Introduction
Achalasia, with an annual incidence of 1/100,000 in
Western countries, is a rare motility disease characterized by
impaired swallowing due to dysfunction of the lower esophageal
sphincter (LES) (1,2). The condition is associated with an
increased risk of esophageal carcinoma (3,4).
Histopathological analyses of myotomy specimens from patients with
idiopathic achalasia demonstrate a degradation of ganglion cells in
the esophageal myenteric plexus (5,6) and
inflammatory cell infiltration. The etiology of achalasia is
unknown, but has been postulated to be related to autoimmunity,
neurodegeneration and viral immunity (7).
The predominant symptoms of achalasia are dysphagia
and regurgitation. In addition, persistent esophageal distension
due to food and fluid retention can lead to fungal and bacterial
overgrowth, and impaired clearance of gastric contents (8). Acid regurgitation can cause chronic
inflammation, dysplasia and even cancer. Meijssen et al
(9) reported that the risk of
esophageal cancer is 33 times greater in patients with achalasia
compared with the general population. Streitz et al
(10) reported that the incidence
of squamous cell carcinoma was 88/100,000 in the patients with
achalasia in their study, which represents a 14.5 times greater
risk than that in the general population after adjustments for age
and sex. In a recent study, Tustumi et al (11) performed a systematic review and
meta-analysis that showed that achalasia cardia is associated with
an increased risk of esophageal cancer, highlighting the need for
strict endoscopic surveillance in patients with achalasia.
A potential contributor to the development of
esophageal cancer in patients with achalasia is aflatoxin (AF)
(12). AF is one of the most
potent toxic, carcinogenic, teratogenic and immunosuppressive
substances that is present naturally in certain foods, particularly
improperly stored foods, such as corn, rice, peanuts, wheat and a
variety of spices (13). AFs
comprise a group of closely related mycotoxins that are produced as
secondary metabolites by several fungi, namely Aspergillus (A.)
flavus, A. nomius and A. parasiticus (14–16).
Although the major AF subtypes (B1, B2,
G1 and G2) are often found together in
varying proportions in different foods, AF B1 is the
predominant subtype with the most potent carcinogenic effect
(17).
Since 2010, per oral endoscopic myotomy (POEM) has
been used as an effective treatment option to relieve esophageal
food retention in patients with achalasia (18), and is increasingly replacing
pneumatic dilatation, Botox injection and Heller myotomy as a
treatment for achalasia (19–22).
Furthermore, Minami et al (23) reported that POEM may reduce the
risk of esophageal carcinoma in patients with achalasia.
On this basis, it was hypothesized that an agent
present in the food retained in the esophagus may be responsible
for the subsequent development of carcinomas in patients with
achalasia (24). The present study
was designed to determine whether AFs are present in the esophageal
contents of patients with achalasia and whether AFs are related to
the symptomatology of achalasia, particularly the cancer risk.
Materials and methods
Study design and patient
population
The present single-center prospective study
consecutively enrolled 75 patients (age range, 14–81 years; 34
males and 41 females) who underwent POEM at the Endoscopy Center
and Endoscopy Research Institute, Zhongshan Hospital, Fudan
University, between January 2016 and June 2016. Patients were
eligible for enrollment in the study if they were 14–90 years of
age and had recurrent/persistent symptoms of achalasia with an
Eckardt symptom score of ≥4. The Eckardt score is the sum of the
symptom scores for dysphagia, regurgitation, chest pain and weight
loss (25). The diagnosis of
achalasia was based on the absence of peristalsis and the presence
of impaired LES relaxation on established tests (barium swallow,
manometry or esophagogastroduodenoscopy). The exclusion criteria
were pseudo-achalasia, megaesophagus (diameter >7 cm), and
severe cardiopulmonary disease or other serious disease posing
unacceptable surgical risk (26).
Patients with mild or moderate esophagitis that did not induce
esophageal stricture were not excluded. In addition, 30 healthy
volunteers (age, 23–45 years; 18 males and 12 females) were
recruited following healthy examination at the Endoscopy Center and
Endoscopy Research Institute, Zhongshan Hospital, Fudan University,
between April 2016 and September 2016. Esophageal contents samples
obtained from healthy volunteers were used as controls. The
baseline characteristics of the patients are given in Table I.
 | Table I.Patient characteristics before and
after POEM. |
Table I.
