
using NO and NOS kits, respectively, according to the
manufacturer's protocols. The tissues from the mice were
homogenized in Cell and Tissue Lysis Buffer for NO assay (cat. no.
S3090; Beyotime Institute of Biotechnology) and used for NO or NOS
activity detection. The protein concentration was quantified by the
bicinchoninic acid (BCA) method in accordance with the
manufacturer's protocol. The optical density value of each sample
was read by a microplate reader at 550 nm. The resulting data
regarding NO expression and NOS activity were normalized to the
total amount of protein in the samples.
Determination of kidney oxidative
stress
Malondialdehyde (MDA; cat. no. S0131) levels and
superoxide dismutase (SOD; cat. no. S0109), glutathione peroxidase
(GSH-Px; cat. no. S0056) and catalase activities (cat. no. S0051)
were determined in the renal tissue sample homogenates using
commercially available kits (Beyotime Institute of Biotechnology)
according to the manufacturer's protocols.
Evaluation of intracellular NO levels
with DAF-2 DA
GEnCs were seeded into 24-well culture plates at a
density of 1×104 per cm2. Following cell
treatment, the supernatants were removed and subsequently washed 3
times with HEPES buffer at room temperature. The cells were
incubated with DAF-FM DA (5 µM) for 20 min in the dark at 37°C and
rinsed 3 times with HEPES buffer (Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China). The fluorescence within
the cells was detected at λex=495 and λem=515
nm with a fluorescence microscope (Zeiss AG, Oberkochen,
Germany).
Gene analysis
Total RNA was extracted from cultured cells and
kidney tissues using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and quantified by spectrophotometry (NanoDrop
Technologies, Wilmington, DE, USA). cDNA was produced by RNA using
a reverse transcription kit (Takara Biotechnology Co., Ltd.,
Dalian, China) in a 10 µl reaction system containing 2 µl 5X
PrimeScript buffer, 0.5 µl PrimeScript RT enzyme mix I, 0.5 µl
oligo dT primers (50 µM), 0.5 µl random 6 mers (100 µM), 2 µl total
RNA (400 ng), 4.5 µl RNase free dH2O2.
Reverse transcription reaction conditions were as follows: 37°C for
15 min and 85°C for 5 sec. Quantitative polymerase chain reaction
(qPCR) was performed using SYBR Green PCR mix (Takara Biotechnology
Co., Ltd.) on a LightCycler 96 (Roche Applied Science, Mannheim,
Germany). Thermocycling conditions were as follows: denaturing at
95°C for 30 sec and 45 cycles of 95°C for 5 sec and 60°C for 30
sec. The 2−ΔΔCq method (14) was used to calculate relative gene
expression. Gene expression levels were normalized to β-actin. The
forward and reverse primer sequences used for eNOS and β-actin were
as follows: Forward, 5′-AGCGGCTGGTACATGAGTTC-3′ and reverse,
5′-ACAGGGATGAGGTTGTCCTG-3′, and forward, 5′-ACTGCTCTGGCTCCTAGCAC-3′
and reverse, 5′-ACATCTGCTGGAAGGTGGAC-3′, respectively.
Protein analysis
Kidney tissue and harvested cells were lysed on ice
with radioimmunoprecipitation assay (RIPA) buffer (Beyotime
Institute of Biotechnology) and the lysates were centrifuged at
12,000 × g for 15 min at 4°C. The protein concentration was
measured using a BCA assay kit (Beyotime Institute of
Biotechnology). A total of 35 µg protein/lane was loaded on an 10%
SDS-PAGE gel for electrophoresis and then transferred to a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). The
blots were blocked in TBS-T (0.1% Tween 20) buffer with 5% skimmed
milk at room temperature for 1 h and then incubated overnight with
primary antibodies against e-NOS (1:500), phopho-Ser1177
eNOS (1:200) and β-actin (1:500) at 4°C. The blots were then
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:5,000) for 1 h at room temperature. The immunoblots
were visualized by enhanced chemiluminescence (Beyotime Institute
of Biotechnology) and quantified with ImageJ software. The protein
expression levels were normalized to β-actin.
