Introduction
Atopic dermatitis (AD) is a common type of chronic
inflammatory disease of the skin, characterized by erythema,
dryness and recurrent pruritus, which seriously impairs the quality
of daily life (1). The prevalence
of AD has been steadily increasing year-on-year in developed and
developing countries, and it affects up to 15–20% of children and
1–3% of adults (2,3). To the best of our knowledge, the
mechanism underlying AD remains unknown; however, an overactive
immune system is strongly correlated with the natural course of the
disease. In particular, the abnormal activation of T helper (Th)2
cells is associated with progression of the disease, which
contributes to a predominant Th2 response, and an imbalance between
Th1 and Th2 responses (4,5). Increasing infiltration of Th2 cells
and related cytokines, including interleukin (IL)-4, IL-13 and
IL-31, mediates the secretion of immunoglobulin E (IgE). High serum
IgE levels are an important characteristic of AD and are associated
with the severity of allergic diseases (6,7).
Previous studies have demonstrated that regulating the balance
between Th1 and Th2 by shifting it to Th1 dominance can markedly
inhibit the immune reaction, which indicates an effective strategy
for the treatment of AD (8,9).
Mast cells are widely recognized as another crucial
factor responsible for allergic inflammatory reactions (10). A previous study demonstrated that
high IgE levels activate mast cells. The IgE receptor FcεRI sits on
the cell surface and mast cells, as effector cells, release
histamines and other biologically active products that trigger
allergic inflammatory symptoms (11). Therefore, methods that reduce the
number of mast cells and inhibit their activation may ameliorate
the symptoms of AD.
Paeonol (2′-hydroxy-4′-methoxyacetophenone) is a
constituent of the traditional Chinese medicine (TCM) Cortex
Moutan, which has been used to treat inflammatory conditions and
immune disorders due to its numerous pharmacological activities,
including anti-oxidant, anti-inflammatory, anticancer,
apoptosis-inducing and anti-diabetic effects (12,13).
Several animal models and cell experiments have reported that
paeonol may suppress inflammatory responses (14,15).
The results of our previous study demonstrated that paeonol
alleviates psoriasis-like lesions in a mouse model and relieves
inflammatory reactions (16). It
was therefore hypothesized that paeonol may ameliorate allergic
diseases induced by activated Th2 and mast cells. Therefore, the
present study focused on the effects of paeonol on
1-chloro-2,4-dinitrobenzene (DNCB)-induced AD-like lesions and mast
cells (P815 cells), in order to determine the potential efficiency
and underlying mechanism of paeonol for the treatment of AD.
Materials and methods
Experimental animals
A total of 48 female BALB/c mice (age, 6–8 weeks;
weight, 18–22 g) were obtained from Beijing HFK Bioscience Co.,
Ltd. (Beijing, China; certification no. SCXK Jing 2017-0001). The
mice were housed in individual ventilated cages under a 12-h
light/dark cycle at a temperature of 23–25°C and relative humidity
of 55–65%. The mice were fed a standard diet and had free access to
purified water. All experimental procedures were performed in
accordance with the Research and Ethical Guidelines of the
Committee of the International Association for the Study of Pain
(17) and were approved by the
Animal Ethics Committee of Capital Medical University (Beijing,
China).
Materials, reagents and
instruments
Paeonol was provided by the National Institutes for
Food and Drug Control (Beijing, China). DNCB (cat. no. 237329;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in
acetone and olive oil (3:1 v/v). Paeonol was dissolved in normal
saline to achieve various concentrations for oral administration.
Prednisolone (Pred; cat. no. BP464; Sigma-Aldrich; Merck KGaA) was
dissolved in normal saline to a concentration of 10 mg.
TRIzol® was purchased from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The NucleoSpin® RNA
Clean-up kit was obtained from Macherey-Nagel GmbH & Co. KG
(Düren, Germany). The real-time polymerase chain reaction (RT-PCR)
FastStart Universal Master mix was purchased from Roche Diagnostics
(Indianapolis, IN, USA) and the AffinityScript Multiple Temperature
cDNA Synthesis kit was purchased from Agilent Technologies, Inc.
(Santa Clara, CA, USA).
Induction of DNCB-induced AD-like skin
lesions
AD-like skin lesions were induced by repeated
application of DNCB onto the hairless dorsal skin of the mice,
which was based on a previous study (18); the experimental schedule is shown
in Fig. 1. The mice were randomly
divided into six groups (n=8) after being allowed to acclimate for
1 week. The groups were as follows: Control, model, Pred (Pred at
10 mg/kg), paeonol-high (PH; 200 mg/kg), paeonol-medium (PM; 100
mg/kg) and paeonol-low (PL; 50 mg/kg). Preoperative dorsal hair
removal was conducted under isoflurane anesthesia, and pectin feed
containing the analgesic carprofen was available for 3 days
following shaving in individually ventilated cage. The shaved
dorsal skin of the mice was sensitized with 200 µl 1% DNCB every
other day for the first week, excluding the control group in which
acetone and olive oil (3:1 v/v) were topically applied.
Subsequently, DNCB (0.5%) was applied twice a week for a further 3
weeks to stimulate and maintain inflammation. Olive oil was used to
moisturize the skin lesions. Mice in the drug therapy groups were
intragastrically administered the corresponding treatments every
other day alongside DNCB for the last 3 weeks. Meanwhile, the model
group was exposed to identical stimuli alongside saline treatment.
Following 4 weeks, the mice were weighed and anesthetized via
intraperitoneal injection of 70 mg/kg pentobarbital sodium (cat.
no. P3761; Sigma-Aldrich; Merck KGaA), blood samples were collected
from the eye socket, and the mice were sacrificed by cervical
dislocation after 24 h. Samples of blood, ear skin and spleens were
obtained for pathological analysis.
Cell culture
The mouse mastocytoma cell line P815 (China
Infrastructure of Cell Line Resources, Beijing) was cultured in
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
and 1% penicillin-streptomycin (both from Gibco; Thermo Fisher
Scientific, Inc.) in a 5% CO2 atmosphere at 37°C. The
mast cells were seeded in 6-well plates and maintained overnight.
Compound 48/80 (10 µg/ml; C48/80; cat. no. C2313; Sigma-Aldrich;
Merck KGaA) was used to activate the cells and various
concentrations of paeonol (375, 750 and 1,500 µg/ml) were
additionally added to the medium. The cells were co-treated in a 5%
CO2 atmosphere at 37°C. Cells and supernatants were
harvested after 12 h.
