Introduction
Patients with chronic obstructive pulmonary disease
(COPD) suffer from persistent respiratory symptoms and limited
airflow. As an obstructive disease of the lungs, COPD affects
>150 million individuals worldwide and caused 3.2 million deaths
in 2015 globally (1,2). Tobacco smoking-induced lung
inflammation and damage are the most common causes of COPD
(3,4); however, the exact mechanisms
underlying long-term cigarette smoking-induced immune dysregulation
are not completely understood (5,6). It
has been reported that the accumulation of activated innate immune
cells, including macrophages and adaptive immune responses
contribute to the pathophysiology of COPD (7).
In mouse models of cigarette smoke exposure-induced
COPD, emphysematous alterations in the lungs, which are accompanied
by altered lung function and infiltration of inflammatory cells and
T cells, have been identified (8,9).
Clinical studies have reported that oligoclonal T cells accumulate
in the airway wall and lungs of patients with COPD, and the degree
of T-lymphocyte infiltration positively correlates with the
severity of airflow limitation and emphysema (10,11).
Animal studies have also demonstrated that cigarette smoking
induces the accumulation of lymphocytes in the lungs of mice with
emphysema, whereas CD4+ and CD8+ T cell
knockout mice do not display emphysema after long-term exposure to
cigarette smoke (12,13). The aforementioned results suggest
that T cell-mediated adaptive immunity plays an important role
during the development of COPD and emphysema.
Mesenchymal stem cells (MSCs) are multipotent
non-hematopoietic cells that exert immunosuppressive effects on a
number of different immune cells, including dendritic cells
(14), B lymphocytes (15) and T lymphocytes (16–20).
MSCs can secrete a large number of chemokines and immunosuppressive
factors when stimulated by inflammatory factors (21). As a result, lymphocytes can be
recruited by chemotaxis to the location where the MSCs reside and
subsequently inhibited by the high local concentration of
immunosuppressive factors (22).
In particular, it has been reported that bone marrow-derived MSCs
(BMSCs) significantly decrease T-lymphocyte proliferation by
secreting transforming growth factor (TGF)-β1, hepatocyte growth
factor (HGF) (16), prostaglandins
(19), nitric oxide (NO) (20) and inducible nitric oxide synthase
(iNOS) (23), an enzyme that
catalyzes the production of NO. Furthermore, the antiproliferative
effect of rat BMSCs on T lymphocytes has been demonstrated to be
mediated by the induced expression of iNOS and production of NO by
BMSCs (23). A previous study also
suggested that tail vein injection of BMSCs in rats significantly
reduces cigarette smoking-induced downregulation of iNOS expression
in the blood circulation and lungs of rats (24). In addition, adoptive transfer of
BMSCs alleviates lung inflammation and injury, and was associated
with reduced STAT5 phosphorylation in lung-infiltrating T
lymphocytes (24). However,
although it has been reported that iNOS regulates T cell death and
immune memory (25), and rat BMSCs
exert immunoregulatory effects in an animal model of COPD, whether
BMSCs directly inhibit the proliferation of nicotine-exposed T
cells via iNOS expression and inhibition of STAT5 phosphorylation
in T cells is not completely understood.
In the present study, splenic T cells were isolated
from rats after chronic nicotine exposure to establish an in
vitro BMSC:T cell co-culture system. Following treatment of the
co-cultured cells with an iNOS inhibitor or lentivirus
infection-mediated silencing of iNOS in rat BMSCs, the
contributions of iNOS and STAT5 phosphorylation to the
antiproliferative effect of BMSCs on nicotine-exposed T cells were
investigated.
Materials and methods
Animal model of nicotine exposure
A total of 30 male Sprague-Dawley rats (age, 8–10
weeks; weight, ~120 g) were purchased from the Charles River
Laboratories and housed in the specific pathogen-free facility at
the Experimental Animal Center of Shanxi Medical University.
Animals were maintained at room temperature (22±1°C), in 60–70%
humidity, with 12-h light/dark cycles and access to food and water
ad libitum. Nicotine exposure to rats was administered via
cigarette smoke, as previously reported (24). Briefly, rats were placed in an
organic glass passive smoking cage and exposed to the cigarette
smoke of 20 filtered commercial cigarettes for 1 h, twice per day
for 6 days per week, for a total of 24 weeks. Under these
conditions, individual rats were exposed to 11 mg tar and 0.9 mg
nicotine during each 1 h exposure. All animal experiments in the
present study were approved by the Animal Care and Research
Committee of Shanxi Medical University and performed with strict
adherence to the Guide for the Care and Use of Laboratory Animals
(8th edition) (26).
BMSC isolation, culture and
characterization
Male Sprague-Dawley rats (age, 4–6 weeks; weight,
~100 g; n=6; purchased from Charles River Laboratories and housed
as previously described above) were euthanized with CO2
with 10–30% volume displacement rate/min. Bone marrow cells were
isolated from the femurs and tibias of the rats by flushing the
bone marrow cavity with complete DMEM (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.). Cells were inoculated into a cell culture flask
and cultured with 5% CO2 at 37°C for 3 days.
