Introduction
Paraquat (PQ), a widely used herbicide, is highly
toxic to humans and animals (1–3).
Acute PQ poisoning by accidental or voluntary ingestion has toxic
effects on various organs, including the liver, lungs, kidneys and
central nervous system (4–7). Studies have reported that the lungs
are the primary target organ in PQ poisoning, where PQ may
accumulate and cause pulmonary edema, hemorrhage, interstitial
inflammation and fibrosis (8,9).
Approximately 90% of ingested PQ accumulates in the lung tissue
within 4–6 h after poisoning, since pulmonary epithelial cells,
particularly type I and II alveolar epithelial cells, may actively
absorb PQ through an endogenous transport system. Moreover, the
concentration of PQ in lung tissue may be 6–10 times higher than
that in plasma (4). Accumulated PQ
also damages the alveolar epithelium, followed by inflammatory cell
stimulation, fibroblast activation, excessive accumulation and
production of extracellular matrix (ECM) and, finally, progressive
and inexorable pulmonary fibrogenesis (8). Therefore, the most common cause of
PQ-induced mortality is respiratory failure from pulmonary fibrosis
(10). However, the underlying
mechanisms of PQ-induced pulmonary fibrosis remain unclear, and
there are no effective drugs or measures for the treatment of
PQ-poisoned patients.
Pulmonary fibrosis is an interstitial lung disease
characterized by the accumulation of excess fibrous connective
tissue and lung scarring (11).
The most frequent histopathological patterns of pulmonary fibrosis
is interstitial pneumonia (12,13).
Clinically, steroids and immunosuppressants have been used for the
treatment of lung inflammation and fibrosis (14–17).
However, numerous cases are treatment-resistant and the outcomes
are poor. Therefore, the development of new strategies for the
therapy of pulmonary fibrosis is necessary.
Recently, research on mesenchymal stem cells (MSCs)
derived from different tissues, including bone marrow, skeletal
muscle, cord blood, placenta and adipose tissue, has been performed
in the field of regenerative medicine research (18–20).
Due to the multiple differentiation potential of MSCs, studies on
MSC transplantation have been performed, aiming at potential
clinical applications (21,22).
Moreover, it was demonstrated that MSCs may modulate the immune
response and adjust the microenvironment of the engraftment sites,
improving their efficacy against inflammatory and autoimmune
responses (18,23–25).
Systemic administration of bone marrow-derived MSCs (BMSCs) may
reduce bleomycin-induced inflammatory cell infiltration and lung
fibrosis via the accumulation of MSCs in the pulmonary parenchyma
and large airway (26–30). Therefore, direct delivery of BMSCs
to the lung appears to constitute effective protection against
PQ-induced pulmonary fibrosis.
In the present study, given the unique
characteristics of BMSCs, the therapeutic effects and possible
mechanism of intravenous BMSC transplantation on PQ-poisoned mice
was assessed. Meanwhile, the accumulation and retention of BMSCs in
lung tissues was evaluated. This procedure attenuated lung
inflammation and cell apoptosis following PQ poisoning, indicating
that BMSC transplantation may be a potential strategy for the
treatment of PQ-induced pulmonary fibrosis.
Materials and methods
Ethics statement
The animal experiments were performed according to
the Guide for the Care and Use of Laboratory Animals (The Ministry
of Science and Technology of China, 2006) and all experimental
protocols were approved under the animal protocol no. SYXK (Su)
2015–0019 by the Animal Care and Use Committee of Medical School of
Nanjing University (Nanjing, China).
Cell culture
Mouse lung epithelial cells (MLE-12) were purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (both purchased from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 1% antibiotics (100
U/ml penicillin and 0.1 mg/ml streptomycin). The cells were grown
at 37°C in a 5% CO2 incubator, and were passaged
following trypsinization.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse lung tissues and
MLE-12 cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). RT-qPCR was used to confirm
the expression levels of mRNAs. cDNA was produced according to the
protocol for PrimeScript™ RT Reagent (Takara Bio, Inc., Otsu,
Japan), at 37°C for 15 min and 85°C for 5 sec. RT-qPCR was
performed as described in the method for the FastStart Universal
SYBR Green Master mix (Roche Molecular Systems, Inc., Pleasanton,
CA, USA) on a 7300 Real-time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Each sample was run in triplicate, and
the data were analyzed with the 7300 Sequence Detection Software
version 1.4.0.25 (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Specific primers for mRNAs tested are listed in Table I. The thermocycling conditions were
as follows: 1 cycle of initial denaturation (95°C for 10 min); 40
cycles of denaturation (95°C for 10 sec), annealing and elongation
(60°C for 30 sec). The Cq values were analyzed using the ΔΔCq
method (31), and relative changes
in mRNA levels were calculated by normalization to GAPDH relative
to the control.
 | Table I.Reverse-transcription quantitative
polymerase chain reaction primers and products. |
Table I.
