Introduction
Cardiovascular diseases are associated with a high
rate of mortality in humans. In addition, cardiovascular diseases
are less prevalent in women aged between 20 and 50 years compared
with the corresponding male population. However, in individuals
>50 years old, the incidence of cardiovascular disease is
equivalent in both sexes (1,2).
Previous studies have reported that the presence of estrogens
serves an important role in protection against cardiac injury, thus
suggesting that menopause may be a risk factor for numerous
cardiovascular diseases (3,4).
Studies in animal models have demonstrated that a lack of ovarian
hormones, in particular estrogens, has detrimental effects on
various organs, including the cardiovascular system (5,6).
Hormone replacement therapy (HRT) has therefore been recommended to
postmenopausal women; however, the controversies regarding the
safety of HRT have drawn attention to novel therapies for
postmenopausal women (3,7–9).
Lycium barbarum polysaccharides (LBP) may be
used in TCM to prevent postmenopausal symptoms. Clinical research
has indicated that polysaccharides extracted from Lycium
barbarum may serve an important biological role. LBP is
composed of arabinose, glucose, galactose, mannose, xylose and
rhamnose monosaccharide, etc., and contains various trace elements
and amino acids (10). A previous
study demonstrated that LBP is the main active ingredient of the
TCM medlar, which can regulate immunity and improve age-associated
symptoms, including fatigue, loss of appetite and blurred vision,
and may reduce blood lipid levels and fatty liver disease, and
exert anti-aging effects (11).
Oxidative stress reflects an imbalance between the
systemic manifestation of reactive oxygen species (ROS) and the
ability of the body to readily detoxify reactive intermediates or
to repair the resulting damage (12). Numerous studies have demonstrated
that oxidative stress is an important factor underlying abnormal
cardiovascular system structure (13–15).
In addition, various cardiovascular diseases, including
hypertension, atherosclerosis, myocardial ischemia,
ischemia-reperfusion injury, myocardial hypertrophy and heart
failure, are associated with an increase in ROS generation.
Furthermore, a previous study revealed that estrogen exerts
antioxidative effects, which may serve a role in cardiac protection
(16).
Increased cardiovascular risk in postmenopausal
women may be due to the postmenopausal reduction in estrogen
levels; therefore, the antioxidative effects are weakened and
cardiovascular disease may be initiated. In the present study, LBP
exerted a protective effect against heart failure in rats. In
addition, LBP has been reported to reduce isopropyl
adrenaline-induced heart failure and rat heart mass/weight ratio,
reduce myocardial injury and significantly improve cardiac function
in rats; the underlying mechanism may be associated with an
improvement in antioxidant enzyme activity and a reduction in lipid
peroxide formation (17,18). The present study investigated
whether LBP effects the oxidative stress state and induces
antioxidative effects in the myocardium of ovariectomized (OVX)
rats. In addition, the expression levels of apoptotic proteins and
Akt pathway proteins were also detected in the myocardium. The
present study aimed to explore whether LBP exerts protective
effects against oxidative insult in OVX rats.
Materials and methods
Animals
A total of 30 female Sprague-Dawley rats (aged 10–12
weeks, weight 200±10 g) were purchased from Vital River
Laboratories Co., Ltd., (Beijing, China). The rats were acclimated
for 7 days, prior to use in subsequent experiments, and were housed
in specific pathogen-free conditions (temperature 22±1°C, humidity
50±5%) under a 12-h light/dark cycle. Tap water and chow were
provided ad libitum. All efforts were made to minimize
suffering, and procedures were performed under chloral hydrate (300
mg/kg, i.p.) anesthesia when necessary. The present study was
approved by and followed the guidelines of the Animal Ethics
Committee of Liaoning University of Traditional Chinese Medicine
(Shenyang, China; permit no. 2011-167).
Experimental protocol
The adult female Sprague-Dawley rats were randomly
divided into the following five groups (n=6/group): i) Sham
operation group, in which a small region of fat was removed via
bilateral paraspinal incisions, and rats were fed with tap water
for 12 weeks; ii) OVX group, in which the ovaries of the rats were
exteriorized and removed via bilateral paraspinal incisions, and
rats were fed tap water for 12 weeks; ii) estradiol valerate group
(Est), in which rats were fed estradiol valerate (0.105 mg/kg) for
12 weeks following OVX; iv) high-dose LBP group (LBP-H), in which
rats were fed LBP (250 mg/kg) for 12 weeks following OVX; v)
low-dose LBP group (LBP-L), in which rats were fed LBP (125 mg/kg)
following OVX. All procedures were performed under 10% chloral
hydrate anesthesia (300 mg/kg). Following surgery, all rats
received prophylactic antibiotic therapy (penicillin G procaine;
4,000 IU/kg i.m.). Daily vaginal smears were collected from all
rats, as previously described (19). This procedure allowed for the phase
of the estrus cycle to be determined by daily analysis of the types
of cells that sloughed off the vaginal epithelium. With this
approach, four different stages can be observed, as follows:
Proestrus (nucleated epithelial cells), estrus (cornified cells),
metestrus (some cornified cells in addition to nucleated cells and
a large number of leukocytes) and diestrus (leukocyte
infiltration). Collected vaginal fluid was placed on glass slides
and examined by light microscopy. In the Sham group, estrous cycle
regularity was confirmed by the presence of vaginal epithelial
cells characteristic of each of the four aforementioned stages. In
the remaining groups, the absence of the estrous cycle was
confirmed by a permanent diestrus phase.