Patient characteristics before and
after POEM.
| Characteristic | Value |
|---|
| Age, years; median
[IQR] (range) | 40.00 [26.0–51.0]
(14.0–81.0) |
| <18,
n (%) | 2.00 (2.7) |
| ≥18 and
<60, n (%) | 65.00 (86.7) |
| ≥60, n
(%) | 8.00 (10.7) |
| Sex, n (%) |
|
|
Male | 34.00 (45.3) |
|
Female | 41.00 (54.7) |
| Disease duration,
years; median [IQR] (range) | 8.00 [4.0–10.0]
(0.5–30.0) |
| Sigmoid-type
esophagus, n (%) | 9.00 (12) |
| Pretreatment, n
(%) | 21.00 (28) |
| Balloon dilatation,
n (%) | 9.00 (12) |
| Stent placement, n
(%) | 4.00 (5.3) |
| Botox treatment, n
(%) | 3.00 (4) |
| Heller myotomy, n
(%) | 4.00 (5.3) |
| POEM, n (%) | 4.00 (5.3) |
| Balloon dilatation
and POEM, n (%) | 1.00 (1.3) |
| Balloon dilatation
and Heller myotomy, n (%) | 1.00 (1.3) |
| Stent and Botox
treatment, n (%) | 1.00 (1.3) |
| Duration of POEM,
min; median [IQR] (range) | 52.00 [32.0–69.0]
(16.0–154.0) |
| Pre-POEM LES
pressure, mmHg; median [IQR] (range) | 25.10 [17.3–35.8]
(3.9–49.5) |
| Pre-POEM Eckardt
score; median [IQR] (range) | 7.00 [6.0–8.0]
(4.0–10.0) |
| Pre-POEM aflatoxin,
ng/ml; median [IQR] (range) | 13.14 [9.3–15.7]
(0.0–36.8) |
|
Negative, n (%) | 8.00 (10.7) |
|
Positive, n (%) | 67.00 (89.3) |
| Post-POEM LES
pressure, mmHg; median [IQR] (range) | 10.1 [8.0–13.2]
(5.0–16.2) |
| Post-POEM Eckardt
score; median [IQR] (range) | 1.00 [1.0–2.0]
(0.0–7.0) |
| Post-POEM
aflatoxin, ng/ml; median [IQR] (range) | 0.15 [0.0–0.0]
(0.0–6.3) |
|
Negative, n (%) | 73.00 (97.3) |
|
Positive, n (%) | 2.00 (2.7) |
All patients and volunteers provided written
informed consent prior to their inclusion into the study. Written
informed consent was obtained from the parents/guardians of all
minors included in the present study. The study was approved by the
ethics committee of Zhongshan Hospital, Fudan University (approval
no. B2012-089).
Calculation of LES pressure
The pre- and post-myotomy LES pressures were
recorded using a high-resolution manometry system (Sierra
Scientific Instruments Inc.), as described previously (27). In brief, the high-resolution
manometry assembly was placed transnasally, and the manometric
catheter was positioned to record from the hypopharynx to the
stomach with ~5 intragastric sensors. Manometric assessments were
performed with the patient in a supine position and after ≥6 h of
fasting. The manometric protocol included a 5 min period to measure
the basal gastroesophageal junction pressure; 10 water swallows of
5 ml each; and 1 water swallow each of 1 (dry), 10 and 20 ml. All
manometric analyses were performed using Mano-View (version 3.0;
Medtronic); the data tracings were evaluated in the color pressure
topography mode and referenced to the intragastric pressure.
Treatment and sample collection
All patients were treated using POEM, which was
performed by experienced endoscopists at the Endoscopy Center and
Endoscopy Research Institute, Zhongshan Hospital, Fudan University.
The patients underwent gastroscopy examination again at 3 months
after POEM. A proton pump inhibitor (usually omeprazole) and
antibiotics (usually second-generation cephalosporins) were
administered for 1–2 days during the fasting period after POEM.
Patients were usually discharged 2–3 days after POEM if they were
able to swallow liquids and had no other complaints, such as
fever.
Prior to the POEM procedure, samples of the
esophageal contents were collected from the patients with achalasia
by performing saline irrigation of the esophageal lumen, followed
by fluid suction into a sterile container. Using the same method,
esophageal samples were obtained from the healthy volunteers in the
control group. During the POEM procedure, two biopsy specimens of
the esophageal mucosa were collected from each patient. The biopsy
sites were located 2 cm from the cardia, within the esophagus.
Measurement of AF concentration in
esophageal contents
The esophageal luminal contents were examined for
AFs by using ELISA (cat. no. E030502; Shanghai Bangyi Biological
Technology Co. Ltd.). This kit can detect AFs B1,
B2, G1 and G2. The assays were
conducted according to the manufacturer's instructions. Residual
food in the esophagus was aspirated using a regular gastroscope
during the endoscopic examination before POEM. If the residual food
was primarily solid, ~250 ml of sterile normal saline was used to
irrigate the esophagus until it was clean. The original volume of
the samples used in for ELISA was usually 20–30 ml. Samples were
prepared as follows: 35 ml methanol was added to the 15 ml original
sample and shaken at 20–25°C for 15 min with a shaker; the sample
was centrifuged at 1500 × g at 20–25°C for 5 min; 1 ml supernatant
was mixed with 1ml deionized water; 50 µl diluted supernatant was
added to each well and incubated for 2 h at 20–25°C prior to
analysis by ELISA.
