Introduction
Prehypertension is the intermediate period prior to
the pathogenesis of hypertension, as proposed by the seventh report
of the Joint National Committee (JNC) in 2003 (1). It is defined as a systolic blood
pressure (SBP) between 120 and 139 mmHg, and/or a diastolic blood
pressure (DBP) between 80 and 89 mmHg. Prehypertension represents a
high risk of developing hypertension and a number of future
clinical outcomes, such as coronary artery disease, myocardial
infarction, early arteriosclerosis, chronic kidney disease and
heart failure (2–8). Schlaich et al (9) and Taddei et al (10) have demonstrated that endothelial
dysfunction is critical in the development of essential
hypertension. Similarly, the number of senescent endothelial
progenitor cells (EPCs) increased in prehypertensive subjects,
while the nitric oxide (NO) production is reduced (11). Bone marrow-derived circulating EPCs
are involved in the repair of the vascular endothelium, promotion
of neovascularization, restoration of endothelial impairment, and
amelioration of vascular endothelial function (12,13).
A weakened endothelial repair capability, caused by EPC
deactivation, may contribute to prehypertension-associated
cardiovascular events (11,14).
A lower morbidity of cardiovascular disease (CVD) is
observed in premenopausal females in comparison with that in
age-matched males; yet, an increase in the incidence of this
disease has been reported following menopause, which indicates that
estradiol may be a protective factor against CVD (15,16).
Seminal studies have revealed that, in postmenopausal females, the
increased incidence of CVD is associated with endothelial
dysfunction (17,18). Furthermore, apobiotic circulating
EPCs have been detected in prehypertensive patients, which are
closely associated with endothelial dysfunction (11,14).
In vitro, estrogen-treated EPCs exhibited higher migratory
and tube-forming capacities, whereas in vivo studies
indicated that estrogen was unable to affect the number of
circulating EPCs (19). Our
earlier study confirmed that highly active circulating EPCs were
maintained in prehypertensive premenopausal females (20). However, the number and activity of
circulating EPCs in prehypertensive postmenopausal females remain
undetermined, and the association between flow-mediated dilation
(FMD) and the activity of EPCs has yet to be examined.
Earlier studies have demonstrated that nitric oxide
(NO), vascular endothelial growth factor (VEGF) and
granulocyte-macrophage colony-stimulating factor (GM-CSF) modulate
the number and activity of circulating EPCs (21–24).
In our previous study (20), the
correlation of highly active circulating EPCs in prehypertensive
premenopausal females with NO production was confirmed.
Accordingly, the present study aimed to investigate the number and
the function of circulating EPCs in subjects with prehypertension
and normotension. Additionally, potential sex-associated
differences in EPC number and function were examined.
Herein, the levels of NO in the culture medium or
plasma, as well as the levels of VEGF and GM-CSF, were determined.
Finally, the study explored the association of circulating EPCs
with FMD.
Materials and methods
Study population
A total of 80 subjects were enrolled in the present
study, including 20 normotensive postmenopausal females, 20
prehypertensive postmenopausal females, 20 normotensive males and
20 prehypertensive males. The prehypertensive subjects exhibited an
SBP between 120 and 139 mmHg, and/or a DBP between 80 and 89 mmHg,
according to the guidelines established by the eighth report of the
JNC (25). All normotensive
subjects had an SBP of <120 mmHg and a DBP of <80 mmHg, and
presented no cardiovascular risk factors. The subjects recruited
into the present study did not suffer from CVD or metabolic
disease, as determined by assessment of their entire clinical
history and auxiliary examinations. In addition, subject with
conditions that may affect the number of EPCs, such as diabetes
mellitus, malignant disease, uncontrolled infection and smoking,
were excluded. Subjects with previous hysterectomy were also
excluded. All subjects were recruited between January 2016 and
January 2017, from Xiangya Hospital, Central South University
(Changsha, China), and written informed consent was provided for
participation. The experimental protocol was approved by The
Ethical committee of Xiangya Hospital (ethical license ID of human
clinical trial, no. 201503377). The baseline clinical data of the
subjects, which were divided into four groups, are listed in
Table I.
 | Table I.Clinical and biochemical
characteristics of study participants (n=80). |
Table I.
Clinical and biochemical
characteristics of study participants (n=80).
| Characteristic | Normotensive
postmenopausal females (n=20) | Prehypertensive
postmenopausal females (n=20) | Normotensive males
(n=20) | Prehypertensive
males (n=20) |
|---|
| Age (years) | 58.8±2.7 | 57.5±2.8 | 59.2±3.6 | 58.5±3.9 |
| Height (cm) | 158.9±5.5 | 157.9±5.5 |
165.8±5.6b |
167.2±4.7b |
| Weight (kg) | 58.5±3.8 | 59.0±5.9 |
63.0±5.9b |
66.8±8.5b |
| BMI
(kg/cm2) | 23.2±1.8 | 23.6±1.7 | 22.9±1.6 | 23.8±2.5 |
| SBP (mmHg) | 108.2±7.2 |
132.1±4.7a | 109.4±6.1 |
130.1±5.5a |
| DBP (mmHg) | 67.4±4.5 |
82.1±4.4a | 68.8±4.8 |
80.0±4.0a |
| HR (beats/min) | 75.7±8.8 | 73.5±6.3 | 76.7±8.9 | 77.2±7.4 |
| AST (mmol/l) | 21.9±5.3 | 22.5±6.1 | 23.2±5.7 | 24.1±5.2 |
| ALT (mmol/l) | 20.6±5.9 | 20.8±4.7 | 21.2±6.3 | 23.6±5.6 |
| BUN (mmol/l) | 5.5±0.7 | 5.2±1.0 | 5.4±1.0 | 5.5±0.7 |
| Cr (mmol/l) | 68.1±11.5 | 65.9±11.9 | 70.6±11.5 | 71.9±11.8 |
| LDL (mmol/l) | 2.72±0.40 | 2.67±0.43 | 2.57±0.39 | 2.74±0.37 |
| TC (mmol/l) | 4.55±0.67 | 4.46±0.54 | 4.33±0.54 | 4.61±0.53 |
| HDL (mmol/l) | 1.48±0.22 | 1.50±0.22 | 1.52±0.20 | 1.45±0.17 |
| TG (mmol/l) | 1.37±0.19 | 1.35±0.20 | 1.31±0.16 | 1.38±0.18 |
| FPG (mmol/l) | 4.73±0.50 | 4.60±0.47 | 4.45±0.53 | 4.63±0.44 |
| FMD (%) | 7.54±1.77 |
5.72±1.28a |
7.49±1.55b |
5.38±1.46a,b |
Clinical measurements
Serum samples were collected in the morning after
overnight fasting from the study population (n=80; 40 females and
40 males), and the levels of EPCs, total cholesterol (TC),
high-density lipoprotein cholesterol (HDL), low-density lipoprotein
cholesterol (LDL), triglycerides (TG) and estradiol were measured.
