Introduction
Complete atrioventricular (AV) conduction block, a
significant complication of numerous manifestations of heart
disease, is a serious threat to human health. Biological pacemakers
are of particular interest in cardiac conduction studies. Recent
studies (1,2) have focused on the use of cell
transplants and gene transfer for AV conduction block therapy.
However, neither gene transfer nor cellular transplantation have
been able to reproduce the normal function of cardiac conduction.
These methods only provide palliation of complete heart block. In
particular, transgene expression is transient, and the cells do not
remain at the desired injection site. Engineered conduction tissues
(ECTs) are biological conduction tissues fabricated in
vitro. Compared with the aforementioned methods, ECTs allow for
more precisely targeted and reliable regenerative therapy.
Therefore, the transplantation of ECTs may be useful for the
treatment of AV conduction block. However, there is very little
published information regarding the effectiveness of ECTs in
vivo.
In 2006, Choi et al (3) used a tissue engineering approach to
fabricate biocompatible, three-dimensional, collagen-based
constructs that contained fetal rat myoblasts. These experiments
provided proof of the principle that engineered tissue constructs
are able to function as an electrical conduit and may offer a
substitute treatment for conventional pacing therapy. Subsequently,
Hou et al (4) demonstrated
that anastomosis of the right auricle and right ventricle assisted
by MSCs may be a future treatment for patients with complete AV
block. Although the aforementioned studies created engineered
tissue constructs as electrical conduits for AV conduction block
therapy, the tissue constructs did not develop into cardiac
conduction tissue or produce a physiological effect in vivo.
In the two studies, the tissue constructs were used only as a
method to deliver cells abundantly and conveniently to the heart.
Therefore, little is currently known regarding the role that an
engineered conduction tissue may serve in vivo.
ECTs may be fabricated by seeding the appropriate
cells into scaffolds in vitro. Previously, we reported that
Nkx2.5+ cardiac progenitor cells (CPCs) derived from
embryonic heart tubes were able to differentiate into cardiac
cells, including cardiomyocytes, pacemaker cells and endothelial
cells (5–7). In the present study, ECTs were
created by seeding CPCs into a collagen sponge in order to
investigate the feasibility that engineered conduction tissue could
restore the normal rhythm of the heart in rats with
atrioventricular block.
Materials and methods
Engineered conduction tissues were
fabricated using rat CPCs
All the experimental procedures were conducted in
accordance with the institutional guidelines for the care and use
of laboratory animals of the Second Military Medical University
(Shanghai, China) and conformed to the National Institutes of
Health Guide for the Care and Use of Laboratory Animals. The
methods used for culturing Nkx2.5+ CPCs were as
previously described (5,6). In brief, at embryonic day 11 heart
tubes were dissected from a total of 27 female SD rats (14 weeks
old, 270–320 g). The rats were obtained from the laboratory animal
center of Second Military Medical University in Shanghai and housed
in a room at 18–26°C, with 40–70% relative humidity, and a 12-h
light/dark cycle. The animals had free access to food and water.
The heart tubes were disaggregated in trypsin 0.25% EDTA (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were
pelleted by centrifugation at 400 × g for 5 min at room
temperature, resuspended in culture medium with a 105/ml
cell density and seeded in 12-well cell culture plates (Corning
Incorporated, Corning, NY, USA). The medium used was Dulbecco's
modified Eagle's medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), supplemented with 15% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 20 µg/l EGF (R&D Systems,
Inc., Minneapolis, MN, USA), 106 U/l LIF (Chemicon
International, Inc.; EMD Millipore, Billerica, MA, USA), 0.375%
NaHCO3, 1% penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) and 2 mM L-glutamine (Amresco, LLC, Solon, OH,
USA). After 5 or 7 days of primary culture, the cells were isolated
using 0.25% trypsin for further experiments. The Nkx2.5+
CPCs were identified and selected as described previously (5,6).
The CPCs were labeled with 10 µg/ml CM-Dil
(Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min at 37°C and
15 min at 4°C. The ECTs were fabricated by seeding the CPCs in a
collagen sponge (Wuxi Biot Bio-technology, Co., Ltd., Wuxi, China).
A CPC suspension with a density of 107/ml was added to
the bottom of the 6-well culture plate. The collagen sponge was
placed in the cell suspension. After the cell suspension was
absorbed by the sponge, the sponge was inverted so that the
internal porosity of the sponge was completely filled with the cell
suspension. The constructs were transferred into an incubator
maintained at 37°C in 95% humidity and 5% CO2 for 3 h.