Immunohistochemical analysis of
esophageal biopsy specimens
A total of two 4 mm-long esophageal mucosal
specimens were collected during each POEM procedure. The specimens
were fixed using 4% formalin at 20–25°C for 8–12 h,
paraffin-embedded, cut into 3 µm thick sections, mounted on
silane-coated glass slides, dried, deparaffinized with xylene and
rehydrated using a graded ethanol series. For antigen retrieval,
the specimens were boiled for 20 min in 10 mM monosodium citrate
buffer (pH 6.0) for Ki67 immunostaining or 10 mM Tris/EDTA buffer
(pH 9.0) for p53 immunostaining. To quench endogenous peroxidase,
the slides were incubated in a 0.5% solution of
H2O2 in phosphate-buffered citric acid for 20
min at 20°C, washed 3–5 times with TBS (pH 7.4), and incubated
again with TBS containing 10% non-immune rabbit serum (Dako;
Agilent Technologies, Inc.) with 10% human plasma (Ki67; Dako;
Aglient Technologies, Inc.) or 5% bovine serum albumin (p53; Dako;
Aglient Technologies, Inc.) at 20–25°C for 30 min. Afterwards, the
slides were incubated with the primary antibodies anti-Ki67 (cat.
no. M724029; MIB-1; Dako; Agilent Technologies, Inc.) and anti-p53
(cat. no. NCL-L-p53-DO7; DO-7; Leica Biosystems GmbH) at 1:100
dilution for 12 h at 0–4°C. Next, the slides were incubated with
biotin-labeled rabbit anti-mouse secondary antibody (cat. no.
DS9800; Leica Microsystems GmbH) at 20–25°C for 8 min and a
streptavidin-horseradish peroxidase complex (cat. no. DS9800; Leica
Biosystems GmbH). 3-Amino-9-ethylcarbazole was used as the
substrate for detection for 5 min at 20–25°C. The immunochemical
staining was observed using a DM 2000 light microscope
(magnification, ×200; Leica Microsystems GmbH) and was analyzed by
two experienced pathologists who were blinded to the clinical data.
At least 300 nuclei were counted per section, and cells with
moderate-to-intense nuclear staining were counted manually as
immune-positive.
Animal experiments
A total of 12 male Wistar albino rats (weight, ~280
g; age, 8 weeks; Beijing Vital River Laboratory Animal Technology
Co., Ltd.) were used in the present study. The recommendations of
the Guide for the Care and Use of Laboratory Animals of Zhongshan
Hospital, Fudan University were followed, and the animal
experiments were approved by the Medical Faculty Animal Care
Committee of Zhongshan Hospital, Fudan University. The rats were
housed at 18–25°C with 40–70% humidity and 12 h light/dark cycles,
and fed water and food ad libitum. All the rats in the study
were anesthetized prior to cervical dislocation. Ketamine (100
mg/kg) and xylazine (10 mg/kg) were administered by intraperitoneal
injection to anesthetize the rats.
Drugs
AF B1, a nitric oxide (NO) synthase
inhibitor [NG-nitro-L-arginine (L-NNA)], and atropine sulfate were
obtained from Sigma-Aldrich (Merck KGaA). A 2 mg/ml stock solution
of AF was prepared in dimethyl sulfoxide (which does not directly
affect murine-isolated smooth muscle preparations) and stored at
0–4°C. Other drugs were dissolved in ethanol and deionized water
(1:1) as 1 mg/ml stock solutions, and were subsequently diluted in
deionized water before use.
Isolated smooth muscle
experiments
All 12 rats were sacrificed by means of cervical
dislocation, and a 2 cm-long esophageal specimen was excised from
the gastro-esophageal junction through a ~7 cm-long mid-abdominal
incision. Fat and connective tissues were removed from the excised
specimen, and the esophageal lumen was cleaned with Tyrode solution
(NaCl, 8 g/l; CaCl2, 2.5 g/l; KCl, 0.2 g/l;
MgSO4, 0.1 g/l; NaH2PO4, 0.05 g/l;
NaHCO3, 1.0 g/l; and glucose, 1.0 g/l) previously
aerated with 95% O2 and 5% CO2. Then, 1
cm-long full-thickness esophageal segments were hung in a circular
orientation in tissue baths filled with 10 ml Tyrode solution at
37.0°C. The upper ends of the esophageal segments were attached to
an isometric transducer (cat. no. JZJ01; Chengdu Instrument
Factory) and the lower ends preloaded with 1.5 g tension. The
tissues were equilibrated for ~20 min. Tissue contraction was
considered the reference response after equilibrium and at the
beginning of each experiment. The tissues were equilibrated for ~20
min again before the addition of 5, 50 and 500 ng/ml AF. Isometric
tension was recorded and analyzed using the RM6280 system (Chengdu
Instrument Factory). To ensure maximal effect, the esophageal
segments were incubated with each concentration of AF for 3 min.