Reverse-transcription quantitative
polymerase chain reaction primers and products.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| α-SMA |
CCCAGATTATGTTTGAGACCTTC |
ATCTCCAGAGTCCAGCACAATAC |
| Vimentin |
CCTGGAGTCACTTCCTCTGGTTG |
TCTTGCTGGTACTGCACTGTTGC |
| Col1a1 |
CTTCTGGTCCTCGTGGTCTCCCT |
AAGCCTCGGTGTCCCTTCATTCC |
| Fibronectin |
GCACCGACGAAGAGCCCTTACAG |
GCACCATTCAGCGTTGCCCACAG |
| GAPDH |
AGGTCGGTGTGAACGGATTTG |
TGTAGACCATGTAGTTGAGGTCA |
Western blot analysis
Cells and lung tissues were lysed in ice-cold
radioimmunoprecipitation assay extraction buffer (150 mM NaCl, 10
mM Tris-HCl, pH 7.4, 1% Triton X-100, 1% sodium deoxycholate and
0.1% SDS) containing a protease inhibitor cocktail (Roche Molecular
Systems, Inc.) for 30 min. The whole lysates were then centrifuged
at 12,000 × g for 30 min at 4°C and the protein concentration in
the supernatant was determined using a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). The protein samples
were boiled for 10 min and 30 µg aliquots were then subjected to
SDS-PAGE (12% gel) and transferred to a polyvinylidene difluoride
membrane. The membrane was blocked with 5% bovine serum albumin
(Thermo Fisher Scientific, Inc.) at 37°C for 1 h, and incubated
with the following primary antibodies at 4°C overnight: Rabbit
anti-α-SMA (at a 1:3,000 dilution; cat. no. ab5694),
anti-E-cadherin (at a 1:1,000 dilution, cat. no. ab76055), rabbit
anti-Vimentin (at a 1:5,000 dilution, cat. no. ab92547), and mouse
anti-GAPDH (at a 1:3,000 dilution; cat. no. ab9485). All primary
antibodies were purchased from Abcam, Cambridge, UK. Next, the
membrane was further incubated with horseradish
peroxidase-conjugated goat anti-rabbit/mouse immunoglobulin G
(Boster Biological Technology, Pleasanton, CA, USA) at 37°C for 1
h. GAPDH was used as the reference protein. Immunoreactive protein
bands were visualized with an enhanced chemiluminescence detection
kit (GE Healthcare, Chicago, IL, USA) and detected using an Odyssey
Scanning System (LI-COR Biosciences, Lincoln, NE, USA). The
expression levels were quantified with ImageJ (version 1.48,
National Institutes of Health, Bethesda, MD, USA).
PQ-induced pulmonary fibrosis model
and BMSC recruitment detection
The recombinant adenovirus vectors carrying a green
fluorescent protein (GFP) reporter gene (Ad-GFP) were obtained from
Cyagen Biosciences, Inc. (cat. no. mubmx-01101; Santa Clara, CA,
USA). BMSCs, purchased from Cyagen Biosciences (cat. no.
mubmx-01101), were treated with 100 µl Ad-GFP at a concentration of
1×109 TU/ml for 16 h. Following this step, stem cell
culture medium (cat. no. MUBMX-90011) containing adenoviral
particles was replaced with fresh medium. After 48 h, BMSCs were
prepared for transplantation.
C57BL/6 mice (male; 8 weeks old; 20 g) were randomly
divided into four groups (n=10 for each group). Animals were
maintained at 19–24°C, 40–50% humidity, with a 12 h light/dark
cycle and were fed a chow diet with free access to drinking water
in the animal facilities of the Medical School of Nanjing
University. Pulmonary fibrosis was induced by intraperitoneal
administration of PQ at the concentration of 20 mg/kg. BMSCs
(2×106 cells) were injected via the tail vein on day 7.
