Introduction
Human skin is the largest organ of the body with a
number of important physiological functions including sensation,
absorption, secretion, excretion and protection, and participates
in various metabolic processes in organisms, maintaining the
stability of the internal environment (1,2).
Extensive damage to skin integrity caused by trauma or disease may
result in severe disability or even mortality (3); therefore, identifying targets for the
prevention and treatment of chronic non-healing wounds is a major
scientific problem that requires further investigation.
Cutaneous wound repair is a multi-step process
involving inflammation, re-epithelialization and tissue remodeling.
The re-epithelialization of keratinocytes (KCs) is a crucial
component of wound repair, remodeling the epidermal barrier through
the migration, proliferation and differentiation of epithelial
cells near the edge of the wound (4). Defects or delays in
re-epithelialization are essential in the complex or slow healing
of wounds (5). The results of a
chronic wound biopsy have revealed that the epithelial cells in the
re-epithelialization stage are active and overproliferated, but
surprisingly had no apparent occurrence in cell migration (6). However, the persistent
re-epithelialization of the wound easily results in abnormal
healing, resulting in scar hyperplasia, secondary infection or
deformity, seriously affecting the health of patients (7). Consequently, it is important to
investigate the molecular mechanism of the KC re-epithelialization
process, and identify a fast and effective way to regulate this
process and to achieve the purpose of preventing and treating
refractory wounds in the clinic.
Forkhead box O3a (FoxO3a), a FoxO protein member of
the Forkhead transcription factor family, located on human
chromosome 6q21, is widely distributed in the human body (8). Its transcriptional activity is mainly
regulated by post-translational modifications including
phosphorylation, acetylation, ubiquitination and methylation
(9). Previous studies have
demonstrated that FoxO3a may integrate different signals to
participate in the regulation of internal environment stability and
serve an integral function in embryonic development, follicular
maturation, glucose homeostasis, cell proliferation and apoptosis
and DNA damage repair (10–12).
In addition, FoxO3a functions as an anti-oncogene by promoting
apoptosis, inhibiting proliferation, controlling the
differentiation of tumor cells and suppressing angiogenesis through
interactions with other transcription factors in the process of
tumorigenesis (13). Upregulation
of FoxO3a expression substantially inhibits the invasion and
migration abilities of colonic carcinoma cells (14). In prostate cancer cells, FoxO3a
negatively regulates the epithelial-mesenchymal transition (EMT)
process by mediating the classical Wnt signaling pathway (15). In the field of wound healing, one
previous study has revealed that a notable decrease in FoxO3a
expression, in wounded human and mouse skin subsequent to an
injury, was observed (16) and
FoxO3a knockout mice have been reported to have a substantially
accelerated rate of wound healing (17). However, whether FoxO3a serves a
similar regulatory function in KC EMT in wound re-epithelialization
has not been reported.
The present study investigated the expression of
FoxO3a in human chronic and acute wound tissues, and the mechanism
of FoxO3a in wound re-epithelialization at a molecular level. The
results may provide a novel entry point for investigating the
mechanism of chronic refractory wound healing.
Materials and methods
Patients and clinical specimens
A total of 20 human chronic (at least 4 weeks after
injury) and acute wound (within one week after injury) specimens
were collected according to the protocol that was ethically
approved by the Research Ethics Committee of The Second Affiliated
Xinqiao Hospital of Army Medical University (Chongqing, China)
between January 2016 and October 2016, and written informed consent
was obtained from each patient prior to surgery. The patients
included 8 males and 12 females, with a median age of 40 years
(range, 8–72 years). All specimens were immediately snap-frozen in
liquid nitrogen and maintained at −80°C until subsequent use.
Immunohistochemistry staining
For immunohistochemical analysis of FoxO3a
expression, the tissues were fixed in 10% formalin for 24 h at room
temperature and embedded in paraffin. 4-µm thick consecutive
sections were cut using microtome, deparaffinized with xylol and
rehydrated with graded ethanol solutions (100, 95, 80 and 50%).
Endogenous peroxidase activity was blocked using 3% hydrogen
peroxide for 10 min at room temperature. The sections were then
subjected to antigen retrieval for 5 min by heating at 100°C in 10
mM of citric acid, pH 6.0. Subsequent to blocking non-specific
binding with normal goat serum (1:20; cat. no. C0265; Beyotime
Institute of Biotechnology) at room temperature for 20 min, the
sections were then incubated with anti-FoxO3a primary antibody
(1:100; cat. no. ab12162; Abcam) at 4°C overnight. The next day,
following three washes with phosphate buffered saline (PBS),
sections were incubated with a goat anti-rabbit horseradish
peroxidase (HRP)-conjugated secondary antibody (1:100; cat. no.