Subsequently, the unattached cells were removed and the remaining
cells were incubated for 9–10 days. At 80–90% confluency,
BMSC-enriched cells displayed a typical long fusiform shape and
were passaged at a ratio of 1:2 or 1:3. BMSCs at passage 3 were
used for subsequent experiments. Flow cytometric staining of
surface markers (CD34+CD45−CD90−)
was performed to evaluate the purity of the BMSCs, as previously
described (27). BMSCs with
>90% purity were used for the co-culture with nicotine-exposed
rat T cells.
T-cell isolation and culture
The spleens were also aseptically isolated from the
Sprague-Dawley rats (age, 32–34 weeks) that had been chronically
exposed to nicotine for 24 weeks and euthanized as described above.
Spleen tissues were repeatedly cut into small pieces with
ophthalmic scissors to generate a splenocyte homogenate in
RPMI-1640 medium (Wuhan Boster Biological Technology, Ltd.)
supplemented with 10% FBS. The homogenate was passed through a
100-µm cell strainer and the subsequent cell suspension was
subjected to density gradient centrifugation at 20°C and 400 × g
for 20 min with a lymphocyte separation solution (TBD Sciences),
according to the manufacturer's protocol. Cells in the lymphocyte
layer were transferred to a separate tube and washed twice with
complete RPMI-1640 medium at 400 × g and 20°C for 5 min. Cells were
inoculated into 75-cm2 cell culture flasks at a cell
density of 1.0×106/ml and T-cell stimulating agent ConA
(5 µg/ml; Sigma-Aldrich; Merck KGaA) and T-cell growth factor
interleukin-2 (20 ng/ml; PeproTech, Inc.) were added to the medium.
Following incubation for 3 days, the T cells began growing and
displayed a round or elliptical shape, which was similar to what
has been previously described of activated cells (28).
BMSC and T cell co-culture
BMSCs in the logarithmic growth phase were seeded
into 96-well plates (100 µl/well) at the following densities:
1×103, 2×103, 4×103,
8×103 and 16×103 cells/well. BMSCs were
incubated overnight to allow cell attachment. Subsequently, T
lymphocytes isolated from the spleens of nicotine-exposed rats were
added to the wells (3.2×105 cells/well). The
corresponding ratios of BMSCs to T lymphocytes were: 1:320, 1:160,
1:80, 1:40 and 1:20, respectively. The total volume of the BMSC/T
cell co-culture system in each well of the 96-well plate was 200
µl. After co-culture for 48 h, the suspended T lymphocytes were
transferred to another 96-well plate. Cells were observed using an
inverted phase contrast light microscope (magnification, ×4 or ×10)
and cell growth was measured. In certain cases, different
concentrations (0.5, 1, 5, 10 or 20 µM) of the iNOS inhibitor
N-nitro-L-arginine methylester (L-NAME; Selleck Chemicals) were
added to the co-culture at the same time as the T cells. In other
cases, lentivirus-infected (control or iNOS knockdown-targeting)
BMSCs were used for the co-culture with T cells.
Cell proliferation evaluation using
the cell counting kit-8 (CCK-8) assay
The antiproliferative effect of BMSCs on T cells was
determined using the CCK-8 assay (Dojindo Molecular Technologies,
Inc.), according to the manufacturer's protocol. Briefly, after
BMSCs and T cells were co-cultured for 48 h, T cells were
transferred to a new plate, 20 µl CCK-8 reagent was added to each
well and cells were incubated for 4 h at 37°C. The optical density
value of each well was determined at a wavelength of 450 nm with a
reference wavelength of 600 nm using a microplate reader. The assay
was repeated three times and the samples were assayed in
triplicate.
Knockdown of iNOS in BMSCs using a
small hairpin (sh)RNA lentivirus
The shRNA sequence (5′-CCACTAACAGTGGCAACAT-3′) and a
random negative control sequence (5′-CGAGGGCGACTTAACCTTAGG-3′) were
cloned into the pLV(shRNA)-EGFP/Puro-U6 lentiviral vector (Cyagen
Biosciences, Inc.). The iNOS-specific shRNA sequence was located at
the coding sequence locus of the NOS2 gene (GenBank accession no.
NM_012611.3). 293T cells (American Type Culture Collection) were
plated onto 10-cm dishes at a density of 6×106
cells/dish and were co-transfected with control lentiviral vector
(CV; 10 µg/dish) or iNOS-knockdown lentiviral vector (iNOS-shRNA;
10 µg/dish) together with helper plasmids pMD2.G (Addgene, Inc.; 5
µg/dish) and psPAX2 (Addgene, Inc.; 5 µg/dish) using Lipofectamine
2000® transfection reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Vector
construction, verification by sequencing, virus packaging and
collection of the corresponding viral supernatants following 48 h
of transfection were performed by Cyagen Biosciences, Inc.
Subsequently, 2.5×105 BMSCs/well at 70% confluence were
seeded into 6-well plates and were infected with the lentiviral
supernatant at a multiplicity of infection of 30. Following 48 h of
infection, BMSCs were used for subsequent experiments.