All subjects avoided ingesting alcohol or caffeine for half a day
prior to specimen collection. Subjects receiving drug treatments,
including antiplatelet agents, anti-inflammatory drugs and
hypolipidemic agents, were excluded from the study to reduce
potential external effects on the number and activity of
circulating EPCs.
EPC isolation, culture and flow
cytometric analysis
The number of EPCs in the serum was tested according
to previous studies (20,24,26–28).
Briefly, Ficoll density gradient centrifugation was used to isolate
the peripheral blood mononuclear cells, and then cells were
suspended in Endothelial Cell Growth Medium 2 (500 ml; Lonza Group,
Ltd., Basel, Switzerland) supplemented with 2% fetal bovine serum
(FBS; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Next, the
cell suspension (2.5×106/ml) was transferred into
25-cm2 cell culture flasks (Corning, Inc., Corning, NY,
USA), coated with fibronectin (Clonetics Corporation, San Diego,
CA, USA), and incubated at 37°C in a humidified atmosphere
containing 5% CO2. After 4 days, nonadherent cells were
discarded, while adherent cells were maintained for a further 7
days, and these cells were then utilized in subsequent
experiments.
After the 7-day culture, endothelial marker proteins
were assessed by flow cytometry. Cell suspension (100 µl) was
incubated for 40 min at 4°C with the following primary antibodies:
Fluorescein isothiocyanate (FITC) anti-human CD45 (1:10; cat. no.
FHF045-025; 4A Biotech, Co., Ltd., Beijing, China), phycoerythrin
(PE)-Cy7 anti-human CD34 (1:10; cat. no. FHN034-025; 4A Biotech,
Co., Ltd.) and PE-conjugated anti-kinase-insert domain receptor
(KDR; 1:20; cat. No. FHK309-025; 4A Biotech, Co., Ltd.). Following
erythrocyte lysis, the remaining cells were washed with
phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde
for 10 min at 37°C. Flow cytometric analysis was conducted with an
ACEA NovoCyte™ flow cytometer (ACEA Biosciences, San Diego, CA,
USA), and the results were analyzed with NovoExpress software™
(ACEA Biosciences). The number of circulating EPCs was determined
according to the ratio of
CD45−CD34+KDR+ cells per 100
peripheral blood mononuclear cells.
In order to determine the EPC phenotype, mononuclear
cells (2.5×106/ml) were plated on cell culture flasks
with endothelial cell growth medium (EGM™−2; Lonza
Group, Ltd., Basel, Switzerland). Following 7 days of culturing,
the attached endothelial cell-like cells were incubated with
DiI-labeled acetylated LDL (Molecular Probes; Thermo Fisher
Scientific, Inc.) for 1 h at 37°C. Subsequent to fixing in 4%
paraformaldehyde for 30 min at 37°C, the cells were incubated with
FITC-labeled lectin (Sigma-Aldrich; Merck KGaA) for 4 h at 37°C.
Following incubation with FITC-labeled lectin, the samples were
observed under a phase-contrast fluorescence microscope
(magnification, ×200). Cells presenting double-positive
fluorescence were identified as differentiating EPCs by two
independent researchers blinded to the study groups.
EPC migration and proliferation
assay
The EPC migration and proliferation assays were
conducted as previously described (20,24,27–29).
In order to determine the cell proliferation, EPCs were harvested
by centrifugation at 438 × g for 5 min at 4°C and
resuspended in 500 µl EGM-2. A total of 2×104 EPCs/well
were added into the upper chamber of a modified Boyden chamber
(24-well Costar Transwell plate; pore size, 8 µm; Corning, Inc.),
while 500 µl EGM-2 supplemented with 50 ng/ml VEGF was added to the
lower chamber. Following incubation at 37°C for 24 h, the lower
side of the filter was washed with PBS and fixed with 2%
paraformaldehyde for 10 min at 37°C. Cell nuclei were then stained
with DAPI. Cells that had migrated into the lower chamber were
manually counted in three random fields under a fluorescence
microscope by two independent blinded investigators.
EPC proliferation was determined using an MTT assay.
Briefly, EPCs were digested with 0.25% trypsin and then cultured in
serum-free medium in a 96-well culture plate (2,000 cells/well) for
24 h. Next, the EPCs were supplemented with 10 µl MTT (5 g/l;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and incubated for a
further 4 h. The supernatant was then aspirated and discarded.