A0208; Beyotime Institute of Biotechnology) for 30 min at room
temperature. Hematoxylin was used as the nuclear counterstain for 3
min at room temperature and 3,3′-diaminobenzidine as the chromogen.
Finally, sections were observed under a light microscope
(magnification, ×200; Nikon Corporation).
Cell culture
The human KC HaCaT cell line was purchased from the
Type Culture Collection of the Chinese Academy of Sciences and
cultured in high glucose Dulbecco's modified Eagle's medium (DMEM;
Hyclone; GE Healthcare Life Sciences) supplemented with 10% fetal
bovine serum (FBS; Hyclone; GE Healthcare Life Sciences), 100 U/ml
penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA)
in an incubator with 5% CO2 at 37°C. For stimulation
experiments, HaCaT cells were seeded in 6-well plates at a density
of 2×105/well. Before stimulation with recombinant
transforming growth factor-β1 (TGF-β1; 10 ng/ml; cat. no. P01137;
R&D Systems, Inc.), the cells were incubated with serum-free
DMEM for 12 h at 37°C. Subsequent to stimulation for 48 h at 37°C,
western blot analysis was performed to analyze EMT-related gene and
FoxO3a expression.
Regulation of FoxO3a expression by
Lentivirus 3×flag-FoxO3a (lenti-3×flag-FoxO3a) and FoxO3a small
interfering (si)-RNA
The lenti-3×flag-FoxO3a and control vector
lenti-3×flag were provided by Shanghai GenePharma Co., Ltd., and
used to establish the HaCaT cells with FoxO3a overexpression. HaCaT
cells were seeded in 6-well plates at a density of
2×105/well and infected with the constructed
lentiviruses (flag-FoxO3a), at a multiplicity of infection of 10,
with 8 µg/ml polybrene. The siRNA sequences for FoxO3a and the
negative control (NC) were 5′-GCUGUCUCCAUGGACAAUATT-3′ and
5′-UUCUCCGAACGUGUCACGUTT-3′, respectively. siRNA (80 nM) was
transfected into HaCaT cells using Lipofectamine™ RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Subsequent to infection or transfection,
cells were incubated for 48 h at 37°C, then they were harvested and
reverse transcription-quantitative (RT-q)PCR and western blot
analysis were performed to analyze FoxO3a expression.
Wound scratch assay
A scratch wound healing assay was performed to
evaluate the migration of cells. Following infection or
transfection, the cells (1×106/well) were collected and
seeded in 6-well plates. When the cells merged into a monolayer, a
sterile 200 µl pipette tip was used to create a straight scratch on
the surface of the cell layer and the cells were cultured in a
serum-free DMEM. Following 24 h of incubation at 37°C, the cells
were washed with PBS twice, the wound healing areas were imaged
under an inverted microscope (magnification, ×200) and the wound
widths were analyzed using ImageJ 1.48 software (National
Institutes of Health).
Cell viability assay
A Cell Counting Kit (CCK)-8 cell viability assay
(Dojindo Molecular Technologies, Inc.) was used to verify that
FoxO3a caused a change in the growth of HaCaT cells according to
the manufacturer's protocol. Briefly, HaCaT cells were plated into
96-well plates at a density of 1×103 cells/well and
cultured in a humidified 37°C, 5% CO2 incubator for 2
days. Then, 10 µl CCK-8 reagent was added to each well, and the
absorbance was detected at 450 nm using a microplate reader.
RT-qPCR
The total RNA of human wound (chronic and acute)
tissues and HaCaT cells was extracted using TRIzol®
regent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol, and 1 µg RNA was reverse transcribed
(reaction conditions: 70°C for 10 min, 42°C for 60 min and 70°C for
10 min) into cDNA using a Reverse Transcriptase kit (Promega
Corporation). GAPDH served as an internal control. The following
primers were used: FoxO3a forward, 5′-AAGCCAGCTACCTTCTCTTCCA-3′ and
reverse, 5′-GTGGCAAGTCAGTCCGAACTGA-3′; and GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. The target gene expression was
examined on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc.) and qPCR was performed using the
SYBR® Premix Ex Taq™ II (Takara Biotechnology Co.,
Ltd.). The thermocycling reaction conditions were as follows: 93°C
for 2 min, followed by 40 cycles of 93°C for 1 min, 55°C for 30 sec
and 72°C for 1 min. Finally, gene expression data were evaluated
according to the 2−ΔΔCq method (18).
