Introduction
Bisphenol A (BPA) is a xenoestrogen (XE) that is
extensively produced for the manufacture of polycarbonate plastic
(1). Ubiquitous exposure to BPA is
associated with a series of health problems, including obesity,
diabetes and disorders of the reproductive system (2–4).
Previous studies have reported that BPA may also affect liver
function, and high levels of urinary BPA are associated with
non-alcoholic fatty liver disease in adults in the United States
(5–8). Another study reported that serum
aspartate transaminase and alanine transaminase (ALT) levels were
not altered following exposure to low level BPA (5 mg/kg), but were
significantly increased following exposure to high level BPA (50
mg/kg) in rats (9).
Liver ischemia/reperfusion (I/R) injury occurs
during a number of clinical events, including liver
transplantation, partial hepatectomy and hemorrhagic shock
(10). The pathophysiological
processes of liver I/R injury are multifactorial and characterized
by various clinical manifestations ranging in severity from
asymptomatic elevation of serum transaminases to hepatic failure
(11). Despite improvements in
perioperative management and surgical techniques, liver I/R injury
continues to be an important clinical issue. Previous studies have
demonstrated that 17-estradiol (E2) protects the liver against I/R
injury by reducing microvascular dysfunction and inflammatory
responses (12), increasing
vasodilator nitric oxide (NO) production (13) and preventing impaired Kupffer cell
activity (14). Furthermore, a
previous study reported that E2 provides hepatoprotection against
I/R injury by downregulating the angiotensin II (Ang II)/Ang II
type I receptor (AT1R) signaling pathway (15). However, the role of BPA during
liver I/R injury has not been previously reported.
Previous studies have demonstrated that BPA can
attenuate the physiological function of estrogen and inhibit the
production of testosterone (16,17).
Moreover, BPA can reduce the activity of multiple hepatoprotective
enzymes, including catalase, total glutathione S-transferase, total
glutathione peroxidase and total superoxide dismutase (18). Therefore, the present study aimed
to investigate whether BPA disrupted E2-mediated hepatic protection
against I/R injury, and to identify the possible underlying
mechanisms using a rat model.
Materials and methods
Animals
Male Sprague-Dawley rats (weight, 190–210 g; age,
9–10 weeks old) were purchased from the Animal Center of Xi'an
Jiaotong University. Animals were kept in standard housing
conditions at 22±2°C with 12-h light/dark cycles, 45–60% humidity,
and free access to food and water. Animal experiments were
conducted according to the National Institutes of Health Guidelines
on the Use of Laboratory Animals (12). The present study was approved by
the Xi'an Jiaotong University Health Science Center Ethics
Committee.
Model of total hepatic I/R
Previous studies have demonstrated that short
periods (60 min) of global liver ischemia result in reversible cell
injury, in which liver oxygen consumption returns to control levels
when the oxygen supply is reestablished following ischemia
(19). However, reperfusion
following prolonged warm ischemia (>120 min) results in
irreversible cell damage (19).
Therefore, the present study established a 60 min global liver
ischemia model, which simulated the clinical situation of warm
ischemia after the Pringle maneuver (20) in hepatic surgery. Rats were fasted
for 16–18 h prior to the operation. Rats were anesthetized using
64% N2O, 32% O2 and 4% isoflurane and
anesthesia was maintained using 65% N2O, 32%
O2 and 3% isoflurane. Rats were ventilated
endotracheally during the operation. A median incision was made in
the upper abdomen and the hepatic pedicle was clamped using a
non-invasive microvascular clip to induce total liver I/R. After 60
min, the clamp was removed to allow reperfusion. Sham controls
received the laparotomy procedure without hepatic pedicle clamping.
After closing the abdominal cavity, rats were allowed to recover
with free access to food and water. At 24 h post-reperfusion, rats
were euthanized by overdose with intravenous sodium pentobarbital
(100 mg/kg) followed by exsanguination. Subsequently, the liver
tissues and 5 ml venous blood drawn from the inferior vena cava
were harvested for subsequent experiments.
Experimental design
A total of 56 rats were randomly divided into the
following seven experimental groups (n=8/group): i) Sham; ii) I/R;
iii) Sham + BPA; iv) I/R + BPA; v) I/R + E2; vi) I/R + E2 + BPA;
and vii) I/R + E2 + BPA + Losartan (LOS). BPA (Tokyo Chemical
Industry Co., Ltd.) and E2 (Sigma-Aldrich; Merck KGaA) were
dissolved in DMSO, and diluted in saline. The final concentration
of DMSO was <0.1%. BPA (4 mg/kg) or E2 (4 mg/kg) were
administered intravenously when the laparotomy procedure began. LOS
(30 mg/kg; Wuhan Boster Biological Technology, Ltd.) was
administered intraperitoneally when the laparotomy procedure began.
The Sham and I/R groups received only dilution vehicle
(saline).
Histological assessment of I/R
injury
Formalin-fixed (10%, 24 h, room temperature)
paraffin-embedded liver tissues were cut into 5 µm-thick sections,
and stained with hematoxylin (15 min) and eosin (5 min) at room
temperature to estimate the severity of I/R injury. Sections were
examined for the following signs: Nuclear pyknosis, loss of
hepatocellular borders or areas of necrosis. Stained sections were
observed in at least 10 randomly selected fields with a light
microscope (magnification, ×400) and examined for the following
signs: Nuclear pyknosis, loss of hepatocellular borders or areas of
necrosis. Sections were scored using according to a previously
described formula: Necrotic area/total area of the field (21), and analyzed using Image-Pro Plus
software (version 6.0; Media Cybernetics, Inc.).
Measurement of serum hepatic damage
markers
At 24 h post-reperfusion, harvested blood samples
were centrifuged at 3,000 × g for 5 min at 4°C for serum separation
and serum concentrations of ALT, alkaline phosphate (ALP) and total
bilirubin (TBIL) were detected using the AU400 automated chemistry
analyzer according to the manufacturer's protocol (Olympus
Corporation).