Western blotting
BMSCs and T cells were harvested and sonicated at
the frequency of 20 kHz for 10 sec at 4°C in
phenylmethanesulfonylfluoride-containing RIPA lysis buffer (Thermo
Fisher Scientific, Inc.) and then incubated with RIPA lysis buffer
for 30 min on ice. Subsequently, cell lysates were centrifuged at
10,000 × g for 15 min at 4°C. Total protein in the supernatant was
quantified using the bicinchoninic acid reagent kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Proteins (30–50 µg) were separated by 10% SDS-PAGE and transferred
onto nitrocellulose membranes. Subsequently, membranes were blocked
with 5% skim milk for 2 h at room temperature and incubated
overnight at 4°C with primary antibodies targeted against: iNOS
(1:250; cat. no. ab49999; Abcam), STAT5 (1:500; cat. no. ab230670;
Abcam), phosphorylated STAT5 (1:1,000; cat. no. 05-495; EMD
Millipore) and β-actin (1:500; cat. no. MA1115; Wuhan Boster
Biological Technology, Ltd.). Following the primary incubation,
membranes were washed with TBS containing 0.5% Tween 20 and
incubated for 2 h at room temperature with a horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody
(1:10,000; cat. no. TA130003; OriGene Technologies, Inc.). Protein
bands were visualized using an enhanced chemiluminescence kit (EMD
Millipore). Blots were performed in triplicate and protein
expression was quantified using AlphaView version 3.4 software
(ProteinSimple) with β-actin as the loading control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from BMSCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. RNA quality was
confirmed by 1% agarose gel electrophoresis. Total RNA (1 µg) was
reverse transcribed into cDNA at 37°C for 15 min using the
PrimeScript® RT Master Mix Perfect Real Time Reagent kit
(Takara Bio, Inc.), according to the manufacturer's protocol.
Subsequently, qPCR was performed using FastStart Universal SYBR
Green Master (ROX; Sigma-Aldrich; Merck KGaA) and an AB7500 RT-PCR
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following primer pairs were used for qPCR: iNOS forward,
5′-CACCTTGGAGTTCACCCAGT-3′ and reverse, 5′-ACCACTCGTACTTGGGATGC-3′;
β-actin forward, 5′-GTCAGGTCATCACTATCGGCAAT-3′ and reverse,
5′-AGAGGTCTTTACGGATGTCAACGT-3′. The following thermocycling
conditions were used for qPCR: Initial denaturation for 10 min at
95°C; 40 cycles of denaturation for 5 sec at 95°C and annealing and
extension for 1 min at 60°C; followed by a melting curve analysis.
RT-qPCR was repeated three times and each sample was tested in
triplicate. mRNA expression levels were quantified using the
2−ΔΔCq method (29) and
normalized to the internal reference gene β-actin.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 13.0; SPSS, Inc.). Data are expressed as the mean
± standard deviation of ≥3 experimental repeats. Multiple
comparisons were performed using one-way ANOVA followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
