Introduction
Breast cancer is the most common cancer type among
women worldwide, and it remains the second leading cause of
cancer-associated mortality in women. In a previous study, it was
identified that breast cancer consists of heterogeneous cell
populations, and is derived from genetic aberrations and
environmental factors (1). Further
findings indicated that stem cells may be involved in
carcinogenesis and that breast cancer is, at least in part, a stem
cell-based disease (2). As a
certain number of residual cancer cells can survive after
chemotherapy, and have self-renewal and differentiation capacity,
these cells may initiate a new tumor and cause relapse. These
tumor-initiating cells are known as cancer stem cells (CSCs)
(2–4). Therefore, determining the role of
CSCs in primary tumorigenesis is critical for making appropriate
treatment decisions for patients with breast cancer. In this
regard, certain methods have been developed to collect breast CSCs,
including cell sorting according to cell surface expression of
cluster of differentiation (CD)44+/CD24–
(5).
The mammosphere assay was established based on the
spheroid model (6), and
mammospheres represent a pre-cancerous state and act as a surrogate
indicator for the presence of CSCs (7). The mammosphere model partially
resembles breast tumorigenesis (8,9).
Notably, only epithelial cells survive in mammosphere suspension
cultures, whereas other cells die via apoptosis, which is due to
the higher self-renewal capacity of stem cells compared to other
cells (10–12).
A previous study indicated that multicellular
mammospheres have clone-initiating abilities and they reform
following trypsin-mediated dissociation (8). Therefore, microsphere culture systems
may be used to study the functions and tumorigenic aspects of CSCs,
and assess the efficacy of therapeutic agents. In addition, some
studies have indicated that human epidermal growth factor receptor
2 (HER2) mediates the self-renewal and proliferation of CSCs
(13,14). It is estimated that 25% of patients
with breast cancer present with aberrant expression of the gene
encoding for HER2, which confers enhanced drug resistance (15) and increases the risk of mortality
(16). HER2 activation may
stimulate downstream signaling pathways, including the
phosphoinositide-3 kinase (PI3K)/AKT serine/threonine kinase
(17), mitogen-activated protein
kinase (MAPK)/extracellular signal-regulated kinase (18) and cyclooxygenase-2 (COX-2)
(19) signaling pathways, thereby
contributing to tumor and drug resistance.
However, HER2-positive tumors are usually
heterogeneous and have a cellular phenotype that is consistent with
CD44+/CD24– CSCs. Overexpression of the
intragenic HER2 enhancer in mammospheres and increased levels of
the stem-cell marker aldehyde dehydrogenase increases the
proportion of stem/progenitor cells compared with normal mammary
epithelial cells (20). Previous
findings indicated that cell surface HER2 expression was critical
for mammosphere formation and maintenance of two-dimensional cell
lines (21,22). Defects in HER2 function led to a
pro-survival phenotype, and downregulated signaling pathways that
mediated cell proliferation and chemoresistance in breast cancer
(23–25). A study also demonstrated that
activation of the estrogen receptor (ER) on the cell membrane
affects the HER2 signaling pathway and is a mechanism by which
estrogen promotes proliferation (26).
To further understand the function of breast CSCs it
is reasonable to assess mammospheres; however, the molecular
mechanisms underlying mammosphere function remain elusive. In the
present study, the effects by which estradiol (E2) increases the
formation of mammospheres by MCF-7 breast cancer cells were
examined and the underlying mechanisms were investigated. It was
hypothesized that E2 mediates mammosphere formation through the
HER2/COX-2 signaling pathway. The aim of the present study was to
establish a therapeutic target that may contribute to the
development of future clinical treatment strategies for eliminating
breast CSCs.
Materials and methods
Cell culture and mammosphere
generation
The MCF-7 human breast cancer cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in minimum essential medium (MEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in a humidified atmosphere with 5% CO2. For
generation of mammosphere cultures, 1×105 MCF-7 cells/ml
were cultured in serum-free MEM supplemented with 10 ng/ml basic
fibroblast growth factor (bFGF; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 20 ng/ml epidermal growth factor (EGF;
Sigma-Aldrich; Merck KGaA) and 2% B-27 cell culture supplement
(Invitrogen; Thermo Fisher Scientific, Inc.). The media was
replaced during the 2 days of culture at 37°C and continue for 7 to
14 days, respectively, non-adherent spherical clusters were
observed by light microscope (Nikon Eclipse TE 300; Nikon
Corporation, Tokyo, Japan) and these were identified as MCF-7
mammosphere cells (hereafter referred to as MCF-7 MS cells)
(27–29). In addition, 1×105 cell
were cultured in 6 well plate and when density reached ~80%, the
cells were treated with 1×10−6 M E2 (Sigma-Aldrich;
Merck KGaA) or 1×10−6 M Benzyl butyl phthalate (BBP;
Sigma-Aldrich; Merck KGaA) and maintained in culture for
experimental processes.
Flow cytometry
Flow cytometric analysis of CD44-, CD24- and
5-bromo-2′-deoxyuridine (BrdU)-stained cells, was used to identify
MCF-7 and MCF-7 MS cells. For the analysis of cell surface
expression of CD44 and CD24, 1×106 cells/ml were
incubated in the dark at 4°C for 30 min with fluorescein
isothiocyanate (FITC)-conjugated monoclonal antibody against CD24
(1:400, cat. no. 555427; BD Biosciences, Franklin Lakes, NJ, USA)
or phycoerythrin-conjugated antibody against CD44 (1:400; cat. no.
550989; BD Biosciences) in staining buffer (3% FBS + 0.01% Sodium
azide). The staining buffer was removed and the cells washed twice
with phosphate buffered saline (PBS; Sigma-Aldrich; Merck KGaA) to
perform the blocking stain and the stained cells were analyzed on a
BD FACSCalibur™ flow cytometer (BD Biosciences).
Cell proliferation was analyzed using the BrdU Cell
Proliferation kit (EMD Millipore, Billerica, MA, USA), according to
the manufacturer's protocol. The BrdU incorporation assay
quantifies newly synthesized DNA in the S phase of the cell cycle.
Briefly, cells were seeded in 6-well plates at a cell density of
1×104 cells/well and treated with 5 ng BrdU for 24 h at
37°C. Subsequently, the cells were harvested and fixed in 70%
ethanol for 10 min at 37°C. In addition, total DNA was stained with
2.5 µg/ml 7-aminoactinomycin D (7-ADD) for 15 min at 37°C. The DNA
synthesized by replicating cells was detected by flow cytometric.