Statistical analyses
All data were presented as the mean ± standard error
of the mean of three experimental repeats. SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA) was used to analyze the data.
One-way analysis of variance and the subsequently
Student-Newman-Keuls' test was used to determine the differences
among groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Role of PF in the progression of
DN
The model control group and PF group mice exhibited
significantly (P<0.01) lower body weight compared with the
control mice. Pretreatment with different doses of PF did not
increase body weight in diabetic mice, compared with the model
control group (Fig. 1A). The
parameters of blood glucose level and ratio of kidney weight to
body weight were significantly increased in the model group and PF
group, compare with the control (P<0.01). The elevated levels of
blood glucose and the increased ratio of kidney weight to body
weight in diabetic mice were not attenuated by different PF
treatments for 12 weeks (Fig. 1B and
C). In the model control group, the parameters of albuminuria
and creatinine and blood urea nitrogen levels were significantly
increased (P<0.01), and PF treatment at 200 and 400 mg/kg body
weight was capable of decreasing significantly (P<0.05) these
effects in diabetic mice (Fig.
1D-F).
Effect of PF on oxidative stress
parameters
Oxidative stress markers, including CAT, SOD, GSH
and MDA, have been associated with the development of diabetic
nephropathy. The effects of PF on these antioxidant parameters were
investigated in STZ-induced diabetic mice at the end of the 12-week
treatment period (Fig. 2). The
model group mice indicated a significant (P<0.05) decrease in
the activities of CAT, SOD and GSH-Px and an increase in the MDA
levels of the kidney tissue samples compared with those detected in
the control samples. Furthermore, treatment of diabetic mice with
medium and high doses of PF significantly (P<0.05) reversed the
decrease in SOD, CAT and GSH-Px activities (Fig. 2A-C). Concomitantly, PF
significantly attenuated the increase detected in the MDA levels
(P<0.05; Fig. 2D) of the kidney
tissue samples, compared with those of the control group.
Histological changes
Representative images of kidney tissue sections that
were stained with H&E and PAS are presented in Fig. 3. H&E-staining of the kidney
tissues derived from model control mice indicated significantly
increased glomerular area (P<0.05) and thickening of Bowman's
capsules compared with those of the control mice (Fig. 3A and C). However, treatment of
diabetic mice with 200 and 400 mg/kg PF significantly (P<0.05)
attenuated the increased glomerular area and restored the renal
cytoarchitecture. PAS staining results indicated that model mice
exhibited increased glomerular mesangial matrix hyperplasia,
basement membrane thickness and the glomerulosclerotic index. The
treatment of diabetic mice with PF significantly attenuated
mesangial expansion and basement membrane thickness, and reduced
the glomerulosclerotic index (P<0.05; Fig. 3B and D). This result suggested that
PF could delay the progression of DN.
PF increases eNOS expression and NO
levels
The results obtained by electron microscopy
indicated that PF treatment further decreased diabetes-induced
glomerular basement membrane thickening and endothelial injury
(Fig. 4A). Previous studies have
reported that inhibition of NO production aggravates the
development of DN (15–17). The levels of NO in renal tissues
were assessed in order to evaluate whether PF exerts renoprotective
effects. NO levels in renal tissues of model control group were
significantly (P<0.05) decreased compared with the control group
(Fig. 4B), whereas treatment of
mice with PF restored these alterations. qPCR and western blotting
were employed to analyze eNOS expression levels in the renal
tissues of each animal group. The data indicated a decrease in the
mRNA and protein levels in the kidney tissues of model control mice
compared with those of the normal control mice (Fig. 4C and E). The mice treated with PF
exhibited a significant increase in the expression levels of eNOS
(P<0.05), compared with those in the model control group
(Fig. 4C, E and F). Subsequently,
the activity of NOS in the renal tissues of each group of mice was
detected. PF increased the ability of NOS and consequently NO
production compared with that of the model control group (Fig. 4D). To further elucidate the
mechanism responsible for the induction of eNOS activity, the
effects of PF on the levels of eNOS-ser1177
phosphorylation in mouse renal tissues were evaluated. PF further
increased eNOS-ser1177 phosphorylation levels in the
kidney tissue of diabetic mice (Fig.