Subsequently, the base medium with 10 µg/l bFGF (R&D Systems,
Inc.) was added to the 6-well culture plate to immerse the
constructs; the medium was changed every 3 days, and the cells were
cultured for 2 weeks. The base medium contained DMEM supplemented
with 15% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 0.375% NaHCO3, 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) and 2 mM L-glutamine (Amresco,
LLC). The control groups were blank collagen sponges (BCSs) not
seeded with CPCs. After 14 days, the ECTs and BCSs were harvested
and used for subsequent implantation.
In vivo implantation in the rat
atrioventricular junction area
A total of 70 male SD rats (18 weeks old) weighing
between 350 and 400 g were prepared for surgery. The rats were
obtained from the laboratory animal center of Second Military
Medical University in Shanghai and housed in a room at 18–26°C,
with 40–70% relative humidity, and a 12-h light/dark cycle. The
animals had free access to food and water. The animals were divided
into three groups: The ECTs (n=26), BCSs (n=24) and Sham (n=20).
Their anterior right-sided chests were opened in layers at the
fifth intercostal space. Following incision of the pericardium
above the right atrium, the epicardium of the atrium and the
ventricle near the aorto-atrioventricular triangle was carefully
removed (3). The prepared ECTs
were implanted into the atrioventricular junction area by surgical
thoracotomy. The implantation site in the myocardium below the
epicardial surface was marked with 7-0 polypropylene suture
(Yangzhou Fuda Medical Devices, Co., Ltd., Yangzhou, China). The
chests were closed, and the animals were left to recover from
anesthesia of 300 mg/kg chloral hydrate (Capot Chemical Co., Ltd.,
Hangzhou, China) and then returned to their cages. Throughout the
surgery, the animals were ventilated with a respirator. A limb lead
(left hind limb, right and left forelimb) surface electrocardiogram
(ECG) monitored the cardiac rhythm using an MPA 2000 multiple
biological signal analysis system (Shanghai Alcott Biotech, Co.,
Ltd., Shanghai, China).
Histological staining
The tissues surrounding the implantation site were
excised and fixed in 4% paraformaldehyde for 36 h at room
temperature. Next, the tissues were embedded in paraffin and
sectioned at 4 or 6 µm. Following incubation at 60°C for 1 h, the
sections were immersed in xylene for 10 min and then in fresh
xylene for a further 10 min. The sections were treated to remove
wax. The sections were then successively rehydrated in 100, 95 or
80% ethanol and purified water for 3 min each for hematoxylin and
eosin, Masson's trichrome and immunohistochemical staining. For the
hematoxylin and eosin staining, the sections were stained with
hematoxylin for 10 min, differentiated with ethanol hydrochloride
and transferred to eosin solution for 2 min, all at room
temperature. The vessel (diameter, >30 µm) density was
calculated as the number of vessels/mm2. The density
measure was determined from 6 randomly selected microscopic fields
by a blinded observer (8).
Masson's trichrome staining (Abcam, Cambridge, MA, USA) was
performed according to the manufacturer's protocols. For
immunohistochemical staining, the sections were incubated in fresh
3% hydrogen peroxide at room temperature for 10 min to remove
endogenous peroxidase blocking buffer. Then the sections were
sufficiently eluted with PBS and goat serum (Thermo Fisher
Scientific, Inc.) was added dropwise onto the slices at room
temperature for 30 min. Excess solution was discarded and the
sections were incubated at 4°C overnight with primary antibodies,
including rabbit anti-rat CD-31 (cat. no. BA2966; 1:200),
Factor-VIII (cat. no. PB0273; 1:100) and VEGFR2 (cat. no. A00901;
1:500; Wuhan Boster Biological Technology, Ltd., Wuhan, China),
followed by treatment with goat anti-rabbit peroxidase-conjugated
secondary antibody (cat. no. ab6721; 1:1,000; Abcam) for 1 h at
room temperature for visualization with 3-diaminobenzidine
tetrahydrochloride. Additionally, the nuclei of the cultured cells
were stained with hematoxylin for 10 min at room temperature.
Subsequently, the sections were dehydrated and mounted with
resinene. Finally, microscopic analysis was performed using an
Olympus BH-2 light microscope (Olympus Corporation, Tokyo,
Japan).
Immunofluorescence staining
To detect cardiac marker proteins of the
implantation tissues, immunofluorescence staining was performed.