Subsequent to mixing the EPC preparation with 200 µl dimethyl
sulfoxide by shaking for 10 min, the optical density value at 490
nm was measured (20,24,28,29).
Detection of plasma NO, VEGF and
GM-CSF levels
The Griess method was used to determine the inactive
metabolite of NO in the plasma, as reported in earlier studies
(20,24), and the results are expressed as
µmol NOx of NO3-/NO2- per liter of
medium. In addition, in order to measure the levels of VEGF and
GM-CSF in the plasma, high-sensitivity enzyme-linked immunosorbent
assay (ELISA; R&D Systems, Inc., Wiesbaden, Germany) was
conducted according to the manufacturer's protocol.
Detection of NO, VEGF and GM-CSF
secretion by EPCs
EPCs were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
20% FBS (Sigma-Aldrich; Merck KGaA) for 48 h. Subsequently, in
order to measure the levels of NO, VEGF and GM-CSF secretion in the
conditioned media of EPCs, the Griess method and ELISA assays were
conducted in accordance with the aforementioned protocol.
FMD
The assessment of FMD was performed as described in
previous studies (30,31). The brachial artery FMD was assessed
by a trained investigator with high-resolution ultrasonography
using a 5–12-MHz linear transducer on an HDI 5000 system (Philips
Medical Systems, Inc., Bothell, WA, USA). After a 15-min rest, the
brachial artery was studied at 20–100 mm proximal to the
antecubital fossa in supine position. An upper-forearm
sphygmomanometer cuff was inflated to raise the pressure to 250
mmHg, and this was maintained for 5 min. The FMD was then
calculated as the percentage of increase in the mean diastolic
diameter reactive to hyperemia at 55–65 sec after deflation to
baseline. After a further 15 min, 400 µg sublingual glyceryl
trinitrate was administered, and the diastolic diameter was
measured again after 5 min to determine the endothelial independent
dilation.
Statistical analysis
All data were analyzed using the SPSS statistical
software package, version 11.0 (SPSS, Inc., Chicago, IL, USA), and
are presented as the mean ± standard deviation. Two-way analysis of
variance was used to compare between the four groups. When a
significant F-value was observed, the Newman-Keuls method was
applied as a post hoc test to identify differences among mean
values. Univariate correlations were examined by Pearson's
correlation coefficient method. A P-value of <0.05 was
considered to denote a difference that was statistically
significant.
Results
Clinical baseline characteristics
The essential characteristics of the study
population are summarized in Table
I. No marked differences were observed with regard to the
patient age and body mass index among the subjects. The SBP and DBP
values were markedly lower in normotensive males and normotensive
postmenopausal females as compared with those in prehypertensive
subjects of the same sex, respectively. However, the levels of TC,
HDL, LDL, TG and plasma glucose were similar among the four groups.
Furthermore, FMD in prehypertensive postmenopausal females was
significantly lower in comparison with that in normotensive
postmenopausal females. Similarly, FMD was lower in prehypertensive
males as compared with that in normotensive males. Additionally,
FMD in postmenopausal females was increased compared with male
subjects, regardless of the level of blood pressure.
Number and activity of circulating
EPCs
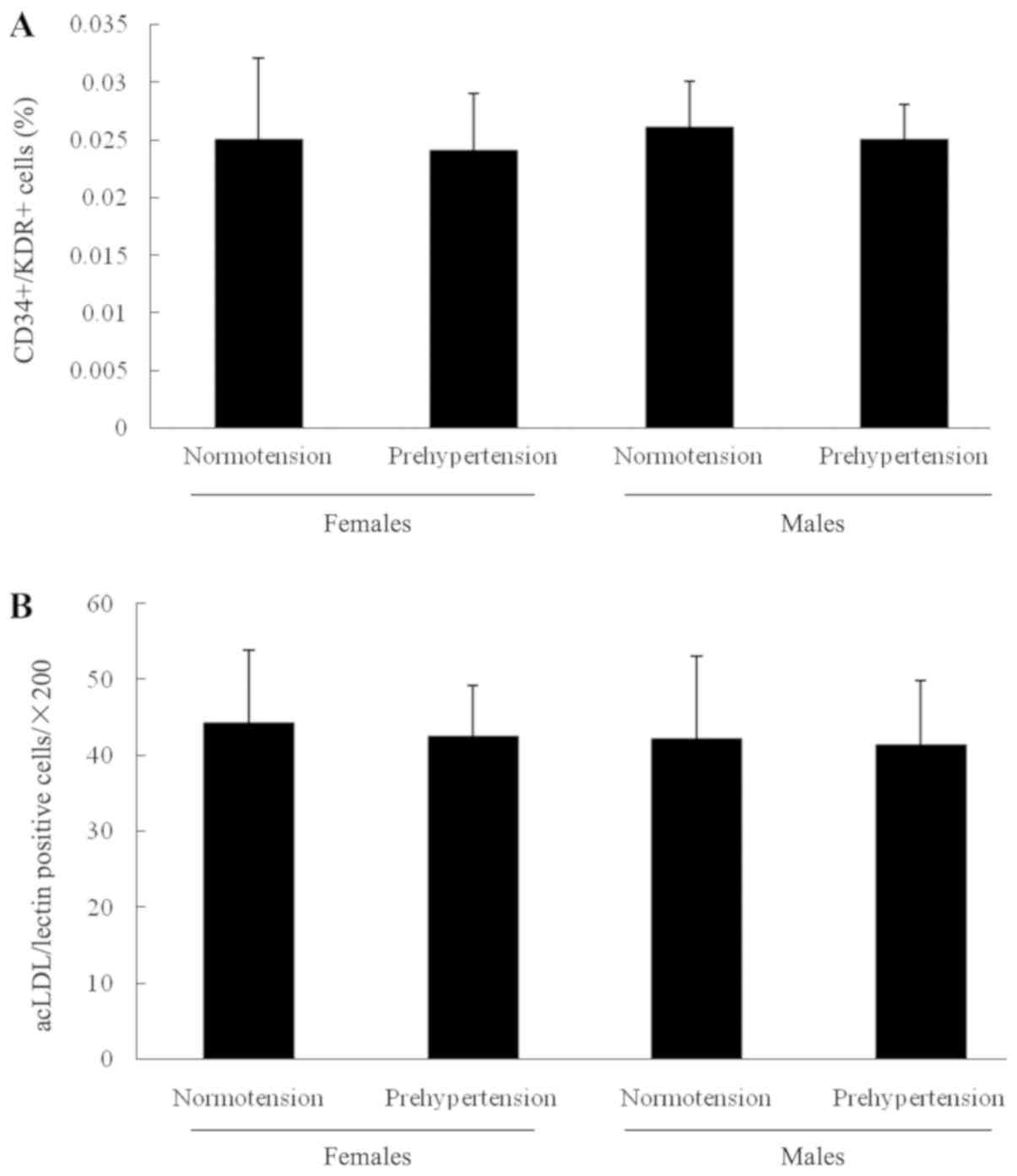
To determine whether the circulating EPC was
associated with prehypertension, their number and activity was
determined. The results shown in Fig.