Hormone assays
The level of 17β-Estradiol (E2) in serum was
assessed using an enzyme-linked immunosorbent assay kit (cat. no.
10006315; MultiSciences Biotech Co., Ltd., Hangzhou, China)
according to manufacturer's protocol. Absorbance was measured at
450 nm within 15 min (Multiskan FC; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Measurement of ROS, malondialdehyde
(MDA), glutathione peroxidase (GSH-px), superoxide dismutase (SOD)
and catalase (CAT) activities
The rats were anaesthetized with 10% chloral
hydrate, and sacrificed by decapitation after 13 weeks. A 1–2 cm
skin incision was first made, 0.5 cm below the rib and 1 cm next to
the spine. Then the subcutaneous tissue, muscle and peritoneum were
cut in turn. Following the opening of the peritoneal cavity, the
rat kidney was identified and a white cellulite beneath it,
exposing the soybean-size glandular spheres (the ovaries) which
were removed by wire. Finally, following the confirmation that
there was no intra-abdominal peritoneal bleeding, the peritoneum
and skin were closed layer by layer, and the operating area
cleaned. Following the surgery, in a warm environment, the rats
were released into cages on wakening and given intraperitoneal
injections of penicillin 160,000 units/rat three days after the
surgery to prevent infection. The myocardium (100 mg) was
homogenized in cold saline. The homogenate was then centrifuged at
900 × g for 15 min. The activities of ROS (cat. no. E004), MDA
(cat. no. A003-2), GSH-px (cat. no. A005), SOD (cat. no. A001-1)
and CAT (cat. no. A007-2; all from Nanjing Jiancheng Biological
Engineering Institute, Nanjing, China) were determined using these
assay kits according to the manufacturer's protocols.
Hematoxylin and eosin (H&E)
staining of myocardium
Myocardium samples were fixed in 4% paraformaldehyde
for 24 h at room temperature and stained with H&E, according to
standard techniques. Briefly, the samples were embedded in paraffin
and sections (5-µm) were obtained. The samples were then dewaxed
with xylene, rehydrated through an alcohol gradient and were
stained with H&E for light microscopy. Images were captured
using a light microscope linked to a digital charge-coupled device
camera (Olympus Corporation, Tokyo, Japan).
Protein extraction and western
blotting
Total cellular proteins were extracted from heart
tissues using Radioimmunoprecipitation Lysis Buffer (Beyotime
Institute of Biotechnology, Shanghai, China). Protein concentration
was measured using a Bicinchoninic Acid Protein Assay kit (Beijing
Dingguo Changsheng Biotechnology Co. Ltd., Beijing, China). Western
blotting was conducted to assess the protein expression levels of B
cell lymphoma-2 (Bcl2), Bcl2-associated X protein (Bax), cleaved
caspase-9, cleaved caspase-3 and phosphorylated (p)-protein kinase
B (Akt). Briefly, 40 µg protein samples were separated by 10%
SDS-PAGE, after which the proteins were transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then incubated overnight (4°C) with
antibodies against β-actin (cat. no. Sc-130300; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), Bax (cat. no. Sc-4239; Santa
Cruz Biotechnology, Inc.), Bcl2 (cat. no. Sc-509; Santa Cruz
Biotechnology, Inc.), caspase-9/cleaved caspase-9 (cat. no. 9504;
Cell Signaling Technology, Inc., Danvers, MA, USA),
caspase-3/cleaved caspase-3 (cat. no. 9509; Cell Signaling
Technology, Inc.), Akt (cat. no. 9662, Cell Signaling Technology,
Inc.) and p-Akt (cat. no. 9667, Cell Signaling Technology, Inc.).
The dilution of β-actin, Bax and Bcl2 antibodies was 1:500, and for
caspase-9/cleaved caspase-9, caspase-3/cleaved caspase-3, Akt and
p-Akt antibodies 1:1,000. The membranes were then incubated with a
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (cat. no. Sc-2004; 1:3,000; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Protein bands were visualized
using an enhanced chemiluminescence kit (Thermo Fisher Scientific,
Inc.). Protein expression was normalized to β-actin. ImageJ
software version 1.45 (National Institutes of Health, Bethesda, MD,
USA) was used to perform densitometric analysis.
Statistical analysis
All experiments were carried out at least in
duplicate. Data are presented as the mean ± standard deviation.