Measurement of Ang II
Rat liver tissue was homogenized and total protein
was extracted using T-PER™ tissue protein extraction buffer
(Pierce; Thermo Fisher Scientific, Inc.) containing protease
inhibitors. Protein concentration was determined using the
Coomassie brilliant blue method. Serum and hepatic Ang II levels
were measured using an Ang II ELISA kit (cat. no. ABIN416068; Uscn
Life Science Inc.), according to the manufacturer's protocol.
Western blotting
The hepatic tissue expression levels of AT1R were
detected using the western blotting. Total protein was extracted
using T-PER™ tissue protein extraction buffer (Pierce; Thermo
Fisher Scientific, Inc.) containing protease inhibitors. Protein
concentration was determined using the Coomassie brilliant blue
method. The mass of the proteins loaded in per lane was 50 µg.
Protein was separated by 10% SDS-PAGE and transferred onto
nitrocellulose membranes (GE Healthcare Life Sciences). The
membranes were blocked with 5% bovine serum albumin (cat. no.
CSB-NP009501B; CUSA Bio.) at 37°C for 1 h, and washed three times
with ice-cold PBS. Subsequently, the membranes were incubated at
37°C for 2 h with the following primary antibodies: Anti-AT1R (cat.
no. sc-31181; 1:1,000; Santa Cruz Biotechnology, Inc.) and
anti-β-actin (cat. no. sc-130656; 1,000; Santa Cruz Biotechnology,
Inc.). Following primary incubation, the membranes were incubated
with a horseradish peroxidase-conjugated donkey anti-rabbit IgG
secondary antibody (cat. no. NA934; 1:1,000; GE Healthcare Life
Sciences) for 1 h at 37°C. Protein bands were visualized using an
enhanced chemiluminescence detection system (GE Healthcare Life
Sciences) and the UVP BioSpectrum500 imaging system (UVP, LLC).
Protein expression was quantified using Quantity One software
(version 4.6.9; Bio-Rad Laboratories, Inc.) with β-actin as the
loading control.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analyses were performed using SPSS software (version 24.0; IBM
Corp.). Comparisons among groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
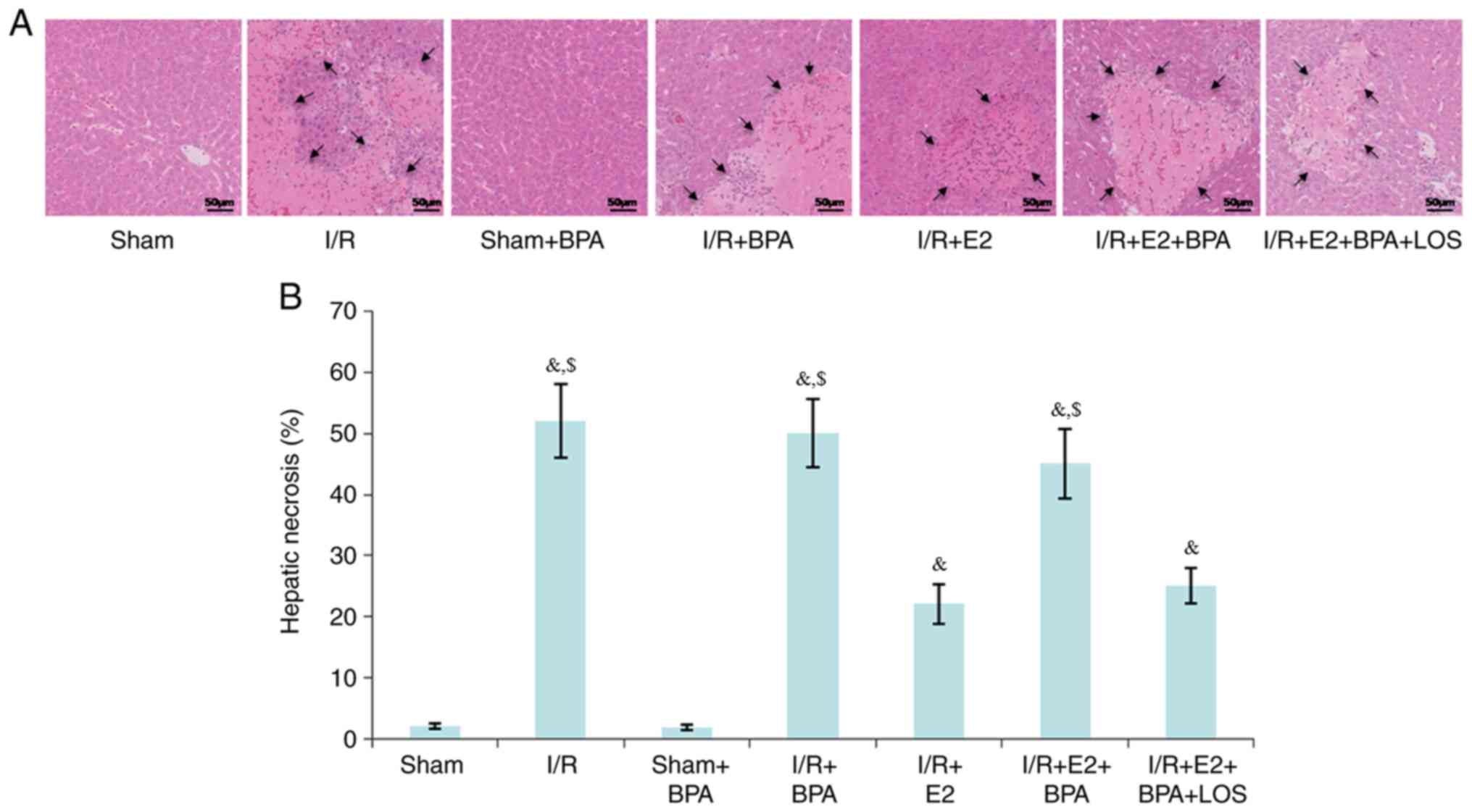
Histological changes
At 24 h post-reperfusion, liver tissues were
harvested. No significant morphological changes were observed
between the Sham and Sham + BPA groups. However, severe necrosis,
extensive nuclear pyknosis and loss of intercellular borders were
detected in the I/R and I/R + BPA groups. Compared with the I/R and
I/R + BPA groups, the I/R + E2 group displayed a significantly
reduced percentage of hepatic necrosis (52±6 vs. 22±3.2% and 50±5.5
vs. 22±3.2%, respectively; P<0.05; Fig. 1); however, BPA treatment decreased
E2-mediated hepatoprotective effects (45±5.6%; P<0.05 vs. I/R +
E2 group). Furthermore, it was demonstrated that LOS treatment
significantly reversed the negative effect of BPA on E2-mediated
hepatoprotection (25±3%; P<0.05 vs. I/R + E2+BPA group; Fig. 1).