Dynamic evaluation of dermatitis
Lesion severity scoring was used to estimate the
severity of the dermatitis on the back regions of the mice each
week; this included scoring of the erythema/hemorrhage, edema,
excoriation/erosion and scaling/dryness (18). The score for each was determined as
follows: No symptoms, 0; mild symptoms, 1; moderate symptoms, 2;
and severe symptoms, 3. The dermatitis score was calculated as the
sum of the individual scores. Scratching behavior was recorded at
the same time.
Histopathological alterations
Dorsal skin and ear samples were fixed in 10%
buffered formalin for 24 h at room temperature, embedded in
paraffin and sectioned into 5-µm slices. The skin sections were
stained with hematoxylin and eosin (H&E) according to the
manufacturer's protocol (cat. no. G1120; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China), and the general tissue
features were observed under a light microscope (Zeiss GmbH, Jena,
Germany; magnification, ×200). Toluidine blue staining (cat. no.
G1436; Beijing Solarbio Science & Technology Co., Ltd.) was
applied for measurement of mast cell infiltration, and the number
of mast cells was counted in five randomly selected fields of view
under a light microscope (magnification, ×200). All procedures were
performed according to the manufacturer's protocol at room
temperature.
Flow cytometry
Spleens were harvested and weighed under sterile
conditions. Single-cell suspensions were obtained after the
isolated spleens were minced through a 70-µm mesh, in order to
determine proportional changes in the Th1/Th2 balance. A total of
1×106 cells were suspended in fixation/permeabilization
solution for 20 min at 4°C (Cytofix/Cytoperm Solution kit; BD
Biosciences, Franklin Lakes, NJ, USA) and were then stained with
fluorescein isothiocyanate (FITC)-conjugated mouse anti-cluster of
differentiation (CD)4 (1:200; cat. no. 35-0041; Tonbo Biosciences,
San Diego, CA, USA). Phorbol myristate acetate (10 ng/ml) in PBS
and ionomycin (1 µg/ml) in PBS were used to stimulate the cells for
intracellular cytokine detection at 37°C and 5% CO2 for
6 h. Subsequently, allophycocyanin (APC) anti-interferon (IFN)-γ
(1:1,000; cat. no. 20-7311) and phycoerythrin anti-IL-4 (1:400;
cat. no. 50-7041; both from Tonbo Biosciences) antibodies were
applied for 20 min at room temperature. Samples were analyzed using
a flow cytometer (FACSCalibur) and CellQuest Pro 5.1 software (both
from BD Biosciences). In order to assess the effects of paeonol on
immune function, the spleen index was calculated as: Spleen index =
(spleen weight/body weight) ×100.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was conducted to detect the mRNA expression
levels of various cytokines, according to the manufacturers'
protocols, and the conditions were similar to those described in a
previous study (16). Total RNA
was isolated from the excised skin using TRIzol® and
purified using the NucleoSpin® RNA Clean-up kit.
Following generation of cDNA using the AffinityScript Multiple
Temperature cDNA Synthesis kit according to the manufacturer's
protocol. The real-time PCR FastStart Universal Master Mix (Roche
Diagnostics) was used to determine the relative expression levels
of genes with an ABI 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The gene-specific
primers are listed in Table I. The
cycle parameters were as follows: 95°C for 10 min, followed by 50
cycles at 95°C for 15 sec and 60°C for 60 sec. β-actin was used as
a reference gene to normalize the data, which were quantitatively
analyzed using the 2−ΔΔCq method (19).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Target gene | Direction | Sequence
(5′-3′) | Amplicon size
(bp) |
|---|
| IL-4 | Forward |
TCGTCTGTAGGGCTTCCAAGGTGCT | 166 |
|
| Reverse |
GTGGACTTGGACTCATTCATGGTGC |
|
| IL-13 | Forward |
GTCAACAACCCACAGGTCCAG | 108 |
|
| Reverse |
TCAGCAGCGACTCCTTTTCC |
|
| IL-31 | Forward |
CCTCAGACTACCTCAACCGTTCC | 191 |
|
| Reverse |
AGGCTCCCTCTTCAGGACCAG |
|
| TSLP | Forward |
CTCAATCCTATCCCTGGCTG | 129 |
|
| Reverse |
TGCCATTTCCTGAGTACCGT |
|
| β-actin | Forward |
GCCTTCCTTCTTGGGTAT | 97 |
|
| Reverse |
GGCATAGAGGTCTTTACGG |
|
Western blot analysis
In order to investigate the possible mechanism
underlying the effects of paeonol on AD, the expression levels of
proteins associated with the mast cell signaling pathway were
analyzed. The skin samples and P815 cells were lysed in
radioimmunoprecipitation assay lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) supplemented with phenylmethane
sulfonyl fluoride (Thermo Fisher Scientific, Inc.) and protein
concentrations were estimated using the bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). Subsequently, 30 µg
total protein was loaded in each lane. Proteins were separated
using 10% SDS-PAGE and were transferred onto polyvinylidene
fluoride membranes by electroblotting at 4°C. Following blocking
using 5% bovine serum albumin (Amresco, LLC, Solon, OH, USA) for 30
min at room temperature, the membrane fractions were incubated with
mouse anti-p-p38 mitogen-activated protein kinase (MAPK; 1:1,000;
cat. no. 9216), rabbit anti-p-ERK1/2 (1:2,000; cat. no. 4370),
rabbit anti-p38 MAPK (1:1,000; cat. no. 8690), mouse anti-ERK1/2
(1:2,000; cat. no. 4696) (all from Cell Signaling Technology,
Inc.), mouse anti-GAPDH (1:20,000; cat. no. YM3029; ImmunoWay
Biotechnology Company, Plano, TX, USA), rat anti-IL-4 (1:1,000;
cat. no. sc-32242), mouse anti-IL-13 (1:1,000; cat. no. sc-393365)
(both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit
anti-IL-31 (1:1,000; cat. no. ab102750; Abcam, Cambridge, MA, USA)
and rabbit anti-TSLP (1:500; cat. no. ab188766; Abcam) primary
antibodies at 4°C overnight. Subsequently, IRDye 700DX- or
800DX-conjugated secondary antibodies immunofluorescence was
assessed using an Odyssey infrared imaging system (LI-COR
Biosciences, Lincoln, NE, USA). The secondary antibodies used were
the following: Goat anti-mouse immunoglobulin G (IgG; conjugated to
Alexa Fluor Plus 800; 1:10,000; cat. no. A32730; Invitrogen; Thermo
Fisher Scientific, Inc.), goat anti-rabbit IgG (conjugated to Alexa
Fluor Plus 680; 1:10,000; cat. no. A32734; Invitrogen; Thermo
Fisher Scientific, Inc.) and goat anti-rat IgG (conjugated to Alexa
Fluor Plus 680; 1:10,000; cat. no. A21096; Invitrogen; Thermo
Fisher Scientific, Inc.). Image Pro Plus (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA) was used for
densitometry.