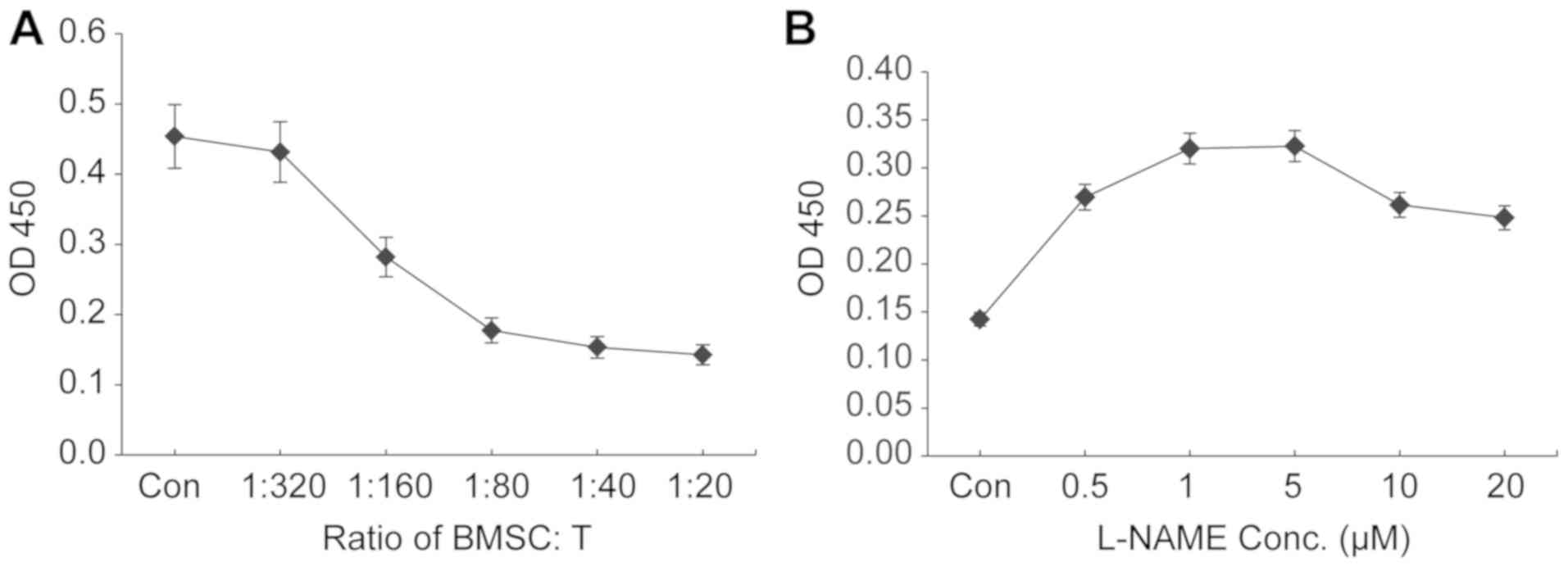
Co-culture of higher ratios of BMSCs:T
cells results in increased suppression of T-cell proliferation
The present study aimed to establish a BMSC:T cell
co-culture system for evaluating the antiproliferative effect of
BMSCs on T cells in vitro. To determine the most suitable
ratio of BMSCs:T cells that resulted in the most potent inhibition
of T-cell proliferation, rat BMSCs were co-cultured with rat
splenocyte-derived T cells at various ratios. An increasing number
of BMSCs (1×103−16×103 cells/well) were
seeded into 96-well plates and co-cultured with a fixed number of T
cells isolated from nicotine-exposed rats (3.2×105
cells/well). An increase in the number of BMSCs increased the
inhibition of T-cell proliferation, as indicated by the CCK-8 assay
(Fig. 1A). At a BMSC:T cell ratio
of 1:20, maximal proliferation inhibition was observed (>90%
compared with the control group; Fig.
1A). The results suggested that a higher ratio of BMSCs:T cells
resulted in increased inhibition of T-cell proliferation. Due to
the limited capacity of the 96-well plate, 1:20 was the highest
ratio that could be investigated in the present study; therefore,
the ratio of 1:20 BMSCs:T cells was selected for subsequent
experiments.
iNOS inhibitor L-NAME at 5 µM displays
the maximal ability to reverse the antiproliferative effects of
BMSCs
To investigate the effect of iNOS on the suppressive
function of BMSCs, the culture medium of the BMSC:T-cell co-culture
system was supplemented with the iNOS inhibitor L-NAME at various
concentrations. When BMSCs and T cells were co-cultured at the
ratio of 1:20, addition of L-NAME (starting from 0.5 µM) reversed
BMSC-mediated inhibition of T-lymphocyte proliferation in a
concentration-dependent manner with L-NAME concentrations ≤5 µM,
and L-NAME at 5 µM displayed the maximal reversal effect (Fig. 1B). Compared with L-NAME at 5 µM,
higher concentrations of L-NAME (10 and 20 µM) resulted in
decreased proliferation of co-cultured T cells; therefore, L-NAME
at a dose of 5 µM was used for further investigation of
iNOS-mediated mechanisms underlying the antiproliferative effects
of BMSCs on T cells.
Functional inactivation of iNOS by
inhibitor treatment or shRNA-mediated knockdown reverses the
suppressive effects of BMSCs
To further investigate the role of iNOS in mediating
the antiproliferative effect of BMSCs on T cells, an iNOS
shRNA-expressing lentivirus was generated. BMSCs were infected with
either CV or shRNA-iNOS. Following incubation for 48 h, transfected
cells were co-cultured with T lymphocytes isolated from
nicotine-exposed rats at the ratio of 1:20. BMSCs in the control
and CV groups significantly inhibited the proliferation of T
lymphocytes. By contrast, treatment with the iNOS inhibitor L-NAME
or silencing of iNOS expression significantly reversed the
antiproliferative effects of BMSCs (Fig. 2).