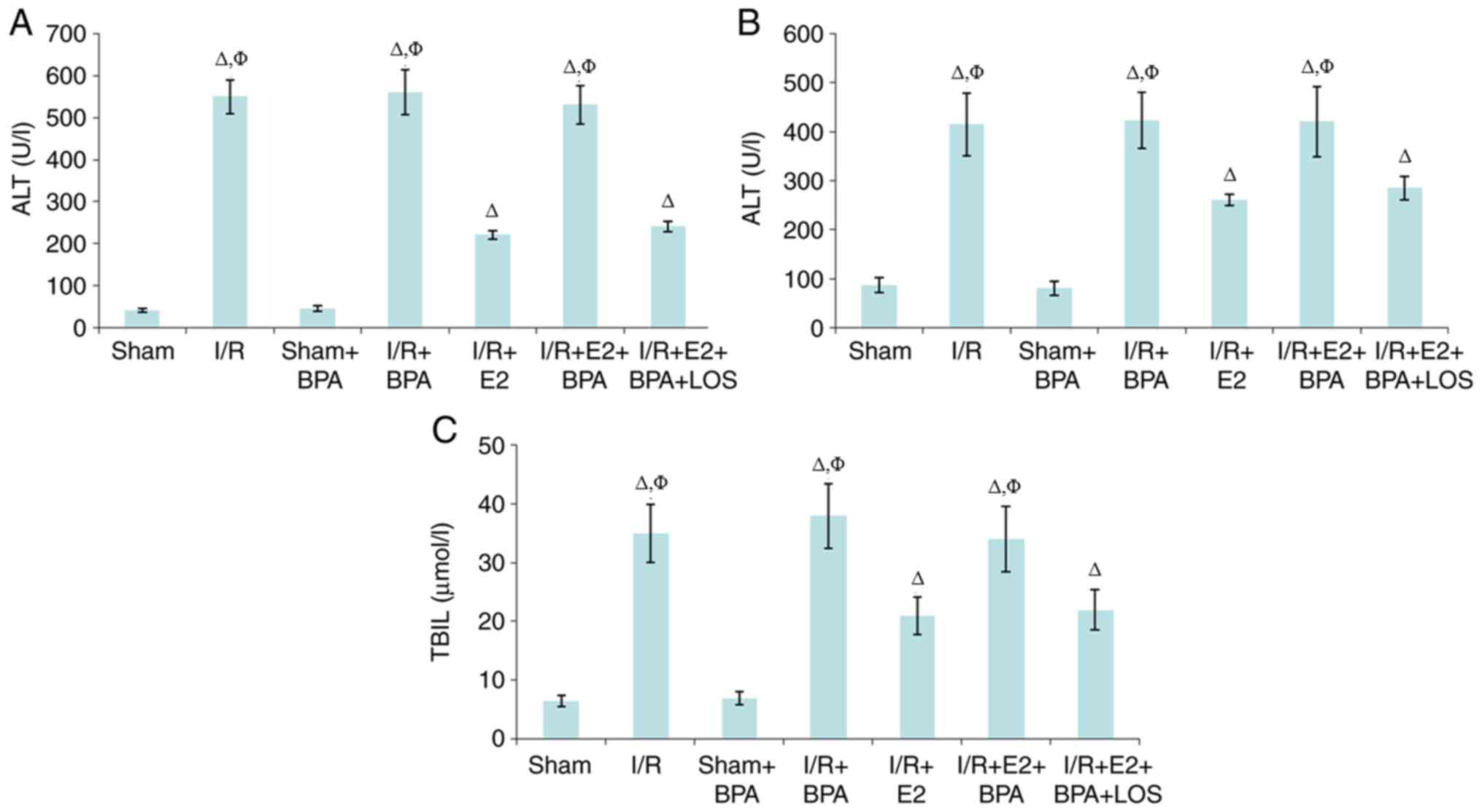
Liver function
Serum concentrations of ALT, ALP and TBIL in the
Sham + BPA group were not significantly different at 24 h
post-reperfusion compared with the Sham group (all P>0.05).
However, the I/R and I/R + BPA groups displayed significantly
higher serum concentrations levels of ALT, ALP and TBIL compared
with the Sham and Sham + BPA groups (P<0.05). Moreover, compared
with the I/R and I/R + BPA groups, the I/R + E2 group displayed
significantly reduced levels of the serum markers (all P<0.05);
however, BPA treatment significantly inhibited the hepatoprotective
activity of E2 against I/R injury (all P<0.05 vs. I/R + E2
group). In addition, the results suggested that LOS treatment
reversed the negative effect of BPA on E2 in the rat model of liver
I/R injury (Fig. 2).