Cell viability assay
Cell viability was measured using the Cell Counting
kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan),
according to the manufacturer's protocol. Briefly, P815 cells were
seeded in 96-well plates (1×104 cells/well) with various
concentrations of paeonol for 12, 24 and 48 h, and were then
maintained in growth media in an atmosphere containing 5%
CO2 at 37°C for 2 h. The mean optical density of the
cells in each group was used to identify the non-toxic
concentration of paeonol.
ELISA
The concentrations of IgE and inflammatory cytokines
in the serum and supernatants were determined. Whole blood was
collected and allowed to clot for 30 min at room temperature. Whole
blood was centrifuged at 1,200 × g for 15 min and the serum samples
were then stored at −80°C until use. The serum levels of IgE and
inflammatory cytokines were determined using corresponding ELISA
kits (IgE, cat. no. CSB-E07983m; IL-4, cat. no. CSB-E04634m;
Cusabio Technology LLC, Houston, TX, USA), according to the
manufacturer's protocols.
P815 cells were seeded in 12-well culture plates at
a density of 1×106 cells/ml and pretreated with various
concentrations (375, 750 and 1,500 µg/ml) paeonol for 24 h,
followed by treatment with C48/80 (1 µg/ml) for 2 h. The expression
levels of TNF-α (cat. no. CSB-E04741m) and histamine (cat. no.
CSB-E07043m) (both from Cusabio Technology LLC) in the supernatants
were determined using mouse ELISA kits, according to the
manufacturer's protocols. Standard curves were generated using
purified recombinant TNF-α and histamine at various dilutions.
Statistical analysis
All statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). The results are
presented as the mean ± standard deviation. The experiments were
performed at least three independent times. Statistical analysis of
the results was performed using an independent t-test; when three
or more groups were compared, one-way analysis of variance followed
by least-significant difference post hoc test was used. P-values
are two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
Paeonol ameliorates the morphological
features of DNCB-induced AD-like lesions
The skin of the model group exhibited AD-like signs
and symptoms that began with infiltrative erythema, edema, pruritus
and hemorrhage, and were followed by erosion, scratching,
excoriation and dryness, which is similar to the clinical
manifestation of AD. No alterations in the back skin of the control
mice were identified. During the same period, the mice that were
orally administered paeonol exhibited less severe pathological
alterations in a dose-dependent manner (Fig. 2A-C).
 | Figure 2.Oral administration of paeonol
inhibits DNCB-induced AD-like skin lesions in BALB/c mice. (A)
Typical pathological presentation of AD-like lesions induced by
DNCB application. (B) Severity scoring of lesions was assessed
daily using the Lesion severity scoring index. Five signs and
symptoms (itching, erythema, edema, excoriation and dryness) were
each scored on a scale between 0 and 3 (0, none; 1, mild; 2,
moderate; and 3, severe). (C) Scratching behavior was observed
daily for 15 min. Pathological observation of the (D) back skin and
(E) ear tissues (hematoxylin and eosin staining; magnification,
×200; scale bar, 50 µm). (F) Ear swelling and (G) epidermal
thickness in each group. Data are presented as the means ± standard
deviation (n=8). **P<0.01 vs. the control group;
#P<0.05, ##P<0.01 vs. the model group.
AD, atopic dermatitis; DNCB, 1-chloro-2,4-dinitrobenzene; Pae-H,
paeonol-high (200 mg/kg); Pae-L, paeonol-low (50 mg/kg); Pae-M,
paeonol-medium (100 mg/kg); Pred, prednisolone. |
H&E staining of the back skin and ear tissue of
the mice revealed hyperkeratosis, acanthosis and increased
perivascular infiltration of inflammatory cells in the model group
compared with the normal skin structure of the control group.
Paeonol treatment significantly reduced epidermal thickness and the
degree of ear swelling (Fig.
2D-G).
Paeonol redresses the Th1/Th2 cell
balance
In order to investigate the ratio of Th1/Th2 cells
in allergically inflamed skin, spleen cells were collected and the
proportions of Th cell subsets were detected. There was an
increased percentage of the Th2 subset in the model group, whereas
treatment with the high dose of paeonol corrected the imbalance by
inhibiting the Th2 immune reaction (Fig. 3). The percentages of Th1 (CD4
positive and IFN-γ APC positive) or Th2 cells (CD4 positive and
IL-4PE positive) in all lymphocytes are presented in the second
quadrant of the scatter plots, corresponding to double positive
cells (Fig. 3A and D). The
percentages of Th2 (IL-4PE positive and IFN-γ APC negative) and Th1
cells (IFN-γ APC positive and IL-4PE negative) in the CD4 positive
lymphocytes are presented in the first and third quadrant of the
scatter plots, respectively (Fig. 3B
and C). The spleen index in the model group increased compared
with the control group. Paeonol treatment decreased significantly
the spleen index compared with the model group (Fig. 3E).