The amount of BrdU and 7-ADD in the cells was detected using the BD
FACSCalibur™ flow cytometer (BD Biosciences) and
analyzed by WinMDI 2.9 software (Scripps Institute, La Jolla, CA,
USA).
Immunofluorescence microscopy
A total of 1×104 cells/well MCF-7 and
MCF-7 MS cells were cultured on glass slides for 24 h, after which
the cells were fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck
KGaA) at 4°C for 10 min. The fixed cells were blocked with 0.5%
(w/v) Triton X-100 and incubated overnight at 4°C with primary
antibodies against Musashi-1 (1:500; cat. no. sc-135721; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), cytokeratin (CK)7 (1:500;
cat. no. sc-53264; Santa Cruz Biotechnology, Inc.) and CK19 (1:500;
cat. no. SC-6728; Santa Cruz Biotechnology, Inc.). Subsequently,
cells were stained with secondary antibody FITC (1:1,000; cat. no.
sc-65218; Santa Cruz Biotechnology, Inc.) for 60 min at 37°C. Cell
nuclei were stained with DAPI (Sigma-Aldrich; Merck KGaA) for 1 min
at 37°C, and three independent experiments images were acquired
using an immunofluorescence microscope (magnification, ×100).
Cell lysis and western blot
analysis
MCF-7 MS cells were treated with E2 a dose
(1×10−11−1×10−6 M) and time (5 min-48 h)
dependent manner. After, MCF-7 and MCF-7 MS cells were washed with
cold PBS (4°C) and resuspended in RIPA lysis buffer (EMD Millipore,
Billerica, CA, USA) containing a protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA). The cells were incubated on ice for 30
min and the lysate was centrifuged at 12,000 × g for 10 min at 4°C.
The supernatant was collected and the protein concentration was
determined by the Bradford method. Proteins were separated by 10%
polyacrylamide SDS-PAGE and electrophoretically transferred onto a
polyvinylidene difluoride membrane (EMD Millipore) for 90 min at
37°C. Non-specific binding protein was blocked by 5% non-fat dry
milk with PBS for 1 h. The membrane was then stained with primary
antibodies against HER2 (1:500; cat. no. sc-08; Santa Cruz
Biotechnology, Inc.), COX-2 (1:500; cat. no. sc-19999; Santa Cruz
Biotechnology, Inc.) and β-actin (1;500; cat. no. sc-47778; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. The secondary
antibodies goat anti-mouse IgG or anti-rabbit IgG (1:500; Santa
Cruz Biotechnology, Inc.) were incubated at 37°C for 1 h. The
intensity of protein bands was assessed via enhanced
chemiluminescence using the Western Lightning Plus-ECL kit
(PerkinElmer, Inc., Waltham, MA, USA). In addition, ER antagonist
Fulvestrant (ICI 182780, Sigma-Aldrich; Merck KGaA), MAPK
inhibitors (SB203580 and PD98059, Sigma-Aldrich), a PI3K inhibitor
(wortmannin; Sigma-Aldrich; Merck KGaA) and a HER2 inhibitor
(Tyrphostin; AG-825, Sigma-Aldrich; Merck KGaA) were used to
analyze the signalling pathway.
Colony formation assay
A total of 1×103 MCF-7 cells were seeded
in 6-well plates and 1×10−6 M E2 or 1×10−6 M
BBP was maintained in the culture medium. The culture medium was
replaced every 3 days and cells were maintained in culture for 14
days. The cells were fixed with 4% paraformaldehyde for 10 min at
4°C and then stained with 0.01% crystal violet (Sigma-Aldrich;
Merck KGaA) for 30 min at 37°C to enable counting of colonies. The
numbers of visible colonies in the control and MCF-7 MS cells were
counted under a fluorescence microscope (Nikon Eclipse TE 300;
Nikon Corporation, Tokyo, Japan; magnification, ×100).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from MCF-7 and MCF-7 MS
cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). Reverse transcription into complementary DNA was
performed using the Deoxy+ HiSpec Reverse Transcriptase kit
(Yeastern Biotech Co., Ltd., Taipei, Taiwan) according to the
manufacturer's protocol. qPCR was performed using SYBR™
Green Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) on a 7900HT Fast Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following primers were used:
CD24, forward primer: 5′-TCAAGTAACTCCTCCCAGAGTA-3′, reverse primer:
5′-AGAGAGTGAGACCACGAGA-3; CD44, forward primer:
5′-CGCTATGTCCAGAAAGGAGAAT-3′, reverse primer:
5′-CTGCTCACGTCATCATCAGTAG-3 and GAPDH, forward primer:
5′-GGTGGCAGAGGCCTTTG-3′. Reverse primer: 5′-TGCCCATTTAGCATCTCCTT-3.
The thermocycling conditions were: 94°C for 5 min; 35 cycles of
94°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec. The relative
gene expression data was analyzed using qPCR and the
2−ΔΔCq method (30).
Statistical analysis
Data are expressed as the mean ± standard deviation
from three independent experiments. Significant differences between
two groups were determined using Student's t-test and comparisons
between two groups were performed using Student's t-test and
one-way analysis of variance using post hoc test to analyze
differences among multiple groups. SPSS statistical software
(version 13.0, SPSS. Inc., Chicago, IL, USA) was used for all
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Mammosphere formation by MCF-7
cells
Previous studies have demonstrated that mammospheres
cultured from breast cancer cell lines express the
CD44+/CD24– biomarker signature (28) and possess side-population
characteristics (31). For MCF-7
MS cell, the MCF-7 cells had attained the ability to grow as
sphere-like mammospheres for 1, 3, 5 or 7 days, they were cultured
in medium with proliferation factors (10 ng/ml bFGF, 20 ng/ml EGF
and 2% B-27) and passaged every 2 days. The diameter of the
mammospheres gradually increased over the 7-day period, reaching
~50 µm (Fig. 1A). Following
mammosphere culture for 7 days, the proportion of
CD44+/CD24– cells was detected by flow
cytometry. As presented in Fig.