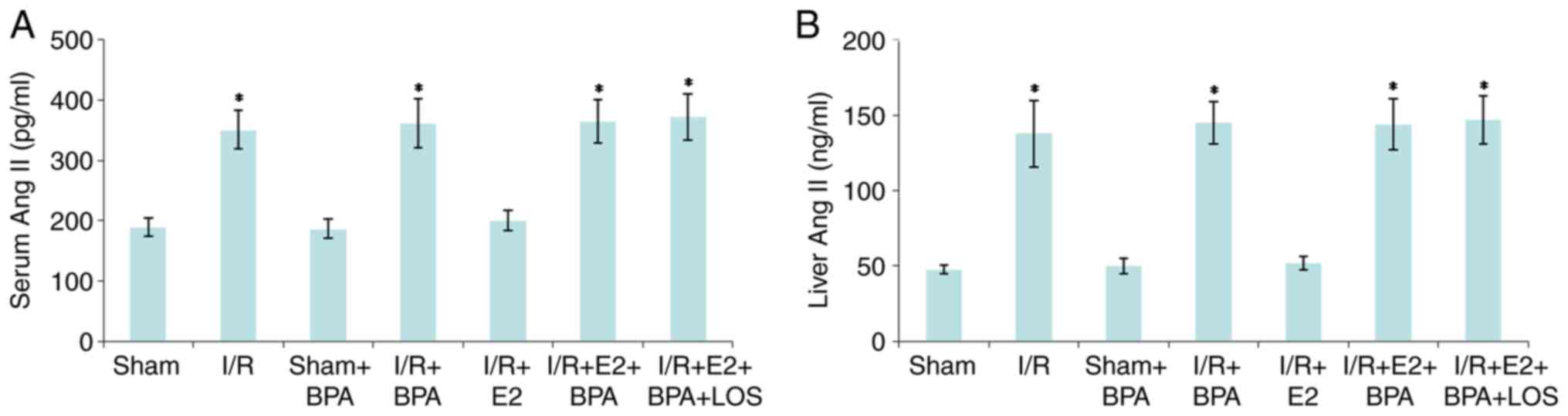
Serum and hepatic Ang II levels
At 24 h post-reperfusion, there were no significant
differences in serum and hepatic Ang II levels between the Sham and
Sham + BPA groups (all P>0.05). By contrast, the levels of serum
and hepatic Ang II in the I/R and I/R + BPA groups were
significantly higher compared with the Sham and Sham + BPA groups
(P<0.05). Furthermore, E2 treatment significantly reduced the
levels of serum and hepatic Ang II compared with the I/R and I/R +
BPA groups (all P<0.05), and BPA treatment inhibited the
hepatoprotective activity of E2 against liver I/R injury (all
P<0.05 vs. I/R + E2 group). However, LOS treatment did not
significantly alter serum and hepatic Ang II levels in the I/R + E2
+ BPA group (all P>0.05 vs. I/R+E2+BPA+LOS group) (Fig. 3).
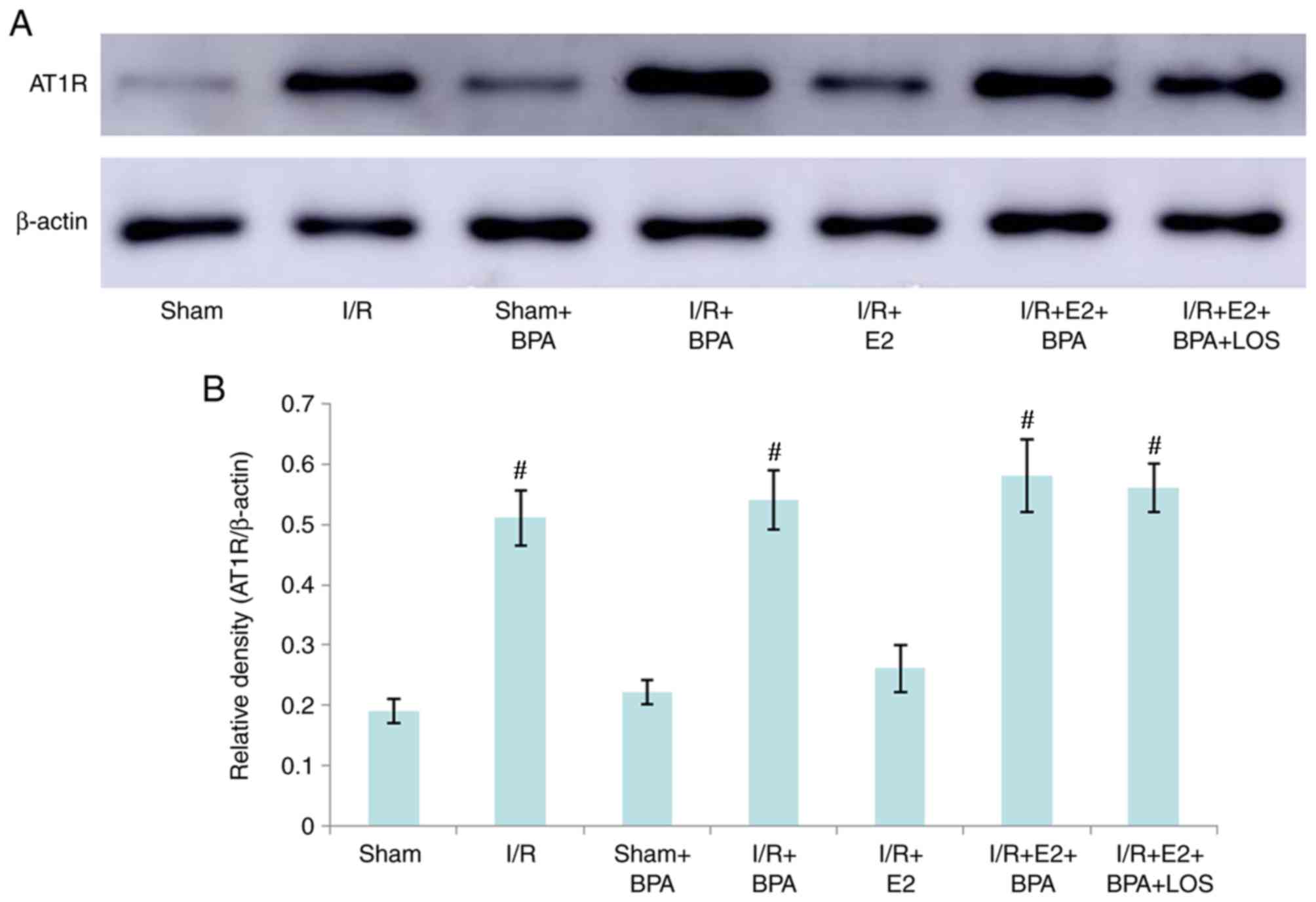
Expression of hepatic AT1R
The hepatic tissue expression level of AT1R was
determined by western blotting. At 24 h post-reperfusion, there
were no significant differences in the expression of AT1R protein
between the Sham and Sham + BPA groups (P>0.05). However,
hepatic AT1R expression levels in the I/R and I/R + BPA groups were
significantly higher compared with the Sham and Sham + BPA groups
(P<0.05). The results indicated that E2 treatment significantly
decreased hepatic AT1R expression levels compared with the I/R and
I/R + BPA groups (all P<0.05); however, BPA treatment also
inhibited E2-mediated effects on AT1R expression (P<0.05 vs.