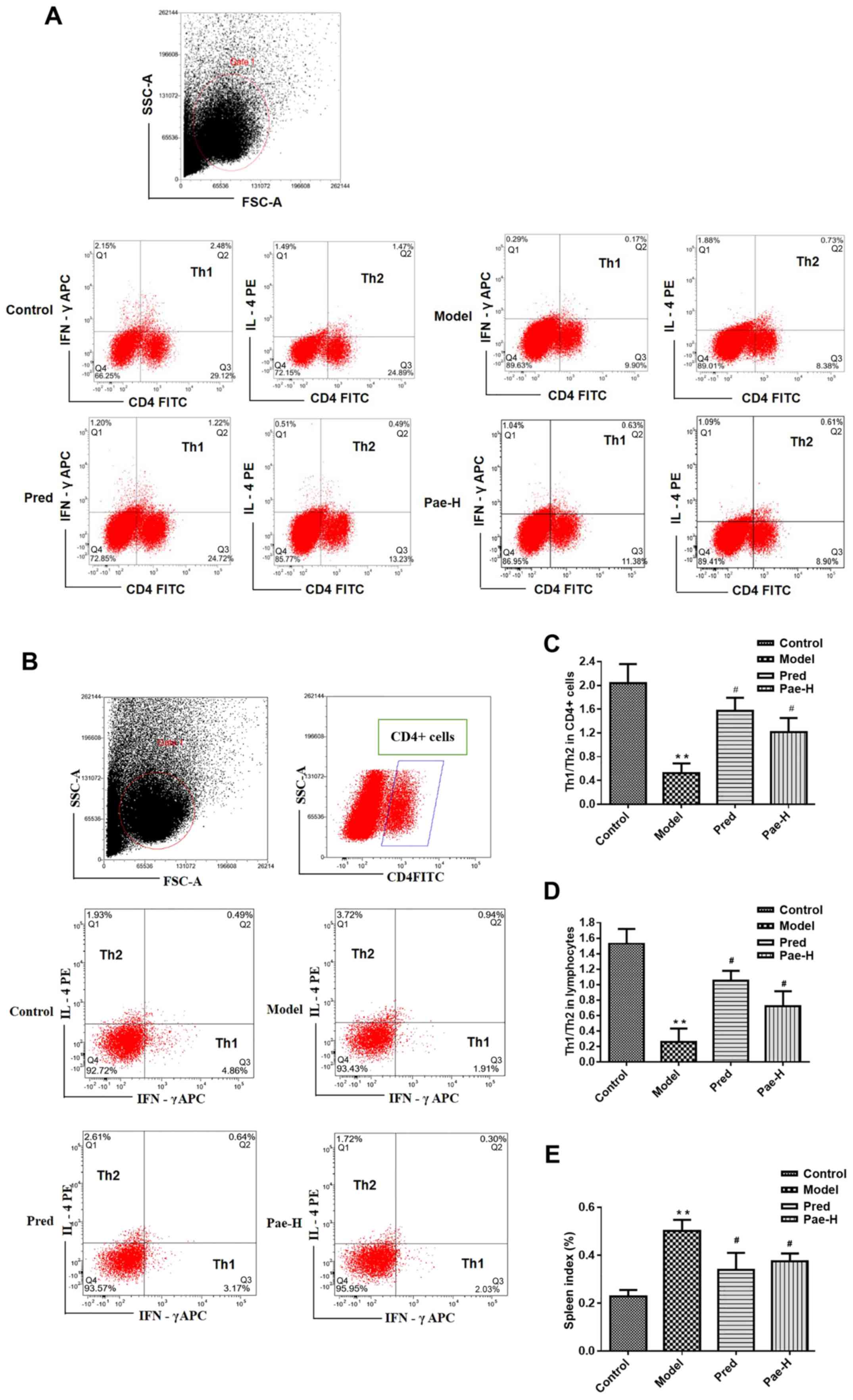 | Figure 3.Paeonol redresses the Th1/Th2 cell
imbalance and inhibits immune responses. (A) Ratio of Th1/Th2
subset cells in the (A) spleen and (B) CD4+ lymphocytes.
Statistical analysis of the (C) spleen and (D) CD4+
lymphocyte data in (A and B), respectively. (E) Spleen index.
**P<0.01 vs. the control group; #P<0.05 vs. the
model group. APC, allophycocyanin; CD4, cluster of differentiation
4; FITC, fluorescein isothiocyanate; FSC, forward scatter; IFN-γ,
interferon-γ; IL, interleukin; Pae-H, paeonol-high (200 mg/kg); PE,
phycoerythrin; Pred, prednisolone; SSC, side scatter; Th, T
helper. |
Paeonol reduces the mRNA and protein
expression levels of pro-inflammatory and immune cytokines in
dorsal skin lesions
Alterations in the mRNA and protein expression
levels of IL-4, IL-13, IL-31 and thymic stromal lymphopoietin
(TSLP) were analyzed in order to elucidate the anti-inflammatory
and immunosuppressive effects of paeonol. The mRNA expression
levels of IL-4, IL-13, IL-31 and TSLP were increased in the model
group compared with in the control group. Conversely, treatment
with a high dose of paeonol suppressed DNCB-induced cytokine
expression, and the protein expression levels of IL-4 and TSLP were
also decreased in the paeonol-treated group (Fig. 4).
Paeonol downregulates mast cell
infiltration in the dermis, and IgE and inflammatory cytokine
levels in the serum
The DNCB-induced skin lesions exhibited increased
infiltration of mast cells compared with in the control group,
whereas administration of a high dose of paeonol markedly reduced
the number of mast cells compared with in the model group.
Treatment with the medium and low doses of paeonol had no
significant effect (Fig. 5A and
B).
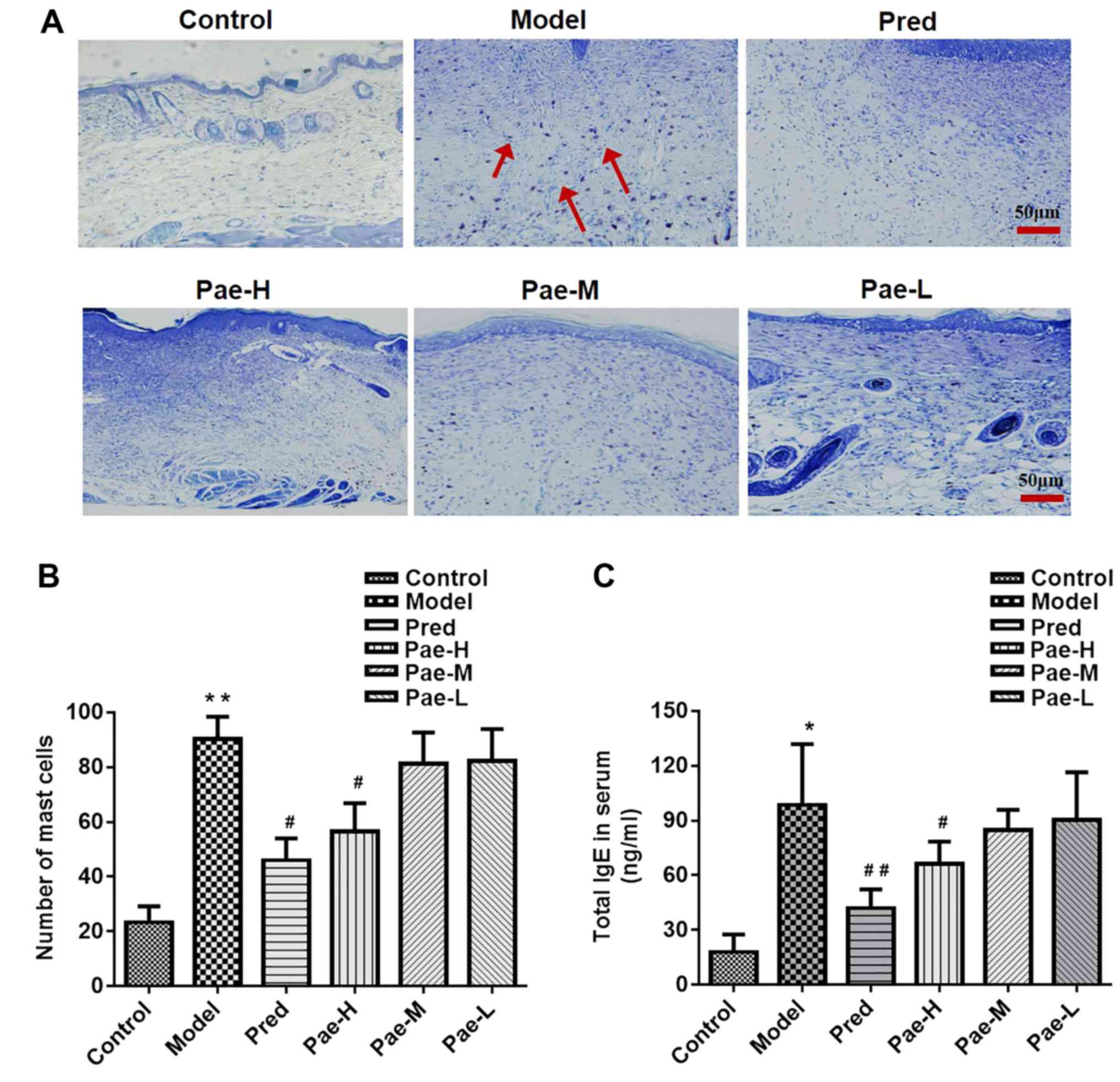 | Figure 5.Paeonol reduces the number of mast
cells in the dermis, and the concentration of IgE and inflammatory