Mice receiving the same volume of saline without BMSCs were used
for control. The two controls of mice injected with saline and mice
injected saline and transplanted with BMSCs-Ad-GFP cells. The two
PQ experimental groups consisted of mice injected with PQ, and
another injected with PQ and with BMSCs-Ad-GFP cells. The mice
(n=6) were sacrificed at day 7 and 14 after PQ treatment, and the
lungs were harvested for histological analysis.
Histology
Lung tissue was fixed overnight with 4%
paraformaldehyde at 4°C, dehydrated in an ascending alcohol
gradient, embedded in paraffin and cut into 5 µm sections. The
sections were stained with hematoxylin and eosin (H&E), and
Masson's trichrome (Solarbio, Beijing, China) were performed
according to standard procedures. Paraffin-embedded sections (5 µm)
were deparaffinized with xylene (room temperature, twice for 5 min
each) before being rehydrated in water using an ethanol gradient.
For H&E staining, lung sections were stained with hematoxylin
(1 min) and eosin (2 min) at room temperature. For Masson's
trichrome staining, lung sections were stained with hematoxylin (1
min), Ponceau S (5 min) and Aniline blue (5 min) at room
temperature. Pathological changes in the lungs were observed under
a light microscope (Olympus Corporation, Tokyo, Japan). To
visualize the efficacy of BMSC transplantation, the lung sections
were washed with PBS and stained with 1 µg/ml DAPI at 37°C for 6
min (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
fluorescence images were captured using a confocal fluorescence
microscope (Olympus Corporation).
Hydroxyproline (HDP) content
assay
Lung tissue weighing 50 mg was homogenized with 1 ml
ddH2O, and mixed with 1 ml NaOH (10 N) in a
pressure-tight, screw-capped polypropylene vial that was heated at
120°C for 1 h. The supernatant was centrifuged at 10,000 × g for 10
min at 4°C according to the instructions of the hydroxyproline
assay kit (cat. no. K226; BioVision, Inc., Milpitas, CA, USA). The
absorbance at 550 nm was measured by a spectrophotometer, and the
HDP content of each lung tissue was calculated.
ELISA
Cytokines in bronchoalveolar lavage fluid (BALF)
were detected by using ELISA kits for mouse tumor necrosis factor-α
(TNF-α; cat. no. MTA00B), interleukin (IL)-1β (cat. no. MLB00C),
IL-6 (cat. no. D6050) and IL-10 (cat. no. DY417; R&D Systems
China Co., Ltd., Shanghai, China), according to the manufacturer's
instructions (32). Briefly, a
total of 100 µl BALF supernatant was added into a 96-well plate and
incubated for 1 h. Then, 100 µl enzyme-linked antibodies were added
for 30 min at 37°C. Following three washes with washing buffer, the
chromogenic reagent was added and incubated for 30 min. To
terminate the reaction, 50 µl 2 M H2SO4 was
added to each well. Absorbance was determined at 450 nm.
Flow cytometry analysis
To determine the numbers of BMSCs that translocated
into the lung tissues, mouse lung parenchyma tissues were digested
after cutting with a razor blade, as described previously (33). The digested material was filtered
and depleted of red blood cells using Ammonium Chloride Potassium
buffer (cat. no. A1049201; Thermo Fisher Scientific, Inc.). GFP
positive cells were detected by flow cytometry and analyzed by
FlowJo software version 7.6.1 (Tree Star, Inc., Ashland, OR,
USA).
Cell apoptosis was measured by an Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
staining kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. MLE-12 cells from different groups
were collected following centrifugation at 1,000 × g at 4°C for 5
min. Cells were washed twice with cold PBS and re-suspended in 500
µl binding buffer. Each cell sample was stained with 5 µl Annexin
V-FITC and 5 µl PI, and incubated in the dark at 25°C for 15 min.
Flow cytometry was performed on a FACSCalibur™ flow cytometer (BD
Biosciences, Franklin, Lakes, NJ, USA) and the data were analyzed
with FlowJo software 7.6.1 (Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). The data are
presented as the mean ± standard deviation (n=3). Differences were
analyzed with one-way analysis of variance, using a Tukey-Kramer
t-test to perform multiple comparisons among different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PQ induces pulmonary fibrosis
The development of pulmonary fibrosis in mice
treated with 20 mg/kg PQ was assessed by H&E (Fig. 1A) and Masson's trichrome staining
(Fig. 1B), and the HDP content
assay for collagen (Fig. 1C).