Subsequently, 20 min before the final and highest dose of AF (500
ng/ml), the cholinergic inhibitor atropine sulfate (0.2 µg/ml) or
the NO synthase inhibitor L-NNA (0.2 µg/ml) was added to the
bath.
Statistical analysis
Data were expressed as mean ± standard error of the
mean. All statistical analyses were conducted using SPSS v13.0
software (SPSS, Inc.). Continuous data were compared using
Student's t-test between two groups. One-way ANOVA followed by
Bonferroni's test was applied to assess the differences among
groups. The pre-operative values of AF, Ki67 and p53 were
subtracted from the post-operative values to represent the changes
of these indexes after POEM. Subsequently, bivariate Pearson
correlation analysis was used to measure relationships between two
variables. All tests were two sided, and P<0.05 was considered
to indicate a statistically significant difference.
Results
AF is found in esophageal contents and
eliminated after POEM in most patients with achalasia
A total of 75 patients were enrolled in this study.
Their baseline characteristics are shown in Table I. Before POEM, AF was detected in
the esophageal contents of 67 of the 75 patients with achalasia.
These samples exhibited a yellow-green fluorescence upon
irradiation with 365 nm violet light, which indicated the presence
of AF (Fig. 1A and B); in
contrast, the control group did not show any fluorescence (Fig. 1C and D). Notably, at 3 months after
POEM, AF was detected in only 2 samples, and both of these patients
still had dysphagia and retention symptoms.
While performing the POEM procedure, retained foods
were first removed from the esophagus (Fig. 2A). In one patient, after the
esophagus had been cleared, a large sheet erosion was incidentally
found in the esophageal mucosa, and this area was biopsied
(Fig. 2B). The pathological report
showed esophageal squamous epithelium with high-grade dysplasia,
and the patient underwent endoscopic submucosal dissection in the
Endoscopy Center and Endoscopy Research Institute, Zhongshan
Hospital, Fudan University after a few weeks.
Reduced Ki67 and p53 immunoreactivity
after POEM
To assess markers associated with cancer
development, immunohistochemical analyses of the esophageal
specimens was performed. The mean percentage of Ki67-immunopositive
cells was significantly lower in the esophageal mucosa samples
taken after POEM compared with the samples collected before POEM,
with 27.8% [95% (CI), 25.98–29.70] before compared with 20.7% (95%
CI, 19.78–24.03) after (P=0.04; Fig.
3). Similarly, the mean percentage of p53-positive cells
significantly decreased after POEM [2.14% (95% CI, 1.85–2.41) vs.
1.45% (95% CI, 1.22–1.68); P=0.03; Fig. 4].
However, no correlation was observed between AF and
Ki67 (R=−0.142, P=0.223; Fig.
S1A), or between AF and p53 (R=0.179, P=0.124; Fig. S1B) prior to POEM. Similarly,
following POEM, no correlation was observed between AF and Ki67
(R=−0.174, P=0.135; Fig. S2A), or
between AF and p53 (R=0.098, P=0.401; Fig. S2B).
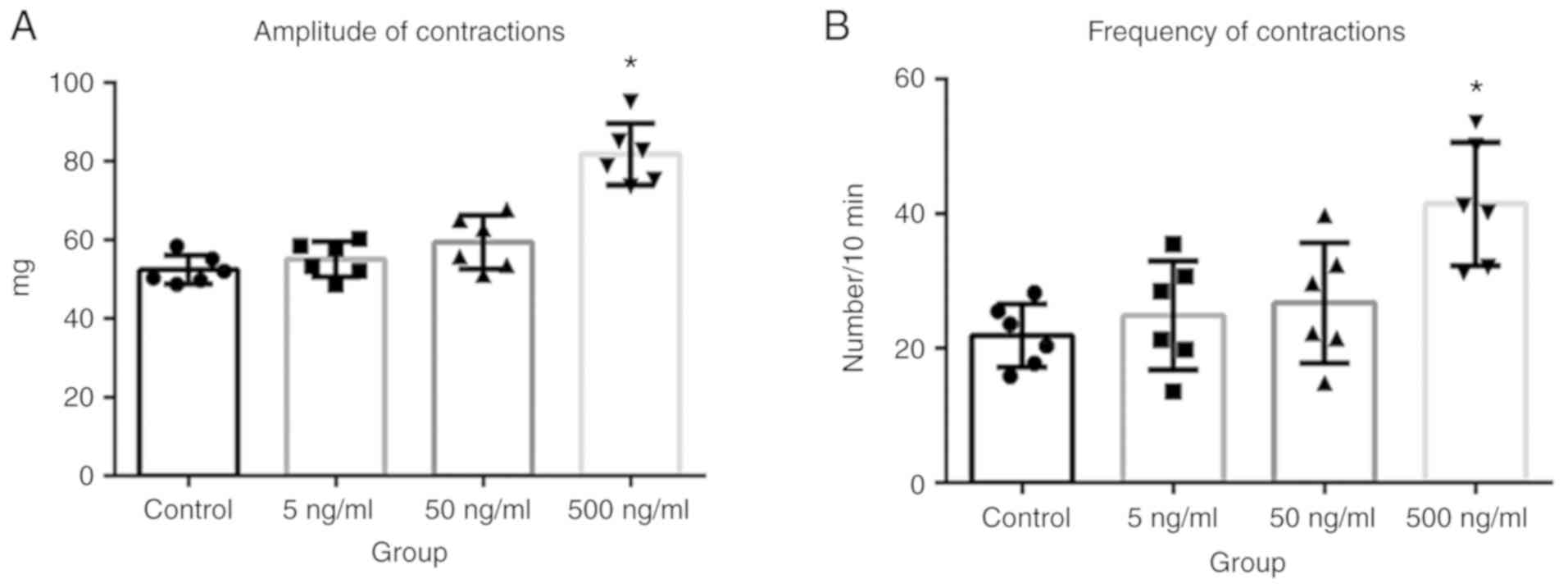
AF increases the amplitude and
frequency of LES contractions in vitro
To determine whether AF affected LES contractions in
patients with achalasia, in vitro experiments were performed
using esophageal segments excised from adult Wistar albino rats. It
was found that both the frequency and amplitude of LES contractions
significantly increased after the application of 500 ng/ml AF
B1 (Fig. 5A and B;
P<0.05). This AF-induced increase in esophageal tissue
contractions was blocked by cholinergic inhibition. The bath
application of atropine sulfate (0.2 µg/ml) significantly inhibited
the amplitude (Fig. 6A) and
frequency (Fig. 6B) of LES
contractions in the presence of AF (both P<0.05). However, the
same dose of the NO synthase inhibitor L-NNA had no such effect.
Altogether, these data demonstrated that LES contractions increased
in the presence of AF, and that this increase was rapidly