cytokines in the serum. (A) Mast cell infiltration in the dorsal
skin was determined using toluidine blue staining (magnification,
×200; scale bar, 50 µm). Red arrows indicate the stained mast
cells. (B) Mast cell counts in the different groups. Serum levels
of (C) IgE, and the inflammatory cytokines (D) IL-4 and (E)
histamine in the different groups, as measured using an ELISA. Data
are presented as the means ± standard deviation (n=8). *P<0.05,
**P<0.01 vs. the control group; #P<0.05,
##P<0.01 vs. the model group. IgE, immunoglobulin E;
IL-4, interleukin-4; Pae-H, paeonol-high (200 mg/kg); Pae-L,
paeonol-low (50 mg/kg); Pae-M, paeonol-medium (100 mg/kg); Pred,
prednisolone. |
High levels of serum IgE were detected in the
DNCB-treated group, and high-dose paeonol reduced these levels
(Fig. 5C). The serum levels of the
inflammatory factors IL-4 and histamine were higher in the model
group than in the control group. High-dose paeonol markedly reduced
the levels of the inflammatory cytokines compared with in the model
group (Fig. 5D and E).
Paeonol decreases p-p38 and p-ERK
proteins in a mouse model of AD
The present study further explored how paeonol
suppresses mast cells and investigated the effects of paeonol on
MAPK signaling in skin lesions. The results revealed that the
protein expression levels of p-p38 and p-ERK were reduced in the
paeonol-high dose group compared with in the model group (Fig. 6A and B).
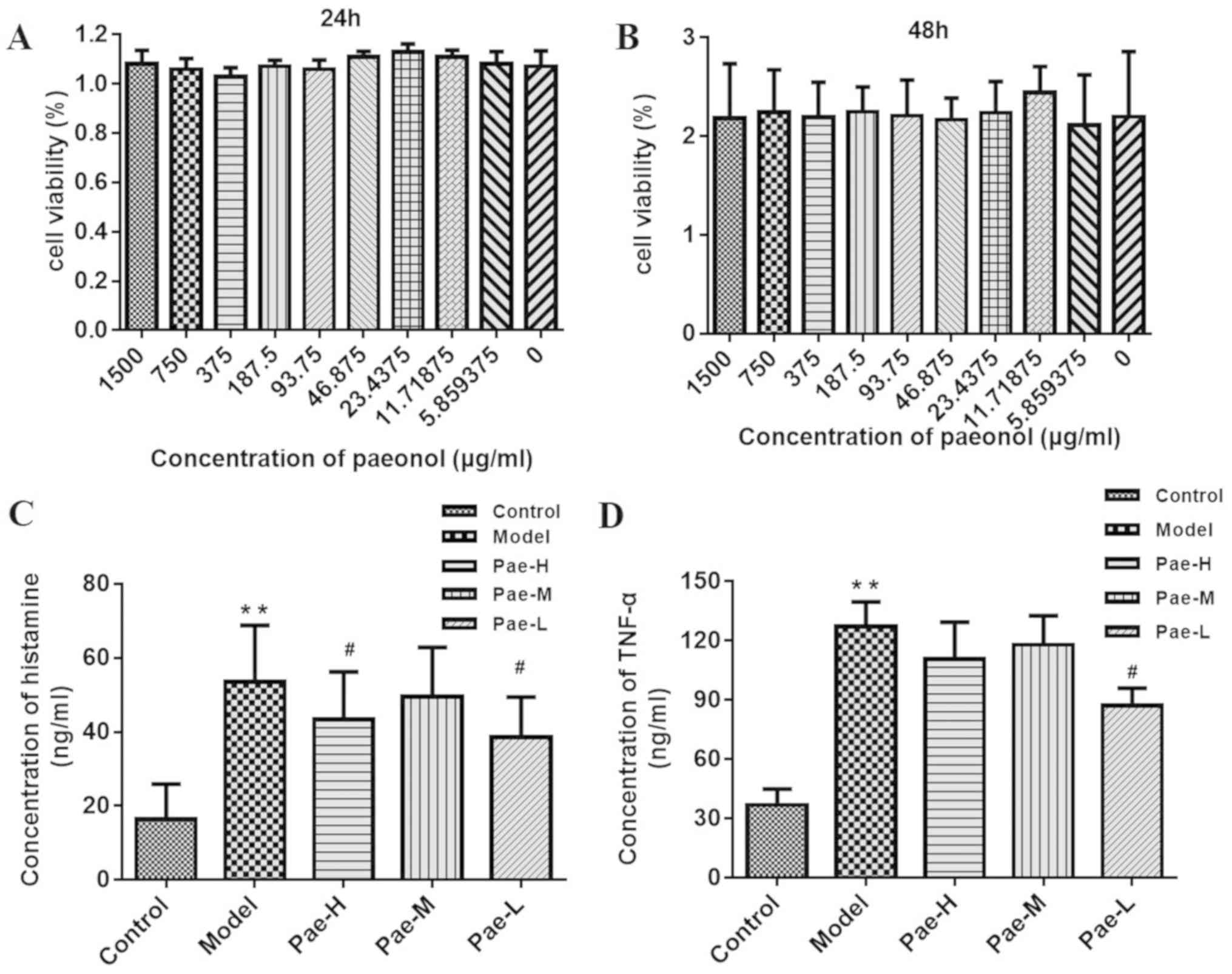
Paeonol reduces the levels of
histamine and TNF-α secreted by P815 cells
This study investigated whether paeonol affected the
viability of P815 cells using the CCK-8 assay; the results
demonstrated that paeonol did not exert a cytotoxic effect on P815
cells (Fig. 7A and B). Safe
concentrations were used in subsequent experiments. Compared with
the levels in untreated P815 cells, the concentrations of histamine
and TNF-α in the C48/80-treated groups were markedly increased.