1 indicated that there was no significant difference in
circulating EPCs between normotensive and prehypertensive
postmenopausal females, or between normotensive and prehypertensive
males. In addition, no notable difference was detected in
circulating EPCs between normotensive males and females
(postmenopausal), or between prehypertensive males and females
(postmenopausal). Furthermore, the number of EPCs determined by the
cell culture assay exhibited no significant difference among the
four groups.
The migration and proliferation of EPCs were then
examined, and the results are shown in Fig. 2. As compared with normotensive
postmenopausal females or males, the migration of EPCs was
attenuated in the prehypertensive postmenopausal females and males,
respectively. Similarly, the proliferation of EPCs in normotensive
subjects was significantly higher compared with that of
prehypertensive subjects in the same sex group, respectively.
However, no marked difference in migration and proliferation of
circulating EPCs was observed between normotensive males and
females, or between prehypertensive males and females.
Plasma levels of NO, VEGF and
GM-CSF
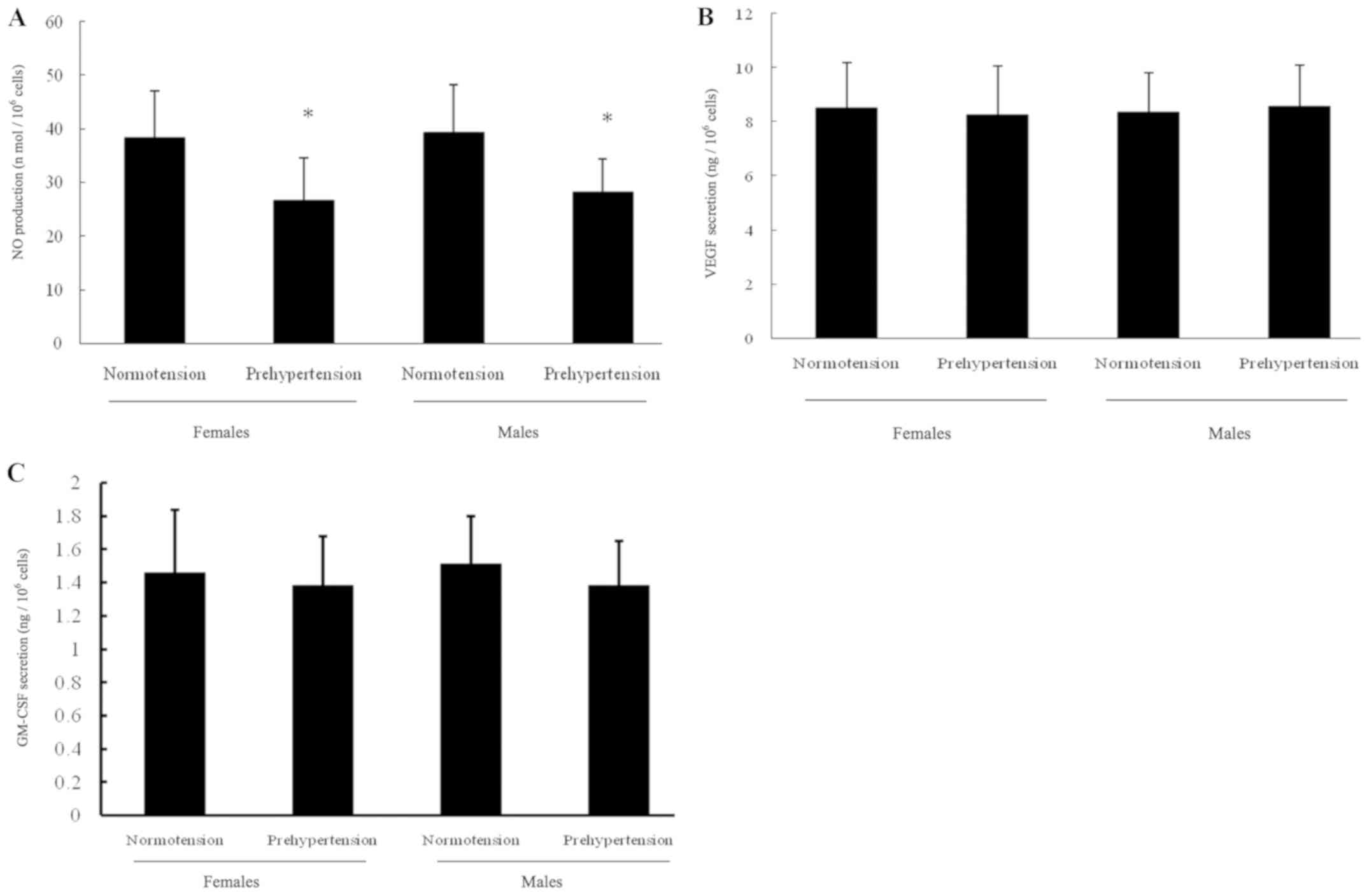
To investigate the mechanism leading to differences
in the activity of circulating EPCs, the levels of certain factors
present in the plasma influencing the function of EPCs were
examined. The levels of NO, VEGF and GM-CSF detected in the plasma
of the four groups are displayed in Fig. 3. As shown in Fig. 3A, the plasma NO level in
postmenopausal females with prehypertension or prehypertensive
males was significantly lower compared with that in normotensive
postmenopausal females or normotensive males, respectively.