4E and F). These results suggested that PF could affect the
expression levels and activity of eNOS.
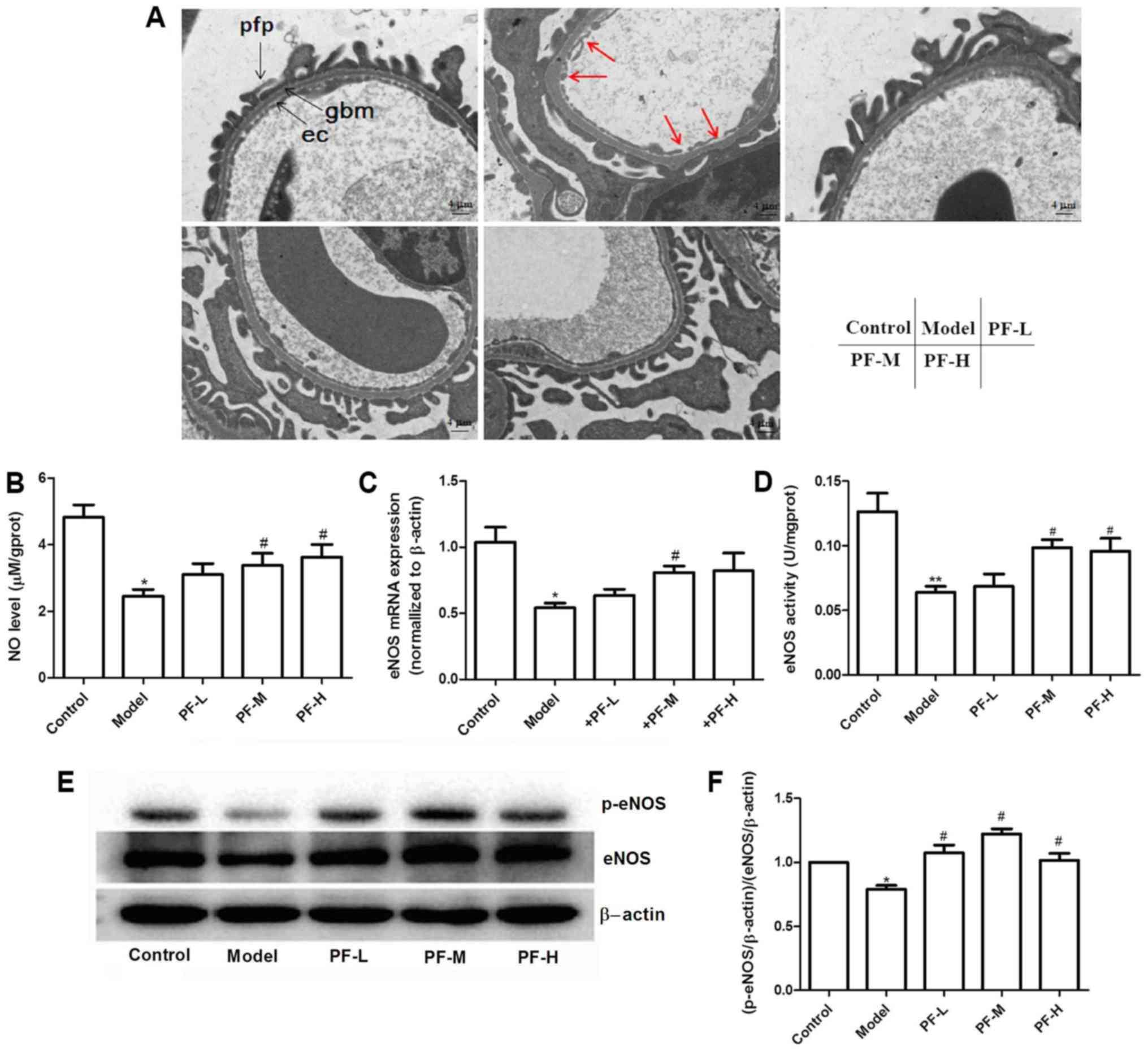 | Figure 4.Diabetic mice exhibit decreased
eNOS/NO levels and PF reverses this condition in renal tissues. (A)
Electron micrographs of glomerular capillary wall (at original
×5,000 magnification). Red arrows indicate endothelial cell damage.