There was a marked reduction in histamine and TNF-α in the
paeonol-pretreated groups compared with in the model group. The
inhibitory effect in the low-dose group was significant for both
histamine and TNF-α (Fig. 7C and
D).
Paeonol inhibits protein expression
levels of p-p38 and p-ERK in activated P815 cells
Western blot analysis was performed to investigate
the effects of paeonol on MAPK signaling in mast cells. The results
demonstrated that the protein expression levels of p-p38 and p-ERK
were reduced in the paeonol-treated groups compared with in the
C48/80-treated group. Consistent with the results of the ELISA, a
low dose of paeonol induced a more powerful effect than the other
two doses. These results indicated that paeonol may exert its
therapeutic action by blocking the p38/ERK/MAPK signaling pathway
(Fig. 8).
Discussion
Paeonol is an active component extracted from the
TCM Cortex Moutan, which is widely used and has high clinical
development prospects (20,21).
Numerous pharmacological and clinical studies have confirmed the
biological properties of paeonol, including anti-inflammatory,
anti-bacterial and antitumor effects (22,23).
Our previous study demonstrated that oral administration of paeonol
results in anti-inflammatory and immunoregulatory effects in BALB/c
mice with psoriasis-like lesions (16). In order to further investigate the
pharmacological activities of paeonol, a series of experiments were
performed on damaged skin.
AD is an allergic and inflammatory skin disorder
that results in itching and recurrent eczematous lesions, and
seriously affects quality of life (24). In addition to genetic and
environmental factors, immunological disarrangement appears to
serve a significant role in the pathogenesis of AD (25). A previous study demonstrated that
increased infiltration of Th cells and related cytokines
contributes to the release of high levels of histamine and numerous
pro-inflammatory factors, thus resulting in itching, dry skin and
eczema (26). Topical therapy is
common practice to alleviate clinical symptoms; however, the side
effects of this treatment include thinner and more sensitive skin.
On the basis of previous studies, it was hypothesized that oral
administration of paeonol may be an effective treatment for
allergic disease, and in vivo and in vitro
experiments were designed to investigate this.
In the present study, an AD-like mouse model was
established via topical application of DNCB, which is a sensitizer
that is used worldwide for chemically inducing contact dermatitis.
The animals that were subjected to repeated DNCB challenge
exhibited clinical and immunological presentations that were
similar to human AD. The irritated skin and ears of the animals
progressively developed into clear allergic reactions with various
symptoms including dryness, scales and pruritus, followed by
erythema, swelling and erosion. Subsequently, experiments to
investigate the anti-atopic effect of paeonol were conducted.
Paeonol markedly improved the skin lesions, with a reduction in the
SCORAD scores and frequency of scratching. Histological examination
of the skin revealed a thicker epidermis and increased inflammatory
infiltration compared with in the control group, whereas these
pathological alterations were significantly ameliorated by oral
administration of paeonol in a dose-dependent manner. Ear thickness
was also measured, in order to confirm the effectiveness of
paeonol. The H&E staining results revealed a thicker ear dermis
in the model group, whereas the paeonol-treated groups exhibited a
significant reduction in thickness compared with in the model
group. These results demonstrated that DNCB may induce damage to
the epidermis and dermis, whereas paeonol exhibited clear
anti-atopic activity, and was involved in regulating the abnormal
condition of the skin.
The immune dysfunction that results from a
disturbance in the Th1/Th2 balance serves a role in the progression
of allergic inflammation (27).
Therefore, the proportion of Th1 and Th2 cells in the spleen and
lymphocytes of the animals in the present study was detected. The
results revealed that the proportion of Th1 cells was markedly
reduced following exposure to DNCB. Paeonol significantly regulated
this effect by inhibiting the Th2 immune response.
Various inflammatory cytokines are involved in
regulating and directing the nature of AD, including IL-4, IL-13,
IL-31 and TSLP, and they are predominantly Th2-derived cytokines
(28,29). IL-4 and IL-13, which act as the key
drivers for isotype switching to IgE, generation of inflammatory
factors and receptor expression on the surface of mast cells,
commonly activate IL-4 receptor (IL-4R) and subsequently
downregulate skin barrier proteins, thus impairing the skin barrier
(30,31). Therapies that target IL-4R and lead
to the inhibition of the IL-4 and IL-13 signaling pathways are key
treatment targets in the complex pathological mechanism of AD
(32,33). TSLP, which is capable of eliciting
a powerful immune response, is secreted by the epithelial cells of
damaged skin. Released TSLP results in priming of resident
dendritic cells, which induces susceptibility to AD and Th2 immune
deviation (29). IL-31 is thought
to serve a critical role in the pathogenesis of AD, particularly in
mediating skin pruritus by transmitting the itch sensation to the
central nervous system (34,35).
Consistent with previous studies, increased mRNA expression levels
of IL-4, IL-13, IL-31 and TSLP were detected in AD-like mouse skin
in the present study (36,37). Furthermore, there was a reduction
in IL-4, IL-13, IL-31 and TSLP mRNA expression following paeonol
treatment. These findings provide further evidence to suggest that
paeonol may downregulate the Th2 immune response.
Localized mast cells serve a key role in the
development of allergic diseases and the activated state of mast
cells may be responsible for signs of dermatitis (38). Toluidine blue staining in the
present study revealed an increased number of mast cells in the
skin lesions of the model group, whereas paeonol treatment
significantly reduced the number in a dose-dependent manner.
