Introduction
Dental implants and ankylosed teeth cannot be moved
by orthodontic force, which highlights the importance of
periodontal ligament cells (PDLCs) in orthodontic tooth movement
(1).
PDLCs are mechanically sensitive cells that may be
induced by mechanical force, and they express a series of
cytokines, including macrophage colony stimulating factor (M-CSF),
receptor activator of NF-κB ligand (RANKL), osteoprotegerin (OPG)
and inflammatory factors (2,3). The
combined functional interventions that result from these factors
serve to control the development of alveolar bone (4,5).
However, the processes underlying the transduction of the
associated mechanical signals to elicited biological signals, which
is mediated by PDLCs, remain to be elucidated.
To date, 2 mechanotransduction mechanisms in the
cells have captured considerable attention. First, attention has
focused on the cytoskeletal-integrin-focal adhesion pathway, which
receives and delivers the signals following transformation of
microfilaments and microtubules (6,7).
Second, attention has also focused on the mechanically sensitive
ion channels (MSCs) on the surface of the cytomembrane, which
transduce signals by increasing membrane permeability of the ion
channels and triggering the influx of extracellular calcium
(8). It has been proposed that the
activation of calcium ion channels and subsequent calcium influx is
associated with cytoskeletal transformation on induction by stress
stimuli (9). However, this process
was contradicted by Cox et al (10), which states that the integrity of
the cytoskeleton is irrelevant in the context of Piezo1 ion channel
function. The functional roles played by MSCs in orthodontic
force-induced PDLC activation and the relationship between these
two types of mechanotransduction have been poorly studied.
Piezo 1 and transient receptor potential cation
channel subfamily V member 4 (TRPV4) are two typical MSCs that have
received widespread attention from the research community. Piezo1
was first identified in a mouse neuroblastoma cell line; it was
determined to respond to mechanical stimuli in as little as 5 msec
and trigger calcium influx into the cells (11). A distinctive feature of the
microscopic structure of Piezo1 is the flexible blades region,
which is proposed to rotate and expose the central ion-conducting
pore under mechanical stimulus (12).
Distinct from Piezo1, TRPV4 was initially recognized
as an osmotically activated channel (13). Further studies identified that
TRPV4 could be activated by fluid shear stress and phorbol ester
(14,15). However, the gating mechanisms of
TRPV4 remain to be elucidated. Although Piezo1 and TRPV4 are found
in several mechanically sensitive cells (16–18),
the downstream signal transduction pathways remain unknown.
Mitogen-activated protein kinase (MAPK) refers to a
group of protein kinases that are associated with Piezo1 and the
TRPV4 channel (19,20). It has been identified that an
ERK1/2 inhibitor decreased the expression of Piezo1 in neonatal rat
ventricular myocytes, whereas this effect was not observed when p38
and JNK inhibitors were applied (21). Additionally, the p38 inhibitor
SB203580 enhanced the expression of TRPV4 in the dorsal root
ganglion (22). Collectively,
these observations suggest that MAPKs may participate in signal
transduction pathways downstream of MSCs under conditions of
mechanical loading.
In the present study, human primary PDLCs were
subjected to stretch using a Flexcell device, leading to a model of
stress-induced transformation. The roles played by MSCs in PDLC
mechanotransduction were functionally analyzed by deconstructing
the cytoskeleton using cytochalasin D (cytoD), or by blocking the
Piezo1 channel using GsMTx4 or the TRPV4 channel using GSK205. The
expression profiles of the MAPK signaling pathway in PDLCs when
both of the MSCs were specifically blocked by targeted inhibition
was also investigated.
Materials and methods
Cell culture
Human PDLCs were obtained from premolars that were
extracted from 4 young donors for orthodontic consultation and
treatment at the Jiangsu Stomatological Hospital. All donors were
healthy ethnic Han Chinese females between 12 and 14 years old. The
donors and their legal guardians were fully informed of the purpose
of this study and provided written informed consent. All human
experimental protocols were approved by the Ethics Committee of
Shanghai Tenth People's Hospital [policy no. 2008 (20)]. The periodontal ligament was
scraped from the root surfaces of the teeth and digested with
collagenase type I (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C.
Cells were collected and resuspended in low-glucose DMEM (HyClone;
GE Healthcare Life Sciences) that was supplemented with 15% FBS
(ScienCell Research Laboratories, Inc.), 100 U/ml penicillin-G and
100 µg/ml streptomycin sulfate (HyClone; GE Healthcare Life
Sciences). Cells were passaged when they reached ~90% confluence,
and those from passages 3–5 were used in subsequent
experiments.
Primary mouse osteoblasts were isolated from 20
2-3-day-old BALB/c neonatal female mice (Beijing Vital River
Laboratory Animal Technology); animals were sacrificed on arrival.
All animal experimental protocols were approved by the Ethics
Committee of Shanghai Tenth People's Hospital (policy no.
SHDSYY-2017-2473). The calvarial bones of the mice were cut into
fractions and digested using 0.25% trypsin for 30 min and 1 mg/ml
collagenase type II for 10 min (Sigma-Aldrich; Merck KGaA) at 37°C.
Following digestion, the fractions were resuspended in DMEM
supplemented with 10% FBS and incubated at 37°C in a humidified
atmosphere of 95% air and 5% CO2. Cells were passaged
when they reached 90% confluence, and those from passages 3–5 were
used in subsequent experiments.
Cell loading
Human periodontal ligament cells (hPDLCs) were
plated in BioFlex culture plates (type I collagen-coated; Flexcell
International Corporation) and divided into four groups. The first
was a control group, in which cells were loaded onto a Flexcell
tension system (FX-5000T, Flexcell International Corporation)
without any treatment. The second were cells in the cytoD group,
which were pretreated with cytoD (Sigma-Aldrich; Merck KGaA) at a
concentration of 5 µg/ml to depolymerize F-actin in the cells and
incubated at 37°C for 20 min, cells were refreshed by addition of
fresh culture medium without cytoD. For the third (GSK205) and
fourth (GsMTx4) groups, fresh medium respectively containing 30 µM
GSK205 (cat. no. 616522; Merck KGaA) and 500 nM GsMTx4 (cat. no.
ab141871; Abcam) were added separately before cell loading to
suppress the TRPV4 and Piezo1 channels, and cells were subjected to
these compounds during loading Next, the plates were set on the
FX-5000T. Then, 15% stretch was applied onto the cells for 4, 8 and
12 h. According to the study of Lu et al (9) on three different cell types, a
stretch time duration of <3 sec fails to initiate calcium
influx. Consequently, a 0.25-Hz square waveform of periodic
loading, which persisted for 3 sec with a 1-sec release, was
selected for the present study.