Alterations in lung structure are visible in Fig. 1A. Lung tissue sections from the
control group exhibited no evidence of inflammation or epithelial
damage. As expected, lung tissue sections from mice treated with PQ
displayed extensive cellular thickening of the interalveolar septa,
interstitial edema, inflammatory cell infiltration, increased
interstitial cells with a fibroblastic appearance, and
fibrogenesis. The results demonstrated that the alveolar structure
was damaged, with extensive collagen deposition between days 7 and
14. The results of the Masson's trichrome staining and HDP content
assay also indicated that PQ may have induced excessive collagen
deposition in lung tissue. The expression of fibrosis-associated
genes, including α-smooth muscle actin (α-SMA), collagen type 1 α1
chain (Col1a1), vimentin and fibronectin, was significantly
increased following PQ administration compared with the control
group (Fig. 1D).
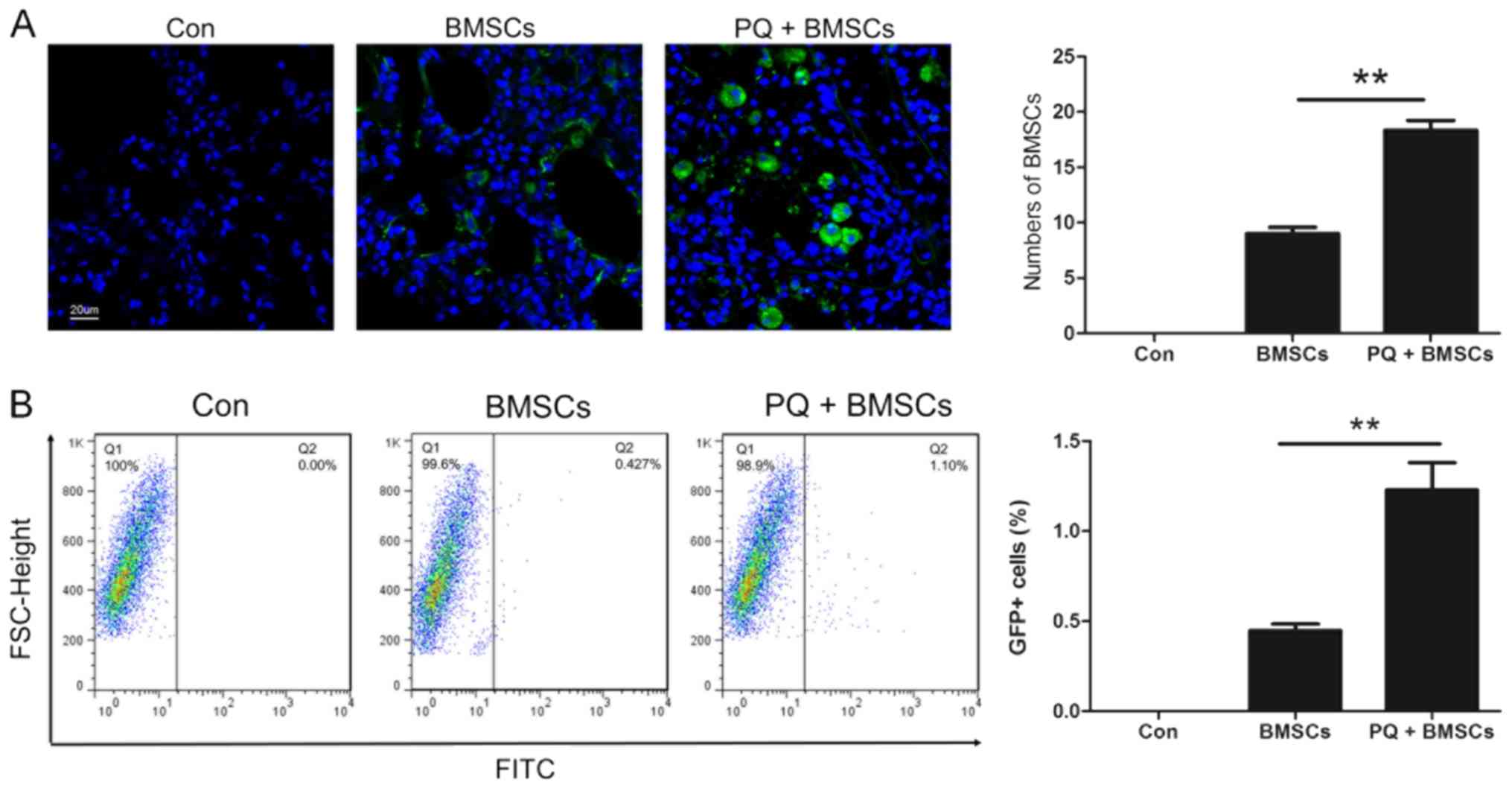
BMSCs were recruited to lung
tissues
In order to confirm whether BMSCs may be recruited
to the lung tissues during the development of PQ-induced pulmonary
fibrosis, BMSCs were transfected with adenovirus vectors carrying
the GFP reporter gene (Ad-GFP). These cells were subsequently
transplanted into mice via injection through the tail vein on day 7
following either saline or PQ injection. A number of GFP-positive
BMSCs were observed in the lung tissues (Fig. 2A). Compared with the mice injected
with saline, the number of BMSCs recruited in the lung tissues of
PQ-poisoned mice was significantly elevated, indicating that PQ
poisoning may have promoted the recruitment of BMSCs to injured
lung tissues. This result was further confirmed by flow cytometry
(Fig. 2B).
BMSCs suppress PQ-induced pulmonary
fibrosis by reducing lung inflammation
In order to determine the potential therapeutic
effects of BMSCs on PQ-induced pulmonary fibrosis, this model was
reproduced and it was demonstrated that BMSC transplantation
significantly reduced the severity of pulmonary fibrosis, as
assessed by H&E, Masson staining and HDP content assay
(Fig. 3A-C). BMSC injection may
have effectively increased the survival rate of mice poisoned with