The implantation tissues were excised, fixed with 4% PFA for 36 h
at room temperature and dehydrated in 30% sucrose in
phosphate-buffered saline. Subsequently, the tissues were embedded
in Tissue-Tek O.C.T compound (Sakura Finetech USA, Inc., Torrance,
CA, USA) and sectioned at 5 micrometers at −26°C. The frozen
sections were placed on poly-L-lysine-coated glass slides and
stored in a −20°C freezer. For the immunofluorescence staining, the
sections were sufficiently eluted with PBS and goat serum (Thermo
Fisher Scientific, Inc.) was added dropwise onto the sections at
room temperature for 30 min. Excess solution was discarded and the
tissues were incubated with rabbit anti-connexin-40 (cat. no.
36-4900; 1:200; Invitrogen; Thermo Fisher Scientific, Inc.),
connexin-43 (cat. no. C6219; 1:150; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), connexin-45 (cat. no. PA5-79311; 1:200;
Invitrogen; Thermo Fisher Scientific, Inc.), HCN2 (cat. no.
ab19346; 1:200; Abcam), Hcn4 (cat. no. AB5808; 1:200; Chemcon
International, Inc.; EMD Millipore) and cTnT (cat. no. ab45932;
1:200; Abcam) at 4°C overnight. The control staining was performed
by omitting the primary antibody. Following the sections being
incubated with the FITC-conjugated anti-rabbit IgG secondary
antibodies (cat. no. F0382; 1:200; Sigma-Aldrich; Merck KGaA) for
60 min at room temperature, fluorescence imaging was visualized
using an Olympus IX70 fluorescence microscope (Olympus
Corporation).
Atrioventricular block (AVB)
A complete AVB was created at days 20, 60 and 90
after the implantation of ECTs and BCSs by ethanol, as previously
described by Lee et al (9).
In brief, the rats were anesthetized by 300 mg/kg chloral hydrate
(Capot Chemical Co., Ltd.) and 15 µl 70% ethanol was injected into
the myocardium 3 mm below the epicardial surface. The injection
point was close to the epicardial fat pad between the aortic root
and the right atrial wall of the rats. The reagents were injected
twice in 10 min. Six-lead surface ECGs (Alcott Biotech Co., Ltd.,
Shanghai, China) were used to monitor the cardiac rhythm.
Statistical analysis
Statistical analysis was conducted with SPSS version
21.0 (IBM Corp., Armonk, NY, USA). The average vessel (diameter
>30 µm) densities, presented as the mean ± standard error of the
mean, were compared using Dunnett's test. The recovery rate was
compared using the χ2 test, followed by Fisher's exact
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Implanted ECTs are capable of
surviving and vascularizing in the heart
The heart was exposed by right-sided thoracotomy at
the fifth intercostal space. Samples of 5×2×2 mm ECTs or BCSs were
implanted into the AV groove. The implanted site was positioned
within the epicardial layer adjacent to the aorto-atrioventricular
triangle (Fig. 1A). Following a
period of growth in vivo, the implanted ECTs were adequately
combined with the host's myocardium. The implanted ECTs were
associated with the atrium and the ventricle (Fig. 1B and C). The tissues surrounding
the implantation site were excised and sectioned at 4 or 6
micrometers. Next, the sections were stained with H&E. The
results revealed that a large number of cells were gathered at the
junction between the implants and the heart at days 20 and 40 after
transplantation (Fig. 2A and B),
but there were few cell clusters observed at the junction at days
60 and 90 after transplantation (Fig.