Calcium measurements
hPDLCs in all groups were washed three times in PBS
following the stretch procedure. Then, the cells were treated with
1 µM Fluo-4AM (Beyotime Institute of Biotechnology) and incubated
for 20 min in the dark at 37°C. Afterwards, the cells were washed
three times in PBS and observed by fluorescence microscopy
(DMI3000B; Leica Microsystems GmbH). The fluorescence intensity was
measured using ImageJ 2X 2.1.4.6 software (National Institutes of
Health). To do the measurements, the image was first converted to
8-bit grayscale and then inverted. The threshold was then set to
ensure that only the cell regions were selected, and measurements
were performed to obtain the area of the cells, the integrated
intensity of the region and the average gray value of the selected
region. The average gray value of each image was used for
statistical analysis.
Cytoskeletal fluorescence
staining
hPDLCs were washed three times in PBS following
stretching and fixed in 4% paraformaldehyde (Beijing Leagene
Biotech Co Ltd.) for 15 min at room temperature. Thereafter, the
cells were permeabilized in 0.5% Triton X-100 for 15 min at room
temperature and then rinsed three times in PBS. ActinGreen™ 488
ReadyProbes® Reagent (Thermo Fisher Scientific, Inc.)
was added to all plates, after which they were incubated at room
temperature for 30 min in the dark. Finally, cells were washed
three times in PBS and observed by fluorescence microscopy at ×200
magnification (DMI3000B; Leica Microsystems GmbH). The average gray
value of the cells were also measured by the method described
above.
Reverse transcription-quantitative
(RT-q)PCR
After 0, 4, 8 or 12 h loading, total RNA was
extracted from the PDLCs using the Tiangen RNAprep pure kit
(Tiangen Biotech Co., Ltd.). cDNA was obtained with the
PrimeScript™ RT Master Mix reagent (Takara Bio, Inc.) following the
manufacturer's instructions. The cDNA was added to a 20-µl system
for qPCR using the SYBR Green Reaction kit (Roche Diagnostics)
using the ABI Prism 7300 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The reaction was carried out under
the following conditions: 94°C for 2 min, then 40 cycles of 94°C
for 30 sec and 55°C for 1 min. The comparative 2−∆∆Cq
method was used to calculate the fold-change of mRNA (23). Primers used are listed in Table I.
 | Table I.Sequences of primers used in the
present study. |
Table I.
Sequences of primers used in the
present study.
| mRNA | Primer
sequence |
|---|
| RANKL | Forward:
5′-ACCGACATCCCATCTGGTT-3′ |
|
| Reverse:
5′-GCCATCCTGATTAACTATTAGTT-3′ |
| OPG | Forward:
5′-AAGCCTTCTCTAACCTCTCC-3′ |
|
| Reverse:
5′-GCCCTCGCTTATGATCTGTC-3′ |
| COX2 | Forward:
5′-TTGAAATGGCAGTTGATTCCTTT-3′ |
|
| Reverse:
5′-TATCCTCTTTCTCAGGGTGCTTG-3′ |
| Piezo1 | Forward:
5′-GGCAACATGAGGGAGTTCATTAACTC-3′ |
|
| Reverse:
5′-TTCTCCGTCAGGTAGTTGACAATGTG-3′ |
| TRPV4 | Forward:
5′-GGCAACTTCCTCACCAAGA-3′ |
|
| Reverse:
5′-GGGTATTTCTTCTCTGTCTCT-3′ |
| GAPDH | Forward:
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
|
| Reverse:
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
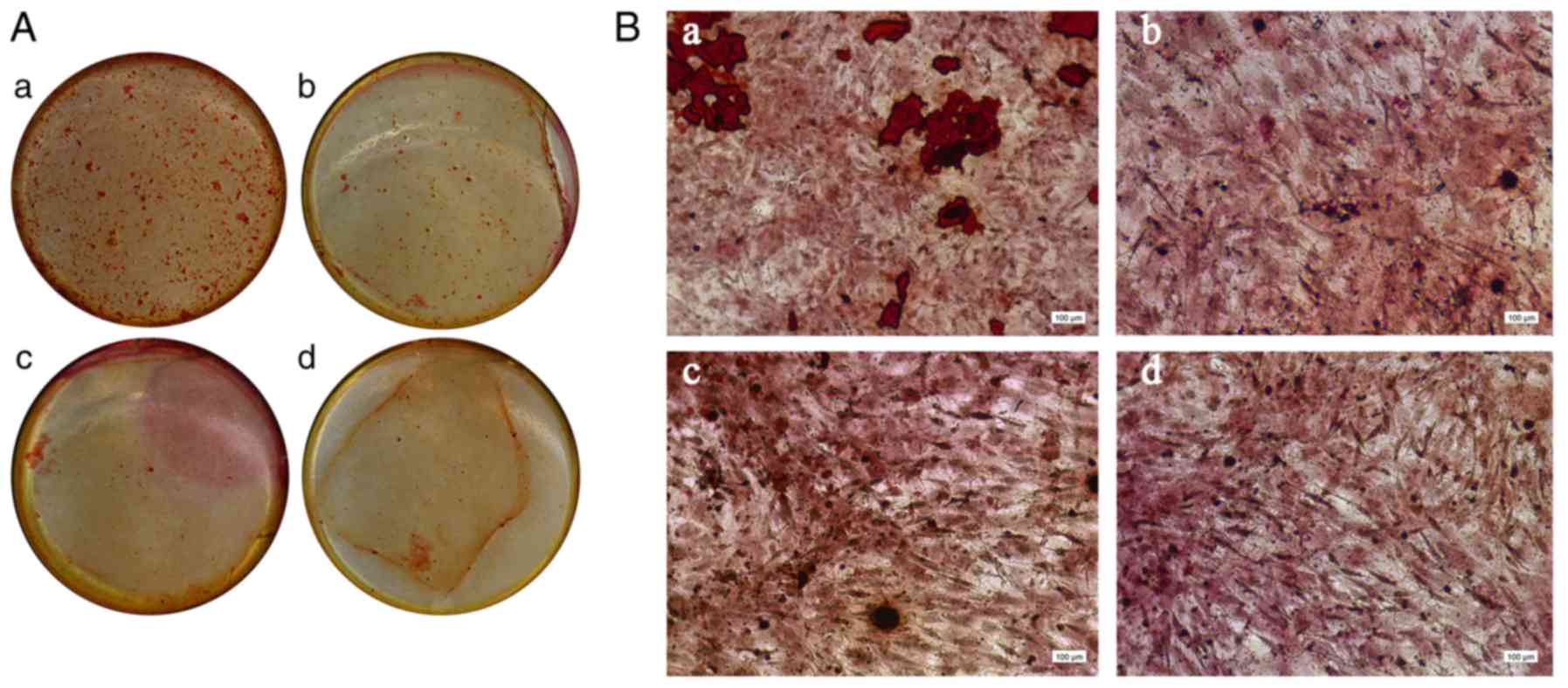
Osteoblast mineralization
induction
Murine osteoblasts that were derived from passages
3–5 were seeded into 24-well plates. Culture media from all four
PDLC treatment groups, which were obtained following loading, were
added to the plates when the cells reached ~90% confluence. The