1B, the proportion of CD44+/CD24– cells
reached 17.26±0.46% for MCF-7 MS cells, which was >40-fold
higher compared with MCF-7 cells (0.37±0.026%). The gene expression
of these biomarkers was also quantified by RT-qPCR. The results
indicated that the expression levels of CD44 were increased whereas
expression levels of CD24 were decreased in MCF-7 MS cells compared
with MCF-7 cells (Fig. 1C). In
addition, flow cytometry was used to investigate whether treatment
with 1×10−6 M E2 or 1×10−6 M benzyl butyl
phthalate (BBP) would affect the proportion of
CD44+/CD24– cells among MCF-7 MS cells. The
results revealed a significant increase in the proportion of
CD44+/CD24– cells after MCF-7 MS cells were
treated with E2 and BBP for 24 h (Table I). BBP is a plasticizer, which
exhibits weak estrogenic activity (32). Previous studies by our group
indicated that BBP could mediate cancer cell proliferation and
angiogenesis in human breast cancer cell lines through the ER
(26,32). In the present study, the results
demonstrated that MCF-7 MS cells expressed
CD44+/CD24– and that the proportion of
CD44+/CD24– cells was increased upon
treatment with estrogen.
 | Table I.Proportion of
CD44+/CD24– cells among MCF-7 and MCF-7 MS
cells. |
Table I.
Proportion of
CD44+/CD24– cells among MCF-7 and MCF-7 MS
cells.
| Treatment
groups |
CD44–/CD24+ (%) |
CD44+/CD24– (%) |
|---|
| MCF-7 |
|
|
|
Control | 5.04±0.46 | 0.37±0.46 |
| E2 | 5.03±0.02 | 0.86±0.012 |
|
BBP | 5.06±0.06 | 0.47±0.086 |
| MCF-7 MS (7 day
culture) |
|
|
|
Control | 2.42±0.01 |
13.26±0.46a |
| E2 | 1.04±0.05 |
19.0±0.36b |
|
BBP | 1.03±0.09 |
16.57±0.42b |
| MCF-7 MS (14 day
culture) |
|
|
|
Control | 1.25±0.10 |
80.63±0.80a |
| E2 | 0.67±0.04 |
89.79±0.37c |
|
BBP | 0.78±0.04 |
86.04±0.67c |
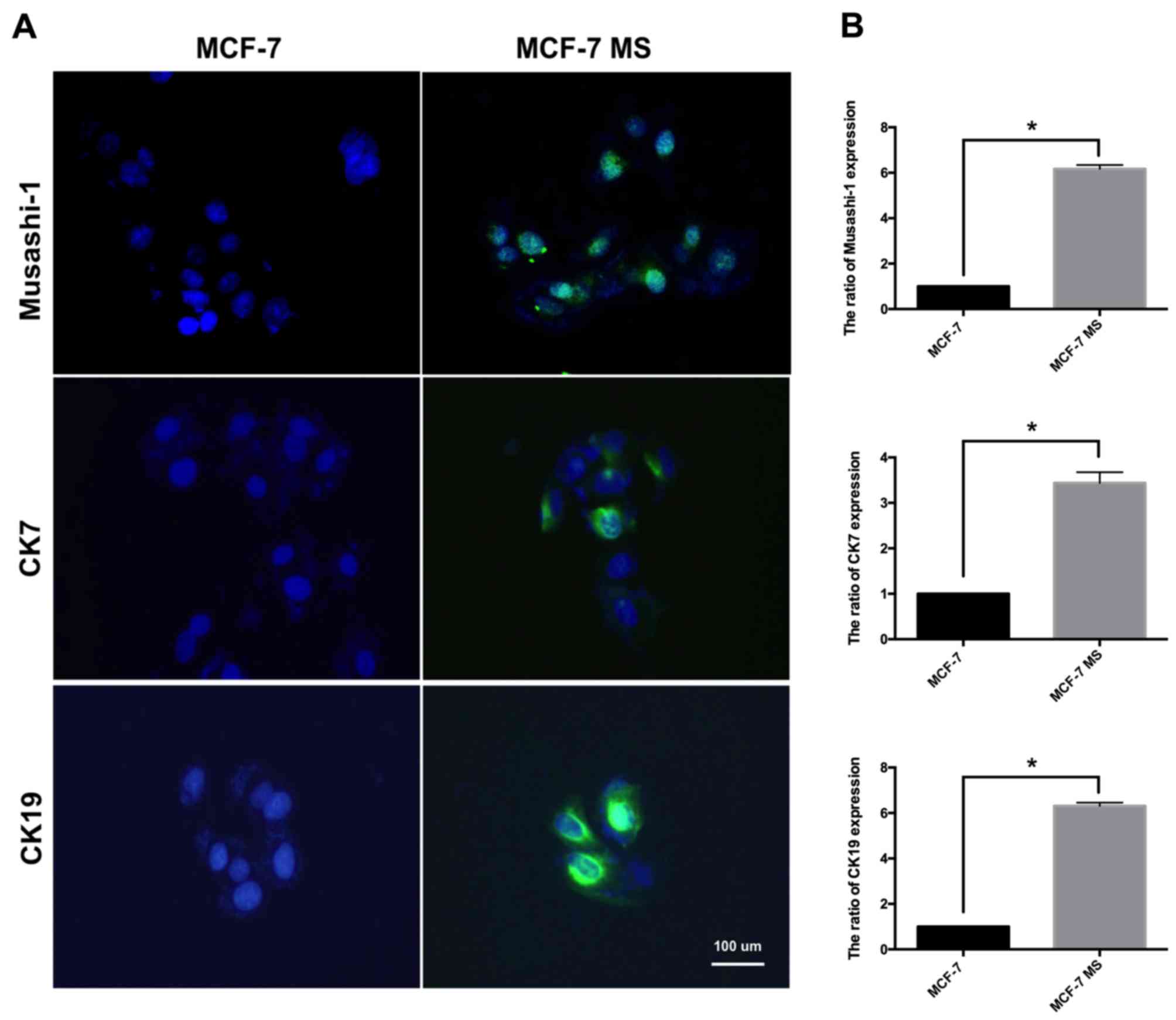
Expression of stem cell markers in
MCF-7 MS cell lines
Next, the expression of additional stem cell markers
in MCF-7 MS cells was measured. Musashi-1, CK7 and CK19 are markers
of progenitor stem cells (33–35).
Musashi-1 mediates the self-renewal of stem cells and is
overexpressed in a variety of tumor types (36). CK is a known CSC marker that
indicates poor prognosis. CK7 and CK19 in particular are associated
with signaling that regulates the epithelial-mesenchymal transition
(37,38). Following mammosphere culture for 14
days, immunofluorescence and RT-qPCR were used to detect the
expression of these stem cell markers in MCF-7 and MCF-7 MS cells.