I/R+E2 group). Additionally, LOS treatment did not alter hepatic
AT1R expression in I/R + E2 + BPA rats (P>0.05 vs. I/R+E2+BPA
group) (Fig. 4).
Discussion
BPA is an endocrine disrupting chemical (EDC) widely
used in various industries, including dentistry (1). BPA has attracted attention due to its
threat to human health, as it can alter the expression of neural
genes including oxytocin and vasopressin, leading to abnormal
social behaviors (22).
Ang II receptors are present in two forms: AT1R and
AT2R (23). The physiological
function of Ang II and AT1R, as well as their role in the
pathogenesis of certain diseases are not completely understood.
However, compared with AT1R, it is difficult to investigate the
functions of AT2R, at least in part due to the relatively low
expression levels in cells (23).
Previous studies investigating Ang II and its receptors have
primarily focused on the Ang II/AT1R axis (24–27);
therefore, the present study investigated the effects of BPA on
hepatic damage and the Ang II/AT1R signaling pathway in a rat model
of liver I/R injury. A previous study reported that 0.4 mg/kg/day
BPA in rats is close to the current reference daily limit for human
exposure by the U.S. Environmental Protection Agency (28). In another study assessing the
effects of BPA on the cognitive function of rats, it was indicated
that 0.4 mg/kg/day BPA caused a significant decline in spatial
memory; however, anxiety-like behavior was only observed in the
high-dose BPA group (4 mg/kg/day) (28). A previous study investigating the
effects of perinatal maternal exposure to BPA on the behavior of
rat offspring, it was reported that male offspring in the 4 mg/kg
group displayed significantly lower responses compared with control
rats (29); therefore, 4 mg/kg BPA
was used in the present study.
It has been reported that 25 mg/kg/day BPA leads to
increased serum levels of liver enzymes and defects in the
morphology of the liver in rats (30). Moreover, Kazemi et al
(31) indicated that 5 µg/kg BPA
induced reactive oxygen species production and increased
antioxidant gene expression in rats; however, the morphological and
functional responses of the liver were not investigated. In the
present study, 4 mg/kg BPA did not affect the liver microstructure
and enzymes in the Sham or I/R groups. Furthermore, it was
demonstrated that there were no significant differences in serum
Ang II levels, hepatic Ang II levels and AT1R protein levels
between rats treated with or without BPA alone. Therefore, the
results suggested that 4 mg/kg BPA may not affect liver function or
the Ang II/AT1R signaling pathway in healthy or I/R-injured
livers.
The hepatoprotective effect of E2 against I/R injury
has been previously reported in rodent models (32–34).
The possible mechanisms underlying the actions of E2 include:
Apoptosis inhibition (21);
increasing serum NO levels and decreasing serum tumor necrosis
factor-α levels (35); regulating
the expression of heat shock protein (36); modulating the activities of
mitogen-activated protein kinase (37); and downregulating the Ang II/AT1R
signaling pathway (16). Moreover,
previous clinical studies have demonstrated that female livers are
more tolerant to I/R injury compared with male livers, which may be
explained by E2 (38,39). BPA, a well-characterized XE,
interacts with estrogen receptors to act as an agonist or
antagonist via estrogen receptor-dependent signaling pathways;
therefore, BPA plays a role in the pathogenesis of several
endocrine disorders, including female and male infertility,
precocious puberty and hormone dependent tumors (40). It has been hypothesized that BPA
may have a negative effect on the protective effect of E2 against
hepatic I/R injury. The present study examined whether BPA
disrupted E2-mediated hepatic protection against I/R injury, and
the possible underlying mechanisms. The results suggested that E2
protected the liver against I/R injury by attenuating hepatic
necrosis, and lowering serum levels of ALT, ALP and TBIL.
Furthermore, BPA, as an EDC, abolished certain hepatoprotective
activities of E2 by aggravating hepatic necrosis, and increasing
the release of ALT, ALP and TBIL, as demonstrated by biochemical
and histological analyses.
Ang II is the major effector or peptide of the
renin-angiotensin system (41).