media was changed every three days. After 10 days of culture, cells
were fixed in 95% ethanol at room temperature for 15 min, and then
stained with 0.2% Alizarin red at room temperature for 5 min.
Western blot analysis
Cells obtained from the control group, the cytoD
group and the GSK205 and GsMTx4 groups were washed in PBS following
loading, following which they were lysed and their total protein
was extracted using a whole-cell lysis kit (Wanleibio Co., Ltd.).
Protein concentration was determined with an enhanced bicinchoninic
acid protein assay reagent kit (cat. no. P0009; Beyotime Institute
of Biotechnology). Proteins were subjected to electrophoresis in a
12% SDS polyacrylamide gel (40 µg protein per lane) and then
transferred on to a PVDF immunoblotting membrane (EMD Millipore).
Then, 5 µl molecular weight standards (cat. no. 26616; Fermentas;
Thermo Fisher Scientific, Inc.) was loaded onto the gels as a
reference. The membrane was blocked in 5% skimmed milk for 2 h at
room temperature and incubated with cyclooxygenase-2 (COX2; 1:500;
cat. no. ab179800; Abcam), RANKL (1:500; cat. no. ab65024; Abcam)
and OPG (1:500; cat. no. ab183910; Abcam) primary antibodies at 4°C
overnight. The membrane was washed six times in Tris-buffered
saline containing 0.05% Tween 20 (TBST) for 5 min per wash. The
membrane was then incubated in secondary antibodies (1:1,000; cat.
no. WLA023; Wanleibio, Co., Ltd.) for 45 min at 37°C and washed six
times in TBST. The membrane-immobilized protein bands were detected
with chemiluminescent detection reagents (cat. no. WBKLS0100; EMD
Millipore) in the WD-9413 fluorescence imaging system (Beijing
Liuyi Biotechnology Co., Ltd.). The gray values for the visible
bands were analyzed with the Gel-Pro Analyzer software 4.0 (Meyer
Instruments, Inc.).
Expression profiling using protein
array
Whole-cell proteins extracted from PDLCs in all four
groups after the loading procedure were screened for their protein
expression profiles using a Human Phospho-MAPK array kit (cat. no.
ARY002B; R&D Systems, Inc.) according to the manufacturer's
instructions, variations of >20% are considered potential
candidates in the mechanotransduction of PDLCs.
Statistical analysis
Each experiment was repeated three times
independently and data are presented as the mean ± SD. Statistical
analyses were performed by one-way analysis of variance using
GraphPad Prism 6 software (GraphPad Software, Inc.). Tukey's
analysis was performed for multiple comparison tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Increased mRNA expression of COX2,
RANKL, OPG, Piezo1 and TRPV4 in PDLCs following periodic mechanical
loading
The mRNA levels of key biomarkers, including COX2,
RANKL and OPG, were increased after periodic loading at 0.25 Hz,
indicating that the PDLCs were activated after stretch (Fig. 1). Additionally, the increased
expression of TRPV4 and Piezo1 suggested that both ion channels
were related to PDLC activation following loading (Fig. 1). However, it was noted that while
most of the expression levels peaked at 8 h, additional stretch
loading reduced PDLC activation, which could be attributed to the
plate membrane losing its elasticity. Another reason for this
observation may have been due to adaptation of the mechanosensitive
cells and attenuation of the mechanotransduction process following
loading, as was discussed in our previous study (24).