PQ (Fig. 3D). In addition, it was
observed that BMSC treatment may have attenuated inflammatory
responses by decreasing the expression of pro-inflammatory factors,
including TNF-α, IL-1β, IL-6 and IL-10 (Fig. 3E). These results suggested that
BMSCs may prevent PQ-induced pulmonary fibrosis through
inflammatory response suppression.
BMSCs protect pulmonary epithelial
cells from PQ-induced epithelial-mesenchymal transition (EMT) and
apoptosis
It has been reported that EMT of pulmonary
epithelial cells serves a critical role in the development of
pulmonary fibrosis (34). In the
present study, MLE-12 cells were co-cultured with or without BMSCs,
followed by PQ treatment. As demonstrated in Fig. 4A, PQ may have induced the
expression of α-SMA and vimentin, which was decreased when MLE-12
cells were co-cultured with BMSCs. Conversely, the epithelial
marker E-cadherin was decreased with PQ treatment, which was
increased when MLE-12 cells were co-cultured with BMSCs. In
addition, co-culture of BMSCs with MLE-12 cells may have suppressed
PQ-induced epithelial cell apoptosis (Fig. 4B). These results indicated that the
therapeutic effects of BMSCs on PQ-induced pulmonary fibrosis may
be mediated by inhibition of apoptosis and the EMT process of
epithelial cells.
Discussion
PQ is an herbicide with high toxicity in human lung
tissues, and causes mortality by inducing pulmonary fibrosis. It
has been reported that the clinical course of PQ-induced pulmonary
fibrosis may be divided into three phases: The early inflammatory
phase, the proliferative phase characterized by fibroblast
proliferation, and the phase of collagen deposition (35,36).
Among these phases, the inflammatory response serves a critical
role in the pathogenesis of pulmonary fibrosis. A large number of
inflammatory cytokines, particularly TNF-α, IL-1β, IL-6,
transforming growth factor β1 (TGF-β1) and platelet-derived growth
factor (PDGF) are known to be associated with the initiation and
development of pulmonary fibrosis (37,38).
TGF-β1, IL-1β and TNF-α may inhibit lung injury repair, promote
lung cell apoptosis and finally induce pulmonary fibrosis (38,39).
Reduced pulmonary inflammation may therefore contribute to the
recovery from lung injury (40,41).