Increased serum IgE levels are the hallmark of allergic disease and
trigger the activation of mast cells (39). Enhanced expression levels of IgE
and IL-4 were observed in the serum following chemical stimulation
in the present study, further validating the feasibility of the
animal model. Furthermore, the anti-inflammatory and anti-allergy
activities of paeonol were determined. Therefore, the underlying
mechanism was subsequently investigated, and the protein expression
levels of p-p38 and p-ERK were detected in vivo; the
expression levels of these proteins were significantly reduced in
the high-dose paeonol-treated group.
Allergic reactions are characterized by activation
of mast cells and they are a key cause of the condition worsening
(40). To the best of our
knowledge, there are no studies regarding the effects of paeonol on
mast cells or the allergic response. Following the results obtained
from the animal experiments of the present study, the effects on
inflammatory effector cells were investigated. Activated mast cells
express and release pro-inflammatory cytokines, including TNF-α
(41). The in vitro
experiments demonstrated that pretreatment with paeonol inhibited
the expression of TNF-α with no cytotoxic effect. Histamine is a
biogenic amine that is primarily stored by mast cells and is
released when they are activated, leading to immediate allergic
symptoms (42). Paeonol diminished
the histamine content in the supernatant of P815 cells. Since the
MAPK signaling cascade is an important signaling pathway in immune
responses (43), the proteins
associated with this transduction pathway were investigated in the
present study. Application of paeonol attenuated the
phosphorylation of p38 and ERK, inhibiting p38 and ERK activation
in the C48/80-stimulated P815 cells, which is essential for the
degranulation of mast cells. Therefore, it was hypothesized that
prevention of mast cell activation by inhibiting the MAPK/ERK/p38
signaling pathway was one critical characteristic of the
anti-allergic activity of paeonol. In vitro, the effects of
paeonol were not revealed to be dose-dependent, and numerous
factors may be responsible for this effect, including the
solubility of paeonol. The present results suggested the molecular
mechanism underlying the effects of paeonol on mast cells. Our
future studies aim to explore the effect of lower dosages within
safe levels, and determine the potential mechanism underlying the
effects of paeonol on C48/80-induced P815 cells. Further studies
are required to determine whether paeonol can inhibit AD via
immunoregulation of T and mast cells.
Numerous studies have identified various biological
effects of paeonol, including inhibition of the proliferation of
SGC-7901 cells, induction of tumor cell apoptosis in breast cancer,
and anti-inflammatory, cardioprotective and neuroprotective effects
(44,45). Our previous study (16) revealed that paeonol inhibits
inflammation and blocks the progression of psoriasis. To the best
of our knowledge, the present study is the first to determine the
effects of paeonol on epidermal thickness, mast cell activation and
proliferation, and the balance of Th1/Th2 cells in allergic
disease.
In conclusion, to the best of the authors'
knowledge, the present study is the first to demonstrate that
paeonol may be used to treat AD. The application of paeonol may
correct the proportional imbalance of the immune system in order to
treat inflammatory skin disease. The precise mechanism of action of
paeonol on target proteins requires further investigation,
including that which utilizes modern biotechnology, such as
molecular docking.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation Project (grant nos. 81403410 and 81673989) and
the Beijing Municipal Project (grant no.
PXM2017_026273_000001).
Availability of data and materials
All datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM, YW and PL conceived and supervised the study.
YM, ZL and CZ performed the animal experiments. YM, LuZ and XX
performed physiological test and the pathological experiments. YM,
TD and JZ performed cell experiments. YM, NW, YL and LeZ performed
the data analysis. YM drafted the manuscript and made substantial
contributions to the manuscript. YM interpreted the data. All
authors revised and approved the present manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the National Guidelines on Laboratory Research and
were approved by the Animal Ethics Committee of Capital Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sidbury R and Khorsand K: Evolving
concepts in atopic dermatitis. Curr Allergy Asthma Rep. 17:422017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griffiths CE, van de Kerkhof P and
Czarnecka-Operacz M: Psoriasis and atopic dermatitis. Dermatol Ther
(Heidelb). 7:31–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Genuneit J, Seibold AM, Apfelbacher CJ,
Konstantinou GN, Koplin JJ, La Grutta S, Logan K, Perkin MR, Flohr
C and Task Force ‘Overview of Systematic Reviews in Allergy
Epidemiology (OSRAE)’ of the EAACI Interest Group on Epidemiology:
Overview of systematic reviews in allergy epidemiology. Allergy.
72:849–856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guttman-Yassky E, Krueger JG and Lebwohl
MG: Systemic immune mechanisms in atopic dermatitis and psoriasis
with implications for treatment. Exp Dermatol. 27:409–417. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hello M, Aubert H, Bernier C, Néel A and
Barbarot S: Atopic dermatitis of the adult. Rev Med Interne.
37:91–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liour SS, Tom A, Chan YH and Chang TW:
Treating IgE-mediated diseases via targeting IgE-expressing B cells
using an anti-CεmX antibody. Pediatr Allergy Immunol. 27:446–451.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stokes J: Anti-IgE Treatment for disorders
other than asthma. Front Med (Lausanne). 4:1522017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Kouchkovsky DA, Ghosh S and Rothlin CV:
Negative regulation of type 2 Immunity. Trends Immunol. 38:154–167.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osinka K, Dumycz K, Kwiek B and Feleszko
W: Novel therapeutic approaches to atopic dermatitis. Arch Immunol
Ther Exp (Warsz). 66:171–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saluja R, Khan M, Church M and Maurer M:
The role of IL-33 and mast cells in allergy and inflammation. Clin
Transl Allergy. 5:332015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malik K, Heitmiller KD and Czarnowicki T:
An update on the pathophysiology of atopic dermatitis. Dermatol
Clin. 35:317–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin X, Wang J, Xia ZM, Shang CH, Chao QL,
Liu YR, Fan HY, Chen DQ, Qiu F and Zhao F: Antiinflammatory and
anti-oxidative activities of paeonol and its metabolites through
blocking MAPK/ERK/p38 signaling pathway. Inflammation.
39:4344462016. View Article : Google Scholar
|
|
13
|
Liu CM, Yang HX, Ma JQ, Yang W, Feng ZJ,
Sun JM, Cheng C, Li J and Jiang H: Role of AMPK pathway in
lead-induced endoplasmic reticulum stress in kidney and in
paeonol-induced protection in mice. Food Chem Toxicol Oct.