alleviated by the inhibition of cholinergic signaling.
Discussion
The present study measured the AF levels in the
esophageal contents of patients with achalasia before and 3 months
after they underwent POEM. The carcinogen AF was present in most
patients with achalasia before POEM, and was absent in nearly all
patients after POEM. In addition, the absence of AF paralleled the
reductions in Ki67 and p53 immunoreactivity in the esophageal
mucosa seen in biopsies, suggesting that AF accumulation in food
contents retained within the esophagus may contribute to cancer
development in patients with achalasia. In vitro studies
revealed that AF accumulation was associated with an increase in
the contractility of murine esophageal sections, and this increase
was blocked by a cholinergic inhibitor. These findings suggested
that AF is not only linked to markers of esophageal cancer in
patients with achalasia but also a possible contributor to the
symptomatology of achalasia via cholinergic signaling.
In the general population, AF is not detected in the
blood using ELISA or high-performance liquid chromatography, and
very low levels of AF (<0.03 ng/ml) are detected in urine
samples (28). Environmental AF
contamination reportedly ranges between 7.2–125.4 µg/kg, which is
normally too low to cause disease (29). While AF is not found in the
esophagus in the general population, it was detected in most
patients with achalasia enrolled in the present study. Importantly,
minimal levels of AF were detected in these patients after POEM,
when the food retention had been alleviated. This is the first
report, to the best of the authors' knowledge, documenting an
association between AF and achalasia.
Fujii et al (30) reported higher Ki67 positivity in
cancerous tissues compared with non-cancer tissues in patients with
achalasia with concomitant esophageal squamous cancer. Leeuwenburgh
et al (31) found that p53
overexpression might be an early marker of neoplastic progression
in patients with achalasia. With this in mind, the present study
performed immunohistochemical analyses of the esophageal mucosal
specimens collected from the patients with achalasia. Notably,
significant decreases in both these markers were observed after
POEM. These findings implied that patients might be at a reduced
risk of developing esophageal carcinoma after they have been
treated with POEM and their symptoms have resolved. In the present
study, AF was detected in the esophageal contents of 67 of the 75
patients with achalasia prior to POEM, but in only 2 patients
following POEM. Therefore, it is not appropriate to directly use
bivariate Pearson correlation analysis for the post-operative data.
Thus, the pre-operative values of AF (also Ki67 and p53) were
subtracted from the post-operative values to represent the changes
of these indexes after POEM, then bivariate Pearson correlation
analysis was used to assess the relationships between these
indexes. However, no correlation was observed between AF and
Ki67/p53 before or after POEM. The samples for AF ELISA were
collected using gastroscopy. Sterile normal saline was used to
flush food residue in the esophagus prior to its complete removal
by suction. This may have contributed to the negative result from
dilution effects that may have lowered the AF concentration.
AF is known to induce both acute and chronic
toxicity in numerous species (32). The in vitro experiments of
the present study further demonstrated that AF directly influenced
murine esophageal contractility, which is consistent with previous
reports concerning the gastrointestinal systems of animals
(33). AF has been shown to induce
abdominal pain, diarrhea, vomiting and severe erosions (34). The present study found that a 500
ng/ml concentration of AF B1 increased both the
amplitude and frequency of LES contractions, and that these effects
were driven by cholinergic signaling, as they were rapidly blocked
by atropine sulfate.