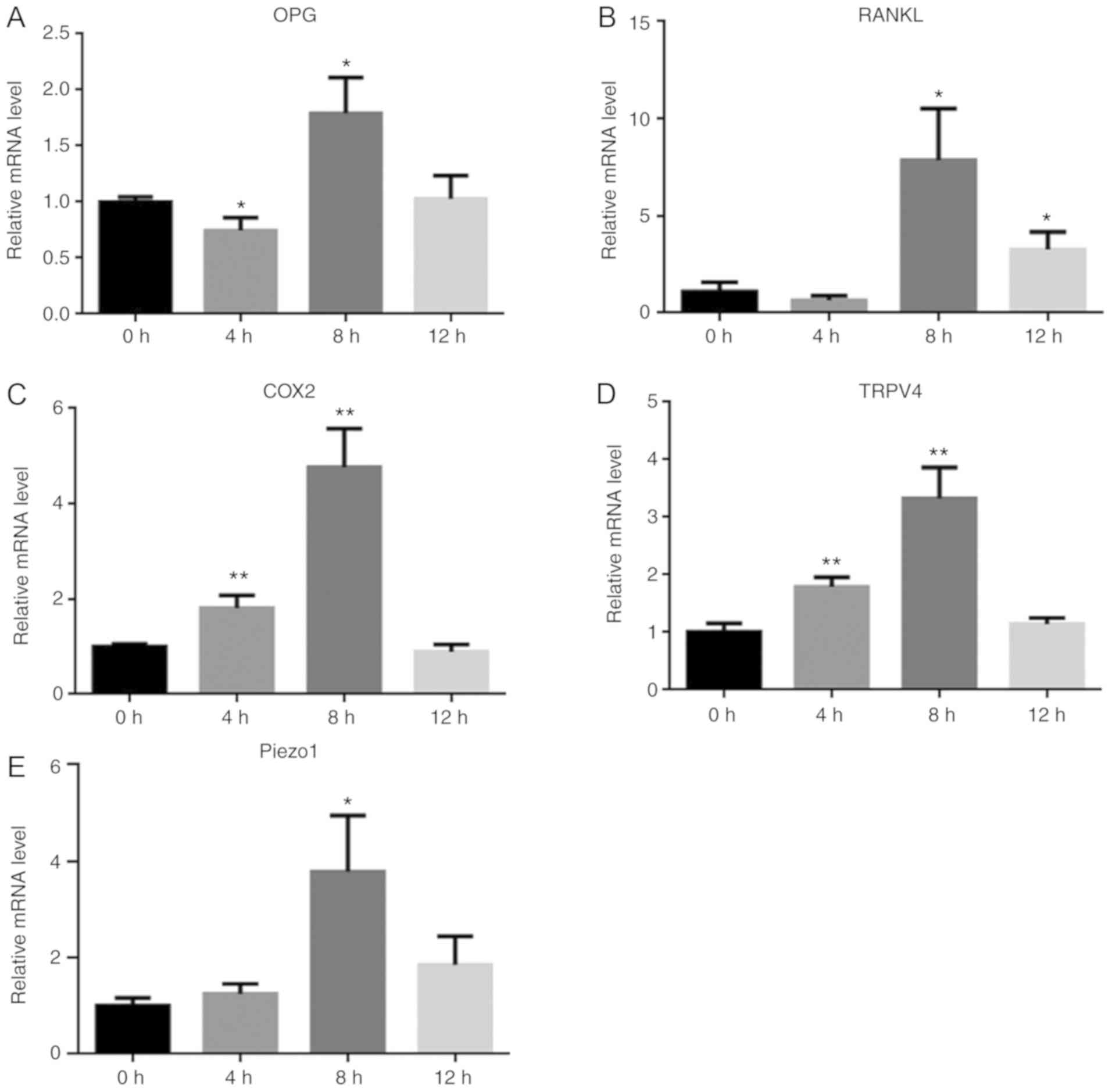 | Figure 1.Stretch loading induces the mRNA
expression of COX2, RANKL, OPG, TRPV4 and Piezo1 in PDLCs. mRNA
expression patterns of COX2, RANKL, OPG, TRPV4 and Piezo1 in PDLCs
following loading. (A) OPG, (B) RANKL, (C) COX2, (D) TRPV4 and (E)
for Piezo1. *P<0.05, **P<0.01 vs. 0 h. PDLC, periodontal
ligament cell; COX2, cyclooxygenase-2; RANKL, receptor activator of
NF-κB ligand; OPG, osteoprotegerin; TRPV4, transient receptor
potential cation channel subfamily V member 4. |
GsMTx4 and GSK205 do not interfere
with cytoskeletal reconstruction in PDLCs, while CytoD does not
affect calcium influx following loading
Fluorescent images showed that the intracellular
calcium concentrations of PDLCs treated with GsMTx4 and GSK205
decreased compared with the control group following loading;
however, cells that were treated with cytoD exhibited a
fluorescence intensity similar to that of the control group
(Fig. 2). This result suggested
that the roles played by MSCs were independent of cytoskeletal
integrity. The fluorescent images of the cytoskeleton showed that
in cells that were pretreated with cytoD, a wrinkled appearance was
noted and the intensity of the fluorescence decreased. By contrast,
neither GsMTx4- nor GSK205-treated cells exhibited any differences
in fluorescence intensity or cell morphology (Fig. 3).
Destruction of the cytoskeleton and
inhibition of mechanosensitive ion channels may compromise PDLC
activation induced by periodic stretching
The biomarkers COX2, RANKL and OPG were all secreted
by PDLCs after mechanical loading. COX2 is the rate-limiting enzyme
that converts arachidonic acid to prostaglandins (PGs), which can
regulate local inflammation and play a key role in the
reconstruction of alveolar bone under orthodontic forces (25). The ratio of RANKL/OPG directly
controls the balance between osteogenesis and osteoclastogenesis
(26). It has been established
that increased expression of these factors in PDLCs is induced by
mechanical loading (27,28).
In the present study, the observed increases in
biomarker expression were compromised by cytoD, GsMTx4 and GSK205
treatment (Fig. 4), which
indicated that the efficiency of mechanotransduction was reduced.
The results suggested that the cytoskeleton, Piezo1 and TRPV4 may
affect signal transduction in PDLCs after stretch loading. The
culture of osteoblasts with loaded PDLC supernatants also
demonstrated that the supernatant of the cells treated with the
selected inhibitors exhibited a diminished capacity to promote
osteogenesis (Fig. 5).
Roles played by cytoskeletal and
mechanosensitive ion channels in PDLC mechanotransduction may be
mediated by MAPK pathways
Although all three inhibitors decreased PDLC
activation that was otherwise induced by stretch, the profiles of
MAPK-associated proteins in each group were observed to differ.
Following a microarray analysis of phosphorylated MAPKs, the three
groups of cells that were treated by the inhibitors were compared
with the control group. Fold changes in the phosphorylation of
proteins in each group are shown in Fig. 6.