2C and D). It was suggested that these cells may be
inflammatory cells. The number of cells (red staining) increased
significantly, and the collagen sponges (slight staining) were
gradually degraded in the implanted ECTs between 20 and 90 days
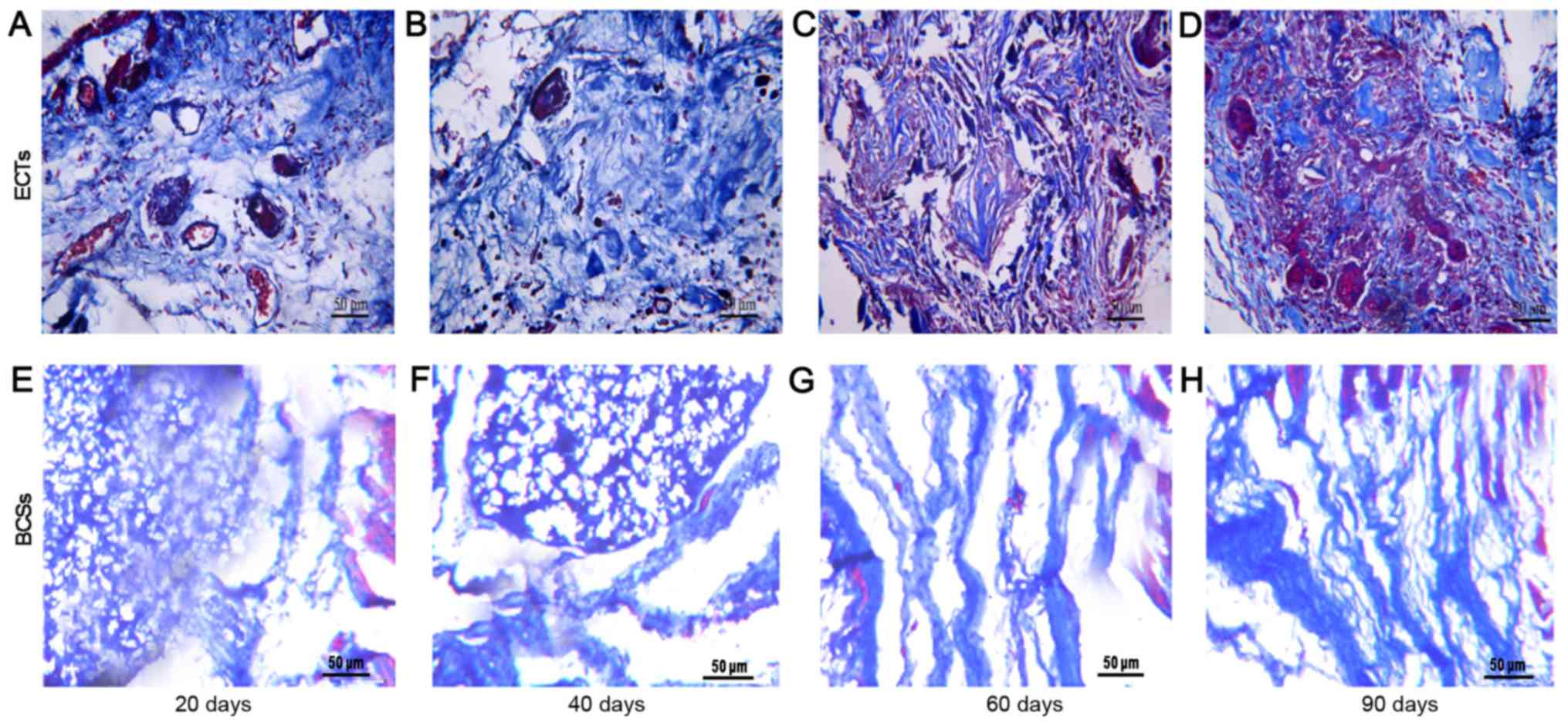
post-transplantation (Fig. 3). The
scaffold at the recipient site was completely degraded at 90 days
post-transplantation. The transplanted tissues formed a cell-matrix
structure similar to the host tissue at days 60 and 90 after
transplantation. By contrast, there were fewer cells in the BCSs
that failed to form a cell-matrix structure similar to the host
tissue (Figs. 2 and 3). The vessel densities of the
transplanted tissues were calculated, and the results revealed that
the vessel number in the ECTs was greater than that of the BCSs at
the implantation site at days 20 and 40 (Fig. 3). This observation was consistent
with the result of Masson's trichrome staining, which revealed that
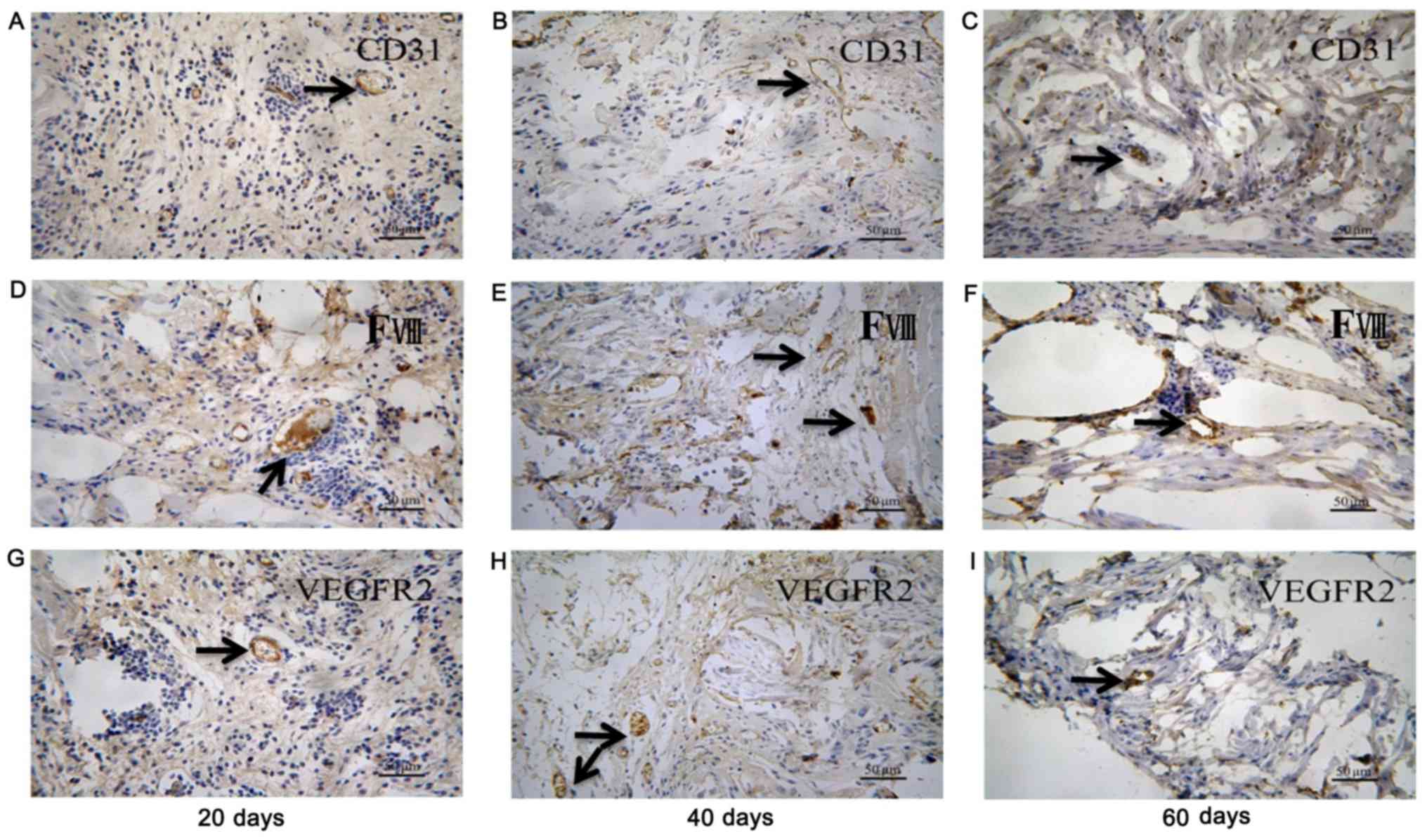
ECTs formed more vessels than BCSs (Figs. 4 and 5). The ECT tissue was stained positive
for CD-31, factor-VIII and VEGFR2 markers, indicating that there
were vessels in the tissue (Fig.
6). It was suggested that the transplanted ECTs were adequately
vascularized at the early stage of transplantation and could
survive in the atrioventricular junction area of the rats. In
addition, a large number of muscle fiber tissues were observed by