ec, endothelial cell; gbm, glomerular basement membrane; pfp,
podocyte foot process. Scale bars: 4 µm. (B) NO levels in renal
tissues. (C) eNOS mRNA expression levels in kidney tissues. (D)
Effect of PF on NOS activity levels. (E) Representative protein
expression levels. (F) p-eNOS/eNOS level. *P<0.05, **P<0.01
vs. the control, #P<0.05 vs. the model. PF,
Piperazine ferulate; L, low; M, medium; H, high; NO, nitric oxide;
eNOS, endothelial nitric oxide synthase; p, phosphorylated. |
PF improves NO levels in the
HG-induced cell model
Previous studies have demonstrated that dysfunction
of GEnCs is a key factor in the progression of DN (18–20).
The effect of PF on NO production was further evaluated in a high
glucose (HG)-treated GEnC model. Initially, the potential effects
of PF on GEnC viability was assessed via MTT assay. The results
indicated that different concentrations (12.5–200 µM) of PF exerted
no effect on cell viability (Fig.
5A). In order to investigate the effect of PF on eNOS
expression levels in vitro, GEnCs were treated with HG (30
mM) for 24 h and mannitol (24.5 mM mannitol plus 5.5 mM glucose)
was used as an osmotic control for the HG. As expected, HG
significantly decreased eNOS mRNA and protein expression levels
(P<0.05), while mannitol exhibited no effect on either of these
markers. In contrast to these observations, preincubation of GEnCs
with PF (100 µM) resulted in an attenuation of the decreased levels
of eNOS mRNA and protein compared with those of the HG group
(P<0.05; Fig. 5B and C).
Whether PF affects NO production in GEnCs by regulating eNOS
activity was further investigated. GEnCs were preincubated with or
without L-NAME, a specific inhibitor of eNOS, for 30 min prior to
incubation of PF at 100 µM for 4 h and subsequently treated with
HG. A decrease in the NO levels of the LAMN+PF+HG group was noted
compared with that of the PF+HG group (Fig. 5D). These results further indicated
that PF affects the concentration of NO by regulating eNOS
activity.
Discussion
DN is a common condition that causes serious health
issues worldwide. Glycemic control, blood pressure control and
inhibition of the renin-angiotensin-aldosterone system have been
demonstrated to reduce the incidence and delay the development of
DN (21,22). However, several patients with DN
still progress to ESRD. Therefore, it is imperative to develop more
effective therapeutic approaches to delay this disease.
Proteinuria, or albuminuria, is considered a key marker of DN,
which is an independent risk factor for the development of kidney
disease (23). Proteins that leak
into the glomerular filtrate produce a toxic effect on tubular
cells. Once tubular cells are damaged, interstitial fibrosis and
inflammation will occur (24). In
DN, renal function damage is also directly reflected by increased
plasma levels of creatinine and urea nitrogen. In the present
study, an STZ-induced diabetic nephropathy model was used to
evaluate the effect of PF on the progression of DN. It was
identified that PF significantly decreased 24-h albuminuria,
creatinine and BUN levels in diabetic mice. Histological sections
of kidney tissue demonstrated that PF alleviated the thickening of
the basement membrane and inhibited mesangial matrix accumulation.
These data highlighted that PF delayed the progression of DN.
Furthermore, oxidative stress is involved in the development of
diabetes complications. In the present study, the model control
group exhibited an increase in the levels of MDA and a decrease in
GSH-Px, SOD and catalase activities in the kidney tissues.