However, the plasma level of NO presented no notable difference
between the two prehypertensive males or postmenopausal females,
and between the two normotensive groups. As exhibited in Fig. 3B, the plasma VEGF level did not
significantly differ in any of the four groups. Similar to VEGF,
the plasma GM-CSF level shown in Fig.
3C exhibited no evident difference among the four groups.
NO, VEGF and GM-CSF secretion by
EPCs
As displayed in Fig.
4, the secretion of NO, VEGF and GM-CSF by EPCs among the four
groups was consistent with their distribution in the plasma. In
brief, NO production in prehypertensive postmenopausal females
declined in comparison with that in normotensive postmenopausal
females. Similar results were observed when comparing
prehypertensive with normotensive males. By contrast, VEGF and
GM-CSF secretion by EPCs did not differ among the four groups.
Correlations of circulating EPC
function and NO level with FMD
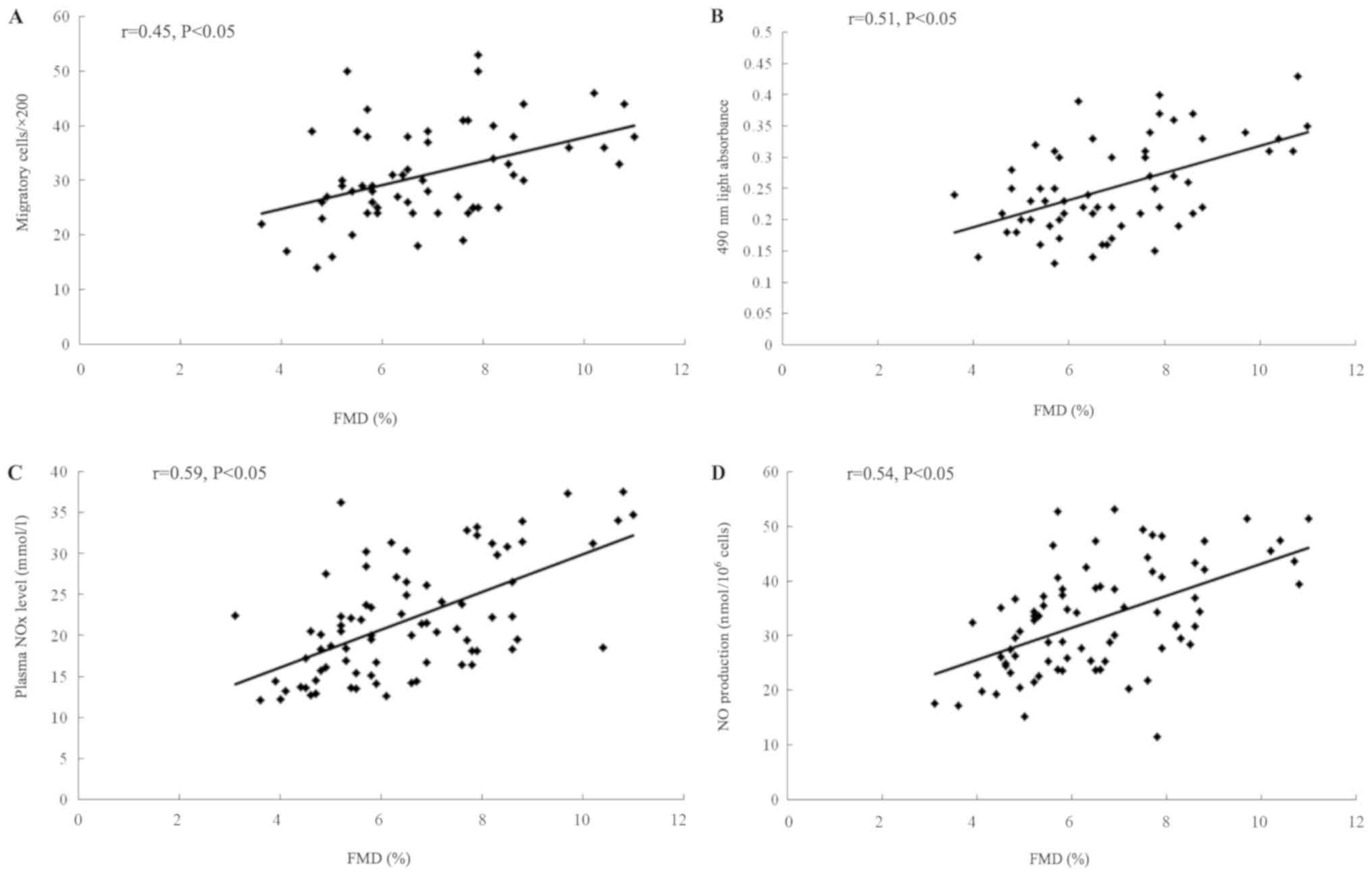
The study then sought to explore the correlation of
FMD with the NO level or circulating EPCs. As exhibited in Fig. 5A, a strong univariate correlation
between FMD and the migratory activity of circulating EPCs was
ascertained. Similarly, as shown in Fig. 5B, the FMD enhancement was
concordant with EPC proliferation promotion. As presented in
Fig. 5C and D, the FMD
significantly increased with the increase in plasma NOx level and
the NO production secreted by EPCs.