Discussion
It has been established that PDLCs are
mechanosensitive cells that secrete multiple cytokines to regulate
the reconstruction of local alveolar bone (28,29).
This mechanism could be considered the foundation of their role in
orthodontic tooth movement (29,30).
PDLCs at the pressure side are capable of continuous RANKL
expression (28). Additionally,
increased expression of PGE2, tumor necrosis factor-α, interleukin
(IL)-1β, IL-2, IL-6, IL-8 and M-CSF has been detected in
periodontal ligament loaded by orthodontic force (31,32).
Tension loading can also activate PDLCs (32).
In the present study, the expression of COX2, RANKL
and OPG in PDLCs was elevated following tension loading, which
corresponds with results obtained from previously published studies
(27). ELISA was not performed in
the present study, as the aim of employing RT-qPCR was to find the
time point of peak molecular expression of the genes of interest.
In addition, changes in mRNA levels were considered to be more
sensitive than the estimation of protein levels by assays such as
ELISA.
Among the biomarkers, COX2 regulates local
inflammation by regulating the synthesis of PGs (25). Increased COX2 expression has been
detected in multiple mechanosensitive cells, and is considered to
be an indication of extraneous stimulation intensity (24). RANKL and OPG are factors that play
opposing roles and both participate in bone metabolism (26,33).
Increases in these factors suggested that PDLCs were activated by
periodic stretch loading. Additionally, increased TRPV4 and Piezo1
expression implied that calcium ion channels may be involved in
mechanotransduction of stretch loading. When PDLCs are subjected to
stress, increased levels of TRPV4 and Piezo1 enable greater
efficiency of the cells in the mechanotransduction process and
regulate their biological adaptation to the environment (34). Similar feedback regulation can be
detected in cytoskeletal reconstruction following loading, as
reported in our previous study (24). The strengthening of mechanical
sensors in the cells following loading may be attributable to
positive feedback mechanisms, which requires further
investigation.
The roles played by the cytoskeleton in the process
of stress-strain-cell signal transduction have been established
(35–37). MSCs, particularly TRPV4 and Piezo1,
have also been considered key factors in mechanotransduction
(8,38). It has been suggested that
mechanical loading can induce calcium influx and calcium-dependent
cytoskeletal reorganization (39).
However, it remains to be determined as to whether cytoskeletal
transformation induced by stress can activate the calcium ion
channels on the cytomembrane, or whether the calcium influx that is
initiated by calcium ion channels can regulate cytoskeletal
reorganization through downstream signaling pathways.
A previous study employing the patch-clamp assay
revealed that the integrity of the cytoskeleton is not essential
for the functioning of the Piezo1 channel (10). Conversely, certain investigators do
not trust the patch-clamp database, as the surfaces of the
cytomembrane are subjected to continuous tension forces during an
experimental study, which may lead to results that differ from
those found in normal cells (34).
By contrast, another study found decreased calcium influx when
induced pluripotent stem cells were pretreated with cytoD prior to
application of 20% stretch force; such effects were not observed
when 10–15% stretch was used (9).
In the present study, 15% stretch was loaded onto
the PDLCs, and no effect on calcium influx was found by treatment
with cytoD, consistent with the abovementioned studies.
Additionally, such contradictions as are described in previous
studies may imply that the Piezo1 channel, unlike TRPV4, is not
involved in cytoskeletal reconstruction. In support of this,
Matthews et al (40)
reported that the force applied to β-1 integrins could activate the
TRPV4 ion channel. In summary, no meaningful interactions between
the cytoskeleton and these two ion channels were detected by the
present study.
Fluxes in calcium ion concentrations play a critical
role in cell-mediated biological activities that are regulated by
calcium ion channels at the cytoplasmic membrane (41,42),
thus enabling MSCs to regulate the cell cycle, proliferation,
differentiation and apoptosis through calcium-dependent pathways
upon application of a mechanical force (43). In the present study, administration
of Piezo1 and TRPV4 antagonists decreased intracellular calcium
concentrations following loading. The antagonists also attenuated
the activation of three important biomarkers, RANKL, OPG and COX2,
in PDLCs. The results suggested that both Piezo1 and TRPV4 are key
factors in PDLC mechanotransduction. Jin et al (8) reported the effect of Piezo1 in this
process; however, the mechanical loading in that study was
performed under conditions of static pressure. This, combined with
the results of the present study, suggests that both tension and
pressure forces are recognized by the Piezo1 channel, which could
initiate the expression of key cellular signals. By contrast, TRPV4
is a special ion channel that possesses similar permeability for
the divalent cations Ca2+, Sr2+,
Mg2+ and Ba2+; however, under physiologic
conditions, Ca2+ is the predominant ion that migrates
through the TRPV4 channel (13,44).
Son et al (38) studied the role of TRPV4 in PDLCs
and found that when TRPV4 was activated by osmotic pressure,
calcium influx was otherwise evoked. Meanwhile, although the
expression of RANKL was increased, it was found that OPG expression
remained unchanged (38). In the
present study, the expression levels of both RANKL and OPG were
decreased. This could be formally attributed to the fact that the
mechanical force applied in the present study was a periodic
stretch force. These conditions differed from those reported in the
previous study (38) and could
have resulted in diverse effects on TRPV4. Analysis of existing
data has revealed that, although both Piezo1 and TRPV4 participate
in mechanotransduction, they differ in terms of permeability, an
ability to activate conditions and in their downstream signaling
pathways.
That being considered, the MAPK family of signal
transducing pathways consists of three major cascades, ERK, JNK and
p38, all of which participate in cell activation by mechanical
stress (45–47). However, it remains to be elucidated
whether mechanical signals that are transduced by MSCs are indeed
delivered by MAPK in PDLCs. It has been reported that the opening
of Piezo1 induced by mechanical force can initiate chondrocyte
apoptosis through ERK1 and ERK2 signaling pathways (20).
In the present study, no significant difference was
found in the phosphorylation of ERK1/2 when the Piezo1 channel was
inhibited. By contrast, a significant increase in glycogen synthase
kinase (GSK)3α/β phosphorylation was observed. As GSK3 is a
negative regulator of ERK1/2 (48), this might imply that GsMTx4
downregulates the ERK1/2 signaling pathway via the activation of
GSK. Additionally, GSK3β contributes to β-catenin activation via
Ras suppression (49).