Western blot analysis
Total protein from human wound (chronic and acute)
tissues and HaCaT cells was extracted using RIPA Lysis reagent
(cat. no. P0013K; Beyotime Institute of Biotechnology) and
quantified via the bicinchoninic acid assay method. The isolation
of cytoplasmic and nuclear proteins was performed according to
methods published previously (19). Western blot analysis was performed
as previously described (20).
Briefly, total protein (40 µg/lane) was separated by 10% SDS-PAGE
(cat. no. P0690; Beyotime Institute of Biotechnology), transferred
to nitrocellulose membranes (cat. no. FFP33; Beyotime Institute of
Biotechnology). The membranes were blocked with 5% skim milk for 1
h at room temperature and then probed overnight using primary
antibodies at 4°C, including anti-FoxO3a (1:500; cat. no. ab12162),
anti-β-catenin (1:1,000; cat. no. ab2365), anti-Histone H3 (nuclear
marker; 1:2,000; cat. no. ab1791; all Abcam), anti-matrix
metalloproteinase 1 (MMP-1; 1:1,000; cat. no. 54376), anti-MMP-9
(1:1,000; cat. no. 13667), anti-tissue inhibitor of
metalloproteinase 1 (TIMP-1; 1:1,000; cat. no. 8946),
anti-E-cadherin (1:500; cat. no. 3195), anti-N-cadherin (1:500;
cat. no. 13116; all Cell Signaling Technology, Inc.), anti-vimentin
(1:1,000; cat. no. AF1975) and anti-GAPDH (1:3000; cat. no. AF1186;
both Beyotime Institute of Biotechnology). Subsequently, the
membranes were incubated for 1 h at 37°C with a goat anti-rabbit
HRP-conjugated secondary antibody (1:2,000; cat. no. 7074; Cell
Signaling Technology, Inc.). Finally, proteins were visualized
using an enhanced chemiluminescent detection reagent (Beyotime
Institute of Biotechnology) and the blots were analyzed using
ImageJ 1.48 software (National Institutes of Health).
Immunofluorescence
For immunofluorescence, HaCaT cells
(2×105) were plated into a confocal small dish, washed
twice with PBS, fixed with 4% paraformaldehyde for 10 min at room
temperature, permeabilized with 0.5% Triton-X-100/PBS for 5 min and
blocked with 1% BSA for 1 h at room temperature. The cells were
then washed again with PBS twice, and stained with the appropriate
primary antibodies: Anti-E-cadherin (1:200; cat. no. 3195; Cell
Signaling Technology, Inc.); and anti-vimentin (1:100; cat. no.
AF1975; Beyotime Institute of Biotechnology) overnight at 4°C.
Followed by incubation with the appropriate secondary antibodies:
Alexa Fluor® 488-conjugated goat anti-rabbit IgG (1:250;
cat. no. ab150077; Abcam); and Alexa Fluor 555-Labeled Donkey
anti-Rabbit IgG (1:250; cat. no. P0179; Beyotime Institute of
Biotechnology) for 1 h at 37°C, respectively. Subsequent to washing
twice with PBS, Hoechst staining was performed at room temperature
for 3 min and the fluorescence was visualized with a confocal
microscope (magnification, ×400).
Statistical analysis
All experimental data are presented as the mean ± SD
and were analyzed using GraphPad Prism 7 software (GraphPad
Software, Inc.). Statistical differences were obtained via paired
or unpaired Student's t-test between two groups, and a one-way
ANOVA followed by Tukey's post hoc test amongst multiple groups.
P<0.05 was considered to indicate a statistically significant
difference, and each experiment was conducted in triplicate.
Results
Expression and distribution of FoxO3a
in human chronic and acute wound tissues
In the initial set of experiments, in order to
characterize the FoxO3a expression pattern in human chronic and
acute wound tissues, immunohistochemistry was performed on sections
of cutaneous samples collected post-wounding. As presented in
Fig. 1A, the expression of FoxO3a
was substantially upregulated in the chronic wounds compared with
the acute wound tissues. Consistent with these results, the present
study also analyzed the expression levels of FoxO3a using RT-qPCR
and western blot analysis, and the results revealed that FoxO3a
mRNA expression levels were significantly increased in the chronic
wounds compared with the acute wound tissues (P<0.01), as was
the protein expression (Fig. 1B and
C).