The immunofluorescence staining and RT-qPCR results indicated that
Musashi-1, CK7 and CK19 were significantly increased in MCF-7 MS
cells compared with in MCF-7 cells (Fig. 2A and B).
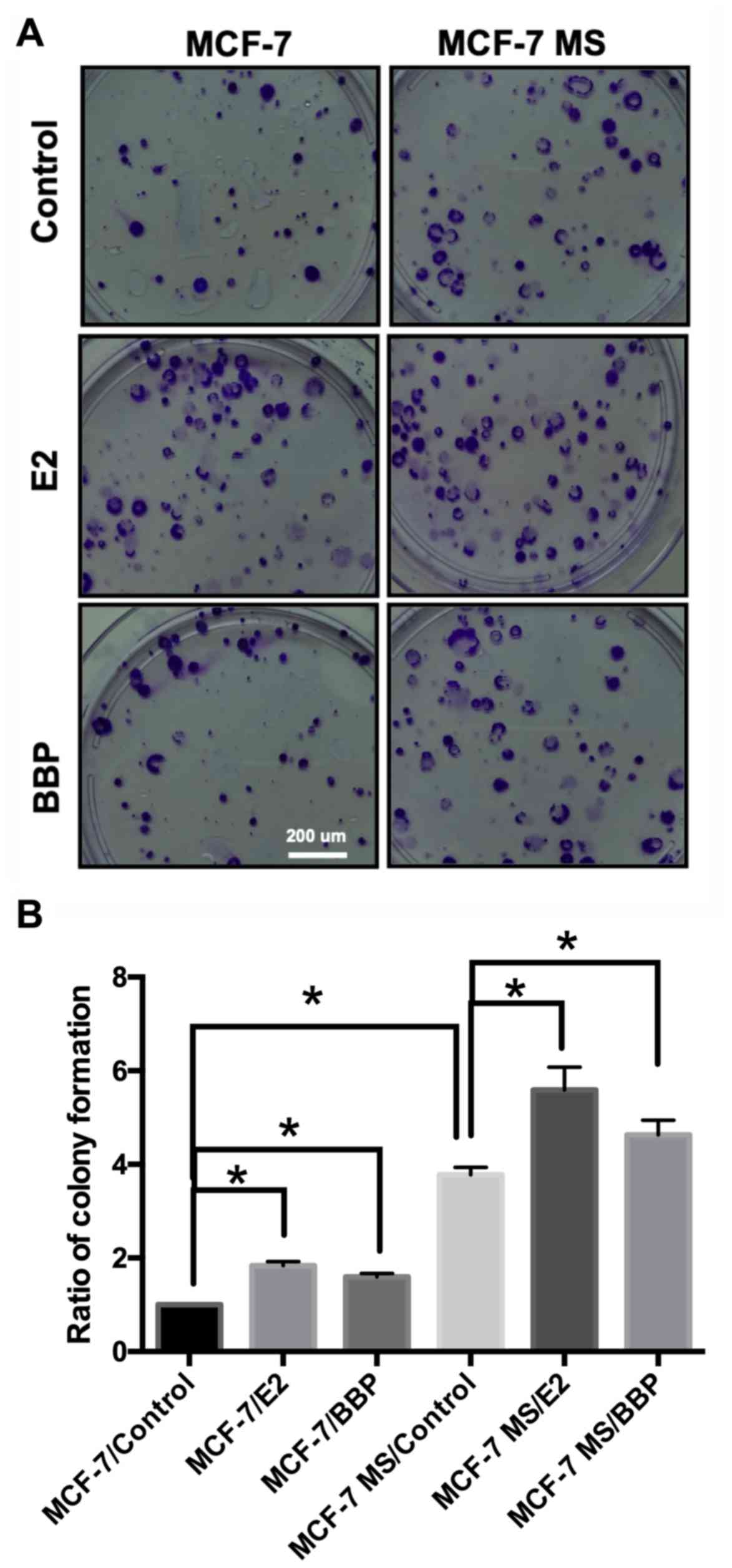
E2 and BBP induce colony formation by
MCF-7 MS cells
A colony formation assay was used to measure the
colony-forming ability of MCF-7 MS cells. Colony-forming ability is
a sensitive in vitro indicator of undifferentiated CSCs
(39). Following mammosphere
culture for 14 days, the cells were treated with 1×10−6
M E2 and 1×10−6 M BBP, and maintained in culture for a
further 14 days. The results revealed that treatment with E2 and
BBP increased colony formation compared with control (Fig. 3A). The visible number of colonies
in the control and treatment groups was counted under a microscope.
The number of colonies was quantified and found that E2 and BBP
significantly induced colony formation in MCF-7 and MCF-7 MS cells
(Fig. 3B).
E2 induces COX-2 expression through
HER2 in MCF-7 MS cells
To date, it has remained elusive whether E2
stimulates the HER2 signaling pathway in MCF-7 MS cells. In the
present study, western blotting was used to identify HER2 signaling
in MCF-7 MS cells. The results revealed that the protein levels of
HER2 and COX-2 were upregulated in MS cells compared with in MCF-7
cells (Fig. 4A). COX-2 is a
downstream gene of HER2 (40) and
regulates the proliferation of breast cancer cells (41). Following mammosphere culture for 14
days, MCF-7 MS cells were treated with E2 at different
concentrations (1×10−11−1×10−6 M) and for
different periods of time (5 min-48 h), and the expression levels
of COX-2 were assessed. The results indicated that E2 increased the
expression of COX-2 in a dose- and time-dependent manner (Fig. 4B and C). To confirm whether the
cellular levels of COX-2 were dependent on ER, MCF-7 MS cells were
treated with E2 and the ER antagonist Fulvestrant (ICI 182780).
Notably, treatment with ICI repressed the E2-mediated induction of
COX-2 in MCF-7 MS cells (Fig. 4D).
Furthermore, two MAPK inhibitors (SB203580 and PD98059), a PI3K
inhibitor (wortmannin) and a HER2 inhibitor (Tyrphostin; AG-825)
were used to analyze the signaling involved in E2-mediated
induction of COX-2 in MCF-7 MS cells. All the inhibitors repressed
E2-mediated induction of COX-2 (Fig.