Furthermore, GSK3β can also directly inhibit the transcription
factors c-Fos and c-Jun (50). The
results of microarray analysis also showed increased
phosphorylation of JNK3, JNK Pan and MAPK kinase (MKK)3 and 6 after
Piezo1 channel attenuation. Among these, MKK3 and 6 are members of
the p38-mediated signaling pathway, which catalyze p38
phosphorylation (51). Further,
the JNK signaling pathway can activate c-Jun (52). It might be hypothesized that when
the Piezo1 channel is blocked, the ERK-related signaling pathway is
also inhibited. Increased functional activation of both JNK and p38
may be attributable to cellular compensation mechanisms under
conditions where the ERK signaling pathway was compromised.
In contrast to the Piezo1 channel, when the TRPV4
ion channel was blocked by GSK205, increased phosphorylation of
ERK, JNK, AKT and p70S6 kinase was observed. In addition,
initiation of the AKT pathway is known to result from mechanical
stimuli (53). The absence of
calcium in the extracellular matrix has been noted to attenuate AKT
phosphorylation (54). Until now,
evidence supporting an interaction between TRPV4 and the MAPK
signaling pathway in mechanotransduction, especially in PDLCs, has
been lacking.
It has been reported that blocking of the ERK1/2
signal transduction pathway may prevent increases in TRPV4 channel
activity induced by hypoosmotic stress in chondrocytes (55). Conversely, the effects of TRPV4
inhibition on MAPK sub-members have rarely been reported. As, in
the present study, no significant suppressive effect was observed
in the context of MAPK signaling molecules, it might be concluded
that the signals received and transduced by TRPV4 were not
delivered via the MAPK-mediated signaling pathways.
Microarray analysis also revealed that
phosphorylation of ERK and p70S6 was enhanced, whereas that of p38α
was diminished following loading and under conditions when the
stress fibers were disrupted by cytoD. This observation suggests
that p38 may participate in signal delivery via the cytoskeletal
network. As reported by Qi et al (56), interference of stress fiber
assembly by Rac1 knockdown may dampen p38 phosphorylation in
vascular smooth muscle cells loaded by cyclic strain. Another study
revealed the roles of p38 signaling in PDLC mechanotransduction
loaded with mechanical stress (57).
There were certain limitations in the present study.
Protein assays are an efficient way to screen out potential
candidates in the mechanotransduction of PDLCs; the roles of the
proteins need to be verified via western blotting or in vivo
experiments. Furthermore, it has been established that several
signaling molecules, including MAPK, Wnt and Notch, are involved in
the process of mechanotransduction (58,59);
however, the present study only focused on the MAPK pathway. Due to
insufficient time and financial constraints, verification
experiments could not be performed in the present study. Further
investigations into these pathways will be conducted in the
future.
In summary, the present study revealed the roles of
Piezo1 and TRPV4 in the mechanotransduction of hPDLCs. In addition,
it was suggested that both Piezo1 and TRPV4 may impart their
functional activities via different signaling pathways distinct
from actin cytoskeletal rearrangement. Furthermore, the present
study indicated that the ERK and p38 signaling pathways might
participate in mechanotransduction mediated by the Piezo1 channel
and actin cytoskeleton, respectively. However, a similar role was
not observed for the TRPV4 channel. Other signal transduction
pathways might be engaged as a compensatory mechanism under
conditions when an individual pathway might be compromised.
Acknowledgements
The authors would like to thank Dr Weibing Zhang
(Affiliated Stomatological Hospital of Nanjing Medical University,
Nanjing, Jiangsu, China) for his assistance with the
experiments.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81830031 and 81570959), the
State Key Lab of Reproductive Medicine of Nanjing Medical
University (grant no. JX116GSP20171416), the Priority Academic
Program Development of Jiangsu Higher Education Institutions (grant
no. PAPD-2018-87), the Natural Science Foundation of Jiangsu
Province (grant nos. BL2014073 and 15KJA320002) and the Jiangsu
Provincial Key Medical Discipline (grant no. zdxka2016026).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS and LW were responsible for the study design and
implementation. SG and LS performed the cell culture, mechanical
loading, patient consent and documentation. PCR, western blotting
and data analysis were conducted by CZ and YP. YS was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Informed written consent was obtained from the legal
guardians of all donors. All experimental protocols were approved
by the Ethics Committee of Shanghai Tenth People's Hospital [policy
nos. 2008 (20) and
SHDSYY-2017-2473].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Proffit WR, Fields HW and Sarver DM:
Contemporary Orthodontics. 5th. Mosby; St. Louis: 2012
|
|
2
|
Chang M, Lin H, Fu H, Wang B, Han G and
Fan M: MicroRNA-195-5p regulates osteogenic differentiation of
periodontal ligament cells under mechanical loading. J Cell
Physiol. 232:3762–3774. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng L, Zhang Y, Kou X, Yang R, Liu D,
Wang X, Song Y, Cao H, He D, Gan Y and Zhou Y: Cadherin-11
modulates cell morphology and collagen synthesis in periodontal
ligament cells under mechanical stress. Angle Orthod. 87:193–199.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H and Zhang D: Effects of
periodontal ligament cells on alveolar bone metabolism under the
action of force and inflammatory factors and its molecular
mechanisms. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 39:432–437.
2017.PubMed/NCBI
|
|
5
|
Li Y, Jacox LA, Little SH and Ko CC:
Orthodontic tooth movement: The biology and clinical implications.