Silencing FoxO3a facilitates, while
its overexpression inhibits, the growth and migration of HaCaT
cells in vitro
To further clarify whether FoxO3a is associated with
KC re-epithelialization following injury, the present study used a
FoxO3a overexpressing lentivirus vector and FoxO3a siRNAs to
perform FoxO3a gain- and loss-of-function studies in HaCaT cells.
The efficiency of FoxO3a expression was then detected using RT-qPCR
and western blot analysis (Fig. 2A and
B). In addition, CCK-8 and wound scratch assays were performed
to investigate the function of FoxO3a in the growth and migration
capacity of HaCaT cells. As presented in Fig. 2C and E, FoxO3a silencing in HaCaT
cells significantly enhanced cell growth compared with NC cells
(P<0.05), and the cell migration capacity was increased.
Conversely, a significantly lower growth rate and inhibited
motility capacity were observed in the Lenti-Flag-FoxO3a group
compared with the control (P<0.05; Fig. 2D and F). These results suggest that
the depletion of FoxO3a results in the notable enhancement of the
growth and migration abilities of KCs in the re-epithelialization
process.
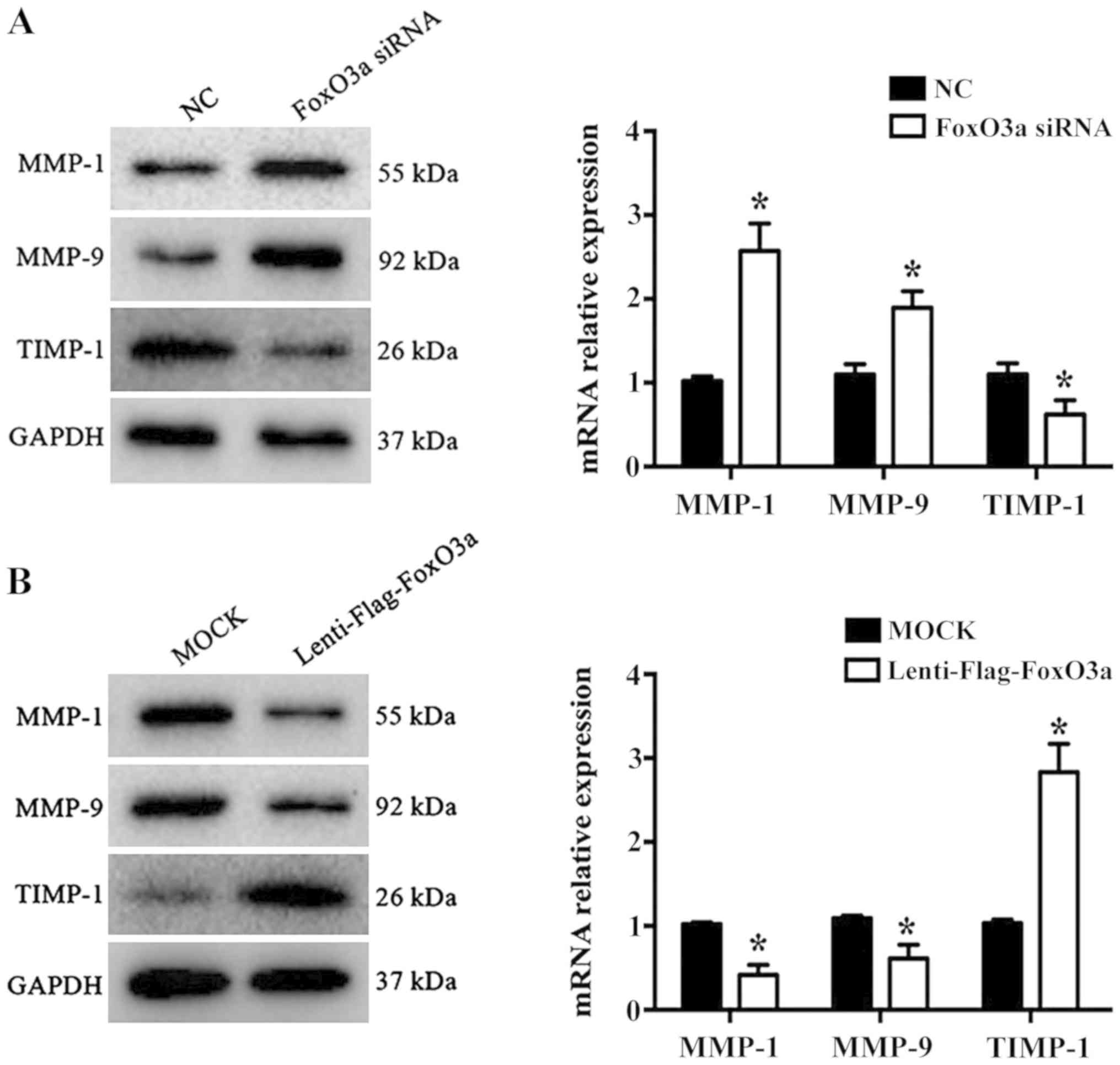
FoxO3a depletion promotes MMP-1 and
MMP-9, and inhibits TIMP-1, expression
Wound repair is a highly complex process that
requires the participation of numerous different extracellular
matrix (ECM) components, cells and soluble media (21). KCs promote cell migration,
re-epithelialization and neovascularization by secreting MMPs
during the proliferative phase of wound healing. A previous study
demonstrated that MMP-1 and MMP-9 are significantly increased in
chronic wound healing, while TIMP-1 levels are significantly
decreased (22). In the present
study, the results revealed that the mRNA levels and protein
expression of MMP-1 and MMP-9 were significantly increased
(P<0.05), while TIMP-1 was significantly decreased in the FoxO3a
siRNA group compared with the NC group (P<0.05; Fig. 3A). Conversely, the opposite results
were observed in FoxO3a-enhanced HaCaT cells (Fig. 3B). These data suggested that FoxO3a
may regulate the expression of MMP-1, MMP9 and TIMP-1 to control
ECM degradation in HaCaT cell migration.
Construction of the EMT model of HaCaT
cells and FoxO3a expression downregulation
In the early stage of wound healing, KCs acquire the
characteristics of interstitial cells to enhance the motor ability
and then migrate to the reconstructed area under cytokine action
(23). At the molecular level, the
expression of interstitial cell markers including N-cadherin and
vimentin were increased, and the epithelial cell marker E-cadherin
protein was decreased (24). In
the present study, 10 ng/ml TGF-β1 was added to the HaCaT cells,
and the results revealed that the expression of E-cadherin was