 | Figure 2.iNOS inhibitor treatment and
shRNA-mediated knockdown of iNOS significantly reverse the
antiproliferative effect of BMSCs on T cells. T cells were
co-cultured with control, shRNA-CV-transduced,
shRNA-iNOS-transduced or L-NAME-treated rat BMSCs at a ratio of
1:20. T cells cultured alone were considered as the negative
control. **P<0.01, as indicated. iNOS, inducible nitric oxide
synthase; shRNA, small hairpin RNA; BMSCs, bone marrow-derived
mesenchymal stem cells; CV, control lentivirus; L-NAME,
N-nitro-L-arginine methylester; OD, optical density; Ctrl,
control. |
Antiproliferative effect of BMSCs is
dependent on iNOS production by BMSCs and is associated with a
reduction in STAT5 phosphorylation in T cells
A previous study on the transfusion of BMSCs into
nicotine-exposed rats indicated that the suppressive roles of BMSCs
in autoimmune responses were associated with reductions in serum
and lung iNOS expression levels and smoking-induced STAT5
phosphorylation in lung tissue-derived lymphocytes (24). To investigate whether STAT5
phosphorylation was also associated with the antiproliferative
effects of BMSCs in the direct BMSC: T-cell co-culture system, the
expression levels of iNOS in BMSCs and STAT5 in T cells were
assessed. As determined by RT-qPCR, compared with the corresponding
control groups, the L-NAME and shRNA-iNOS groups displayed
significantly downregulated iNOS expression levels in BMSCs
(Fig. 3A). Consistently, the
western blotting results also indicated that the protein expression
levels of iNOS were significantly reduced in the L-NAME and
shRNA-iNOS groups compared with the corresponding control groups
(Fig. 3B and C). Furthermore, the
level of STAT5 phosphorylation was significantly increased in T
cells co-cultured with BMSCs with reduced iNOS expression compared
with the corresponding control groups (Fig. 4A). The increased expression of
phosphorylated STAT5 resulted in significantly increased ratios of
phosphorylated STAT5/total STAT5 expression in the L-NAME and
shRNA-iNOS groups compared with the corresponding control groups
(Fig. 4B). Collectively, the
results suggested that iNOS expression in BMSCs was negatively
associated with STAT5 phosphorylation in T cells in the co-culture
system. Furthermore, the results indicated that iNOS production in
BMSCs and reduced STAT5 phosphorylation in T cells contributed to
the antiproliferative effects of BMSCs.
Discussion
In previous years, the role of T cell-mediated
immune regulation in the pathogenesis of COPD has gained increasing
attention. Long-term cigarette smoking is a risk factor for COPD
and lung-infiltrating T lymphocytes play an indispensable role
during the development of COPD and emphysema (30,31).
MSCs with strong immunosuppressive properties have been reported to
attenuate COPD and emphysema progression in animal models and human
clinical trials (32–34). In the present study, the
suppressive effect of rat BMSCs on the proliferation of splenic T
cells isolated from chronic nicotine-exposed rats was investigated
in an in vitro BMSC and T cell co-culture system. The
results indicated that the suppressive function of BMSCs on T-cell
proliferation was dependent on the expression of iNOS in BMSCs and
reduced STAT5 phosphorylation in T cells.
A previous study reported that chronic cigarette
smoke exposure induces immune dysregulation in the lungs of rats
and adoptive transfer of BMSCs significantly attenuates the
imbalance of proinflammatory and anti-inflammatory factors, reduces
the antibody response to lung elastin antigen, and attenuates
chronic inflammatory damage in the lungs (24). Furthermore, analysis of the cell
cycle demonstrated that T-lymphocyte proliferation in the lungs of
rats treated with BMSC adoptive transfer following exposure to
cigarette smoke is arrested at the G0/G1
phase, which correlates with the reduced level of inflammatory
mediators and local inflammatory responses (24). However, the molecular mechanisms
underlying MSC-mediated inhibition of T-lymphocyte proliferation
and immune response regulation are not completely understood
(16,17,35–38).
The hypothesis that a soluble molecule mediates the inhibitory
effect of MSCs on T cells remains controversial. Certain previous
studies have reported that TGF-β, HGF, indoleamine 2,3-dioxygenase
(IDO) and prostaglandin E2 mediate the suppressive effects of MSCs
on T cells (16,38,39).
By contrast, other previous studies have reported that MSCs inhibit
T lymphocytes via contact-dependent mechanisms (18,40)
and cellular stress induction (41). Previous studies conducted by Su
et al (42) and Shi et
al (43) revealed that the key
molecule mediating immunosuppression by MSCs is species dependent;
MSCs from monkeys, pigs and humans suppress immune responses via
IDO, whereas MSCs from mice, rats, rabbits and hamsters mediate
immune responses via iNOS. MSCs derived from iNOS knockout mice
displayed significantly reduced antiproliferative effects on T
lymphocytes (20). In addition, it
has been indicated that the antiproliferative effect of rat BMSCs
on T lymphocytes is mediated by the induction of iNOS expression
and production of NO by BMSCs (23). Therefore, the present study aimed
to determine whether BMSCs directly inhibited the proliferation of
nicotine-exposed T cells via iNOS expression, while the effects of
other soluble factors, including TGF-β and prostaglandins, require
further investigation. Consistently, a previous study also
demonstrated that tail vein injection of BMSCs significantly
reduced the downregulation of iNOS levels in the blood circulation
and lungs of rats chronically exposed to cigarette smoke (24). The critical role of iNOS in
mediating the suppressive function of BMSCs was further suggested
by the results of the direct cell co-culture experiments performed
in the present study. Functional inactivation of iNOS by
shRNA-mediated iNOS silencing or treatment with an iNOS inhibitor
significantly reversed the suppressive function of BMSCs during
co-culture with T cells. In the present study, BMSCs at passage 3
were used and the co-culture period was relatively short (24 or 48
h), which suggested that the antiproliferative effect of BMSCs was
not due to their differentiation potentials. Similarly, previous
studies have reported that cell stemness does not explain the
MSC-mediated repair of a number of tissues (44,45).