Kaohsiung J Med Sci. 34:207–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roca-Cusachs P, del Rio A, Puklin-Faucher
E, Gauthier NC, Biais N and Sheetz MP: Integrin-dependent force
transmission to the extracellular matrix by alpha-actinin triggers
adhesion maturation. Proc Natl Acad Sci USA. 110:E1361–E1370. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou J, Aponte-Santamaria C, Sturm S,
Bullerjahn JT, Bronowska A and Grater F: Mechanism of focal
adhesion kinase mechanosensing. PLoS Comput Biol. 11:e10045932015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin Y, Li J, Wang Y, Ye R, Feng X, Jing Z
and Zhao Z: Functional role of mechanosensitive ion channel Piezo1
in human periodontal ligament cells. Angle Orthod. 85:87–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu J, Lee YK, Ran X, Lai WH, Li RA, Keung
W, Tse K, Tse HF and Yao X: An abnormal TRPV4-related cytosolic
Ca2+ rise in response to uniaxial stretch in induced
pluripotent stem cells-derived cardiomyocytes from dilated
cardiomyopathy patients. Biochim Biophys Acta Mol Basis Dis.
1863:2964–2972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cox CD, Bae C, Ziegler L, Hartley S,
Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA and
Martinac B: Removal of the mechanoprotective influence of the
cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat
Commun. 7:103662016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coste B, Mathur J, Schmidt M, Earley TJ,
Ranade S, Petrus MJ, Dubin AE and Patapoutian A: Piezo1 and Piezo2
are essential components of distinct mechanically activated cation
channels. Science. 330:55–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P,
Li R, Gao N, Xiao B and Yang M: Architecture of the mammalian
mechanosensitive Piezo1 channel. Nature. 527:64–69. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strotmann R, Harteneck C, Nunnenmacher K,
Schultz G and Plant TD: OTRPC4, a nonselective cation channel that
confers sensitivity to extracellular osmolarity. Nat Cell Biol.
2:695–702. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Güler AD, Lee H, Iida T, Shimizu I,
Tominaga M and Caterina M: Heat-evoked activation of the ion
channel, TRPV4. J Neurosci. 22:6408–6414. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao X, Wu L and O'Neil RG:
Temperature-modulated diversity of TRPV4 channel gating: Activation
by physical stresses and phorbol ester derivatives through protein
kinase C-dependent and -independent pathways. J Biol Chem.
278:27129–27137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Danielczok JG, Terriac E, Hertz L,
Petkova-Kirova P, Lautenschlager F, Laschke MW and Kaestner L: Red
blood cell passage of small capillaries is associated with
transient Ca2+-mediated adaptations. Front Physiol.
8:9792017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Servin-Vences MR, Richardson J, Lewin GR
and Poole K: Mechanoelectrical transduction in chondrocytes. Clin
Exp Pharmacol Physiol. 45:481–488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugimoto A, Miyazaki A, Kawarabayashi K,
Shono M, Akazawa Y, Hasegawa T, Ueda-Yamaguchi K, Kitamura T,
Yoshizaki K, Fukumoto S and Iwamoto T: Piezo type mechanosensitive
ion channel component 1 functions as a regulator of the cell fate
determination of mesenchymal stem cells. Sci Rep. 7:176962017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blythe NM, Muraki K, Ludlow MJ,
Stylianidis V, Gilbert HTJ, Evans EL, Cuthbertson K, Foster R,
Swift J, Li J, et al: Mechanically activated Piezo1 channels of
cardiac fibroblasts stimulate p38 mitogen-activated protein kinase
activity and interleukin-6 secretion. J Biol Chem. 294:17395–17408.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XF, Zhang Z, Li XD, Wang TB and Zhang
HN: Mechanism of the Piezo1 protein-induced apoptosis of the
chondrocytes through the MAPK/ERK1/2 signal pathway. Zhonghua Yi
Xue Za Zhi. 96:2472–2477. 2016.(In Chinese). PubMed/NCBI
|
|
21
|
Liang J, Huang B, Yuan G, Chen Y, Liang F,
Zeng H, Zheng S, Cao L, Geng D and Zhou S: Stretch-activated
channel Piezo1 is up-regulated in failure heart and cardiomyocyte
stimulated by AngII. Am J Transl Res. 9:2945–2955. 2017.PubMed/NCBI
|
|
22
|
Qu YJ, Zhang X, Fan ZZ, Huai J, Teng YB,
Zhang Y and Yue SW: Effect of TRPV4-p38 MAPK pathway on neuropathic
pain in rats with chronic compression of the dorsal root ganglion.
Biomed Res Int. 2016:69789232016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Tan S, Shen Y, Chen R, Wu C, Xu Y,
Song Z and Fu Q: Inhibition of FSS-induced actin cytoskeleton
reorganization by silencing LIMK2 gene increases the
mechanosensitivity of primary osteoblasts. Bone. 74:182–190. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanzaki H, Chiba M, Shimizu Y and Mitani
H: Periodontal ligament cells under mechanical stress induce
osteoclastogenesis by receptor activator of nuclear factor kappaB
ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner
Res. 17:210–220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boyce BF and Xing L: The RANKL/RANK/OPG
pathway. Curr Osteoporos Rep. 5:98–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garlet TP, Coelho U, Silva JS and Garlet
GP: Cytokine expression pattern in compression and tension sides of
the periodontal ligament during orthodontic tooth movement in
humans. Eur J Oral Sci. 115:355–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim T, Handa A, Iida J and Yoshida S:
RANKL expression in rat periodontal ligament subjected to a
continuous orthodontic force. Arch Oral Biol. 52:244–250. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanjanamekanant K, Luckprom P and Pavasant
P: Mechanical stress-induced interleukin-1beta expression through
adenosine triphosphate/P2X7 receptor activation in human
periodontal ligament cells. J Periodontal Res. 48:169–176. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu HD, Wang BK, Wan ZQ, Lin H, Chang ML
and Han GL: Wnt5a mediated canonical Wnt signaling pathway
activation in orthodontic tooth movement: Possible role in the
tension force-induced bone formation. J Mol Histol. 47:455–466.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alhashimi N, Frithiof L, Brudvik P and
Bakhiet M: CD40-CD40L expression during orthodontic tooth movement
in rats. Angle Orthod. 74:100–105. 2004.PubMed/NCBI
|
|
32
|
Grieve WG 3rd, Johnson GK, Moore RN,
Reinhardt RA and DuBois LM: Prostaglandin E (PGE) and interleukin-1
beta (IL-1 beta) levels in gingival crevicular fluid during human
orthodontic tooth movement. Am J Orthod Dentofacial Orthop.