Statistical analysis was performed with one-way analysis of
variance followed by Kruskal Wallis test using the GraphPad Prism 5
software package (GraphPad, La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
LBP increases the serum levels of E2
and modifies the OVX-induced alterations in cardiac tissue
In order to confirm that OVX was successful, serum
E2 levels were measured. A significant reduction in serum E2 levels
was observed in the OVX group compared with in the Sham group
(P<0.01), thus indicating that OVX was successful. Treatment
with high-dose LBP and estradiol valerate increased serum E2 levels
compared with in OVX rats (P<0.01). However, there was no
significant difference in E2 levels in the serum between the LBP-L
and OVX groups (Fig. 1A). In the
Sham group, myocardial fibers were arranged regularly with clear
striations, without any damage or necrosis in the tissue (Fig. 1B). Histopathological sections of
the OVX group displayed disorganized fibers and increased
dilatation of intercellular spaces. Conversely, high-dose LBP and
estradiol valerate modified the OVX-induced alterations in cardiac
tissue (Fig. 1C-F).
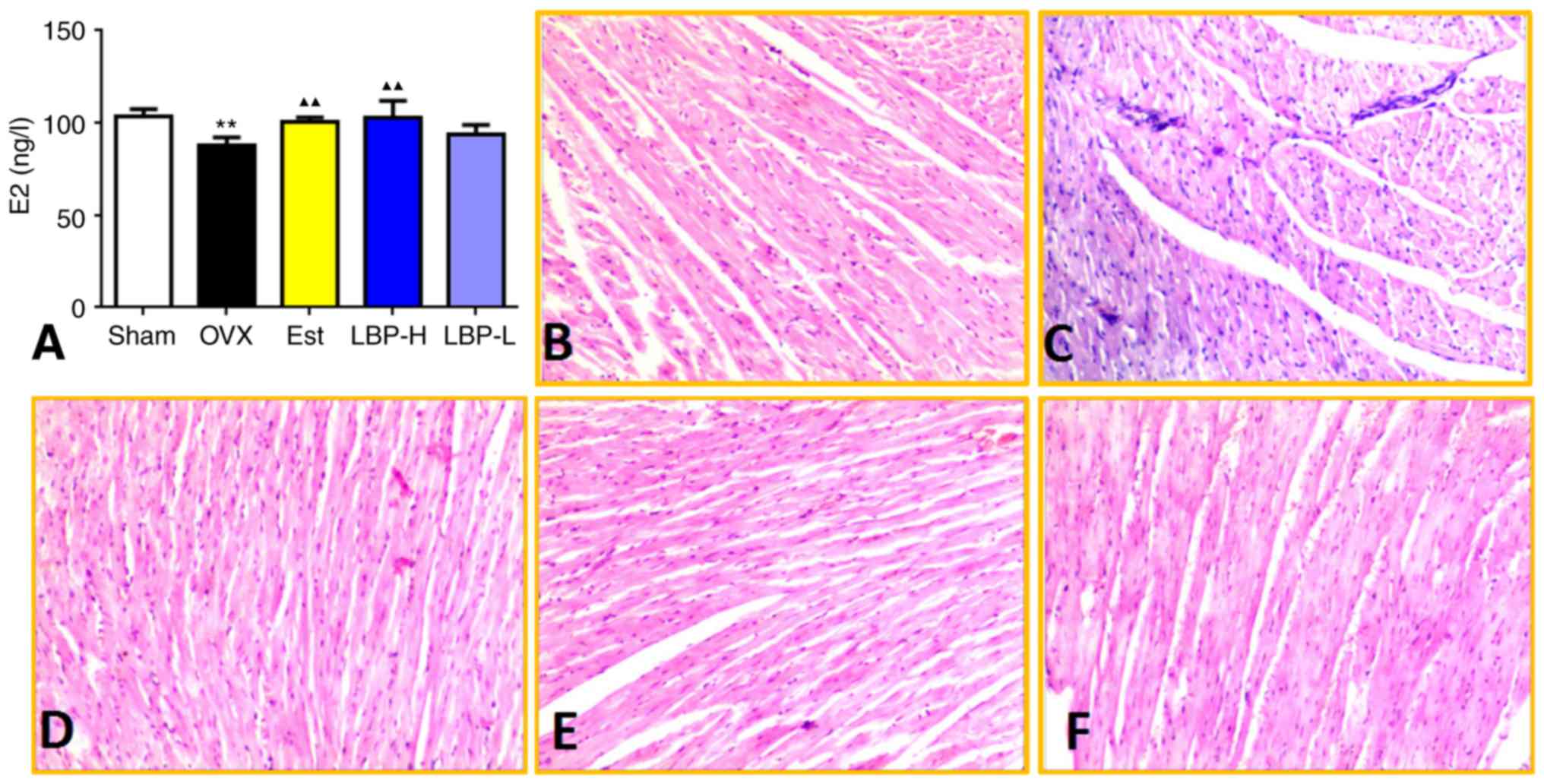 | Figure 1.Effects of LBP on serum E2 levels and
histopathological examination of cardiac tissue. (A) Effects of LBP
therapy on serum E2 levels. A significant reduction in serum E2
levels was observed in OVX rats, whereas E2 levels were increased
in the LBP-H group compared with in the OVX group (n=6/group).