Optimal therapies for pulmonary fibrosis remain
controversial. There is no effective agent that improves the
survival of patients with pulmonary fibrosis (42). To date, lung transplantation is the
only effective treatment for pulmonary fibrosis (43). However, lung transplantation also
has its own disadvantages, namely the shortage of organs and
complications associated with long-term immunosuppression (44). Therefore, exploring new effective
therapies to suppress or reverse pulmonary fibrosis is essential
for decreasing the morbidity and mortality of patients with
pulmonary fibrosis. Recently, it was reported that cells derived
from bone marrow, particularly MSCs, may repopulate the lung and
repair the injured lung tissues, which is a promising treatment for
lung injury (45,46). MSCs are multipotent cells capable
of differentiating into different cell lines, and display
anti-proliferative, immunomodulatory and anti-inflammatory effects
(47). The protective roles of
BMSCs have been attributed to different mechanisms. Tracking of
labeled cells has demonstrated that MSCs localize primarily to the
lung following intravenous administration (48), engrafting into the injured lung and
trans-differentiating into lung epithelium (26,49).
In the present study, it was demonstrated that BMSCs may engraft
into injured lung tissue and reduce the lung epithelium damage,
which is beneficial for lung repair following PQ exposure. Although
the precise mechanism of action of MSCs remains elusive (25), in a number of clinical trials
(50,51) it was demonstrated that MSCs may
have the ability to produce paracrine factors that may mitigate
tissue damage (52). The paracrine
factors released by MSCs may alter the microenvironment and
contribute to a repairing effect in pulmonary fibrosis (50). The present results demonstrated
that BMSCs may have attenuated lung injury by inhibiting the
production of inflammatory cytokines in lung tissue, and also by
reducing lung epithelial cells apoptosis in vitro.
Therefore, these findings illustrate that BMSC-based therapies for
PQ-induced lung injury may be clinically relevant, as these cells
target inflammatory pathways and reduce epithelium damage.
In conclusion, the present observations provided
evidence that intravenous BMSC transplantation elevated the
survival rate and suppressed the development of PQ-induced
pulmonary fibrosis, by accumulating in the lung interstitium and
reducing pulmonary inflammation. BMSCs retarded the secretion of
pro-inflammatory cytokines, namely TNF-α and IL-1β, in PQ-poisoned
lung tissues, and protected pulmonary epithelial cells from
PQ-induced apoptosis. Taken together, the present study provides a
novel strategy for the treatment of PQ-induced pulmonary
fibrosis.
Acknowledgements
Not applicable.
Funding
This study was supported by Scientific Projects of
Yancheng (grant no. YK2015013).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JC performed the majority of the experiments; LS and
LZ provided the statistical analysis, interpretation of data and
contributed to some experiments. JC and YD contributed to the
manuscript drafting and revision, designed the study and analyzed
the data. All the authors contributed to the manuscript preparation
and gave final approval of the submitted manuscript.
Ethics approval and consent to
participate
The animal experiments were performed according to
the Guide for the Care and Use of Laboratory Animals (The Ministry
of Science and Technology of China, 2006) and all experimental
protocols were approved under the animal protocol number SYXK (Su)
2015–0019 by the Animal Care and Use Committee of Medical School of
Nanjing University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee SK, Ameno K, In SW, Yang JY, Kim KU,
Koo KS, Yoo YC, Ameno S and Ijiri I: Levels of paraquat in fatal
intoxications. Int J Legal Med. 112:198–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu
CW, Hsu HH and Yen TH: Improved survival in severe paraquat
poisoning with repeated pulse therapy of cyclophosphamide and
steroids. Intensive Care Med. 37:1006–1013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang RL, Tang X, Wu X, Xu R, Yu KL and Xu
K: The relationship between HIF-1α expression and the early lung
fibrosis in rats with acute paraquat poisoning. Zhonghua Lao Dong
Wei Sheng Zhi Ye Bing Za Zhi. 30:273–277. 2012.(In Chinese).
PubMed/NCBI
|
|
4
|
Forman HJ, Aldrich TK, Posner MA and
Fisher AB: Differential paraquat uptake and redox kinetics of rat
granular pneumocytes and alveolar macrophages. J Pharmacol Exp
Ther. 221:428–433. 1982.PubMed/NCBI
|
|
5
|
Naito H and Yamashita M: Epidemiology of
paraquat in Japan and a new safe formulation of paraquat. Human
Toxicol. 6:87–88. 1987. View Article : Google Scholar
|
|
6
|
Rose MS, Smith LL and Wyatt I: Evidence
for energy-dependent accumulation of paraquat into rat lung.