122:87–94. 2018. View Article : Google Scholar
|
|
14
|
Choy KW, Mustafa MR, Lau YS, Liu J,
Murugan D, Lau CW, Wang L, Zhao L and Huang Y: Paeonol protects
against endoplasmic reticulum stress-induced endothelial
dysfunction via AMPK/PPAR delta signaling pathway. Biochem
Pharmacol. 116:51–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MH, Lin AH, Lee HF, Ko HK, Lee TS and
Kou YR: Paeonol attenuates cigarette smoke-induced lung
inflammation by inhibiting ROS-sensitive inflammatory signaling.
Mediators Inflamm. 2014:6518902014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng Y, Wang M, Xie X, Di T, Zhao J, Lin
Y, Xu X, Li N, Zhai Y, Wang Y and Li P: Paeonol ameliorates
imiquimod-induced psoriasis-like skin lesions in BALB/c mice by
inhibiting the maturation and activation of dendritic cells. Int J
Mol Med. 39:1101–1110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi JH, Kim HG, Jin SW, Han EH, Khanal T,
Do MT, Hwang YP, Choi JM, Chun SS, Chung YC, et al: Topical
application of Pleurotus eryngii extracts inhibits
2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice
by the regulation of Th1/Th2 balance. Food Chem Toxicol. 53:38–45.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charlton E: Committee on ethical issues of
the international association for the study of pain. Pain.
63:277–278. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, Zhong S, Kong R, Shao H, Wang C,
Piao H, Lv W, Chu X and Zhao Y: Paeonol alleviates
interleukin-1beta-induced inflammatory responses in chondrocytes
during osteoarthritis. Biomed Pharmacother. 95:914–921. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JH, Seo CS and Shin HK: Development of
validated determination of the eleven marker compounds in
Gyejibokryeong-hwan for the quality assessment using HPLC analysis.
Arch Pharm Res. 38:52–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu TH, Cao SW and Yu YY: Synthesis,
characterization and biological evaluation of paeonol
thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int
J Biol Macromol. 62:589–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scheerer C and Eyerich K: Pathogenesis of
atopic dermatitis. Hautarzt. 69:191–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Köberle M and Biedermann T: Microbiome,
atopic eczema and blockade of type 2 immunity. Hautarzt.
69:197–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soumelis V: Molecular and cellular
discoveries in inflammatory dermatoses. J Eur Acad Dermatol
Venereol. 31:3–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashimoto Y, Arai I, Nakanishi Y, Sakurai
T, Nakamura A and Nakaike S: Scratching of their skin by NC/Nga
mice leads to development of dermatitis. Life Sci. 76:783–794.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eyerich K and Novak N: Immunology of
atopic eczema: Overcoming the Th1/Th2 paradigm. Allergy.
68:974–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manti S, Chimenz R, Salpietro A, Colavita
L, Pennisi P, Pidone C, Sturiale M, Arrigo T, Miraglia Del Giudice
M, Salpietro C and Cuppari C: Atopic Dermatitis: Expression of
Immunological Imbalance. J Biol Regul Homeost Agents. 29:13–7.
2015.PubMed/NCBI
|
|
29
|
Lawrence MG, Steinke JW and Borish L:
Cytokine-targeting biologics for allergic diseases. Ann Allergy
Asthma Immunol. 120:376–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DE, Clark AK, Tran KA and Shi VY: New
and emerging targeted systemic therapies: A new era for atopic
dermatitis. J Dermatolog Treat. 29:364–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mc Cormick SM and Heller NM: Commentary:
IL-4 and IL-13 receptors and signaling. Cytokine. 75:38–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hurdayal R and Brombacher F: The role of
IL-4 and IL-13 in cutaneous Leishmaniasis. Immunol Lett.
16:179–183. 2014. View Article : Google Scholar
|
|
33
|
Di Lernia V: Therapeutic strategies in
extrinsic atopic dermatitis: Focus on inhibition of IL-4 as a new
pharmacological approach. Expert Opin Ther Targets. 19:87–96. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Danso MO, van Drongelen V, Mulder A, van
Esch J, Scott H, van Smeden J, El Ghalbzouri A and Bouwstra JA:
TNF-alpha and Th2 cytokines induce atopic dermatitis-like features
on epidermal differentiation proteins and stratum corneum lipids in
human skin equivalents. J Invest Dermatol. 134:1941–1950. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nygaard U, Hvid M, Johansen C, Buchner M,
Fölster-Holst R, Deleuran M and Vestergaard C: TSLP, IL-31, IL-33
and sST2 are new biomarkers in endophenotypic profiling of adult
and childhood atopic dermatitis. J Eur Acad Dermatol Venereol.
30:1930–1938. 2016.PubMed/NCBI
|
|
36
|
Han H, Roan F and Ziegler SF: The atopic
march: Current insights into skin barrier dysfunction and
epithelial cell-derived cytokines. Immunol Rev. 278:116–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cianferoni A and Spergel J: The importance
of TSLP in allergic disease and its role as a potential therapeutic
target. Expert Rev Clin Immunol. 10:1463–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bağci IS and Ruzicka T: IL-31: A new key
player in dermatology and beyond. J Allergy Clin Immunol.
141:858–866. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Otsuka A, Nomura T, Rerknimitr P, Seidel
JA, Honda T and Kabashima K: The interplay between genetic and
environmental factors in the pathogenesis of atopic dermatitis.
Immunol Rev. 278:246–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Silvestre MC, Sato MN and Reis VMSD:
Innate immunity and effector and regulatory mechanisms involved in
allergic contact dermatitis. An Bras Dermatol. 93:242–250. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Campana R, Dzoro S, Mittermann I, Fedenko
E, Elisyutina O, Khaitov M, Karaulov A and Valenta R: Molecular
aspects of allergens in atopic dermatitis. Curr Opin Allergy Clin
Immunol. 17:269–277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Korošec P, Gibbs BF, Rijavec M, Custovic A
and Turner PJ: Important and specific role for basophils in acute
allergic reactions. Clin Exp Allergy. 48:502–512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mukai K, Tsai M, Saito H and Galli SJ:
Mast cells as sources of cytokines, chemokines, and growth factors.
Immunol Rev. 282:121–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kawakami T, Kashiwakura J and Kawakami Y:
Histamine-releasing factor and immunoglobulins in asthma and
allergy. Allergy Asthma Immunol Res. 6:6–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Patterson H, Nibbs R, McInnes I and
Siebert S: Protein kinase inhibitors in the treatment of
inflammatory and autoimmune diseases. Clin Exp Immunol. 176:1–10.
2014. View Article : Google Scholar : PubMed/NCBI
|