4E), suggesting that E2 upregulated cellular COX-2 levels
through ER/HER2/MAPK/PI3K signaling in MCF-7 MS cells.
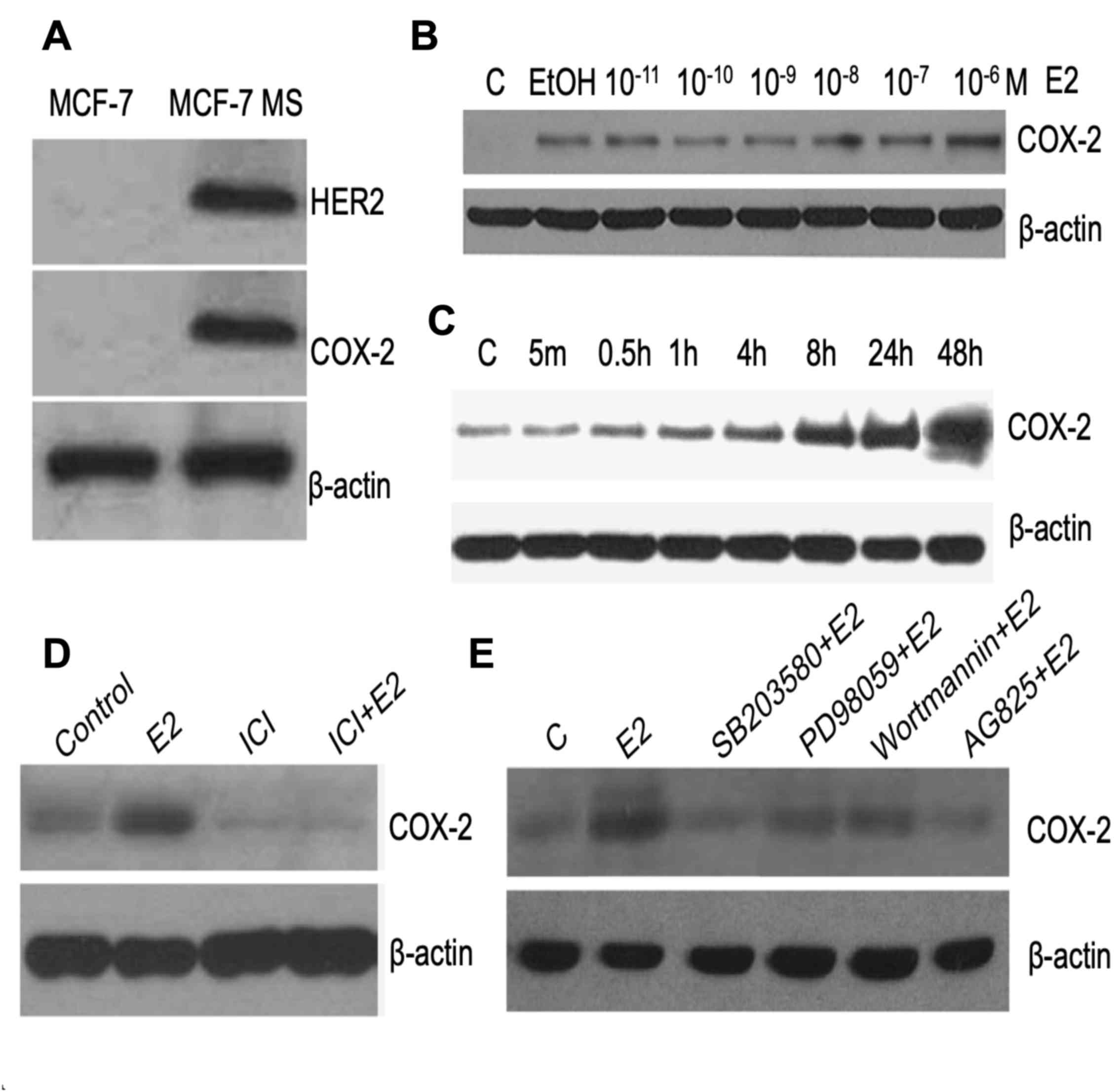 | Figure 4.E2 induces COX-2 expression in MCF-7
MS cells through the ER/HER2/MAPK/PI3K signaling pathway. (A)
Western blot analysis of HER2 and COX-2 in MCF-7 and MCF-7 MS
cells. (B) COX-2 levels were assessed by western blotting after
MCF-7 MS cells were treated with different concentrations of E2
(1×10−11−1×10−6 M). (C) MCF-7 MS cells were
treated 1×10−6 M for various durations (5 min-48 h) and
COX-2 protein levels were measured. MCF-7 MS cells were treated
with 1×10−6 M E2 alone or in combination with (D)
1×10−6 M ER inhibitor ICI182780, (E) 3×10−5 M
MAPK inhibitor SB203580, 2×10−5 M MAPK inhibitor
PD98059, 5×10−7 M PI3K inhibitor wortmannin or
5×10−6 M HER2 inhibitor AG-825, and COX-2 was detected
by western blotting. β-actin was used as the loading control. The
images are representative of 3 independent experiments. C, control;
COX-2, cyclooxygenase-2; E2, estradiol; ER, estrogen receptor;
EtOH, ethanol; HER2, human epidermal growth factor receptor 2; ICI,
ICI182780; MAPK, mitogen-activated protein kinase; MS, mammosphere;
PI3K, phosphoinositide-3 kinase. |
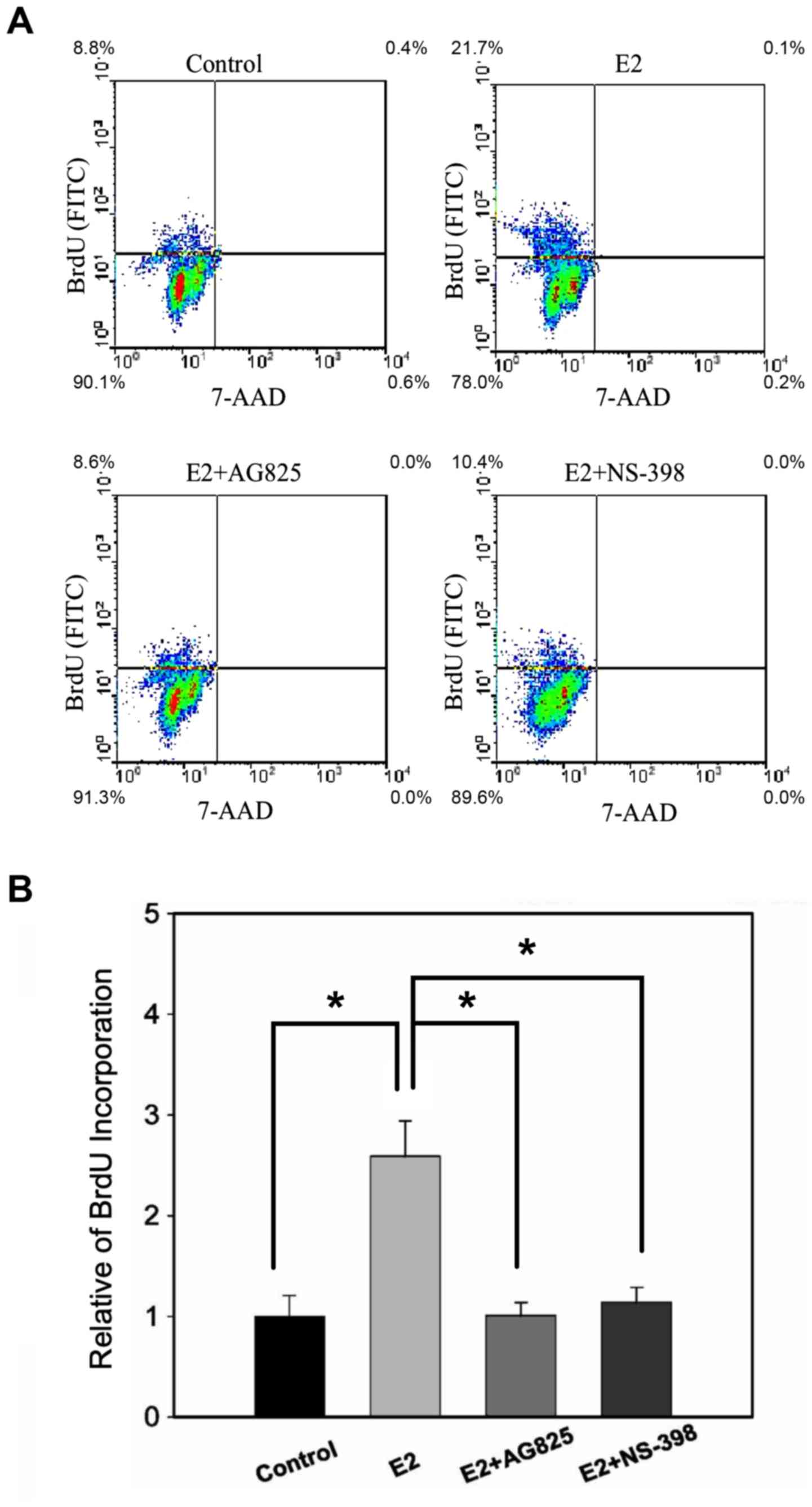
E2 induces the proliferation of MCF-7
MS cells through COX-2
To determine whether E2 induces cell proliferation
through COX-2 in MCF-7 MS cells, MCF-7 MS cells that had been in
mammosphere culture for 14 days were treated with E2 plus HER2
antagonist AG-825 or COX-2 antagonist NS-398, and BrdU
incorporation was measured by flow cytometry. Indeed, both
E2+AG-825 and E2+ NS-398 inhibited the proliferation of E2-treated
MCF-7 MS cells (Fig. 5A and B),
suggesting that E2 induced proliferation of MCF-7 MS cells through
HER2/COX-2.
Discussion
The present study demonstrated that E2 increased the
proportion of CD44+/CD24−MCF-7 MS cells and
induced cell proliferation through ER/HER2/MAPK/PI3K/COX-2
signalling, which is consistent with a previous study, which
indicated that E2 promotes cancer cell proliferation (42). CD44 is a hemagglutinin-binding
glycoprotein surface marker, which is overexpressed in numerous
solid malignancies and CSCs. There is evidence indicating that
monoclonal antibodies against CD44 may be a favourable therapeutic
strategy for the clinical treatment of cancer (43). The mammosphere culture model used
in the present study was convenient and fast in obtaining
CD44+ cells, which may aid in the development of