substantially decreased, whereas the expression of vimentin and
N-cadherin protein were increased (Fig. 4A); consistent results were observed
in HaCaT cells upon FoxO3a downregulation (Fig. 4B). Furthermore, the expression of
FoxO3a protein in the TGF-β1 group exhibited a decreasing trend
compared with the control group. It was indicated that the EMT
model was successfully established, and FoxO3a was regarded as a
negative regulatory factor that participated in the TGF-β1
induced-EMT process of HaCaT cells.
Overexpression of FoxO3a suppresses
TGF-β1 induced-EMT and β-catenin activation
To further investigate the potential mechanism of
FoxO3a-induced cell migration, changes in the expression of TGF-β1
induced-EMT markers were examined in FoxO3a-upregulated HaCaT
cells. The results of the immunofluorescence and western blot
assays demonstrated that the expression of E-cadherin in HaCaT
cells decreased substantially, and the expression of vimentin
increased, compared with the control cells (Fig. 5A and B). These data proved that the
upregulation of the transcription factor FoxO3a was able to
suppress the EMT process of HaCaT cells.
In addition, the western blot analysis results
further revealed that the expression of β-catenin, a key molecule
of the Wnt pathway, was increased in the cytoplasm and nucleus once
HaCaT cells were treated with FoxO3a siRNA (Fig. 5C). By contrast, the expression of
β-catenin in the cytoplasm and nucleus was substantially decreased
once HaCaT cells were infected with lenti-flag-FoxO3a (Fig. 5D). Collectively, the present data
demonstrated that the downregulation of FoxO3a accelerated the
growth and migration of HaCaT cells in vitro, which notably
affected the EMT process and increased the nuclear translocation of
β-catenin.
Discussion
Re-epithelialization is an intricate, dynamic
process that is dominated by KC cells and coordinated by multiple
factors (25). The migration of
KCs is regarded as a key speed-limiting step in the initiation of
re-epithelialization, which is closely associated with the
occurrence of chronic refractory wounds. Therefore, it is of great
importance to identify the key regulatory factors that regulate the
process of KC migration and initiate re-epithelialization for the
rapid and effective healing of chronic refractory wounds. The data
obtained in the present study revealed that FoxO3a is abundantly
expressed in human chronic wound tissues compared with those in
acute wound tissues. Further analyses revealed that silencing
FoxO3a enhanced cell growth and caused a marked increase in the
migration of HaCaT cells. Consistent with the expected results, the
opposite result was observed in the FoxO3a overexpression group
compared with the control. These results suggest that FoxO3a may
negatively regulate the wound healing process.