**P<0.01 vs. the Sham group; ▲▲P<0.01 vs. the OVX
group. (B-F) Representative photomicrographs of cardiac tissue from
the Sham, OVX, Est, LBP-H and LBP-L groups, respectively.
Histopathological sections of the OVX group displayed disorganized
fibers and increased dilatation of intercellular spaces, whereas
these alterations were modified in the LBP-H and Est groups.
Hematoxylin and eosin staining; magnification, ×20. E2,
17β-estradiol; Est, estradiol valerate group; LBP, Lycium
barbarum polysaccharides; LBP-H, high-dose LBP group; LBP-L,
low-dose LBP group; OVX, ovariectomy group. |
LBP recovers the antioxidant status in
OVX rats
As illustrated in Fig.
2, various parameters associated with oxidative stress were
detected. Enhanced ROS and MDA activity was detected in the
myocardium of the OVX group (P<0.01). Administration of a
high-dose of LBP exerted a significant protective effect on OVX
rats (P<0.01). In addition, administration of estradiol valerate
exerted a significant protective effect on MDA only in OVX rats
(P<0.01). GSH-px CAT and SOD are markers of the antioxidant
defense system. As presented in Fig.
2, OVX resulted in a decrease in GSH-px and CAT activity
(P<0.01), whereas in the LBP-H and Est groups GSH-px and CAT
were significantly increased in the myocardium compared with in OVX
rats (P<0.05). No alterations in SOD were detected among the
various groups. These findings indicated that administration of LBP
may recover the antioxidant status in OVX rats.
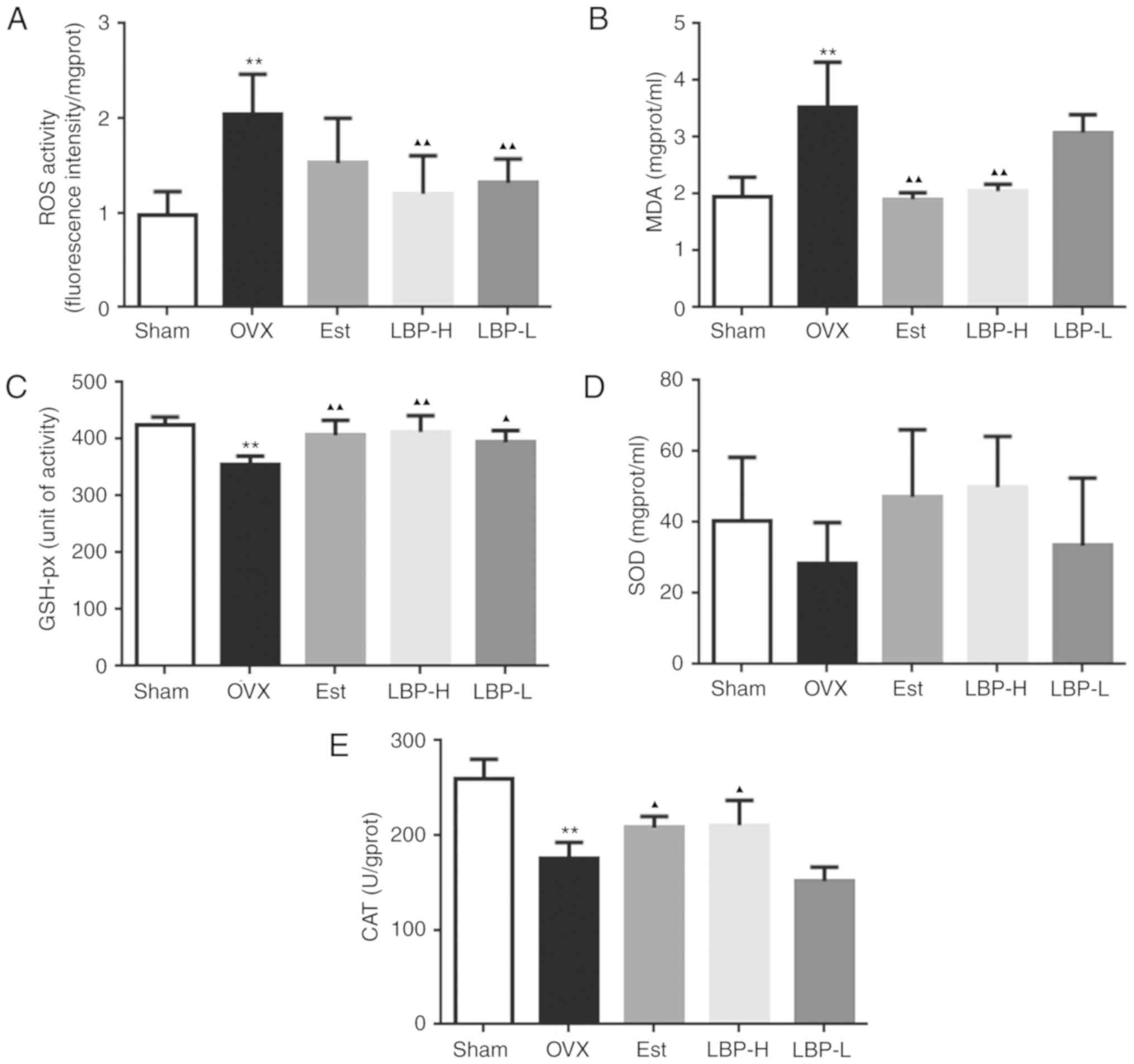 | Figure 2.Effects of LBP and OVX on oxidative
status in the myocardium of rats. (A and B) Effects of LBP on ROS
and MDA activity. A significant increase in ROS and MDA was
detected in the myocardium of OVX rats. Conversely, in the LBP-H
group ROS and MDA activity was significantly decreased compared
with in the OVX group. (C-E) Effects of LBP on GSH-px, SOD and CAT
activity. A significant decrease in GSH and CAT was detected in the
myocardium of OVX rats. Conversely, in the LBP-H group GSH and CAT
activity was significantly increased compared with in the OVX group
(n=6/group). **P<0.01 vs. the Sham group; ▲P<0.05,
▲▲P<0.01 vs. the OVX group. CAT, catalase; Est,
estradiol valerate group; GSH-px, glutathione peroxidase; LBP,
Lycium barbarum polysaccharides; LBP-H, high-dose LBP group;
LBP-L, low-dose LBP group; MDA, malondialdehyde; OVX, ovariectomy
group; ROS, reactive oxygen species; SOD, superoxide dismutase. |
LBP alleviates apoptosis in OVX
rats
Bax, as a proapoptotic protein, Bcl2, as an
anti-apoptotic protein, and caspase-9 and caspase-3 are important
indicators of apoptosis. As demonstrated in Fig. 3, OVX resulted in an increase in
Bax, cleaved caspase-9 and cleaved caspase-3 expression in the
myocardium (P<0.01). Administration of high-dose LBP and
estradiol valerate exerted a significant protective effect on OVX
rats, as evidenced by the decreased expression of apoptotic