clinical drugs in the future.
E2 is a steroid hormone that mediates various cell
processes through the ER. The levels of E2 have an important role
in breast cancer development and are associated with increased risk
of breast cancer in women (44).
Previous findings demonstrated that E2 significantly increases the
percentage of cells in the S-phase during culture, and activates
the phosphorylation of PI3K and MAPK in human mesenchymal stem
cells (45). E2 also affects
adipogenesis and osteogenesis through the ER (46). Therefore, regulation of stem cell
differentiation by E2 is required for human tissue
regeneration.
ER, HER2, MAPK, PI3K and COX-2 also have an
important role in cell proliferation and stem cell
differentiation/self-renewal. Side-population cells are stem cells
that have a high drug efflux function and exhibit a 6-fold enriched
expression of ER compared with non-side-population cells (47). In ER-positive breast cancer, HER2
is a CSC-selective marker for regulating self-renewal and
proliferation (48). In addition,
COX-2 is the rate-limiting enzyme for catalyzing the formation of
prostaglandins and promoting cell proliferation in cancer. A
previous study demonstrated that COX-2 is highly expressed in
hematopoietic stem cells and mediates stem cell self-renewal and
differentiation (49). Another
study indicated that the COX-2-specific inhibitor celecoxib
provided no clinical benefits for ER-positive patients with
advanced disease, but had a greater effect in ER-negative patients
(50). Therefore, the mechanisms
of COX-2 and ER in breast cancer remains to be elucidated, and
further clarification on their association is required to pave way
for the development of novel drugs for the clinical treatment of
breast cancer.
Furthermore, COX-2 is involved in osteogenesis by
inducing the expression of core-binding factor α1 and
osterix, which mediate normal skeletal repair and stem cell
differentiation (51). In
addition, alterations of the MAPK/PI3K signaling pathway are
involved in metastatic progression linked with CSC/progenitor cells
(52). Therefore, it is indicated
that ER, HER2, MAPK, PI3K and COX-2 have an important role in
breast CSC self-renewal, differentiation and proliferation in
mammospheres.
The mammosphere culture system based on human cancer
cell lines can be relatively easily established and has been well
characterized. Stem-like cells may be induced by growth factors and
stem cell surface markers are identifiable by flow cytometry.