Discussion
In the present study, it was demonstrated that the
proliferation and the migration of EPCs were decreased in
prehypertensive postmenopausal females relative to the normotensive
postmenopausal females, although there was no significant change in
the number of EPCs. This phenomenon was also observed between
normotensive and prehypertensive males. The data also verified that
the NO level in the plasma and NO secretion by EPCs declined in
both prehypertensive postmenopausal females and age-matched males,
as compared with the corresponding normotensive groups. This
indicated that circulating NO level and the NO secretion function
of EPCs may be connected to pathophysiological processes of
prehypertension. In addition, the positive correlation of FMD with
both circulating EPC function (migration and proliferation) and NO
production was confirmed. These results are concordant with the
findings of our previous study (20). The previous study reported that an
increased number and activity of EPCs in premenopausal females in
comparison with age-matched males. However, in the present study,
there was no evidence that the activity and number of EPCs differed
between prehypertensive males and prehypertensive postmenopausal
females, which implied that prehypertensive postmenopausal females
may have the same cardiovascular risk as age-matched males. Thus,
it can be inferred that, as females enter menopause, the EPC
function may decrease, which may result in an increase in the
cardiovascular risk owing to estradiol decline.
In patients with prehypertension, the migratory and
proliferative abilities of circulating EPCs are attenuated,
suggesting that EPCs are involved in the pathogenesis of
prehypertension through affecting endothelial repair (11,14).
It is widely accepted that prehypertensive premenopausal females
have more active circulating EPCs in comparison with age-matched
males, indicating that sex difference may serve a dominant role in
prehypertension-associated endothelial dysfunction at a young age
(32–35). Our previous study has demonstrated
the improved activity of circulating EPCs in prehypertensive
premenopausal females as compared with that in young males, which
was associated with higher NO levels in the plasma and NO
production by circulating EPCs (20). There is a rational hypothesis is
that estradiol may be involved in vascular protection through
regulating EPC function. In the present study, attenuated activity
of EPCs was observed in prehypertensive postmenopausal females,
which appeared to disagree with the findings of our previous study
in premenopausal females (20).
Furthermore, the alterations in EPC function between
prehypertensive and normotensive subjects were detected in males
and females. The present results revealed that vascular dysfunction
in prehypertensive postmenopausal females may be linked to
decreasing EPC-mediated endogenous endothelial repair capacity
caused by the decrease in the estradiol-protective effects against
CVD.
Numerous studies have indicated that NO, VEGF and
GM-CSF can modulate the amount and activity of circulating EPCs
(21–24). Endogenous NO biosynthesis has a
significant effect on the biological function of EPCs (36). The modulation of NADPH oxidase 2 in
human EPCs can restore its physiological function and properties
(37). During prehypertension, NO
production by early EPCs, which is associated with EPC-mediated
endothelial repair capacity (38,39),
is markedly decreased (11). In
our previous study, it was confirmed that the preserved NO
production was associated with elevated circulating EPCs in
prehypertensive premenopausal females, rather than VEGF and GM-CSF
(20). In the present study, it
was further demonstrated that the NO level and NO production in
prehypertensive postmenopausal females were similar to those of
prehypertensive age-matched males. Furthermore, the VEGF or GM-CSF
levels did not differ among the groups, suggesting that the
attenuated EPC function in prehypertensive postmenopausal females
was independent of alterations in VEGF and GM-CSF levels.
The mechanism of lower NO secretion by EPCs has yet
to be defined. However, estrogen may account for the difference in
endothelial NO release between males and females (40–42),
which may promote NO production via the upregulation of endothelial
NO synthase (eNOS) expression, protection against destabilization
of eNOS mRNA (43), exertion of
antioxidant effects (44),
activation of the PI3K/Akt pathway (45), and upregulation of Mas receptor
(46). In addition, it is presumed
that the mechanism may be involved in eNOS expression, which should
be discussed in further studies. Apparent discrepancies in EPC
function between prehypertensive postmenopausal females and
prehypertensive premenopausal females may be due to the pivotal
role of estradiol in EPC function protection. Along with the
results of previous studies, it can be concluded that the gender
difference in EPC function disappears when females enter
post-menopause. Furthermore, it is considered that EPC-mediated
endothelial repair capacity and endothelium-dependent dilation are
impaired in postmenopausal females as a result of the decrease in
the estradiol-protective effects against CVD. Thus, the
perimenopausal period may be critical for early intervention in
prehypertension therapy.
In conclusion, the current study demonstrated that
the gender difference in endothelial function and circulating EPCs
disappeared in prehypertensive postmenopausal females, which may be
associated with the decline in NO production. The attenuated
endogenous endothelial repair capacity partly elucidates the
decreased endothelial protection in postmenopause. The present
findings may provide novel targets for rescuing endothelial
dysfunction accompanied by postmenopause and prehypertension.