proteins (P<0.05). In addition, OVX resulted in a decrease in
Bcl2 expression in the myocardium (P<0.01). However, Bcl2
expression was enhanced in the myocardium of the LBP-H and Est
groups compared with in OVX rats (P<0.01).
 | Figure 3.Effects of LBP and OVX on apoptotic
protein expression in the myocardium. The expression levels of
apoptotic proteins were determined by western blotting. A
significant increase in Bax, cleaved caspase-9 and cleaved
caspase-3, and a decrease in Bcl2 expression were detected in the
myocardium of OVX rats. In the LBP-H group, the expression levels
of Bax, cleaved caspase-9 and cleaved caspase-3 were decreased, and
Bcl2 expression was increased compared with in the OVX group
(n=3/group). **P<0.01 vs. the Sham group; ▲P<0.05,
▲▲P<0.01 vs. the OVX group. Bax, Bcl2-associated X
protein; Bcl2, B cell lymphoma-2; Est, estradiol valerate group;
LBP, Lycium barbarum polysaccharides; LBP-H, high-dose LBP
group; LBP-L, low-dose LBP group; OVX, ovariectomy group. |
LBP alleviates apoptosis via the Akt
signaling pathway in OVX rats
The Akt signaling pathway is involved in apoptosis.
As illustrated in Fig. 4, OVX
resulted in a decrease in the phosphorylation of Akt in the
myocardium (P<0.01). Administration of high-dose LBP resulted in
a significant increase in the expression of p-Akt (P<0.01).
Discussion
The present study demonstrated that LBP can improve
antioxidant status, ameliorate oxidative stress-induced cell
apoptosis and increase phosphorylation of Akt in the myocardium of
OVX rats. OVX-induced cardiac injury suggests that estrogens may be
associated with the maintenance of normal cardiac function. This
relationship has already been suggested by other studies (20,21).
Estrogen has also been reported to provide cardiac protection in
various models of cardiac disease (22,23).
Oxidative stress represents an imbalance between the
production and manifestation of ROS and their detoxification.
Oxidative stress may serve an important pathophysiological role
during the menopause, and is considered one of the main causative
factors of various cardiovascular disorders, including
postmenopausal cardiovascular disorders (24,25).
A number of studies have demonstrated that OVX may result in a
decrease in antioxidative biomarkers and an increase in MDA content
(oxidative biomarker) in the myocardium (26,27).
SOD, CAT and GSH comprise the antioxidant defense system; SOD and
CAT are important components of this system. SOD is considered the
most important antioxidant enzyme that provides defense against
oxidative stress, particularly oxygen radicals. SOD scavenges
superoxide by converting it to peroxide, which in turn is destroyed
by CAT. Therefore, SOD and CAT act in a mutually supportive way
with antioxidant enzymes to provide a protective defense against
ROS. GSH-px catalyzes the reductive action of GSH to
H2O2, in order to protect the integrity and
functions of the plasma membrane (28–30).