Although the factors contributing to achalasia have
not yet been fully elucidated, evidence suggests that ganglionitis
due to aberrant immune responses to viral infections underlies the
loss of esophageal neurons, particularly in genetically susceptible
populations (35,36). The loss of inhibitory tone and the
subsequent retention of ingested food can lead to regurgitation,
chest pain, dysphagia and weight loss, as well as an increased risk
of esophageal carcinoma (25,37–39).
Thus, the main pathophysiology of achalasia may be the degradation
of ganglion cells in the esophageal myenteric plexus. Although the
absence of NO synthase in the myenteric plexus of the LES has been
proposed as the primary factor (40), the results of the present study
suggested a more important role for the cholinergic system.
However, other studies have reported that AF can inhibit NO
synthase (41–43). Therefore, it is hypothesized that
the AF-mediated inhibition of NO synthase may have a long-term
effect on the LES, while AF has a direct and acute effect on LES
via the cholinergic signaling pathway. However, verification using
in vivo studies will make these results more convincing.
The present study has certain limitations. First, in
the preliminary studies, a number of contaminants were found in
food samples, including nitrates, Candida albicans,
sterigmatocystin and Staphylococcus aureus. However, only AF
was assessed in the present study, as AF is one of the most potent
toxic and carcinogenic substances that is naturally present in
improperly stored foods. Other carcinogens should be assessed in
future studies. Second, the diet of the patients was not
controlled, and the patients were not asked to provide diet
information. Third, as mentioned earlier, saline irrigation and
suction may not be an ideal method for the collection of esophageal
contents due to dilution effects. An appropriate and efficient
alternative method to collect samples for AF measurement without
any bias needs to be found. Finally, animal models of achalasia are
difficult to establish. Commonly used methods include esophageal
ligation (44), but it is
difficult to simulate the complex changes of achalasia using this
method. Infection with Trypanosoma americanum (T.
americanum) has been used to imitate the pathophysiology of
achalasia in animals (45,46), but this method might not be
ethically acceptable, and T. americanum cannot be imported
to China.
In conclusion, the findings of the present study
demonstrated that AF accumulated in the esophageal contents of
patients with achalasia and was eliminated after POEM. The reduced
Ki67 and p53 expression after treatment in these patients showed
that POEM may decrease the risk of esophageal neoplastic
progression. It was hypothesized that the removal of AF after POEM
may be the reason for the reduced expression of these biomarkers.
Furthermore, the present study demonstrated that AF increased the
contractility of the LES, and that this increase can be blocked by
cholinergic inhibitors. Further studies are needed to elucidate how
AF participates in the process of achalasia, the AF dose required
to produce these effects, and whether esophageal AF accumulation is
a cause or result of achalasia, or simply maintains the symptoms of
achalasia. It is also hoped to establish a suitable animal model of
achalasia to study the long-term effects of AF on the LES.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant nos. 81302098,
81370588, 81470811, 81401930 and 81570595) and The Natural Science
Foundation of Shanghai (grant no. 13ZR1452300).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, PG and QL collected the data, performed the
experiments and wrote the manuscript. YZ, JH, MC, WQ, LM, ZR, ZZ,
LY and XC collected and analyzed data. PZ and WC designed the
study, recruited patients and volunteers and made critical
revisions to the manuscript. All authors approved the final version
of the manuscript.
Ethics approval and consent to
participate
All patients and volunteers provided written
informed consent prior to their inclusion into the study. Written
informed consent was obtained from the parents/guardians of all
minors included in the present study. The study was approved by the
ethics committee of Zhongshan Hospital, Fudan University (approval
no. B2012-089). The recommendations of the Guide for the Care and
Use of Laboratory Animals of Zhongshan Hospital, Fudan University
were followed, and the animal experiments were approved by the
Medical Faculty Animal Care Committee of Zhongshan Hospital, Fudan
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sadowski DC, Ackah F, Jiang B and Svenson
LW: Achalasia: Incidence, prevalence and survival. A
population-based study. Neurogastroenterol Motil. 22:e256–e261.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Birgisson S and Richter JE: Achalasia in
Iceland, 1952–2002: An epidemiologic study. Dig Dis Sci.
52:1855–1860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gennaro N, Portale G, Gallo C, Rocchietto
S, Caruso V, Costantini M, Salvador R, Ruol A and Zaninotto G:
Esophageal achalasia in the veneto region: Epidemiology and
treatment. Epidemiology and treatment of achalasia. J Gastrointest
Surg. 15:423–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marlais M, Fishman JR, Fell JM, Haddad MJ
and Rawat DJ: UK incidence of achalasia: An 11 year national
epidemiological study. Arch Dis Child. 96:192–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark SB, Rice TW, Tubbs RR, Richter JE
and Goldblum JR: The nature of the myenteric infiltrate in
achalasia: An immunohistochemical analysis. Am J Surg Pathol.