Previous studies have revealed that Ang II can induce a series of
proinflammatory responses by increasing adhesion molecule
expression (42),
leukocyte-endothelial interaction (43), activator protein 1 and NF-κB
activation (44), reactive oxygen
species production (45) and
proinflammatory cytokine accumulation (46). Moreover, the role of Ang II and its
primary receptor, AT1R, in the process of liver I/R injury has been
previously reported. Alfany-Fernandez et al (47) reported that Ang II receptor
antagonists protect non-steatotic liver grafts against I/R damage.
Furthermore, Sabry et al (48) revealed that the hepatoprotective
effect of Apelin-13 against I/R injury was related to suppression
of the Ang II/AT1R signaling pathway. The results of the
aforementioned studies were consistent with the results of the
present study. In addition, our previous study indicated that E2
hepatoprotection against I/R injury occurs via downregulation of
the Ang II/AT1R signaling pathway (15). However, whether BPA disrupts the
hepatoprotective activity of E2 against I/R injury by modulating
the Ang II/AT1R signaling pathway is not completely understood. A
previous studies has demonstrated that oral administration of BPA
induces high blood pressure in mice by upregulating Ang II
(49). Another in vitro
study reported that following BPA treatment, Ang II expression is
upregulated in vascular smooth muscle cells, and the Ang II
receptor antagonist LOS attenuates BPA-induced cell proliferation
(50). The results of the present
study suggested that although BPA did not significantly affect the
Ang II/AT1R signaling pathway in the rat model of hepatic I/R
injury without E2 treatment, BPA treatment significantly increased
the levels of serum and hepatic Ang II, as well as the expression
of AT1R protein in the liver in the ER-treated rat model of hepatic
I/R injury.
Previous studies have indicated that chronic use of
LOS can decrease the expression of AT1R. Abbasloo et al
(51) reported that treatment with
LOS (10 mg/kg; intraperitoneally) for 5 days reduces the expression
of AT1R in cardiac tissue in a rat model of I/R injury.
Furthermore, Panico et al (52) demonstrated that renal I/R-induced
cardiac levels of AT1R were decreased following LOS treatment (10
mg/kg in drinking water) for 7 days. However, the results of the
present study suggested that LOS treatment did not significantly
alter serum and liver Ang II levels, or liver AT1R protein
expression following hepatic I/R injury; however, LOS reversed the
negative effect of BPA on certain E2-mediated hepatoprotective
activities. A potential explanation for the discrepancies between
the results of the present study and the results of previous
studies could be the different doses and courses of treatments
used. In the present study, LOS was administered once (30 mg/kg;
intraperitoneally) at 24 h before tissue harvesting, which may
alter AT1R function, but not AT1R expression. Therefore, further
studies are required to identify the mechanisms underlying
LOS-mediated regulation of the Ang II/AT1R axis.
In conclusion, to the best of our knowledge, the
present study was the first to suggest that upregulation of the Ang
II/AT1R signaling pathway may play an important role in
BPA-mediated disruption of the hepatoprotective activities of E2
against I/R injury. Moreover, the Ang II/AT1R axis may serve as a
promising target for the development of hepatoprotective strategies
to prevent BPA-induced liver damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science and Technology Co-ordination and Innovation Project of
Shaanxi (grant. no. 2011KTCL03-21) and the National Natural Science
Foundation of China (grant. no. 81373157).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY, YZ and YS analyzed and interpreted the
experimental animal data. ZL and LS performed the histological
examination of the liver. MZ performed the PCR and western
blotting. LY was a major contributor in writing the manuscript. SW
designed the experiments and reviewed the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Xi'an Jiaotong
University Health Science Center Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ehrlich S, Calafat AM, Humblet O, Smith T
and Hauser R: Handling of thermal receipts as a source of exposure
to bisphenol A. JAMA. 311:859–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernández M, Bianchi M, Lux-Lantos V and
Libertun C: Neonatal exposure to bisphenol a alters reproductive
parameters and gonadotropin releasing hormone signaling in female
rats. Environ Health Perspect. 117:757–762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li DK, Zhou Z, Miao M, He Y, Wang J,
Ferber J, Herrinton LJ, Gao E and Yuan W: Urine bisphenol-A (BPA)
level in relation to semen quality. Fertil Steril.
95:625–630.e1-e4. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahalingam S, Ther L, Gao L, Wang W,
Ziv-Gal A and Flaws JA: The effects of in utero bisphenol A
exposure on ovarian follicle numbers and steroidogenesis in the F1
and F2 generations of mice. Reprod Toxicol. 74:150–157. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim D, Yoo ER, Li AA, Cholankeril G, Tighe
SP, Kim W, Harrison SA and Ahmed A: Elevated urinary bisphenol A
levels are associated with non-alcoholic fatty liver disease among
adults in the United States. Liver Int. 39:1335–1342. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eweda SM, Newairy AS, Abdou HM and Gaber
AS: Bisphenol A-induced oxidative damage in the hepatic and cardiac
tissues of rats: The modulatory role of sesame lignans. Exp Ther
Med. 19:33–44. 2020.PubMed/NCBI
|
|
7
|
Lin R, Jia Y, Wu F, Meng Y, Sun Q and Jia
L: Combined exposure to fructose and bisphenol a exacerbates
abnormal lipid metabolism in liver of developmental male rats. Int
J Environ Res Public Health. 16:E41522019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peerapanyasut W, Kobroob A, Palee S,
Chattipakorn N and Wongmekiat O: N-Acetylcysteine attenuates the
increasing severity of distant organ liver dysfunction after acute
kidney injury in rats exposed to bisphenol a. Antioxidants (Basel).