Ji et al (31) demonstrated
that estrogens are able to suppress the overproduction of ROS.
Numerous studies (32–34) have revealed the protective effects
of E2 on GSH synthesis. Therefore, OVX may significantly alter the
stimulatory effects of E2 on GSH synthesis.
In the present study, a significant reduction in
serum E2 levels was observed in OVX rats. Conversely, 12-week
treatment with 250 mg/kg LBP, which has estrogen-like effects,
following OVX improved serum E2 levels. Furthermore, OVX induced an
imbalance in oxidative stress status: ROS and MDA activity was
increased, whereas GSH-px and CAT activity was decreased in cardiac
tissues. LBP treatment decreased ROS and MDA activity, and
increased GSH-px and CAT activity in cardiac tissues. These results
indicated that an important association exists between oxidative
stress and LBP-induced cardioprotective effects.
Lycium barbarum L is a well-known antioxidant
traditional Chinese medicine. The aqueous extract of Lycium
barbarum L has been reported to exert marked antioxidant
activity in vivo and in vitro (35,36).
LBP is considered the main active component in Lycium
barbarum L, which possesses numerous bioactivities, including
anti-aging, anticancer, immunomodulatory and antioxidative effects
(37). Li demonstrated that LBP
(20–50 mg/kg) protects liver and kidney tissue from oxidative
damage in streptozotocin-induced diabetic rats (38). Furthermore, Luo et al
(39) indicated that LBP could
alleviate heat-induced damage of rat testes, and
H2O2-induced DNA damage in mouse testicular
cells, by increasing their resistance to oxidative stress-induced
injury. The results of the present study demonstrated that in rats
treated with LBP (250 mg/kg), OVX-induced oxidative injury in
cardiac tissue was less apparent compared with in the OVX group;
LBP was able to decrease ROS and MDA activity, thus improving
OVX-induced abnormalities. In addition, LBP significantly
(P<0.05) increased CAT and GSH-px activity in the heart tissues
of OVX rats. Taken together, LBP exerted indirect effects to
alleviate OVX-induced cardiomyocyte damage.
Oxidative stress can activate cell apoptosis
signaling, resulting in the induction of apoptosis in various cell
types (40). Bax, as a
proapoptotic protein, and Bcl2, as an anti-apoptotic protein, and
caspase-9 and caspase-3, are important indicators of apoptosis. In
the OVX group, increased Bax and decreased Bcl2 protein expression
was detected in the cardiac muscle, thus indicating that the
cardiomyoctes in these rats were undergoing apoptosis. In addition,
there were significant differences in Bax and Bcl2 protein
expression between the OVX and LBP-H groups. These results
suggested that the balance between anti-apoptotic and proapoptotic
factors was disrupted by OVX. Compared with the OVX group,
cardiomyocyte apoptosis was alleviated by LBP (250 mg/kg). In
addition to Bcl2 and Bax, caspase-3 and caspase-9 deactivation also
contributed to LBP-mediated cardiac protection. These findings
indicated that estrogen and LBP may serve a similar role in
regulating cardiomyocyte function by inhibiting apoptosis.
Previous studies have reported that overproduction
of ROS is associated with three pathways: Extracellular
auto-oxidation, intracellular metabolism by monoamine oxidase and
direct inhibition of the mitochondrial respiratory chain (41,42).
Furthermore, the generation of intracellular ROS suppresses Akt
phosphorylation, which induces activation of caspase-9 and
caspase-3, which finally leads to cell apoptosis (42). In the present study, in the OVX
group, cleaved caspase-3 and caspase-9 protein expression was
increased, indicating the occurrence of cell apoptosis, whereas in
the LBP group, cleaved caspase-3 and caspase-9 protein expression
was decreased in cardiac tissues. In addition, the phosphorylation
of Akt was decreased in the cardiac tissues of the OVX group;
however, following treatment with a high dose of LBP, the
phosphorylation of Akt was improved.
In conclusion, LBP may increase activity levels of
the antioxidative markers CAT and GSH-px, and decrease activity
levels of the oxidative markers MDA and ROS in the myocardium of
OVX rats. Therefore, LBP may ameliorate oxidative stress-induced
cardiac damage in OVX rats. LBP is associated with cardiac
protection by inhibiting the apoptotic signaling pathway in
response to oxidative stress; this finding is associated with the
Akt signaling pathway in the myocardium.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Liaoning
University of Traditional Chinese Medicine Key Laboratory of
Ministry of Education for TCM Viscera-State Theory and Applications
open fund (grant no. F14-231-1-17), the Liaoning University of
Traditional Chinese Medicine College Students' Innovative
Entrepreneurial Training Plan (grant no. 201510162000002), and The
60th China Postdoctoral Science Fundation Grant (grant no.