24:1153–1158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villanacci V, Annese V, Cuttitta A,
Fisogni S, Scaramuzzi G, De Santo E, Corazzi N and Bassotti G: An
immunohistochemical study of the myenteric plexus in idiopathic
achalasia. J Clin Gastroenterol. 44:407–410. 2010.PubMed/NCBI
|
|
7
|
Park W and Vaezi MF: Etiology and
pathogenesis of achalasia: The current understanding. Am J
Gastroenterol. 100:1404–1414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohamed AA, Lu XL and Mounmin FA:
Diagnosis and treatment of esophageal candidiasis: Current updates.
Can J Gastroenterol Hepatol. 20:35851362019.
|
|
9
|
Meijssen MA, Tilanus HW, van Blankenstein
M, Hop WC and Ong GL: Achalasia complicated by oesophageal squamous
cell carcinoma: A prospective study in 195 patients. Gut.
33:155–158. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Streitz JM, Ellis FH, Gibb SP and Heatley
GM: Achalasia and squamous cell carcinoma of the esophagus:
Analysis of 241 patients. Ann Thorac Surg. 59:1604–1609. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tustumi F, Bernardo WM, da Rocha JRM,
Szachnowicz S, Seguro FC, Bianchi ET, Sallum RAA and Cecconello I:
Esophageal achalasia: A risk factor for carcinoma. A systematic
review and meta-analysis. Dis Esophagus. 30:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghasemi-Kebria F, Joshaghani H, Taheri NS,
Semnani S, Aarabi M, Salamat F and Roshandel G: Aflatoxin
contamination of wheat flour and the risk of esophageal cancer in a
high risk area in Iran. Cancer Epidemiol. 37:290–293. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marchese S, Polo A, Ariano A, Velotto S,
Costantini S and Severino L: Aflatoxin B1 and M1: Biological
properties and their involvement in cancer development. Toxins
(Basel). 24:E2142018. View Article : Google Scholar
|
|
14
|
Rawal S, Kim JE and Coulombe R Jr:
Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res
Vet Sci. 89:325–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raisuddin S, Singh K, Zaidi S, Paul BN and
Ray PK: Immunosuppressive effects of aflatoxin in growing rats.
Mycopathologia. 124:189–194. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marin D, Taranu I, Bunaciu RP, Pascale F,
Tudor DS, Avram N, Sarca M, Cureu I, Criste RD, Suta V and Oswald
IP: Changes in performance, blood parameters, humoral and cellular
immune responses in weanling piglets exposed to low doses of
aflatoxin. J Anim Sci. 80:1250–1257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JW, Kim EK, Shon DH and Kim YB:
Natural co-occurrence of aflatoxin B1, fumonsin B1, and ochratoxin
A in barley and maize foods from Korea. Food Addit Contam.
19:1073–1080. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue H, Minami H, Kobayashi Y, Sato Y,
Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H and Kudo S: Peroral
endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy.
42:265–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou PH, Li QL, Yao LQ, Xu MD, Chen WF,
Cai MY, Hu JW, Li L, Zhang YQ, Zhong YS, et al: Peroral endoscopic
remyotomy for failed heller myotomy: A prospective single-center
study. Endoscopy. 45:161–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khashab MA, Messallam AA, Onimaru M,
Teitelbaum EN, Ujiki MB, Gitelis ME, Modayil RJ, Hungness ES,
Stavropoulos SN, El Zein MH, et al: International multi-center
experience with peroral endoscopic myotomy for the treatment of
spastic esophageal disorders refractory to medical therapy (with
video). Gastrointest Endosc. 81:1170–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones EL, Meara MP, Pittman MR, Hazey JW
and Perry KA: Prior treatment does not influence the performance or
early outcome of per-oral endoscopic myotomy for achalasia. Surg
Endosc. 30:1282–1286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue H, Sato H, Ikeda H, Onimaru M, Sato
C, Minami H, Yokomichi H, Kobayashi Y, Grimes KL and Kudo SE:
Per-Oral endoscopic myotomy: A series of 500 patients. J Am Coll
Surg. 221:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minami H, Yamaguchi N, Matsushima K,
Akazawa Y, Ohnita K, Takeshima F, Nakayama T, Hayashi T, Inoue H,
Nakao K and Isomoto H: Improvement of endocytoscopic findings after
per oral endoscopic myotomy (POEM) in esophageal achalasia; does
POEM reduce the risk of developing esophageal carcinoma? Per oral
endoscopic myotomy, endocytoscopy and carcinogenesis. BMC
Gastroenterol. 30:222013. View Article : Google Scholar
|
|
24
|
Wang J and Liu XM: Assessment of dietary
aflatoxin exposure in Chinese residents. Chin J Food Hyg.