Macrophages can inhibit the proliferation of T cells
by NO-mediated reduction of STAT5 phosphorylation in T cells
(46). Similarly, BMSCs displayed
antiproliferative effects on nicotine exposed-T cells in a NO
production-dependent manner in the present study. Moreover, in a
previous in vivo study, adoptive transfer of BMSCs also led
to significantly reduced STAT5 phosphorylation in rat lung T
lymphocytes after chronic direct exposure to cigarette smoke. In
the present study, the direct BMSC:T cell in vitro
co-culture resulted in increased phosphorylated STAT5 and decreased
total STAT5 expression in T cells. Additionally, L-NAME reversed
the reduction of STAT5 phosphorylation induced by BMSCs, which
suggested that NO functionally suppressed STAT5 to inhibit T-cell
proliferation, as STAT5 is a crucial transcription factor for T
cell activation and proliferation (47,48).
However, neither shRNA-mediated silencing of iNOS or L-NAME
treatment completely reversed the BMSC-induced inhibition of T-cell
proliferation, suggesting that NO might not be the only factor
responsible for the antiproliferative effect of BMSCs on T cells.
In the present study, ~80% of T-cell proliferation was restored
after functional inactivation of iNOS, indicating that NO may serve
as the major soluble factor responsible for the suppressive
function of BMSCs. Consistently, BMSCs derived from mice also
inhibit T-cell proliferation via NO production (20), which together with the results of
the present study and a previous study (23) indicated that MSCs from mice and
rats could be categorized as iNOS-utilizing immunosuppressors
(42). Collectively, inhibition of
T-cell proliferation by BMSC-derived NO and subsequent inhibition
of STAT5 phosphorylation in T cells may partially explain
BMSC-mediated alleviation of chronic inflammation and lung injury
in rats exposed to long-term chronic cigarette smoke.
T cells are classified into CD4+ and
CD8+ T cells, depending on the surface expression of CD4
and CD8 molecules. A clinical study revealed that a high number of
CD4+ T cells infiltrate the airway wall and lung
parenchyma of patients with COPD, and the number of CD4+
T cells is positively correlated with the severity of airflow
limitation and emphysema (10).
Maeno et al (49) reported
that cigarette smoke exposure did not induce emphysema-like changes
in CD8+ T cell-deficient mice, suggesting that
CD8+ T cell-mediated inflammation is involved in the
development of COPD and emphysema. However, the precise mechanisms
underlying the regulation of T-lymphocyte differentiation by BMSCs
during the modulation of chronic lung inflammation and injury in
animal models of COPD requires further investigation. A limitation
of the present study was that total T cells were used in the
co-culture experiments. Both CD4+ and CD8+ T
cells have been implicated in the disease progression of COPD;
therefore, characterizing the T cell subtype that is more
susceptible to BMSC-mediation and NO is required to improve the
knowledge of the suppressive function of BMSCs. In addition,
whether CD4+ and CD8+ T cells exhibit
differential STAT5 phosphorylation following NO stimulation or
co-culture with BMSCs also requires further investigation. Another
limitation of the present study was that the T cells were isolated
from rat spleens and not from rat lungs due to low abundance;
therefore, spleen-derived T cells might not fully represent the
phenotype of cigarette smoke-exposed lung-derived T cells.
In summary, an in vitro BMSC:T cell
co-culture system was established and the antiproliferative effect
of rat BMSCs on splenic T cells isolated from rats after long-term
chronic cigarette smoke exposure was investigated. Functional
inactivation of iNOS in BMSCs with shRNA-mediated silencing or
specific inhibitor treatment indicated that BMSC-induced inhibition
of T-cell proliferation was mediated by iNOS expression and
NO-induced reduction of STAT5 phosphorylation in T cells. The
present study provided direct evidence of the suppressive effect of
BMSCs on cigarette-exposed T cells and could, at least partially,
explain the mechanisms underlying the beneficial roles of BMSC
infusion in animal models and patients. Furthermore, the present
study indicated that adoptive transfer of BMSCs may serve as a
promising therapeutic strategy for chronic smoking-induced COPD and
emphysema.
Acknowledgements
The authors would like to thank Dr Huaping Zhang and
Dr Xiaoyan Zhai from the Central Laboratory of Shanxi Medical
University for their insightful discussions. The authors would also
like to thank Dr Jiahui Zhao from the Central Laboratory of Shanxi
Medical University for their technical assistance on western
blotting experiments. The authors would like to thank Dr Rui Wang
from the Physiology Laboratory of Shanxi Medical University for the
critical review and suggestions of the manuscript.