2016M601331).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NY performed the animal breeding, the detection of
experimental indicators and the statistical analysis. NS conducted
the detection of experimental indicators. CL was mainly responsible
for the statistical analysis. GY designed the study. All authors
have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by and followed the
guidelines of the Animal Ethics Committee of Liaoning University of
Traditional Chinese Medicine (Shenyang, China; permit no.
2011-167).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mendelsohn ME and Karas RH: Molecular and
cellular basis of cardiovascular gender differences. Science.
308:1583–1587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossouw JE, Prentice RL, Manson JE, Wu L,
Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL and Stefanick
ML: Postmenopausal hormone therapy and risk of cardiovascular
disease by age and years since menopause. JAMA. 297:1465–1477.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arias-Loza PA, Muehlfelder M and Pelzer T:
Estrogen and estrogen receptors in cardiovascular oxidative stress.
Pflugers Arch. 465:739–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang F, Xiao J, Shen Y, Yao F and Chen Y:
Estrogen protects cardiomyocytes against lipopolysaccharide by
inhibiting autophagy. Mol Med Rep. 10:1509–1512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossouv JE, Prentice RE, Manson JE, Wu L,
Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL and Stefanick
ML: Postmenopausal hormone 1therapy and risk of cardiovascular
disease by age and years since menopause. JAMA. 297:1465–1477.
2007.PubMed/NCBI
|
|
6
|
Babiker FA, DeWindt LJ, Van Eickels M,
Grohe C, Meyer R and Doevendans PA: Estrogenic hormone action in
the heart: Regulatory network and function. Cardiovasc Res.
53:709–719. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zegura B, Keber I, Sebestjen M and Koenig
W: Double blind, randomized study of estradiol replacement therapy
on markers of inflammation, coagulation and fibrinolysis.
Atherosclerosis. 168:123–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller VM and Duckles SP: Vascular actions
of estrogens: Functional implications. Pharmacol Rev. 60:210–241.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knowlton AA and Lee AR: Estrogen and the
cardiovascular system. Pharmacol Ther. 135:54–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ni W, Gao T and Wang H: Anti-fatigue
activity of polysaccharides from the fruits of four Tibetan plateau
indigenous medicinal plants. J Ethnopharmacol. 150:529–535. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao J, Xing F, Huo J, Fung ML, Liong EC,
Ching YP, Xu A, Chang RC, So KF and Tipoe GL: Lycium barbarum
polysaccharides therapeutically improve hepatic functions in
non-alcoholic steatohepatitis rats and cellular steatosis model.
Sci Rep. 4:55872014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giordano FJ: Oxygen, oxidative stress,
hypoxia and heart failure. J Clin Invest. 115:500–508. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saglam H, Kaya E, Cemek M, Ciçek Y, Kulac
M and Karaca S: No apparent correlation between Behçet's disease
and oxidative stress disturbance. Clin Hemorheol Microcric.
44:287–296. 2010.
|
|
14
|
El-Sayyad HI, AI-Haggar MS, EI-Ghawet HA
and Bakr IH: Cardiomyopathy and angiogenesis defects of Wistar rat
fetuses of diabetic and hypercholesterolemic mothers. Nutrition.
28:e33–e43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Q, Len Q, Liu Z and Wang W:
Overexpression of miR-22 attenuates oxidative stress injury in
diabetic cardiomyopathy via Sirt 1. Cardiovasc Ther. 36:2017.doi:
10.1111/1755-5922.12318.
|
|
16
|
Mallat Z, Philip I, Lebret M, Chatel D,
Maclouf J and Tedgui A: Elevated levels of 8-iso-prostaglandin
F2alpha in pericardial fluid of patients with heart failure: A
potential role for in vivo oxidant stress in ventricular dilatation
and progression to heart failure. Circulation. 97:1536–1539. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xin YF, Wan LL and Peng JL: Alleviation of
the acute doxorubicin-induced cardiotoxicity by Lycium barbarum
polysaccharides through the suppression of oxidative stress. Food
Chem Toxicol. 49:259–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu SP and Zhao PT: Chemical
characterization of Lycium barbarum polysaccharides and their
reducing myocardial injury in ischemia/reperfusion of rat heart.
Int J Biol Macromol. 47:681–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poumeau-Delille G: Techniques biologiques
en endocrinologie experimentale chez rat. Masson and Cie. 1953.
|
|
20
|
Bolego C, Cignarella A, Ruzza R, Zaarour
C, Messi E, Zanisi M and Puglisi L: Differential effects of low-
and high-dose estrogen treatments onvascular responses in female
rats. Life Sci. 60:2291–2302. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Persky AM, Green PS, Stubley L, Howell CO,
Zaulyanov L, Brazeau GA and Simpkins JW: Protective effect of
estrogens against oxidativedamage to heart and skeletal muscle in
vivo and in vitro. Proc Soc Exp Biol Med. 223:59–66. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patten RD, Pourati I, Aronovitz MJ, Ali
AA, Eder S, Force T, Mendelsohn ME and Karas RH: 17 Beta-estradiol
differentially affects left ventricular and cardiomyocyte
hypertrophy following myocardial infarction and pressure overload.