Following administration of PF, a restoration of normal kidney
oxidative stress levels was observed as MDA levels decreased and
SOD, GSH-Px and catalase activities increased. It is worth noting
that PF exerted no effect on blood glucose levels of diabetic mice
and that the effect of PF on DN was independent of the blood
glucose levels.
Dysfunction of endothelial cells contributes to the
occurrence and development of DN, and NO is considered a potential
therapeutic target (25). Renal
GEnCs are a crucial component of the basement membrane. They
regulate vasomotor tone, hemostasis and trafficking of leukocytes
(26). GEnCs are part of the
glomerular filtration barrier (GFB) and are used to prevent passage
of macromolecules in the blood (27). eNOS regulates endothelial function
and is involved in the development of DN (28). Diabetic eNOS knockout mice develop
hypertension, albuminuria, mesangial matrix expansion and
Kimmelstiel-Wilson nodules (29–31).
The present in vitro study indicated that high glucose
inhibited endothelial NO levels and eNOS expression and activity
levels, which was reversed by pre-incubation of the cells with PF
(100 µM). Animal experiments further demonstrated that the model
control group mice exhibited a significant downregulation of eNOS
expression levels and that PF-treated diabetic mice demonstrated
increased eNOS expression levels. These results are in line with
previous reports that indicated significant downregulation of eNOS
expression and activity levels in diabetic renal tissues (28,32–34).
However, it has also been reported that the expression of eNOS was
upregulated in diabetic animals (35). The reason for this discrepancy is
not yet clear. However, insulinopenia, the use of different animal
models, the course of diabetes and other factors may be possible
reasons leading to inconsistencies between the results of the
present study and previous findings (36,37).
Taken collectively, the data suggest that PF can improve DN and
that the mechanism is associated with the regulation of the NO
levels and the eNOS expression levels of GEnCs.
Even though a lot of work has been done and useful
information provided on treatment DN with PF in the current study,
this study lacks in vivo experiments with eNOS knockdown
mice to support the conclusions in the mechanism research. In
addition, the effect of PF on the dysfunction of GEnCs induced by
HG is the focus of the present study and there are no other
available microvascular endothelia cell is suitable for the present
study. So, only one type of endothelial cell was used to prove the
conclusion in this study.
In conclusion, it was demonstrated that PF delayed
the development of DN. The mechanism of action was associated with
normalized expression and activity of eNOS. Future clinical studies
are required to confirm the therapeutic effect of PF in patients
with DN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Open-End Fund
for the Valuable and Precision Instruments of the Central South
University (grant no. CSUZC201734) and the National Natural Science
Foundation of China (grant no. 81603171).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published manuscript.
Authors' contributions
YY and LS performed the experiments. JL analyzed the
data and YY wrote the manuscript. LY and DX designed the
experiments and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Animal Experiments of the Central South
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Diabetes Federation (IDF).
IDF Diabetes Atlas (7th). 2017.
|
|
2
|
Balakumar P, Bishnoi HK and Mahadevan N:
Telmisartan in the management of diabetic nephropathy: A
contemporary view. Curr Diabetes Rev. 8:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu YC, Lee PH, Lei CC, Ho C, Shih YH and
Lin CL: Nitric oxide donors rescue diabetic nephropathy through
oxidative-stress-and nitrosative-stress-mediated Wnt signaling
pathways. J Diabetes Investig. 6:24–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hiragushi K, Sugimoto H, Shikata K,
Yamashita T, Miyatake N, Shikata Y, Wada J, Kumagai I, Fukushima M
and Makino H: Nitric oxide system is involved in glomerular
hyperfiltration in Japanese normo- and micro-albuminuric patients
with type 2 diabetes. Diabetes Res Clin Pract. 53:149–159. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiarelli F, Cipollone F, Romano F, Tumini
S, Costantini F, di Ricco L, Pomilio M, Pierdomenico SD, Marini M,
Cuccurullo F and Mezzetti A: Increased circulating nitric oxide in
young patients with type 1 diabetes and persistent
microalbuminuria: Relation to glomerular hyperfiltration. Diabetes.