However, the mammosphere culture system has certain limitations. It
features a heterogeneous population with varying expression levels
of CD44 and CD24 among cells (53). Furthermore, it has been reported
that trastuzumab antibodies have poor penetration in the
mammosphere model (22). In the
future, cells from primary breast cancer may be isolated and
induced to form mammospheres. These mammosphere-forming cells
resembling a cancer stem-like cell population, which are resistant
to clinical drugs may provide useful information for the
development or selection of personalized therapies in the
future.
In conclusion, the present study established an
MCF-7 MS cell model, and revealed that E2 increased the proportion
of CD44+/CD24–MCF-7 MS cells and mediated
cell proliferation via ER/HER2/MAPK/PI3K/COX-2 signaling. This
information may be used to examine the functions and tumorigenic
aspects of stem cells, and assess the efficacy of novel therapeutic
agents.
Acknowledgements
This study was supported with research space,
technical teaching and instrumentation by the Research Center for
Environmental Medicine, Kaohsiung Medical University, Kaohsiung,
Taiwan via The Featured Areas Research Center Program within the
framework of the Higher Education Sprout Project by the Ministry of
Education (MOE) Taiwan.
Funding
The present study was supported in part by the
Ministry of Science and Technology of Taiwan (grant nos. MOST
106–2320-B-037-019 and MOST 105-2314-B-037-052-MY3) and the
Kaohsiung Medical University (Hospital) Research Fund (grant nos.
KMUH105-5M26, KMUH105-M532, KMUH105-5R31, KMUH106-6M35,
KMUH106-6R38 and KMUH106-6R39).
Availability of data and materials
All analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
THH, CHW, CLW and EMT conceived and designed the
experiments. HYC, CYL and CYH performed the experiments. THH, CHW,
CLW and EMT revised the manuscript and interpreting all data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martelotto LG, Ng CK, Piscuoglio S,
Weigelt B and Reis-Filho JS: Breast cancer intra-tumor
heterogeneity. Breast Cancer Res. 16:2102014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dontu G, Liu S and Wicha MS: Stem cells in
mammary development and carcinogenesis: Implications for prevention
and treatment. Stem Cell Rev. 1:207–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fillmore C and Kuperwasser C: Human breast
cancer stem cell markers CD44 and CD24: Enriching for cells with
functional properties in mice or in man? Breast Cancer Res.
9:3032007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheridan C, Kishimoto H, Fuchs RK,
Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S and
Nakshatri H: CD44+/CD24- breast cancer cells exhibit enhanced
invasive properties: An early step necessary for metastasis. Breast
Cancer Res. 8:R592006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell
characteristics. Breast Cancer Res. 10:R102008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Serrano M: The INK4a/ARF locus in murine
tumorigenesis. Carcinogenesis. 21:865–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scheel C, Eaton EN, Li SH, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL and
Weinberg RA: Paracrine and autocrine signals induce and maintain
mesenchymal and stem cell states in the breast. Cell. 145:926–940.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dey D, Saxena M, Paranjape AN, Krishnan V,
Giraddi R, Kumar MV, Mukherjee G and Rangarajan A: Phenotypic and
functional characterization of human mammary stem/progenitor cells
in long term culture. PLoS One. 4:e53292009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cicalese A, Bonizzi G, Pasi CE, Faretta M,
Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP and Pelicci
PG: The tumor suppressor p53 regulates polarity of self-renewing
divisions in mammary stem cells. Cell. 138:1083–1095. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manuel Iglesias J, Beloqui I,
Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon
A, Menendez JA, Dopazo J and Martin AG: Mammosphere formation in
breast carcinoma cell lines depends upon expression of E-cadherin.
PLoS One. 8:e772812013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei B, Zhang XY, Zhou JP, Mu GN, Li YW,
Zhang YX and Pang D: Transcriptome sequencing of HER2-positive
breast cancer stem cells identifies potential prognostic marker.
Tumour Biol. 37:14757–14764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lo PK and Chen H: Cancer stem cells and
cells of origin in MMTV-Her2/neu-induced mammary tumorigenesis.
Oncogene. 32:1338–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alexander PB, Chen R, Gong C, Yuan L,
Jasper JS, Ding Y, Markowitz GJ, Yang P, Xu X, McDonnell DP, et al:
Distinct receptor tyrosine kinase subsets mediate anti-HER2 drug
resistance in breast cancer. J Biol Chem. 292:748–759. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma H, Lu Y, Malone KE, Marchbanks PA,
Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG,
et al: Mortality risk of black women and white women with invasive
breast cancer by hormone receptors, HER2, and p53 status. BMC
Cancer. 13:2252013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harari D and Yarden Y: Molecular
mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene.
19:6102–6114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang KH, Kao AP, Chang CC, Lee JN, Hou MF,
Long CY, Chen HS and Tsai EM: Increasing CD44+/CD24(−) tumor stem
cells and upregulation of COX-2 and HDAC6, as major functions of
HER2 in breast tumorigenesis. Mol Cancer. 9:2882010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korkaya H, Paulson A, Iovino F and Wicha
MS: HER2 regulates the mammary stem/progenitor cell population
driving tumorigenesis and invasion. Oncogene. 27:6120–6130. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pickl M and Ries CH: Comparison of 3D and
2D tumor models reveals enhanced HER2 activation in 3D associated
with an increased response to trastuzumab. Oncogene. 28:461–468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oak PS, Kopp F, Thakur C, Ellwart JW, Rapp
UR, Ullrich A, Wagner E, Knyazev P and Roidl A: Combinatorial
treatment of mammospheres with trastuzumab and salinomycin
efficiently targets HER2-positive cancer cells and cancer stem
cells. Int J Cancer. 131:2808–2819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yarden Y: Biology of HER2 and its
importance in breast cancer. Oncology. 61 Suppl 2:S1–S13. 2001.
View Article : Google Scholar
|
|
24
|
Belsches-Jablonski AP, Biscardi JS, Peavy
DR, Tice DA, Romney DA and Parsons SJ: Src family kinases and HER2
interactions in human breast cancer cell growth and survival.
Oncogene. 20:1465–1475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Ding W, Chen Y, Feng M, Ouyang Y,
Yu Y and He Z: Up-regulation of breast cancer resistance protein
plays a role in HER2-mediated chemoresistance through PI3K/Akt and
nuclear factor-kappa B signaling pathways in MCF7 breast cancer
cells. Acta Biochim Biophys Sin (Shanghai). 43:647–653. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Lee JN,
Chai CY, Hou MF, Chang CC, Long CY, Ko YC and Tsai EM: Phthalates
stimulate the epithelial to mesenchymal transition through an
HDAC6-dependent mechanism in human breast epithelial stem cells.