Masson's trichrome staining of the transplanted ECTs at 60 and 90
days post-transplantation.
The transplanted ECTs develop into a
phenotype similar to cardiac conduction tissues
The expression of connexin-40, connexin-43,
connexin-45, Hcn2, Hcn4 and cTnT in the implanted tissue was
detected by immunofluorescence staining. The results revealed that
the transplanted ECTs exhibited stronger positive staining for
connexin-40, connexin-43 and Hcn2 during the period of 20 to 90
days post-transplantation. By contrast, the transplanted BCSs
exhibited negative staining for the three proteins (Fig. 7A, B and C). The transplanted ECTs
exhibited little positive staining for the molecular markers of
pacemaker cells, including connexin-45 and Hcn4 (Fig. 7D and E). In addition, cTnT, as a
marker of working myocardium, was positive in the transplanted ECTs
and negative in the transplanted BCSs during the period of 20 to 90
days post-transplantation (Fig.
7F).
 | Figure 7.Immunostaining for connexin-40,
connexin-43, HCN2, connexin-45, HCN4 and cTnT in the transplanted
ECTs (n=26) and BCSs (n=24). Representative staining for (A)
connexin-40, (B) connexin-43, (C) HCN2, (D) connexin-45, (E) HCN4
and (F) cTnT in the rats transplanted with ECTs and BCSs at days
20, 60, and 90 after implantation. The transplanted tissue cells
were identified by labeling with CM-Dil (red). Immunostaining for
connexin-40, connexin-43, HCN2, connexin-45, HCN4 and cTnT in the
implantation site was shown by the secondary antibodies (green).