J Cardiac Fail. 14:245–253. 2008. View Article : Google Scholar
|
|
23
|
Tagashira H, Bhuiyan S, Shioda N and
Fukunaga K: Distinct cardioprotective effects of 17β-estradiol and
dehydroepiandrosterone on pressure overload-induced hypertrophy in
ovariectomized female rats. Menopause. 12:1317–1326. 2011.
View Article : Google Scholar
|
|
24
|
Aldini G, Yeum KJ, Niki E, et al:
Biomarkers for antioxidant defense and oxidative damage: Principles
and practical applications. Ames. Wiley-Blackwell. 2010.
|
|
25
|
Castelao JE and Gago-Dominguez M: Risk
factors for cardiovascular disease in women: Relationship to lipid
peroxidation and oxidative stress. Med Hypotheses. 71:39–44. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu X, Tang Z, Cong B, Du J, Wang C, Wang
L, Ni X and Lu J: Estrogens increase cystathionine-γ-lyase
expression and decrease inflammation and oxidative stress in the
myocardium of ovariectomized rats. Menopause. 20:1084–1091. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Z, Wang Y, Zhu X, Ni X and Lu J:
Exercise increases cystathionine-γ-lyase expression and decreases
the status of oxidative stress in myocardium of ovariectomized
rats. Int Heart J. 57:96–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liochev SI and Fridovich I: Arch Biochem
Biophys. 337:115–120. 1997.PubMed/NCBI
|
|
29
|
Wu HT, He XJ, Hong YK, Ma T, Xu YP and Li
HH: Chemical characterization of lycium barbarum polysaccharides
and its inhibition against liver oxidative injury of high-fat mice.
Int J Biol Macromol. 46:540–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aksakal E, Akaras N, Tanboga IH, Kurt M,
Halici Z, Odabasoglu F and Unal B: Relationship between oxidative
stress and cardiomyopathic changes in ovariectomized rats.
Cardiology. 119:235–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji H, Zheng W, Menini S, Pesce C, Kim J,
Wu X, Mulroney SE and Sandberg K: Female protection in progressive
renal disease is associated with estradiol attenuation of
superoxide production. Gend Med. 4:56–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Urata Y, Ihara Y, Murata H, Goto S, Koji
T, Yodoi J, Inoue S and Kondo T: 17Beta-estradiol protects against
oxidative stress-induced cell death through the
glutathione/glutaredoxin-dependent redox regulation of Akt in
myocardiac H9c2 cells. J Biol Chem. 281:13092–13102. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sawicka E and Długosz A: The role of
17β-estradiol metabolites in chromium-induced oxidative stress. Adv
Clin Exp Med. 26:215–221. 2017.PubMed/NCBI
|
|
34
|
Baeza I, Fdez-Tresguerres J, Ariznavarreta
C and De la Fuente M: Effects of growth hormone, melatonin,
oestrogens and phytoestrogens on the oxidized glutathione
(GSSG)/reduced glutathione (GSH) ratio and lipid peroxidation in
aged ovariectomized rats. Biogerontology. 11:687–701. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu SJ, Ng LT and Lin CC: Antioxidant
activities of some common ingredients of traditional Chinese
medicine, Angelica sinensis, Lycium barbarum and Poriacocos.
Phytother Res. 18:1008–1012. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo Q, Cai Y, Yan J, Sun M and Corke H:
Hypoglycemic and hypolipidemic effects and antioxidant activity of
fruit extracts from Lycium barbarum. Life Sci. 76:137–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Potterat O: Goji (Lycium barbarum and L.
Chinense): Phytochemistry, pharmacology and safety in the
perspective of traditional uses and recent popularity. Planta. Med.
76:7–19. 2010.
|
|
38
|
Li XM: Protective effect of Lycium
barbarum polysaccharides on streptozotocin-induced oxidative stress
in rats. Int J Biol Macromol. 40:461–465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo Q, Li Z, Huang X, Sun M and Corke H:
Lycium barbarum polysaccharides: Protective effects against
heat-induced damage of rat testes and
H2O2-induced DNA damage in mouse testicular
cells and beneficial effect on sexual behavior and reproductive
function of hemicastrated rats. Life Sci. 79:613–621. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martindale JL and Holbrook N: Cellular
response to oxidative stress: Signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saito Y, Nishio K, Ogawa Y, Kinumi T,
Yoshida Y, Masuo Y and Niki E: Molecular mechanisms of
6-hydroxydopamine-induced cytotoxicity in PC12 cells: Involvement
of hydrogen peroxide-dependent and -independent action. Free Radic
Biol Med. 42:675–685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujita H, Ogino T, Kobuchi H, Fujiwara T,
Yano H, Akiyama J, Utsumi K and Sasaki J: Cell-permeable cAMP
analog suppresses 6-hydroxydopamine-induced apoptosis in PC12 cells
through the activation of the Akt pathway. Brain Res 1113. 10–23.
2006. View Article : Google Scholar
|


