Nature. 252:314–315. 1974. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Wal NA, van Oirschot JF, van Dijk
A, Verhoef J and van Asbeck BS: Mechanism of protection of alveolar
type II cells against paraquat-induced cytotoxicity by
deferoxamine. Biochem Pharmacol. 39:1665–1671. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venkatesan N: Pulmonary protective effects
of curcumin against paraquat toxicity. Life Sci. 66:PL21–P28.
2000.PubMed/NCBI
|
|
9
|
Tomita M, Okuyama T, Katsuyama H, Miura Y,
Nishimura Y, Hidaka K, Otsuki T and Ishikawa T: Mouse model of
paraquat- poisoned lungs and its gene expression profile.
Toxicology. 231:200–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hagiwara S, Iwasaka H, Matsumoto S and
Noguchi T: An antisense oligonucleotide to HSP47 inhibits
paraquat-induced pulmonary fibrosis in rats. Toxicology.
236:199–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
Pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Behr J: Evidence-based treatment
strategies in idiopathic pulmonary fibrosis. Eur Respir Rev.
22:163–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
American Thoracic Society. Idiopathic
pulmonary fibrosis, . Diagnosis and treatment. International
consensus statement. American Thoracic Society (ATS), and the
European Respiratory Society (ERS). Am J Respir Crit Care Med.
161:646–664. 2000.PubMed/NCBI
|
|
15
|
Kotani T, Makino S, Takeuchi T, Kagitani
M, Shoda T, Hata A, Tabushi Y and Hanafusa T: Early intervention
with corticosteroids and cyclosporin A and 2-hour postdose blood
concentration monitoring improves the prognosis of acute/subacute
interstitial pneumonia in dermatomyositis. J Rheumatol. 35:254–259.
2008.PubMed/NCBI
|
|
16
|
King TE Jr, Bradford WZ, Castro-Bernardini
S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM,
Kardatzke D, Lancaster L, et al: A phase 3 trial of pirfenidone in
patients with idiopathic pulmonary fibrosis. N Engl J Med.
370:2083–2092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richeldi L, du Bois RM, Raghu G, Azuma A,
Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y,
et al: Efficacy and safety of nintedanib in idiopathic pulmonary
fibrosis. New Eng J Med. 370:2071–2082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baer PC: Adipose-derived mesenchymal
stromal/stem cells: An update on their phenotype in vivo and in
vitro. World J Stem Cells. 6:256–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caruso M, Evangelista M and Parolini O:
Human term placental cells: Phenotype, properties and new avenues
in regenerative medicine. Int J Mol Cellular Med. 1:64–74.
2012.
|
|
21
|
Taha MF and Hedayati V: Isolation,
identification and multipotential differentiation of mouse adipose
tissue-derived stem cells. Tissue Cell. 42:211–216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weiss DJ: Stem cells, cell therapies, and
bioengineering in lung biology and diseases. Comprehensive review
of the recent literature 2010–2012. Ann Am Thorac Soc. 10:S45–S97.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiss DJ and Ortiz LA: Cell therapy trials
for lung diseases: Progress and cautions. Am J Respir Crit Care
Med. 188:123–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McNulty K and Janes SM: Stem cells and
pulmonary fibrosis: Cause or cure? Proc Am Thorac Soc. 9:164–171.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phan SH: The myofibroblast in pulmonary
fibrosis. Chest. 122 (Suppl 6):S286–S289. 2002. View Article : Google Scholar
|
|
28
|
Epperly MW, Guo H, Gretton JE and
Greenberger JS: Bone marrow origin of myofibroblasts in irradiation
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:213–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scotton CJ and Chambers RC: Molecular
targets in pulmonary fibrosis: The myofibroblast in focus. Chest.
132:1311–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Investigation.