The nuclei were counterstained with DAPI (blue). ECTs, engineered
conduction tissues; BCSs, blank collagen sponges. |
Transplanted ECTs provide
atrioventricular conduction of the accessory pathway
ECG recordings were used to detect the
atrioventricular conduction of the accessory pathway. The results
revealed that there was an obvious pre-excitation syndrome in the
rats transplanted with ECTs during the period of 20 to 90 days
post-transplantation. By contrast, the rats transplanted with BCSs
did not exhibit any pre-excitation syndrome (Fig. 8).
To assess the functional conduction of ECTs in
vivo, atrioventricular block was created by ethanol injection
to induce atrioventricular node damage (Fig. 9). ECG recordings were used to
monitor the rats with atrioventricular block. The results revealed
that atrioventricular block was not recoverable within 1 h after
the ethanol injection for all the normal rats(Table I). After 1 h, increasing numbers of
rats with atrioventricular block were restored. Consequently, it
was confirmed that atrioventricular block in rats injected with
ethanol could be maintainable or unrecoverable within 1 h. However,
the recovery rate in the rats implanted with ECTs was 61.54% within
1 h after atrioventricular block during the period of 20 to 90 days
post-transplantation (Table II).
The ECG recordings demonstrated that the cardiac rhythm of recovery
was close to normal (Fig. 10). By
contrast, the recovery rate was only 4.17% in the rats implanted
with BCSs, and no rats in the Sham group exhibited atrioventricular
block recovery (Table II). The
recovery rate was significantly higher in the rats in the ECT group
than in those in the BCS group or Sham group.
 | Table I.The time-dependent proportion of
recovery in rats with atrioventricular block (n=85). |
Table I.
The time-dependent proportion of
recovery in rats with atrioventricular block (n=85).
|
|
Time |
|---|
|
|
|
|---|
| Proportion | 1 h | 2 h | 2 d | 4 d | 6 d | 8 d | 10 d | 14 d | 20 d | 40 d | 2 m | 3 m |
|---|
| Recovery/block | 0/15 | 1/7 | 5/7 | 3/7 | 6/7 | 4/6 | 4/6 | 4/6 | 5/6 | 4/6 | 5/6 | 5/6 |
 | Table II.Recovery rate in rats implanted with
ETCs within 1 h following atrioventricular block. |
Table II.
Recovery rate in rats implanted with
ETCs within 1 h following atrioventricular block.
| Group | Recovery | Block | Total | Recovery rate, % |
|---|
| Sham (n=20) | 0 | 20 | 20 | 0 |
| BCSs (n=24) | 1 | 23 | 24 | 4.17 |
| ETCs (n=26) | 16 | 10 | 26 | 61.54 |
Discussion
Complete atrioventricular conduction block is a
serious threat to human health. Despite the utility of electronic
pacemakers in treating this disease, permanent implantation of
devices is associated with certain complications. Recent studies
have focused on the use of cell transplants and gene transfer for
atrioventricular conduction block therapy (10,11).
However, neither gene transfer nor cellular transplantation
reproduces the normal function of atrioventricular conduction. The
aforementioned methods only provide palliation of atrioventricular
conduction block. In particular, transgenic expression is transient
and cells do not remain at the desired injection site. Therefore,
the transplantation of ECTs may be ideal for the treatment of
atrioventricular conduction block. The transplantation of ECTs into
the heart may establish a novel atrioventricular conduction
pathway, with characteristics similar to those of original
conduction. Currently, little is known regarding the
transplantation and functional conduction of ECTs in vivo.
In the present study, ECTs were created by seeding CPCs into
collagen sponges in vitro. Next, ECTs were transplanted into
the atrioventricular junction areas of rats in order to investigate
the feasibility that they may restore atrioventricular block in a
short amount of time.
In the present study, ECG recordings were used to
detect atrioventricular conduction at days 20, 40, 60 and 90 after
transplantation. It was revealed that the specific delta wave of
the pre-excitation syndrome appeared in the rats transplanted with
ECTs at different time points during the transplantation period.
The specific delta wave is mostly caused by the presence of the
Kent bundle. Kent bundles are composed of atrial-like muscle and
are mostly located between the left and right sides of the
atrioventricular groove, connecting the atrial and ventricular
myocytes (12,13). The transplanted ECTs were able to
establish an atrioventricular accessory pathway similar to Kent
bundles between the atria and ventricles of rats.
Cardiac conduction pathway repair using engineered
biological tissues has significant clinical translation potential.