Funding
The present study was supported by the Ministry of
Education (grant no. 2016BY075) and the Health Committee of Shanxi
Province in China (grant no. 201601011).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and XL conceived and designed the study. XL and
PL designed the methodology. XL and PL performed the experiments
and acquired the data. XL analyzed and interpreted the data, wrote
the manuscript and provided supervision. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Research Committee of Shanxi Medical University and performed
with strict adherence to the Guide for the Care and Use of
Laboratory Animals (8th edition).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2015 Disease, Injury Incidence and
Prevalence Collaborators: Global, regional, and national incidence,
prevalence, and years lived with disability for 310 diseases and
injuries, 1990–2015: A systematic analysis for the global burden of
disease study 2015. Lancet. 388:1545–1602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2015 Mortality and Causes of Death
Collaborators, . Global, regional, and national life expectancy,
all-cause mortality, and cause-specific mortality for 249 causes of
death, 1980–2015: A systematic analysis for the global burden of
disease study 2015. Lancet. 388:1459–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Decramer M, Janssens W and Miravitlles M:
Chronic obstructive pulmonary disease. Lancet. 379:1341–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salvi S: Tobacco smoking and environmental
risk factors for chronic obstructive pulmonary disease. Clin Chest
Med. 35:17–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strzelak A, Ratajczak A, Adamiec A and
Feleszko W: Tobacco smoke induces and alters immune responses in
the lung triggering inflammation, allergy, asthma and other lung
diseases: A mechanistic review. Int J Environ Res Public Health.
15(pii): E10332018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu F, Liang CL, Liu H, Zeng YQ, Hou S,
Huang S, Lai X and Dai Z: Impacts of cigarette smoking on immune
responsiveness: Up and down or upside down? Oncotarget. 8:268–284.
2017.PubMed/NCBI
|
|
7
|
Gadgil A and Duncan SR: Role of
T-lymphocytes and pro-inflammatory mediators in the pathogenesis of
chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon
Dis. 3:531–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nurwidya F, Damayanti T and Yunus F: The
role of innate and adaptive immune cells in the immunopathogenesis
of chronic obstructive pulmonary disease. Tuberc Respir Dis
(Seoul). 79:5–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rinaldi M, Maes K, De Vleeschauwer S,
Thomas D, Verbeken EK, Decramer M, Janssens W and Gayan-Ramirez GN:
Long-term nose-only cigarette smoke exposure induces emphysema and
mild skeletal muscle dysfunction in mice. Dis Model Mech.
5:333–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sullivan AK, Simonian PL, Falta MT,
Mitchell JD, Cosgrove GP, Brown KK, Kotzin BL, Voelkel NF and
Fontenot AP: Oligoclonal CD4+ T cells in the lungs of patients with
severe emphysema. Am J Respir Crit Care Med. 172:590–596. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hogg JC, Chu F, Utokaparch S, Woods R,
Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson
HO and Paré PD: The nature of small-airway obstruction in chronic
obstructive pulmonary disease. N Engl J Med. 350:2645–2653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Hulst AI, Vermaelen KY, Brusselle GG,
Joos GF and Pauwels RA: Time course of cigarette smoke-induced
pulmonary inflammation in mice. Eur Respir J. 26:204–213. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
D'Hulst AI, Maes T, Bracke KR, Demedts IK,
Tournoy KG, Joos GF and Brusselle GG: Cigarette smoke-induced
pulmonary emphysema in scid-mice. Is the acquired immune system
required? Respir Res. 6:1472005.PubMed/NCBI
|
|
14
|
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y,
Yu XD and Mao N: Human mesenchymal stem cells inhibit
differentiation and function of monocyte-derived dendritic cells.
Blood. 105:4120–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corcione A, Benvenuto F, Ferretti E,
Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi
GL, Pistoia V and Uccelli A: Human mesenchymal stem cells modulate
B-cell functions. Blood. 107:367–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krampera M, Glennie S, Dyson J, Scott D,
Laylor R, Simpson E and Dazzi F: Bone marrow mesenchymal stem cells
inhibit the response of naive and memory antigen-specific T cells
to their cognate peptide. Blood. 101:3722–3729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: Implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasmusson I, Ringden O, Sundberg B and Le
Blanc K: Mesenchymal stem cells inhibit lymphocyte proliferation by
mitogens and alloantigens by different mechanisms. Exp Cell Res.
305:33–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato K, Ozaki K, Oh I, Meguro A, Hatanaka
K, Nagai T, Muroi K and Ozawa K: Nitric oxide plays a critical role
in suppression of T-cell proliferation by mesenchymal stem cells.
Blood. 109:228–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang M, Yuan Q and Xie L: Mesenchymal stem
cell-based immunomodulation: Properties and clinical application.
Stem Cells Int. 2018:30576242018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yagi H, Soto-Gutierrez A, Parekkadan B,
Kitagawa Y, Tompkins RG, Kobayashi N and Yarmush ML: Mesenchymal
stem cells: Mechanisms of immunomodulation and homing. Cell
Transplant. 19:667–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zinöcker S and Vaage JT: Rat mesenchymal
stromal cells inhibit T cell proliferation but not cytokine
production through inducible nitric oxide synthase. Front Immunol.
3:622012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Wang J, Cao J, Ma L and Xu J:
Immunoregulation of bone marrow-derived mesenchymal stem cells on
the chronic cigarette smoking-induced lung inflammation in rats.