Toxicol Sci. 128:365–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Lv Q, Meng W, Tan Q, Zhang S, Mo X
and Yang X: Comparison of mammosphere formation from breast cancer
cell lines and primary breast tumors. J Thorac Dis. 6:829–837.
2014.PubMed/NCBI
|
|
28
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu YZ, Yan YY, He M, Xiao QH, Yao WF, Zhao
L, Wu HZ, Yu ZJ, Zhou MY, Lv MT, et al: Salinomycin induces
selective cytotoxicity to MCF-7 mammosphere cells through targeting
the Hedgehog signaling pathway. Oncol Rep. 35:912–922. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic.
Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Hsi E,
Suen JL, Hung CH, Lee JN, Chai CY, Wang SC and Tsai EM: n-Butyl
benzyl phthalate promotes breast cancer progression by inducing
expression of lymphoid enhancer factor 1. PLoS One. 7:e427502012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Götte M, Wolf M, Staebler A, Buchweitz O,
Kelsch R, Schüring AN and Kiesel L: Increased expression of the
adult stem cell marker Musashi-1 in endometriosis and endometrial
carcinoma. J Pathol. 215:317–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Michel M, Török N, Godbout MJ, Lussier M,
Gaudreau P, Royal A and Germain L: Keratin 19 as a biochemical
marker of skin stem cells in vivo and in vitro: Keratin 19
expressing cells are differentially localized in function of
anatomic sites, and their number varies with donor age and culture
stage. J Cell Sci. 109:1017–1028. 1996.PubMed/NCBI
|
|
35
|
Larouche D, Hayward C, Cuffley K and
Germain L: Keratin 19 as a stem cell marker in vivo and in vitro.
Methods Mol Biol. 289:103–110. 2005.PubMed/NCBI
|
|
36
|
Nikpour P, Mowla SJ, Forouzandeh-Moghaddam
M and Ziaee SA: The stem cell self-renewal gene, Musashi 1, is
highly expressed in tumor and non-tumor samples of human bladder.
Indian J Cancer. 50:214–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawai T, Yasuchika K, Ishii T, Katayama H,
Yoshitoshi EY, Ogiso S, Kita S, Yasuda K, Fukumitsu K, Mizumoto M,
et al: Keratin 19, a cancer stem cell marker in human
hepatocellular carcinoma. Clin Cancer Res. 21:3081–3091. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
El Sakka D, Gaber MA, Abdou AG, Wahed MA,
Saleh AA and Shehata W: Stem cell markers (Cytokeratin 17 and
Cytokeratin 19) in scarring and nonscarring alopecia. J Cutan
Aesthet Surg. 9:165–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rajendran V and Jain MV: In vitro
tumorigenic assay: Colony forming assay for cancer stem cells.
Methods Mol Biol 1692. 89–95. 2018. View Article : Google Scholar
|
|
40
|
Vadlamudi R, Mandal M, Adam L, Steinbach
G, Mendelsohn J and Kumar R: Regulation of cyclooxygenase-2 pathway
by HER2 receptor. Oncogene. 18:305–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lanza-Jacoby S, Burd R, Rosato FE Jr,
McGuire K, Little J, Nougbilly N and Miller S: Effect of
simultaneous inhibition of epidermal growth factor receptor and
cyclooxygenase-2 in HER-2/neu-positive breast cancer. Clin Cancer
Res. 12:6161–6169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pattarozzi A, Gatti M, Barbieri F, Würth
R, Porcile C, Lunardi G, Ratto A, Favoni R, Bajetto A, Ferrari A
and Florio T: 17beta-estradiol promotes breast cancer cell
proliferation-inducing stromal cell-derived factor-1-mediated
epidermal growth factor receptor transactivation: Reversal by
gefitinib pretreatment. Mol Pharmacol. 73:191–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thapa R and Wilson GD: The importance of
CD44 as a stem cell biomarker and therapeutic target in cancer.
Stem Cells Int 2016. 20872042016.
|
|
44
|
Cauley JA, Lucas FL, Kuller LH, Stone K,
Browner W and Cummings SR: Elevated serum estradiol and
testosterone concentrations are associated with a high risk for
breast cancer. Study of osteoporotic fractures research group. Ann
Intern Med. 130:270–277. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yun SP, Lee MY, Ryu JM, Song CH and Han
HJ: Role of HIF-1alpha and VEGF in human mesenchymal stem cell
proliferation by 17beta-estradiol: Involvement of PKC, PI3K/Akt,
and MAPKs. Am J Physiol Cell Physiol. 296:C317–C326. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hong L, Colpan A and Peptan IA:
Modulations of 17-beta estradiol on osteogenic and adipogenic
differentiations of human mesenchymal stem cells. Tissue Eng.
12:2747–2753. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clarke RB, Spence K, Anderson E, Howell A,
Okano H and Potten CS: A A putative human breast stem cell
population is enriched for steroid receptor-positive cells. Dev
Biol. 277:443–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ithimakin S, Day KC, Malik F, Zen Q,
Dawsey SJ, Bersano-Begey TF, Quraishi AA, Ignatoski KW, Daignault
S, Davis A, et al: HER2 drives luminal breast cancer stem cells in
the absence of HER2 amplification: Implications for efficacy of
adjuvant trastuzumab. Cancer Res. 73:1635–1646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
North TE, Goessling W, Walkley CR,
Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH,
Grosser T, et al: Prostaglandin E2 regulates vertebrate
haematopoietic stem cell homeostasis. Nature. 447:1007–1011. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dirix LY, Ignacio J, Nag S, Bapsy P, Gomez
H, Raghunadharao D, Paridaens R, Jones S, Falcon S, Carpentieri M,
et al: Treatment of advanced hormone-sensitive breast cancer in
postmenopausal women with exemestane alone or in combination with
celecoxib. J Clin Oncol. 26:1253–1259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Schwarz EM, Young DA, Puzas JE,
Rosier RN and O'Keefe RJ: Cyclooxygenase-2 regulates mesenchymal
cell differentiation into the osteoblast lineage and is critically
involved in bone repair. J Clin Invest. 109:1405–1415. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer Res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|