Previous studies have reported that myocardial tissue constructs
may serve as conduits for restoring electrical conduction. The
constructs were explanted into hearts for ex vivo
experiments after 3 days (14,15).
Due to the short amount of time, it is difficult to validate in
detail the structural and functional recoupling of the atrial and
ventricular myocardia in these studies. The results of the present
study provided evidence that the transplanted ECTs gradually
developed an atrioventricular accessory pathway architecture during
the period of 20 to 90 days post-transplantation. Histological
staining confirmed the ECT survival and integration with the host
cardiomyocytes in vivo. The cells and extracellular matrix
were enriched in the transplanted ECTs, and the collagen sponges
were increasingly degraded in this process. In particular, Masson's
trichrome staining revealed that the transplanted ECTs gradually
became homogeneous with the surrounding tissues. The staining is
able to identify the fibrotic change of tissue. Collagen fibers
were shown as blue and muscle fibers were shown as red. A large
number of muscle fiber tissues were observed in the transplanted
ECTs at 60 days post-transplantation. Poor conductive response has
often been observed in fibrotic tissue. However, the number of
fibers surrounding the ECTs was much less than that of native
fibrotic tissue. Consequently, to the best of our knowledge, the
fibrosis surrounding the ECTs did not influence the pre-excitation
pattern. These results provided essential histological evidence
that the transplanted ECTs established an atrioventricular
accessory pathway similar to Kent bundles.
Previously, we reported the characterization of CPCs
derived from embryonic heart tubes (5–7). In
these aforementioned studies, the characterization of CPCs was well
investigated. The results revealed that CPCs are able to
differentiate into cardiac cells, including cardiomyocytes,
conduction cells and endothelial cells. Therefore, ECTs created by
seeding CPCs into collagen sponges were able to develop into
cardiac conduction or myocardial tissues in vivo in the
present study. This deduction was consistent with the results
obtained using immunofluorescence staining, which indicated that,
following being transplanted into the rat hearts, the ECTs located
between the atrial and ventricular myocytes exhibited positive
staining for connexin-40, connexin-43, HCN2 and cTnT during the
period of 20 to 90 days post-transplantation. By contrast, there
was less positive staining for the aforementioned markers in the
transplanted BCSs. As cardiac conducting tissue markers,
connexin-40, connexin-43 and HCN2 were expressed in the
transplanted ECTs. Positive staining for cTnT proved the existence
of myocardial tissues in the transplanted ECTs. As cardiac nodal
tissue markers, connexin-45 and HCN4 were rarely expressed in the
transplanted ECTs, suggesting that the transplanted ECTs developed
into cardiac conduction tissues with certain myocardial components.
Connexins and pacemaker channel remodeling are usually considered a
structural basis for the pattern of atrioventricular electrical
conduction (16). The increased
expression of connexin-40, connexin-43, HCN2 and cTnT provided
adequate support for the atrioventricular accessory pathway in the
present study. These proteins are also the indispensable molecular
bases for atrioventricular electrical conduction (17,18).
ECTs survived and mechanically integrated with the
allogeneic myocardium following transplantation into rat hearts.
The present study also provided evidence for the in vivo
functional integration of ECTs by demonstrating the ability of ECTs
to restore the normal rhythm of the heart in rats with
atrioventricular block. ECTs established an atrioventricular
accessory pathway by which 61.54% of the rats transplanted with
ECTs had restored normal cardiac rhythm in 60 min, while only 4.17%
of the rats transplanted with BCSs had restored rhythm. As
demonstrated in Fig. 10, the
cardiac rhythm of recovery in the rats implanted with ECTs was
close to normal. However, Fig. 8
revealed that pre-excitation patterns were observed intermittently,
not in each beat. We hypothesized that this is because the
pre-excitation electrical signal was interfered with or interrupted
by normal atrioventricular conduction without AV block (Fig. 8). Consequently, the patterns were
observed transiently or intermittently prior to AV block. In
summary, ECTs are a potential substitute therapy for
atrioventricular conduction block.
Acknowledgements
The authors would like to thank Mrs. Sun Aijun and
Mr. Qiao Liang (both of Department of Anatomy, Second Military
Medical University) for providing valuable suggestions.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81371708
and 81571821) and by the Scientific Research and Innovation Project
for College Students of Chongqing Medical University (2016–18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and SS conceived and designed the study and
reviewed and edited the manuscript. WZ and XL performed the
experiments, contributed to the data analyses and image processing,
and wrote the manuscript. All the authors read and approved the
final manuscript for publication.