Acknowledgements
Not applicable.
Funding
The study was financially supported by grants from
the National Natural Scientific Foundation of China (nos.
81570461).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL designed the project. WW, DH, ZW and LL performed
the experiments, and collected, analyzed and interpreted the data,
as well as generated the figures. WW, LL and ZW wrote the
manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by The
Ethical committee of Xiangya Hospital (ethical license ID of human
clinical trial, no. 201503377). Written informed consent was
provided for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chobanian AV, Bakris GL, Black HR, Cushman
WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright
JT Jr, et al: The seventh report of the joint national committee on
prevention, detection, evaluation, and treatment of high blood
pressure: The JNC 7 report. JAMA. 289:2560–2572. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xue H, Wang J, Hou J, Li J, Gao J, Chen S,
Zhu H and Wu S: Prehypertension and chronic kidney disease in
chinese population: Four-year follow-up study. PLoS One.
10:e01444382015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bajpai JK, A P S, A K A, A K D, Garg B and
Goel A: Impact of prehypertension on left ventricular structure,
function and geometry. J Clin Diagn Res. 8:BC07–BC010.
2014.PubMed/NCBI
|
|
4
|
Navarro-Gonzalez JF, Mora C, Muros M,
Garcia J, Donate J and Cazana V: Relationship between inflammation
and microalbuminuria in prehypertension. J Hum Hypertens.
27:119–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Celik T, Yuksel UC, Fici F, Celik M, Yaman
H, Kilic S, Iyisoy A, Dell'oro R, Grassi G, Yokusoglu M and Mancia
G: Vascular inflammation and aortic stiffness relate to early left
ventricular diastolic dysfunction in prehypertension. Blood Press.
22:94–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang
H and Xu D: Prehypertension and incidence of cardiovascular
disease: A meta-analysis. BMC Med. 11:1772013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasan RS, Larson MG, Leip EP, Evans JC,
O'Donnell CJ, Kannel WB and Levy D: Impact of high-normal blood
pressure on the risk of cardiovascular disease. N Engl J Med.
345:1291–1297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasan RS, Larson MG, Leip EP, Kannel WB
and Levy D: Assessment of frequency of progression to hypertension
in non-hypertensive participants in the framingham heart study: A
cohort study. Lancet. 358:1682–1686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schlaich MP, Parnell MM, Ahlers BA, Finch
S, Marshall T, Zhang WZ and Kaye DM: Impaired L-arginine transport
and endothelial function in hypertensive and genetically
predisposed normotensive subjects. Circulation. 110:3680–3686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taddei S, Virdis A, Mattei P, Ghiadoni L,
Sudano I and Salvetti A: Defective L-arginine-nitric oxide pathway
in offspring of essential hypertensive patients. Circulation.
94:1298–1303. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giannotti G, Doerries C, Mocharla PS,
Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA,
Steenken N, Manes C, et al: Impaired endothelial repair capacity of
early endothelial progenitor cells in prehypertension: Relation to
endothelial dysfunction. Hypertension. 55:1389–1397. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aicher A, Zeiher AM and Dimmeler S:
Mobilizing endothelial progenitor cells. Hypertension. 45:321–325.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
MacEneaney OJ, DeSouza CA, Weil BR,
Kushner EJ, Van Guilder GP, Mestek ML, Greiner JJ and Stauffer BL:
Prehypertension and endothelial progenitor cell function. J Hum
Hypertens. 25:57–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moreau KL, Hildreth KL, Meditz AL, Deane
KD and Kohrt WM: Endothelial function is impaired across the stages
of the menopause transition in healthy women. J Clin Endocrinol
Metab. 97:4692–4700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gavin KM, Seals DR, Silver AE and Moreau
KL: Vascular endothelial estrogen receptor alpha is modulated by
estrogen status and related to endothelial function and endothelial
nitric oxide synthase in healthy women. J Clin Endocrinol Metab.
94:3513–3520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denton KM, Hilliard LM and Tare M:
Sex-related differences in hypertension: Seek and ye shall find.
Hypertension. 62:674–677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taddei S, Virdis A, Ghiadoni L, Mattei P,
Sudano I, Bernini G, Pinto S and Salvetti A: Menopause is
associated with endothelial dysfunction in women. Hypertension.
28:576–582. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rudzitis-Auth J, Nenicu A, Nickels RM,
Menger MD and Laschke MW: Estrogen stimulates homing of endothelial
progenitor cells to endometriotic lesions. Am J Pathol.
186:2129–2142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhen Y, Xiao S, Ren Z, Shen HW, Su H, Tang
YB and Zeng H: Increased endothelial progenitor cells and nitric
oxide in young prehypertensive women. J Clin Hypertens (Greenwich).
17:298–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duda DG, Fukumura D and Jain RK: Role of
eNOS in neovascularization: NO for endothelial progenitor cells.