49:1258–1263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knott AB and Bossy-Wetzel E: Impact of
nitric oxide on metabolism in health and age-related disease.
Diabetes Obes Metab. 12 Suppl 2:S126–S133. 2010. View Article : Google Scholar
|
|
7
|
Garsen M, Rops AL, Li J, van Beneden K,
van den Branden C, Berden JH, Rabelink TJ and van der Vlag J:
Endothelial nitric oxide synthase prevents heparanase induction and
the development of proteinuria. PLoS One. 11:e1608942016.
View Article : Google Scholar
|
|
8
|
Nakayama T, Sato W, Kosugi T, Zhang L,
Campbell-Thompson M, Yoshimura A, Croker BP, Johnson RJ and
Nakagawa T: Endothelial injury due to eNOS deficiency accelerates
the progression of chronic renal disease in the mouse. Am J Physiol
Renal Physiol. 296:F317–F327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Pan J, Sun C, Zhou J and Li NA:
Clinical effects of perazine ferulate tablets combined with
eucalyptol limonene pinene enteric soft capsules for treatment of
children with IgA nephropathy. Exp Ther Med. 12:169–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You GQ, Fu P, Xie XS, Liu F and Li J:
Effects of piperazine ferulate on TGF-beta1-induced renal
interstitial fibroblast activation. Sichuan Da Xue Xue Bao Yi Xue
Ban. 39:736–739, 762. 2008.(In Chinese). PubMed/NCBI
|
|
11
|
Yin JP, Fu P, Xie XS, Liu F, Liu F and
Zhou L: Effects of piperazine ferulate on connective tissue growth
factor and extracellular matrix in TGF-beta1 induced mesangial
cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 39:732–735. 2008.(In
Chinese). PubMed/NCBI
|
|
12
|
Chakravarthy H, Beli E, Navitskaya S,
O'Reilly S, Wang Q, Kady N, Huang C, Grant MB and Busik JV:
Imbalances in mobilization and activation of pro-inflammatory and
vascular reparative bone marrow-derived cells in diabetic
retinopathy. PLoS One. 11:e1468292016. View Article : Google Scholar
|
|
13
|
Saito T, Sumithran E, Glasgow EF and
Atkins RC: The enhancement of aminonucleoside nephrosis by the
co-administration of protamine. Kidney Int. 32:691–699. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanetsuna Y, Takahashi K, Nagata M, Gannon
MA, Breyer MD, Harris RC and Takahashi T: Deficiency of endothelial
nitric-oxide synthase confers susceptibility to diabetic
nephropathy in nephropathy-resistant inbred mice. Am J Pathol.
170:1473–1484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prabhakar S, Starnes J, Shi S, Lonis B and
Tran R: Diabetic nephropathy is associated with oxidative stress
and decreased renal nitric oxide production. J Am Soc Nephrol.
18:2945–2952. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamijo H, Higuchi M and Hora K: Chronic
inhibition of nitric oxide production aggravates diabetic
nephropathy in Otsuka Long-Evans Tokushima Fatty rats. Nephron
Physiol. 104:p12–p22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi H, Casalena G, Shi S, Yu L, Ebefors K,
Sun Y, Zhang W, D'Agati V, Schlondorff D, Haraldsson B, et al:
Glomerular endothelial mitochondrial dysfunction is essential and
characteristic of diabetic kidney disease susceptibility. Diabetes.
66:763–778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boels MG, Avramut MC, Koudijs A, Dane MJ,
Lee DH, van der Vlag J, Koster AJ, van Zonneveld AJ, van Faassen E,
Gröne HJ, et al: Atrasentan reduces albuminuria by restoring the
glomerular endothelial glycocalyx barrier in diabetic nephropathy.