Ethics approval and consent to
participate
All the animal experiments were approved by the
Ethics Committee of the Second Military Medical University
(Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cingolani E, Goldhaber JI and Marbán E:
Next-generation pacemakers: From small devices to biological
pacemakers. Nat Rev Cardiol. 15:139–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang X and Li XT: From theory to practice
in biological pacing how to recreate an atrioventricular conduction
pathway. Acad J Sec Mil Med Uni. 38:821–827. 2017.
|
|
3
|
Choi YH, Stamm C, Hammer PE, Kwaku KF,
Marler JJ, Friehs I, Jones M, Rader CM, Roy N, Eddy MT, et al:
Cardiac conduction through engineered tissue. Am J Pathol.
169:72–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou YB, Zou CW, Wang Y, Li DC, Li QB, Li
HX, Zhang HZ, Zhang Q and Fan QX: Establishing a new electrical
conduction pathway by anastomosis of the right auricle and right
ventricle assisted by mesenchymal stem cells in a canine model.
Transplant Proc. 43:3980–3986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Zhang CS, Liu YC, Yang XQ, Xiong
SH, Wen Y, Jiang EP, Li R, Zhang ZY, Liu F and Ye Y: Isolation,
culture and characterization of cardiac progenitor cells derived
from human embryonic heart tubes. Cells Tissues Organs.
190:194–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, Guo JP, Chi YL, Liu YC, Zhang CS,
Yang XQ, Lin HY, Jiang EP, Xiong SH, Zhang ZY and Liu BH:
Endothelin-induced differentiation of Nkx2.5+ cardiac
progenitor cells into pacemaking cells. Mol Cell Biochem.
366:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Shen MR, Xu ZD, Hu Z, Chen C, Chi
YL, Kong ZD, Li ZF, Li XT, Guo SL, et al: Cardiomyocyte
differentiation induced in cardiac progenitor cells by cardiac
fibroblast-conditioned medium. Exp Biol Med (Maywood). 239:628–637.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Li X, Yu X, Li Y, Sun A, Huang C,
Xu F, Guo J, Sun Y, Zhang X, et al: Construction of vascularized
pacemaker tissues by seeding cardiac progenitor cells and
endothelial progenitor cells into Matrigel. Life Sci. 179:139–146.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee RJ, Sievers RE, Gallinghouse GJ and
Ursell PC: Development of a model of complete heart block in rats.
J Appl Physiol (1985). 85:758–763. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho HC: Pacing the heart with genes:
Recent progress in biological pacing. Curr Cardiol Rep. 17:652015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yokokawa M, Ohnishi S, Ishibashi-Ueda H,
Obata H, Otani K, Miyahara Y, Tanaka K, Shimizu W, Nakazawa K,
Kangawa K, et al: Transplantation of mesenchymal stem cells
improves atrioventricular conduction in a rat model of complete
atrioventricular block. Cell Transplant. 17:1145–1155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanna Deschamps E and Hanna EB:
Atrioventricular accessory pathways: Mechanisms,
electrocardiograms, and associated arrhythmias. South Med J.
109:670–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Biase L, Gianni C, Bagliani G and
Padeletti L: Arrhythmias involving the atrioventricular junction.
Card Electrophysiol Clin. 9:435–452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cingolani E, Ionta V, Cheng K, Giacomello
A, Cho HC and Marbán E: Engineered electrical conduction tract
restores conduction in complete heart block: From in vitro to in
vivo proof of concept. J Am Coll Cardiol. 64:2575–2585. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kohl P: Structural and functional
recoupling of atrial and ventricular myocardium: New conduits for
electrical flow. J Am Coll Cardiol. 64:2586–2588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nisbet AM, Camelliti P, Walker NL, Burton
FL, Cobbe SM, Kohl P and Smith GL: Prolongation of
atrio-ventricular node conduction in a rabbit model of ischaemic
cardiomyopathy: Role of fibrosis and connexin remodelling. J Mol
Cell Cardiol. 94:54–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanni J, Maczewski M, Mackiewicz U, Siew
S, Fedorenko O, Atkinson A, Price M, Beresewicz A, Anderson RH,
Boyett MR and Dobrzynski H: Structural and functional alterations
in the atrioventricular node and atrioventricular ring tissue in
ischaemia-induced heart failure. Histol Histopathol. 29:891–902.
2014.PubMed/NCBI
|
|
18
|
Bartos DC, Grandi E and Ripplinger CM: Ion
channels in the heart. Compr Physiol. 5:1423–1464. 2015. View Article : Google Scholar : PubMed/NCBI
|