8:E4972019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peerapanyasut W, Kobroob A, Palee S,
Chattipakorn N and Wongmekiat O: Activation of sirtuin 3 and
maintenance of mitochondrial integrity by N-acetylcysteine protects
against bisphenol A-induced kidney and liver toxicity in rats. Int
J Mol Sci. 20:E2672019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao X, Zhou S, Zong J and Wang Z:
Sevoflurane exerts protective effects on liver ischemia/reperfusion
injury by regulating NFKB3 expression via miR-9-5p. Exp Ther Med.
17:2632–2640. 2019.PubMed/NCBI
|
|
11
|
Song H, Du C, Wang X, Zhang J and Shen Z:
[Erratum] MicroRNA-101 inhibits autophagy to alleviate liver
ischemia/reperfusion injury via regulating the mTOR signaling
pathway. Int J Mol Med. 43:25322019.PubMed/NCBI
|
|
12
|
Burkhardt M, Slotta JE, Garcia P, Seekamp
A, Menger MD and Pohlemann T: The effect of estrogen on hepatic
microcirculation after ischemia/reperfusion. Int J Colorectal Dis.
23:113–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lü P, Liu F, Wang CY, Chen DD, Yao Z, Tian
Y, Zhang JH and Wu YH: Gender differences in hepatic ischemic
reperfusion injury in rats are associated with endothelial cell
nitric oxide synthase-derived nitric oxide. World J Gastroenterol.
11:3441–3445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokoyama Y, Kuebler JF, Matsutani T,
Schwacha MG, Bland KI and Chaudry IH: Mechanism of the salutary
effects of 17beta-estradiol following trauma-hemorrhage: Direct
downregulation of Kupffer cell proinflammatory cytokine production.
Cytokine. 21:91–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Li D, Sun L, Li Z, Yu L and Wu S:
The protective effects of estrogen on hepatic ischemia-reperfusion
injury in rats by downregulating the Ang II/AT1R pathway. Biochem
Biophys Res Commun. 503:2543–2548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Viñas R and Watson CS: Mixtures of
xenoestrogens disrupt estradiol-induced non-genomic signaling and
downstream functions in pituitary cells. Environ Health. 12:262013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desdoits-Lethimonier C, Lesné L,
Gaudriault P, Zalko D, Antignac JP, Deceuninck Y, Platel C,
Dejucq-Rainsford N, Mazaud-Guittot S and Jégou B: Parallel
assessment of the effects of bisphenol A and several of its analogs
on the adult human testis. Hum Reprod. 32:1465–1473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uzunhisarcikli M and Aslanturk A:
Hepatoprotective effects of curcumin and taurine against bisphenol
A-induced liver injury in rats. Environ Sci Pollut Res Int.
26:37242–37253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mendes-Braz M, Elias-Miró M,
Jiménez-Castro MB, Casillas-Ramírez A, Ramalho FS and Peralta C:
The current state of knowledge of hepatic ischemia-reperfusion
injury based on its study in experimental models. J Biomed
Biotechnol. 2012:2986572012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei X, Zheng W, Yang Z, Liu H, Tang T, Li
X and Liu X: Effect of the intermittent Pringle maneuver on liver
damage after hepatectomy: A retrospective cohort study. World J
Surg Oncol. 17:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin FS, Shen SQ, Chen ZB and Yan RC:
17β-estradiol attenuates reduced-size hepatic ischemia/reperfusion
injury by inhibition apoptosis via mitochondrial pathway in rats.
Shock. 37:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nilsson EE, Sadler-Riggleman I and Skinner
MK: Environmentally induced epigenetic transgenerational
inheritance of disease. Environ Epigenet. 4:dvy0162018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azushima K, Morisawa N, Tamura K and
Nishiyama A: Recent research advances in
renin-angiotensin-aldosterone system receptors. Curr Hypertens Rep.
22:222020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng P, Wu Z, Liu H, Shen Y, Yao X, Li X
and Shen Z: Electroacupuncture improved chronic cerebral
hypoperfusion-induced anxiety-like behavior and memory impairments
in spontaneously hypertensive rats by downregulating the ACE/Ang
II/AT1RAxis and upregulating the ACE2/Ang-(1–7)/MasR axis. Neural
Plast. 2020:90760422020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawabe Y, Mori J, Morimoto H, Yamaguchi M,
Miyagaki S, Ota T, Tsuma Y, Fukuhara S, Nakajima H, Oudit GY and
Hosoi H: ACE2 exerts anti-obesity effect via stimulating brown
adipose tissue and induction of browning in white adipose tissue.
Am J Physiol Endocrinol Metab. 317:E1140–E1149. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Figueiredo VP, Barbosa MA, de Castro UGM,
Zacarias AC, Bezerra FS, de Sá RG, de Lima WG, Dos Santos RAS and
Alzamora AC: Antioxidant effects of oral Ang-(1–7) restore insulin
pathway and RAS components ameliorating cardiometabolic
disturbances in rats. Oxid Med Cell Longev. 2019:58689352019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang D, Chai XQ, Magnussen CG, Zosky GR,
Shu SH, Wei X and Hu SS: Renin-angiotensin-system, a potential
pharmacological candidate, in acute respiratory distress syndrome
during mechanical ventilation. Pulm Pharmacol Ther. 58:1018332019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Li T, Zhang L, Wang H and Hu F:
Bisphenol A exposure remodels cognition of male rats attributable
to excitatory alterations in the hippocampus and visual cortex.