MMPs are considered to be a crucial family of cell
migration-associated proteins that degrade collagen and different
components of the ECM, contributing to KC migration and the EMT
during wound healing (26,27). Previous studies have reported that
the function of MMPs is regulated by numerous factors, including
transcription factors, microRNAs and methylation modifications
(28–30). Furthermore, further studies have
demonstrated that the transcription factor FoxO3a is associated
with the negative regulation of the MMP-1 and MMP-9 genes (31,32).
Therefore, the present study further observed that the inhibitory
effect of FoxO3a upregulation on the migration of HaCaT cells was
associated with reduced levels of MMP-1 and MMP-9, and increased
the expression of TIMP-1. By contrast, the knockdown of FoxO3a
substantially upregulated the expression of MMP-1 and MMP-9, and
downregulated the expression of TIMP-1 compared with the control
group. Altogether, these data suggest that FoxO3a contributes to
HaCaT cell migration, partly by regulating the balance of
MMP-1/TIMP-1 expression and controlling ECM degradation during
wound healing.
Numerous reports have indicated that EMT is an
essential condition for KC migration associated with wound healing
(24,33,34).
Extensive previous studies have revealed that TGF-β1 may induce the
expression of migratory integrin in KCs to promote the gene
transcription of downstream molecules, resulting in enhanced
cellular proliferation and migration, and subsequently complete
epithelialization (35–37). A previous clinical study has
revealed that exogenous treatment with TGF-β1 accelerated chronic
wound healing, but it also may induce serious fibroblast effects,
cause abnormal wound healing and form a pathological scar (38). The present study established an EMT
model of HaCaT cells in vitro via TGF-β1 stimulation. The
results revealed that the expression of FoxO3a was decreased
substantially during the process of EMT, suggesting that FoxO3a
participated in the EMT of HaCaT cells, and its expression level
was negatively associated with EMT. Similarly, the depleted
expression of FoxO3a promoted N-cadherin and vimentin expression
but decreased E-cadherin expression in HaCaT cells. In contrast
with these results, the ectopic expression of FoxO3a blocked the
TGF-β1-induced EMT process. Thus, FoxO3a may function as a negative
regulator of KC EMT, inhibiting cell migration and subsequently
affecting the initiation of KC re-epithelialization.
β-catenin is a multifunctional protein involved in
cell-to-cell adhesion, which activates the transcription
transduction system by forming transcription factor complexes with
lymphoid enhancer factor/T-cell factor (TCF) family members,
thereby regulating cell growth and migration (39). Upon cutaneous injury, TGF-β1
induces the accumulation of β-catenin in KC nuclei, and then
functions downstream in the gene transcription of the Wnt pathway,
causing EMT (40). Suppression of
β-catenin expression may notably restrain the TGF-β1-induced EMT
process (41). In addition, a
previous study reported that β-catenin serves a crucial function in
the pathogenesis of chronic refractory skin ulcers, and its
abnormal activity may be one of the reasons for the delayed healing
of wounds (42). Furthermore,
molecular evidence has revealed β-catenin as a master regulator of
the FoxO3a-mediated suppression of EMT. FoxO3a inhibits
β-catenin/TCF transcriptional activity via an indirect or direct
interaction with β-catenin, thereby reducing the expression of
β-catenin target genes, including MMPs (15,43).
Consistent with this result, the overexpression of cytoplasmic and
nuclear β-catenin was observed during the silencing of
FoxO3a-induced accelerated HaCaT migration in the present study,
whereas restoration of FoxO3a levels induced the opposite effect,
as the process of EMT was blocked and the expression of β-catenin
decreased notably, suggesting that FoxO3a may negatively regulate
the expression of β-catenin protein to control the process of EMT
in HaCaT cells.
In conclusion, the present results demonstrated that
the depletion of FoxO3a accelerates wound healing by promoting KC
proliferation and migration during the process of
re-epithelialization, and the mechanism appeared to involve the
activation of EMT and β-catenin. These results provide a promising
therapeutic approach for improving chronic wound healing.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DLF conceived and designed the experiments. TL, JZH
and RSY conducted the experiments. TL and ZYL analyzed the data.