105:369–374. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dougall WC: Molecular pathways:
Osteoclast-dependent and osteoclast-independent roles of the
RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer
Res. 18:326–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sachs F: Mechanical transduction by ion
channels: A cautionary tale. World J Neurol. 5:74–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ingber DE: Tensegrity-based mechanosensing
from macro to micro. Prog Biophys Mol Biol. 97:163–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Chen G, Zheng L, Luo S and Zhao Z:
Osteoblast cytoskeletal modulation in response to compressive
stress at physiological levels. Mol Cell Biochem. 304:45–52. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mammoto A and Ingber DE: Cytoskeletal
control of growth and cell fate switching. Curr Opin Cell Biol.
21:864–870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Son GY, Yang YM, Park WS, Chang I and Shin
DM: Hypotonic stress induces RANKL via transient receptor potential
melastatin 3 (TRPM3) and vaniloid 4 (TRPV4) in human PDL cells. J
Dent Res. 94:473–481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choquet D, Felsenfeld DP and Sheetz MP:
Extracellular matrix rigidity causes strengthening of
integrin-cytoskeleton linkages. Cell. 88:39–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matthews BD, Thodeti CK, Tytell JD,
Mammoto A, Overby DR and Ingber DE: Ultra-rapid activation of TRPV4
ion channels by mechanical forces applied to cell surface beta1
integrins. Integr Biol (Camb). 2:435–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Berridge MJ, Bootman MD and Lipp P:
Calcium-a life and death signal. Nature. 395:645–648. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Benavides Damm T and Egli M: Calcium's
role in mechanotransduction during muscle development. Cell Physiol
Biochem. 33:249–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Strotmann R, Schultz G and Plant TD:
Ca2+-dependent potentiation of the nonselective cation channel
TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol
Chem. 278:26541–26549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ishida T, Peterson TE, Kovach NL and Berk
BC: MAP kinase activation by flow in endothelial cells. Role of
beta 1 integrins and tyrosine kinases. Circ Res. 79:310–316. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kippenberger S, Bernd A, Loitsch S,
Guschel M, Muller J, Bereiter-Hahn J and Kaufmann R: Signaling of
mechanical stretch in human keratinocytes via MAP kinases. J Invest
Dermatol. 114:408–412. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamazaki T, Komuro I, Shiojima I and
Yazaki Y: The molecular mechanism of cardiac hypertrophy and
failure. Ann N Y Acad Sci. 874:38–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Q, Zhou Y, Wang X and Evers BM:
Glycogen synthase kinase-3 is a negative regulator of extracellular
signal-regulated kinase. Oncogene. 25:43–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu S, Fang X, Hall H, Yu S, Smith D, Lu
Z, Fang D, Liu J, Stephens LC, Woodgett JR and Mills GB: Homozygous
deletion of glycogen synthase kinase 3beta bypasses senescence
allowing Ras transformation of primary murine fibroblasts. Proc
Natl Acad Sci USA. 105:5248–5253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Götschel F, Kern C, Lang S, Sparna T,
Markmann C, Schwager J, McNelly S, von Weizsäcker F, Laufer S,
Hecht A and Merfort I: Inhibition of GSK3 differentially modulates
NF-kappaB, CREB, AP-1 and beta-catenin signaling in hepatocytes,
but fails to promote TNF-alpha-induced apoptosis. Exp Cell Res.
314:1351–1366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Terada Y, Nakashima O, Inoshita S,
Kuwahara M, Sasaki S and Marumo F: Mitogen-activated protein kinase
cascade and transcription factors: The opposite role of MKK3/6-p38K
and MKK1-MAPK. Nephrol Dial Transplant. 14 (Suppl 1):S45–S47. 1999.
View Article : Google Scholar
|
|
52
|
Papadopoulou A, Todaro A, Eliades T and
Kletsas D: Effect of hyperglycaemic conditions on the response of
human periodontal ligament fibroblasts to mechanical stretching.
Eur J Orthod. 41:583–590. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Danciu TE, Adam RM, Naruse K, Freeman MR
and Hauschka PV: Calcium regulates the PI3K-Akt pathway in
stretched osteoblasts. FEBS Lett. 536:193–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Divolis G, Mavroeidi P, Mavrofrydi O and
Papazafiri P: Differential effects of calcium on PI3K-Akt and
HIF-1α survival pathways. Cell Biol Toxicol. 32:437–449. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hdud IM, Mobasheri A and Loughna PT:
Effect of osmotic stress on the expression of TRPV4 and BKCa
channels and possible interaction with ERK1/2 and p38 in cultured
equine chondrocytes. Am J Physiol Cell Physiol. 306:C1050–C1057.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qi YX, Qu MJ, Yan ZQ, Zhao D, Jiang XH,
Shen BR and Jiang ZL: Cyclic strain modulates migration and
proliferation of vascular smooth muscle cells via Rho-GDIalpha,
Rac1, and p38 pathway. J Cell Biochem. 109:906–914. 2010.PubMed/NCBI
|
|
57
|
Zheng L, Huang Y, Song W, Gong X, Liu M,
Jia X, Zhou G, Chen L, Li A and Fan Y: Fluid shear stress regulates
metalloproteinase-1 and 2 in human periodontal ligament cells:
Involvement of extracellular signal-regulated kinase (ERK) and P38
signaling pathways. J Biomech. 45:2368–2375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen Y, Yang K, Zhou Z, Wang L, Du Y and
Wang X: Mechanical stress modulates the RANKL/OPG system of
periodontal ligament stem cells via α7 nAChR in human deciduous
teeth: An in vitro study. Stem Cells Int. 2019:53263412019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Manokawinchoke J, Pavasant P and Osathanon
T: Intermittent compressive stress regulates Notch target gene
expression via transforming growth factor-β signaling in murine
pre-osteoblast cell line. Arch Oral Biol. 82:47–54. 2017.
View Article : Google Scholar : PubMed/NCBI
|