Biomed Res Int. 2015:9329232015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vig M, Srivastava S, Kandpal U, Sade H,
Lewis V, Sarin A, George A, Bal V, Durdik JM and Rath S: Inducible
nitric oxide synthase in T cells regulates T cell death and immune
memory. J Clin Invest. 113:1734–1742. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
Washington (DC): National Academies Press (US); 2011
|
|
27
|
Mendicino M, Bailey AM, Wonnacott K, Puri
RK and Bauer SR: MSC-based product characterization for clinical
trials: An FDA perspective. Cell Stem Cell. 14:141–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wulfing C, Sjaastad MD and Davis MM:
Visualizing the dynamics of T cell activation: Intracellular
adhesion molecule 1 migrates rapidly to the T cell/B cell interface
and acts to sustain calcium levels. Proc Natl Acad Sci USA.
95:6302–6307. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barnes PJ and Cosio MG: Characterization
of T lymphocytes in chronic obstructive pulmonary disease. PLoS
Med. 1:e202004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ni L and Dong C: Roles of myeloid and
lymphoid cells in the pathogenesis of chronic obstructive pulmonary
disease. Front Immunol. 9:14312018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Antunes MA, Lapa E Silva JR and Rocco PR:
Mesenchymal stromal cell therapy in COPD: From bench to bedside.
Int J Chron Obstruct Pulmon Dis. 12:3017–3027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Broekman W, Khedoe P, Schepers K, Roelofs
H, Stolk J and Hiemstra PS: Mesenchymal stromal cells: A novel
therapy for the treatment of chronic obstructive pulmonary disease?
Thorax. 73:565–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kruk DMLW, Heijink IH, Slebos DJ, Timens W
and Ten Hacken NH: Mesenchymal stromal cells to regenerate
emphysema: On the horizon? Respiration. 96:148–158. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maitra B, Szekely E, Gjini K, Laughlin MJ,
Dennis J, Haynesworth SE and Koç ON: Human mesenchymal stem cells
support unrelated donor hematopoietic stem cells and suppress
T-cell activation. Bone Marrow Transplant. 33:597–604. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beyth S, Borovsky Z, Mevorach D,
Liebergall M, Gazit Z, Aslan H, Galun E and Rachmilewitz J: Human
mesenchymal stem cells alter antigen-presenting cell maturation and
induce T-cell unresponsiveness. Blood. 105:2214–2219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Groh ME, Maitra B, Szekely E and Koç ON:
Human mesenchymal stem cells require monocyte-mediated activation
to suppress alloreactive T cells. Exp Hematol. 33:928–934. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meisel R, Zibert A, Laryea M, Gobel U,
Daubener W and Dilloo D: Human bone marrow stromal cells inhibit
allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated
tryptophan degradation. Blood. 103:4619–4621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Glennie S, Soeiro I, Dyson PJ, Lam EW and
Dazzi F: Bone marrow mesenchymal stem cells induce division arrest
anergy of activated T cells. Blood. 105:2821–2827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Djouad F, Plence P, Bony C, Tropel P,
Apparailly F, Sany J, Noel D and Jorgensen C: Immunosuppressive
effect of mesenchymal stem cells favors tumor growth in allogeneic
animals. Blood. 102:3837–3844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laing AG, Fanelli G, Ramirez-Valdez A,
Lechler RI, Lombardi G and Sharpe PT: Mesenchymal stem cells
inhibit T-cell function through conserved induction of cellular
stress. PLoS One. 14:e02131702019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su J, Chen X, Huang Y, Li W, Li J, Cao K,
Cao G, Zhang L, Li F, Roberts AI, et al: Phylogenetic distinction
of iNOS and IDO function in mesenchymal stem cell-mediated
immunosuppression in mammalian species. Cell Death Differ.
21:388–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi Y, Su J, Roberts AI, Shou P, Rabson AB
and Ren G: How mesenchymal stem cells interact with tissue immune
responses. Trends Immunol. 33:136–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prockop DJ: ‘Stemness’ does not explain
the repair of many tissues by mesenchymal stem/multipotent stromal
cells (MSCs). Clin Pharmacol Ther. 82:241–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stagg J: Immune regulation by mesenchymal
stem cells: Two sides to the coin. Tissue Antigens. 69:1–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mazzoni A, Bronte V, Visintin A, Spitzer
JH, Apolloni E, Serafini P, Zanovello P and Segal DM: Myeloid
suppressor lines inhibit T cell responses by an NO-dependent
mechanism. J Immunol. 168:689–695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Owen DL and Farrar MA: STAT5 and CD4
+ T cell immunity. F1000Res. 6:322017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tripathi P, Kurtulus S, Wojciechowski S,
Sholl A, Hoebe K, Morris SC, Finkelman FD, Grimes HL and Hildeman
DA: STAT5 is critical to maintain effector CD8+ T cell responses. J
Immunol. 185:2116–2124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maeno T, Houghton AM, Quintero PA,
Grumelli S, Owen CA and Shapiro SD: CD8+ T cells are required for
inflammation and destruction in cigarette smoke-induced emphysema
in mice. J Immunol. 178:8090–8096. 2007. View Article : Google Scholar : PubMed/NCBI
|