RSY contributed the reagents/materials. TL wrote the manuscript.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by The
Research Ethics Committee of The Second Affiliated Xinqiao Hospital
of Army Medical University (Chongquing, China) and written informed
consent was obtained from each patient prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Koppen CJ and Hartmann RW: Advances in
the treatment of chronic wounds: A patent review. Expert Opin Ther
Pat. 25:931–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorg H, Tilkorn DJ, Hager S, Hauser J and
Mirastschijski U: Skin wound healing: An update on the current
knowledge and concepts. Eur Surg Res. 58:81–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Powers JG, Higham C, Broussard K and
Phillips TJ: Wound healing and treating wounds: Chronic wound care
and management. J Am Acad Dermatol. 74:607–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pastar I, Stojadinovic O, Yin NC, Ramirez
H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR and
Tomic-Canic M: Epithelialization in wound healing: A comprehensive
review. Adv Wound Care (New Rochelle). 3:445–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin P and Nunan R: Cellular and
molecular mechanisms of repair in acute and chronic wound healing.
Br J Dermatol. 173:370–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moura LI, Cruz MT and Carvalho E: The
effect of neurotensin in human keratinocytes- implication on
impaired wound healing in diabetes. Exp Biol Med (Maywood).
239:6–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue M and Jackson CJ: Extracellular matrix
reorganization during wound healing and Its impact on abnormal
scarring. Adv Wound Care (New Rochelle). 4:119–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
9
|
Kim CG, Lee H, Gupta N, Ramachandran S,
Kaushik I, Srivastava S, Kim SH and Srivastava SK: Role of Forkhead
Box Class O proteins in cancer progression and metastasis. Semin
Cancer Biol. 50:142–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gurkar AU, Robinson AR, Cui Y, Li X,
Allani SK, Webster A, Muravia M, Fallahi M, Weissbach H, Robbins
PD, et al: Dysregulation of DAF-16/FOXO3A-mediated stress responses
accelerate oxidative DNA damage induced aging. Redox Biol.
18:191–199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Ao X, Ding W, Ponnusamy M, Wu W,
Hao X, Yu W, Wang Y, Li P and Wang J: Critical role of FOXO3a in
carcinogenesis. Mol Cancer. 17:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaanine AH, Kohlbrenner E, Gamb SI,
Guenzel AJ, Klaus K, Fayyaz AU, Nair KS, Hajjar RJ and Redfield MM:
FOXO3a regulates BNIP3 and modulates mitochondrial calcium,
dynamics, and function in cardiac stress. Am J Physiol Heart Circ
Physiol. 311:H1540–H1559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coomans de Brachène A and Demoulin JB:
FOXO transcription factors in cancer development and therapy. Cell
Mol Life Sci. 73:1159–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taylor S, Lam M, Pararasa C, Brown JE,
Carmichael AR and Griffiths HR: Evaluating the evidence for
targeting FOXO3a in breast cancer: A systematic review. Cancer Cell
Int. 15:12015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Yin J, Wang H, Jiang G, Deng M,
Zhang G, Bu X, Cai S, Du J and He Z: FOXO3a modulates WNT/β-catenin
signaling and suppresses epithelial-to-mesenchymal transition in
prostate cancer cells. Cell Signal. 27:510–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roupé KM, Nybo M, Sjöbring U, Alberius P,
Schmidtchen A and Sørensen OE: Injury is a major inducer of
epidermal innate immune responses during wound healing. J Invest
Dermatol. 130:1167–1177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roupé KM, Veerla S, Olson J, Stone EL,
Sørensen OE, Hedrick SM and Nizet V: Transcription factor binding
site analysis identifies FOXO transcription factors as regulators
of the cutaneous wound healing process. PLoS One. 9:e892742014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, He B, Liu X, Li J, Liu Q, Dong W, Xu
Z, Qian G, Zuo H, Hu C, et al: Regulation on toll-like receptor 4
and cell barrier function by Rab26 siRNA-loaded DNA nanovector in
pulmonary microvascular endothelial cells. Theranostics.