Toxicology. 410:132–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Negishi T, Kawasaki K, Takatori A, Ishii
Y, Kyuwa S, Kuroda Y and Yoshikawa Y: Effects of perinatal exposure
to bisphenol A on the behavior of offspring in F344 rats. Environ
Toxicol Pharmacol. 14:99–108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korkmaz A, Ahbab MA, Kolankaya D and
Barlas N: Influence of vitamin C on bisphenol A, nonylphenol and
octylphenol induced oxidative damages in liver of male rats. Food
Chem Toxicol. 48:2865–2871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kazemi S, Mousavi SN, Aghapour F, Rezaee
B, Sadeghi F and Moghadamnia AA: Induction effect of bisphenol A on
gene expression involving hepatic oxidative stress in rat. Oxid Med
Cell Longev. 2016:62985152016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Hu B, Huang H, Tsung A, Gaikwad NW,
Xu M, Jiang M, Ren S, Fan J, Billiar TR, et al: Estrogen
sulfotransferase is an oxidative stress-responsive gene that
gender-specifically affects liver ischemia/reperfusion injury. J
Biol Chem. 290:14754–14764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Vries HA, Ponds FA, Nieuwenhuijs VB,
Morphett A, Padbury RT and Barritt GJ: Evidence that estrogen
receptors play a limited role in mediating enhanced recovery of
bile flow in female rats in the acute phase of liver ischemia
reperfusion injury. Ann Hepatol. 12:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang X, Qin L, Liu J, Tian L and Qian H:
17β-Estradiol protects the liver against cold ischemia/reperfusion
injury through the Akt kinase pathway. J Surg Res. 178:996–1002.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eckhoff DE, Bilbao G, Frenette L, Thompson
JA and Contreras JL: 17-Beta-estradiol protects the liver against
warm ischemia/reperfusion injury and is associated with increased
serum nitric oxide and decreased tumor necrosis factor-alpha.
Surgery. 132:302–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen SQ, Zhang Y and Xiong CL: The
protective effects of 17beta-estradiol on hepatic
ischemia-reperfusion injury in rat model, associated with
regulation of heat-shock protein expression. J Surg Res. 140:67–76.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vilatoba M, Eckstein C, Bilbao G, Frennete
L, Eckhoff DE and Contreras JL: 17beta-estradiol differentially
activates mitogen-activated protein-kinases and improves survival
following reperfusion injury of reduced-size liver in mice.
Transplant Proc. 37:399–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ng IO, Ng M and Fan ST: Better survival in
women with resected hepatocellular carcinoma is not related to
tumor proliferation or expression of hormone receptors. Am J
Gastroenterol. 92:1355–1358. 1997.PubMed/NCBI
|
|
39
|
Yokoyama Y, Nagino M and Nimura Y: Which
gender is better positioned in the process of liver surgery? Male
or female? Surg Today. 37:823–830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Konieczna A, Rutkowska A and Rachoń D:
Health risk of exposure to Bisphenol A (BPA). Rocz Panstw Zakl Hig.
66:5–11. 2015.PubMed/NCBI
|
|
41
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: Physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pueyo ME, Gonzalez W, Nicoletti A, Savoie
F, Arnal JF and Michel JB: Angiotensin II stimulates endothelial
vascular cell adhesion molecule-1 via nuclear factor-kappaB
activation induced by intracellular oxidative stress. Arterioscler
Thromb Vasc Biol. 20:645–651. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yusof M, Kamada K, Gaskin FS and Korthuis
RJ: Angiotensin II mediates postischemic leukocyte-endothelial
interactions: Role of calcitonin gene-related peptide. Am J Physiol
Heart Circ Physiol. 292:H3032–H3037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ruiz-Ortega M, Lorenzo O, Rupérez M,
Blanco J and Egido J: Systemic infusion of angiotensin II into
normal rats activates nuclear factor-kappaB and AP-1 in the kidney:
Role of AT(1) and AT(2) receptors. Am J Pathol. 158:1743–1756.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harrison DG, Cai H, Landmesser U and
Griendling KK: Interactions of angiotensin II with NAD(P)H oxidase,
oxidant stress and cardiovascular disease. J Renin Angiotensin
Aldosterone Syst. 4:51–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, Cai W, Xi W, Sun W, Shen W, Wei T,
Chen X, Sun L, Zhou H, Sun Y, et al: MicroRNA-31 Regulates
immunosuppression in Ang II (Angiotensin II)-induced hypertension
by targeting Ppp6C (Protein Phosphatase 6c). Hypertension.
73:e14–e24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alfany-Fernandez I, Casillas-Ramirez A,
Bintanel-Morcillo M, Brosnihan KB, Ferrario CM, Serafin A, Rimola
A, Rodés J, Roselló-Catafau J and Peralta C: Therapeutic targets in
liver transplantation: Angiotensin II in nonsteatotic grafts and
angiotensin-(1–7) in steatotic grafts. Am J Transplant. 9:439–451.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sabry MM, Ramadan NM, Al Dreny BA, Rashed
LA and Abo El Enein A: Protective effect of apelin preconditioning
in a rat model of hepatic ischemia reperfusion injury; possible
interaction between the apelin/APJ system, Ang II/AT1R system and
eNOS. United European Gastroenterol J. 7:689–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saura M, Marquez S, Reventun P,
Olea-Herrero N, Arenas MI, Moreno-Gómez-Toledano R, Gómez-Parrizas
M, Muñóz-Moreno C, González-Santander M, Zaragoza C and Bosch RJ:
Oral administration of bisphenol A induces high blood pressure
through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB
J. 28:4719–4728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao F, Huang Y, Zhang L and Liu W:
Involvement of estrogen receptor and GPER in bisphenol A induced
proliferation of vascular smooth muscle cells. Toxicol In Vitro.
56:156–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Abbasloo E, Najafipour H and Vakili A:
Chronic treatment with apelin, losartan and their combination
reduces myocardial infarct size and improves cardiac mechanical
function. Clin Exp Pharmacol Physiol. 47:393–402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Panico K, Abrahão MV, Trentin-Sonoda M,
Muzi-Filho H, Vieyra A and Carneiro-Ramos MS: Cardiac inflammation
after ischemia-reperfusion of the kidney: Role of the sympathetic
nervous system and the renin-angiotensin system. Cell Physiol
Biochem. 53:587–605. 2019. View Article : Google Scholar : PubMed/NCBI
|