19:238–240. 2007.(In Chinese).
|
|
25
|
Eckardt V: Clinical presentations and
complications of achalasia. Gastrointest Endosc Clin N Am.
11281–292. (vi)2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pandolfino JE, Kwiatek MA, Nealis T,
Bulsiewicz W, Post J and Kahrilas PJ: Achalasia: A new clinically
relevant classification by high-resolution manometry.
Gastroenterology. 135:1526–1533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eckardt AJ and Eckardt VF: Treatment and
surveillance strategies in achalasia: An update. Nat Rev
Gastroenterol Hepatol. 8:311–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferri F, Brera C, De Santis B, Fedrizzi G,
Bacci T, Bedogni L, Capanni S, Collini G, Crespi E, Debegnach F, et
al: Survey on urinary levels of aflatoxins in professionally
exposed workers. Toxins (Basel). 9:E1172017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yard EE, Daniel JH, Lewis LS, Rybak ME,
Paliakov EM, Kim AA, Montgomery JM, Bunnell R, Abudo MU, Akhwale W,
et al: Human aflatoxin exposure in Kenya, 2007: A cross-sectional
study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess.
30:1322–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujii T, Yamana H, Sueyoshi S, Fujita H,
Tanaka Y, Kubota M, Toh U, Mine T, Sasahara H, Shirouzu K, et al:
Histopathological analysis of non-malignant and malignant
epithelium in achalasia of the esophagus. Dis Esophagus.
13:110–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leeuwenburgh I, Gerrits MM, Capello A, van
den Bogert B, van Dekken H, Steyerberg EW, Siersema PD and Kuipers
EJ: Expression of p53 as predictor for the development of
esophageal cancer in achalasia patients. Dis Esophagus. 23:506–511.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peraica M, Radic B, Lucic A and Pavlović
M: Toxic effects of mycotoxins in humans. Bull World Health Organ.
77:754–766. 1999.PubMed/NCBI
|
|
33
|
Luzi A, Cometa MF and Palmery M: Acute
effects of aflatoxins on guinea pig isolated ileum. Toxicol In
Vitro. 6:525–529. 2002. View Article : Google Scholar
|
|
34
|
Gursoy N, Durmus N, Bagcivan I, Sarac B,
Parlak A, Yildirim S and Kaya T: Investigation of acute effects of
aflatoxin on rat proximal and distal colon spontaneous
contractions. Food Chem Toxicol. 46:2876–2880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gockel HR, Schumacher J, Gockel I, Lang H,
Haaf T and Nöthen MM: Achalasia: Will genetic studies provide
insights? Hum Genet. 128:353–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boeckxstaens GE: Achalasia: Virus-induced
euthanasia of neurons? Am J Gastroenterol. 103:1610–1612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rohof WO, Salvador R, Annese V, Bruley des
Varannes S, Chaussade S, Costantini M, Elizalde JI, Gaudric M,
Smout AJ, Tack J, et al: Outcomes of treatment for achalasia depend
on manometric subtype. Gastroenterology. 144:718–725. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eckardt VF, Kohne U, Junginger T and
Westermeier T: Risk factors for diagnostic delay in achalasia. Dig
Dis Sci. 42:580–585. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
El-Takli I, O'Brien P and Paterson WG:
Clinical diagnosis of achalasia: How reliable is the barium x-ray?
Can J Gastroenterol. 20:335–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghoshal UC, Daschakraborty SB and Singh R:
Pathogenesis of achalasia cardia. World J Gastroenterol.
28:3050–3057. 2012. View Article : Google Scholar
|
|
41
|
Moon EY and Pyo S: Aflatoxin B(1) inhibits
CD14-mediated nitric oxide production in murine peritoneal
macrophages. Int J Immunopharmacol. 22:237–246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moon EY, Rhee DK and Pyo S: Inhibition of
various functions in murine peritoneal macrophages by aflatoxin B1
exposure in vivo. Int J Immunopharmacol. 21:47–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moon EY, Han JJ, Rhee DK and Pyo S:
Aflatoxin B1-induced suppression of nitric oxide production in
murine peritoneal macrophages. J Toxicol Environ Health A.
55:517–530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khajanchee YS, VanAndel R, Jobe BA, Barra
MJ, Hansen PD and Swanstrom LL: Electrical stimulation of the vagus
nerve restores motility in an animal model of achalasia. J
Gastrointest Surg. 7:843–849. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Souza EM, Rivera MT, Araujo-Jorge TC
and de Castro SL: Modulation induced by estradiol in the acute
phase of Trypanosoma cruzi infection in mice. Parasitol Res.
87:513–520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ming Z and Davis CD: CD8+ T
lymphocytes required for enhanced survival of Trypanosoma
cruzi-infected mice at elevated environmental temperature. J
Parasitol. 89:630–632. 2003. View Article : Google Scholar : PubMed/NCBI
|