7:2537–2554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi XF, Chen ZY, Xia JB, Zheng L, Zhao H,
Pi LQ, Park KS, Kim SK, Lee KJ and Cai DQ: FoxO3a suppresses the
senescence of cardiac microvascular endothelial cells by regulating
the ROS-mediated cell cycle. J Mol Cell Cardiol. 81:114–126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tracy LE, Minasian RA and Caterson EJ:
Extracellular matrix and dermal fibroblast function in the healing
wound. Adv Wound Care (New Rochelle). 5:119–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ligi D, Mosti G, Croce L, Raffetto JD and
Mannello F: Chronic venous disease-Part II: Proteolytic biomarkers
in wound healing. Biochim Biophys Acta. 1862:1900–1908. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seeger MA and Paller AS: The roles of
growth factors in keratinocyte migration. Adv Wound Care (New
Rochelle). 4:213–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stoll SW, Rittié L, Johnson JL and Elder
JT: Heparin-binding EGF-like growth factor promotes
epithelial-mesenchymal transition in human keratinocytes. J Invest
Dermatol. 132:2148–2157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu X, Yang L, Zheng Z, Li Z, Shi J, Li Y,
Han S, Gao J, Tang C, Su L and Hu D: Src promotes cutaneous wound
healing by regulating MMP-2 through the ERK pathway. Int J Mol Med.
37:639–648. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schultz GS, Davidson JM, Kirsner RS,
Bornstein P and Herman IM: Dynamic reciprocity in the wound
microenvironment. Wound Repair Regen. 19:134–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krishnaswamy VR, Mintz D and Sagi I:
Matrix metalloproteinases: The sculptors of chronic cutaneous
wounds. Biochim Biophys Acta Mol Cell Res. 1864:2220–2227. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guarneri C, Bevelacqua V, Polesel J,
Falzone L, Cannavò PS, Spandidos DA, Malaponte G and Libra M: NF-κB
inhibition is associated with OPN/MMP-9 downregulation in cutaneous
melanoma. Oncol Rep. 37:737–746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Falzone L, Candido S, Salemi R, Basile MS,
Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M and
Libra M: Computational identification of microRNAs associated to
both epithelial to mesenchymal transition and NGAL/MMP-9 pathways
in bladder cancer. Oncotarget. 7:72758–72766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Falzone L, Salemi R, Travali S, Scalisi A,
McCubrey JA, Candido S and Libra M: MMP-9 overexpression is
associated with intragenic hypermethylation of MMP9 gene in
melanoma. Aging (Albany NY). 8:933–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miao C, Li Y and Zhang X: The functions of
FoxO transcription factors in epithelial wound healing. Australas J
Dermatol. 60:105–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kikuno N, Shiina H, Urakami S, Kawamoto K,
Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M and Dahiya
R and Dahiya R: Knockdown of astrocyte-elevated gene-1 inhibits
prostate cancer progression through upregulation of FOXO3a
activity. Oncogene. 26:7644–7655. 2007. View Article : Google Scholar
|
|
33
|
Haensel D and Dai X:
Epithelial-to-mesenchymal transition in cutaneous wound healing:
Where we are and where we are heading. Dev Dyn. 247:473–480. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HY, Jackson TR and Davidson LA: On the
role of mechanics in driving mesenchymal-to-epithelial transitions.
Semin Cell Dev Biol. 67:113–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsubakihara Y and Moustakas A:
Epithelial-mesenchymal transition and metastasis under the control
of transforming growth factor β. Int J Mol Sci. 19:E36722018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramirez H, Patel SB and Pastar I: The role
of TGFβ signaling in wound epithelialization. Adv Wound Care (New
Rochelle). 3:482–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Philandrianos C, Kerfant N, Jaloux C Jr,
Martinet L, Bertrand B and Casanova D: Keloid scars (part I):
Clinical presentation, epidemiology, histology and pathogenesis.
Ann Chir Plast Esthet. 61:128–135. 2016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar R and Bashyam MD: Multiple oncogenic
roles of nuclear beta-catenin. J Biosci. 42:695–707. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bielefeld KA, Amini-Nik S and Alman BA:
Cutaneous wound healing: Recruiting developmental pathways for
regeneration. Cell Mol Life Sci. 70:2059–2081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao F, Alwhaibi A, Sabbineni H, Verma A,
Eldahshan W and Somanath PR: Suppression of Akt1-β-catenin pathway
in advanced prostate cancer promotes TGFβ1-mediated epithelial to
mesenchymal transition and metastasis. Cancer Lett. 402:177–189.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Nie X, Shi X, Zhao J, Chen Y, Yao
Q, Sun C and Yang J: Regulatory mechanisms of the Wnt/β-catenin
pathway in diabetic cutaneous ulcers. Front Pharmacol. 9:11142018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nawrocki-Raby B, Gilles C, Polette M,
Martinella-Catusse C, Bonnet N, Puchelle E, Foidart JM, Van Roy F
and Birembaut P: E-Cadherin mediates MMP down-regulation in highly
invasive bronchial tumor cells. Am J Pathol. 163:653–561. 2003.
View Article : Google Scholar : PubMed/NCBI
|