Trends Mol Med. 10:143–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Powell TM, Paul JD, Hill JM, Thompson M,
Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, et
al: Granulocyte colony-stimulating factor mobilizes functional
endothelial progenitor cells in patients with coronary artery
disease. Arterioscler Thromb Vasc Biol. 25:296–301. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Wang JM, Chen L, Luo CF, Tang AL
and Tao J: Acute exercise-induced nitric oxide production
contributes to upregulation of circulating endothelial progenitor
cells in healthy subjects. J Hum Hypertens. 21:452–460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
James PA, Oparil S, Carter BL, Cushman WC,
Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML,
MacKenzie TD, Ogedegbe O, et al: 2014 evidence-based guideline for
the management of high blood pressure in adults: Report from the
panel members appointed to the Eighth Joint National Committee (JNC
8). JAMA. 311:507–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Z, Chen L, Su C, Xia WH, Wang Y, Wang
JM, Chen F, Zhang YY, Wu F, Xu SY, et al: Impaired endothelial
progenitor cell activity is associated with reduced arterial
elasticity in patients with essential hypertension. Clin Exp
Hypertens. 32:444–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Z, Xia WH, Su C, Wu F, Zhang YY, Xu
SY, Liu X, Zhang XY, Ou ZJ, Lai GH, et al: Regular exercise-induced
increased number and activity of circulating endothelial progenitor
cells attenuates age-related decline in arterial elasticity in
healthy men. Int J Cardiol. 165:247–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Xia WH, Zhang YY, Xu SY, Liu X,
Zhang XY, Yu BB, Qiu YX and Tao J: Shear stress-induced activation
of Tie2-dependent signaling pathway enhances reendothelialization
capacity of early endothelial progenitor cells. J Mol Cell Cardiol.
52:1155–1163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corretti MC, Anderson TJ, Benjamin EJ,
Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H,
Gerhard-Herman M, Herrington D, et al: Guidelines for the
ultrasound assessment of endothelial-dependent flow-mediated
vasodilation of the brachial artery: A report of the international
brachial artery reactivity task force. J Am Coll Cardiol.
39:257–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sibal L, Aldibbiat A, Agarwal SC, Mitchell
G, Oates C, Razvi S, Weaver JU, Shaw JA and Home PD: Circulating
endothelial progenitor cells, endothelial function, carotid
intima-media thickness and circulating markers of endothelial
dysfunction in people with type 1 diabetes without macrovascular
disease or microalbuminuria. Diabetologia. 52:1464–1473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lemieux C, Cloutier I and Tanguay JF:
Menstrual cycle influences endothelial progenitor cell regulation:
A link to gender differences in vascular protection? Int J Cardiol.
136:200–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoetzer GL, MacEneaney OJ, Irmiger HM,
Keith R, Van Guilder GP, Stauffer BL and DeSouza CA: Gender
differences in circulating endothelial progenitor cell
colony-forming capacity and migratory activity in middle-aged
adults. Am J Cardiol. 99:46–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rousseau A, Ayoubi F, Deveaux C, Charbit
B, Delmau C, Christin-Maitre S, Jaillon P, Uzan G and Simon T:
Impact of age and gender interaction on circulating endothelial
progenitor cells in healthy subjects. Fertil Steril. 93:843–846.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fadini GP, de Kreutzenberg S, Albiero M,
Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M,
Nardelli GB, et al: Gender differences in endothelial progenitor
cells and cardiovascular risk profile: The role of female
estrogens. Arterioscler Thromb Vasc Biol. 28:997–1004. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aicher A, Heeschen C and Dimmeler S: The
role of NOS3 in stem cell mobilization. Trends Mol Med. 10:421–425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Falco E, Carnevale R, Pagano F,
Chimenti I, Fianchini L, Bordin A, Siciliano C, Monticolo R,
Equitani F, Carrizzo A, et al: Role of NOX2 in mediating
doxorubicin-induced senescence in human endothelial progenitor
cells. Mech Ageing Dev. 159:37–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aicher A, Heeschen C, Mildner-Rihm C,
Urbich C, Ihling C, Technau-Ihling K, Zeiher AM and Dimmeler S:
Essential role of endothelial nitric oxide synthase for
mobilization of stem and progenitor cells. Nat Med. 9:1370–1376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorrentino SA, Bahlmann FH, Besler C,
Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A,
Limbourg F, et al: Oxidant stress impairs in vivo
reendothelialization capacity of endothelial progenitor cells from
patients with type 2 diabetes mellitus: Restoration by the
peroxisome proliferator-activated receptor-gamma agonist
rosiglitazone. Circulation. 116:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Darkow DJ, Lu L and White RE: Estrogen
relaxation of coronary artery smooth muscle is mediated by nitric
oxide and cGMP. Am J Physiol. 272:H2765–H2773. 1997.PubMed/NCBI
|
|
41
|
Thompson J and Khalil RA: Gender
differences in the regulation of vascular tone. Clin Exp Pharmacol
Physiol. 30:1–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iwakura A, Luedemann C, Shastry S, Hanley
A, Kearney M, Aikawa R, Isner JM, Asahara T and Losordo DW:
Estrogen-mediated, endothelial nitric oxide synthase-dependent
mobilization of bone marrow-derived endothelial progenitor cells
contributes to reendothelialization after arterial injury.
Circulation. 108:3115–3121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sumi D, Hayashi T, Jayachandran M and
Iguchi A: Estrogen prevents destabilization of endothelial nitric
oxide synthase mRNA induced by tumor necrosis factor alpha through
estrogen receptor mediated system. Life Sci. 69:1651–1660. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wagner AH, Schroeter MR and Hecker M:
17beta-estradiol inhibition of NADPH oxidase expression in human
endothelial cells. FASEB J. 15:2121–2130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hohmann N, Xia N, Steinkamp-Fenske K,
Forstermann U and Li H: Estrogen receptor signaling and the
PI3K/Akt pathway are involved in betulinic acid-induced eNOS
activation. Molecules. 21:E9732016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sobrino A, Vallejo S, Novella S,
Lázaro-Franco M, Mompeón A, Bueno-Betí C, Walther T, Sánchez-Ferrer
C, Peiró C and Hermenegildo C: Mas receptor is involved in the
estrogen-receptor induced nitric oxide-dependent vasorelaxation.
Biochem Pharmacol. 129:67–72. 2017. View Article : Google Scholar : PubMed/NCBI
|