Diabetes. 65:2429–2439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Broekhuizen LN, Lemkes BA, Mooij HL,
Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M,
Stroes ES and Vink H: Effect of sulodexide on endothelial
glycocalyx and vascular permeability in patients with type 2
diabetes mellitus. Diabetologia. 53:2646–2655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallagher H and Suckling RJ: Diabetic
nephropathy: Where are we on the journey from pathophysiology to
treatment? Diabetes Obes Metab. 18:641–647. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim Y and Park CW: New therapeutic agents
in diabetic nephropathy. Korean J Intern Med. 32:11–25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hemmelgarn BR, Manns BJ, Lloyd A, James
MT, Klarenbach S, Quinn RR, Wiebe N and Tonelli M; Alberta Kidney
Disease Network, : Relation between kidney function, proteinuria,
and adverse outcomes. JAMA. 303:423–429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nolin AC, Mulhern RM, Panchenko MV,
Pisarek-Horowitz A, Wang Z, Shirihai O, Borkan SC and Havasi A:
Proteinuria causes dysfunctional autophagy in the proximal tubule.
Am J Physiol Renal Physiol. 311:F1271–F1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng H, Wang H, Fan X, Paueksakon P and
Harris RC: Improvement of endothelial nitric oxide synthase
activity retards the progression of diabetic nephropathy in db/db
mice. Kidney Int. 82:1176–1183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ballermann BJ: Glomerular endothelial cell
differentiation. Kidney Int. 67:1668–1671. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan L, Han Y, Ren W, Jiang J, Wang P and
Hu Z: Advanced glycation endproducts affect the cytoskeletal
structure of rat glomerular endothelial cells via the Ras-related
C3 botulinum toxin substrate 1 signaling pathway. Mol Med Rep.
11:4321–4326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dellamea BS, Leitão CB, Friedman R and
Canani LH: Nitric oxide system and diabetic nephropathy. Diabetol
Metab Syndr. 6:172014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa T, Sato W, Glushakova O, Heinig
M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ
and Croker B: Diabetic endothelial nitric oxide synthase knockout
mice develop advanced diabetic nephropathy. J Am Soc Nephrol.
18:539–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heeringa P, van Goor H, Itoh-Lindstrom Y,
Maeda N, Falk RJ, Assmann KJ, Kallenberg CG and Jennette JC: Lack
of endothelial nitric oxide synthase aggravates murine accelerated
anti-glomerular basement membrane glomerulonephritis. Am J Pathol.
156:879–888. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mohan S, Reddick RL, Musi N, Horn DA, Yan
B, Prihoda TJ, Natarajan M and Abboud-Werner SL: Diabetic eNOS
knockout mice develop distinct macro- and microvascular
complications. Lab Invest. 88:515–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koo JR and Vaziri ND: Effects of diabetes,
insulin and antioxidants on NO synthase abundance and NO
interaction with reactive oxygen species. Kidney Int. 63:195–201.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sönmez MF and Dündar M: Ameliorative
effects of pentoxifylline on NOS induced by diabetes in rat kidney.
Ren Fail. 38:605–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Rasheed NM, Al-Rasheed NM, Attia HA,
Al-Amin MA, Al-Ajmi HN, Hasan IH, Mohamad RA and Sinjilawi NA:
Renoprotective effects of fenofibrate via modulation of LKB1/AMPK
mRNA expression and endothelial dysfunction in a rat model of
diabetic nephropathy. Pharmacology. 95:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sugimoto H, Shikata K, Matsuda M, Kushiro
M, Hayashi Y, Hiragushi K, Wada J and Makino H: Increased
expression of endothelial cell nitric oxide synthase (ecNOS) in
afferent and glomerular endothelial cells is involved in glomerular
hyperfiltration of diabetic nephropathy. Diabetologia.
41:1426–1434. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Komers R and Anderson S: Paradoxes of
nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol.
284:F1121–F1137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding Y, Vaziri ND, Coulson R, Kamanna VS
and Roh DD: Effects of simulated hyperglycemia, insulin, and
glucagon on endothelial nitric oxide synthase expression. Am J
Physiol Endocrinol Metab. 279:E11–E17. 2000. View Article : Google Scholar : PubMed/NCBI
|



