119:1420–1428. 2009. View
Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Husari A, Khayat A, Bitar H, Hashem Y,
Rizkallah A, Zaatari G and El Sabban M: Antioxidant activity of
pomegranate juice reduces acute lung injury secondary to hyperoxia
in an animal model. BMC Res Notes. 7:6642014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gong X, Sun Z, Cui D, Xu X, Zhu H, Wang L,
Qian W and Han X: Isolation and characterization of lung resident
mesenchymal stem cells capable of differentiating into alveolar
epithelial type II cells. Cell Biol Int. 38:405–411. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Strieter RM and Mehrad B: New mechanisms
of pulmonary fibrosis. Chest. 136:1364–1370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smith LL and Rose MS: A comparison of the
effects of paraquat and diquat on the water content of rat lung and
the incorporation of thymidine into lung DNA. Toxicology.
8:223–230. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vijeyaratnam GS and Corrin B: Experimental
paraquat poisoning: A histological and electron-optical study of
the changes in the lung. J Pathol. 103:123–129. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maggini J, Mirkin G, Bognanni I, Holmberg
J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S,
Vermeulen M and Geffner JR: Mouse bone marrow-derived mesenchymal
stromal cells turn activated macrophages into a regulatory-like
profile. PLoS One. 5:e92522010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Anderson P, Souza-Moreira L, Morell M,
Caro M, O'Valle F, Gonzalez-Rey E and Delgado M: Adipose-derived
mesenchymal stromal cells induce immunomodulatory macrophages which
protect from experimental colitis and sepsis. Gut. 62:1131–1141.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim C, Lee JM, Park SW, Kim KS, Lee MW,
Paik S, Jang AS, Kim DJ, Uh S, Kim Y and Park CS: Attenuation of
cigarette smoke-induced emphysema in mice by apolipoprotein A-1
overexpression. Am J Respir Cell Mol Biol. 54:91–102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Li JS, Li WW, Li SY, Tian YG, Lu XF,
Jiang SL and Wang Y: Long-term effects of three Tiao-Bu Fei-Shen
therapies on NF-κB/TGF-β1/smad2 signaling in rats with chronic
obstructive pulmonary disease. BMC Complement Altern Med.
14:1402014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang C, Ding H, Tang X, Li Z and Gan L:
Effect of Liuweibuqi Capsules in pulmonary alveolar epithelial
cells and COPD through JAK/STAT pathway. Cell Physiol Biochem.
43:743–756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Adamali HI and Maher TM: Current and novel
drug therapies for idiopathic pulmonary fibrosis. Drug Des Devel
Ther. 6:261–272. 2012.PubMed/NCBI
|
|
43
|
Whelan TP: Lung transplantation for
interstitial lung disease. Clinics Chest Med. 33:179–189. 2012.
View Article : Google Scholar
|
|
44
|
McShane PJ and Garrity ER Jr: Minimization
of immunosuppression after lung transplantation: Current trends.
Transpl Int. 22:90–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cargnoni A, Gibelli L, Tosini A, Signoroni
PB, Nassuato C, Arienti D, Lombardi G, Albertini A, Wengler GS and
Parolini O: Transplantation of allogeneic and xenogeneic
placenta-derived cells reduces bleomycin-induced lung fibrosis.
Cell Transplant. 18:405–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang YS, Oh W, Choi SJ, Sung DK, Kim SY,
Choi EY, Kang S, Jin HJ, Yang YS and Park WS: Human umbilical cord
blood-derived mesenchymal stem cells attenuate hyperoxia-induced
lung injury in neonatal rats. Cell Transplant. 18:869–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao J, Dennis JE, Muzic RF, Lundberg M and
Caplan AI: The dynamic in vivo distribution of bone marrow-derived
mesenchymal stem cells after infusion. Cells Tissues, Organs.
169:12–20. 2001. View Article : Google Scholar
|
|
49
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elnakish MT, Hassan F, Dakhlallah D, Marsh
CB, Alhaider IA and Khan M: Mesenchymal stem cells for cardiac
regeneration: Translation to bedside reality. Stem Cells Int.
2012:6460382012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rathinasabapathy A, Bruce E, Espejo A,
Horowitz A, Sudhan DR, Nair A, Guzzo D, Francis J, Raizada MK,
Shenoy V and Katovich MJ: Therapeutic potential of adipose stem
cell-derived conditioned medium against pulmonary hypertension and
lung fibrosis. Br J Pharmacol. 173:2859–2879. 2016. View Article : Google Scholar : PubMed/NCBI
|