Introduction
Obesity is one of the causes of type 2 diabetes. In
obesity, adipocytes are hypertrophied, and their functions are
aberrant, leading to glucose intolerance or type 2 diabetes. It is
widely suggested that adipose tissues are poorly oxygenated in
obesity (1–5). Hypoxia activates many genes for
cellular adaptation to hypoxic environments. Hypoxia-inducible
factor (HIF)-1α is a transcription factor that responds to low
oxygen conditions (6). Prolyl
hydroxylase enzymes (PHDs) sense cellular oxygen, leading to
degradation of HIF-1α under normoxic conditions. However, HIF-1α is
stabilized under hypoxic conditions and is transferred to the
nucleus where it activates its target genes (7,8).
HIF-1α is expressed in adipocytes from obese adipose tissue under
hypoxic conditions (4,9). Because HIF-1α regulates glucose
metabolism, cell survival, and inflammation (6), it is expected that HIF-1α expression
in adipocytes is dysregulated causing inflammation in adipose
tissue, thereby inducing the onset of type 2 diabetes. Indeed,
previous reports show expression of HIF-1α in adipocytes leads to
inflammation and fibrosis in adipose tissue, secretion defects of
hormones and cytokines from adipocytes, increased lipid storage,
and whole-body glucose intolerance (5,10–12).
In addition, adipocyte-specific knockout of HIF-1α shows reduced
adipocyte sizes in obese adipose tissues (11,12).
Transgenic HIF-1α mice show increased adipocyte size in
subcutaneous white adipose tissues (5). These results indicate that HIF-1α
participates in adipogenesis.
Lipin1 plays important roles in lipid homeostasis
and metabolism as an enzyme for lipid synthesis and as a nuclear
receptor coactivator (13). The
enzymatic function of Lipin1 is as a phosphatidate phosphatase
(PAP), which catalyzes phosphatidic acid to diacylglycerol,
contributing to lipid storage in adipocytes (14,15).
As a transcriptional coactivator, Lipin1 forms a complex with
peroxisome proliferator-activated receptor (PPAR)α and PPARγ
coactivator 1 (PGC-1) regulating gene expression for fatty acid
oxidation (16). In addition,
Lipin1 is expressed in differentiating pre-adipocytes. Lipin1
activation during differentiation of adipocyte requires the
adipogenic transcription factors PPARγ and CCAAT/enhancer binding
protein α (C/EBPα) (17).
Knockdown of Lipin1 in pre-adipocytes inhibits differentiation into
adipocytes, whereas Lipin1 overexpression enhances adipocyte
differentiation (18,19). Thus, Lipin1 is important for
adipogenesis. Lipin1 is regulated by HIF-1α in cells of
non-adipocyte origin (20).
However, the regulation of Lipin1 by HIF-1α in adipocytes is
unknown.
In the present study, we investigated the HIF-1α
regulation of Lipin1 in adipocytes. Lipin1 expression levels in
epididymal adipose tissue of adipocyte-specific HIF-1α knockout
mice were significantly decreased relative to wild type mice,
indicating that HIF-1α regulates Lipin1. In differentiated 3T3-L1
adipocytes, HIF-1α activation induced Lipin1 and HIF-1α inhibition
reduced Lipin1. However, during differentiation, HIF-1α activation
reduced Lipin1 and HIF-1α inhibition induced Lipin1. Lipin1
expression levels correlated with adipocyte differentiation
efficiency. Together, our results indicate that regulation of
Lipin1 by HIF-1α is different in pre-adipocytes and mature
adipocytes.
Materials and methods
Chemicals and antibodies
3-(5′-hydroxymethyl-2′-furyl)- 1-benzylindazole
(YC-1) and dimethyloxallyl glycine (DMOG) were purchased from
Cayman Chemical. Anti-HIF-1α antibody (Cayman Chemical),
anti-Lipin1 antibody (Cell Signaling), and anti-β-actin antibody
(Cell Signaling) were used.
Adipocyte-specific HIF-1α knockout
mice
All the experimental procedures were performed in
accordance with the guidelines of the Animal Research Committee,
Tokushima University. The protocol was approved by the Animal
Research Committee, Tokushima University (approval no: 14129).
HIF-1α-floxed mice containing loxP sites flanking exons 13–15 of
the HIF-1α gene (21) were crossed
with mice harboring the Cre recombinase under the control of the
aP2 promoter (aP2-Cre mice; a gift from Ronald M. Evans, Salk
Institute for Biological Studies), generating adipocyte-specific
HIF-1α knockout (ahKO) mice. All mice were C57BL/6 and only male
mice were used for experiments. The mice were maintained under
temperature- and light-controlled environmental settings with free
access to water. Six-week-old mice were fed a high fat diet (HFD)
(57% kcal consisting of fat; high fat diet 32 (CLEA Japan)) for 15
weeks. The epididymal fat pads were resected from the wild type and
ahKO mice.
Cell culture
3T3-L1 cells were cultured until confluence, allowed
to grow for 2 days postconfluency, and then differentiated with the
addition of 500 µM 3-isobutyl-1-methylxanthine, 1 µM dexamethasone,
and 10 µg/ml insulin for 2 days. The medium was changed to growth
medium supplemented with 10 µg/ml insulin (differentiation medium)
every 2 days. For chemical treatment of differentiated 3T3-L1
adipocytes, the adipocytes were cultured in serum-free media for 24
h and then treated with 500 µM DMOG or 50 µM YC-1. For measurement
of differentiation efficiency, the area of differentiated
adipocytes was divided by the total area in a microscopic field
taken at magnification, ×100.
Western blot analysis
Epididymal fat pads and cells were lysed in lysis
buffer (20 mM Tris-HCl, pH 8.0), 0.15 M NaCl, 1 mM
phenylmethylsulfonyl fluoride, 1% Triton X-100, protease inhibitor
mixture (2 g/ml aprotinin, 1 µg/ml leupeptin, 2 µg/ml antipain, and
10 µg/ml benzamidine), and phosphatase inhibitor mixture (10 mM
NaF, 60 mM β-glycerophosphate, 10 mM sodium pyrophosphate, and 2 mM
sodium orthovanadate). Proteins were separated on SDS
polyacrylamide gels and electrophoretically transferred to
polyvinylidene fluoride membranes. The membranes were incubated
with a primary antibody overnight at 4°C and probed with an
HRP-conjugated secondary antibody (KPL). Immunoreactive bands were
detected with ECL (GE Healthcare) and visualized by exposing the
membranes to X-ray films (GE Healthcare). The proteins were
quantified by densitometric analysis using ImageJ analysis
software.
Statistical analysis
Data are presented as mean values ± standard error
of the mean (S.E.M.). Statistical significance was assessed using
the Student's t-test or two-way analysis of variance (ANOVA) with
Sidak's multiple comparisons test, where values of P<0.05 were
considered to indicate a statisically significant difference. Prism
version 6.0h (GraphPad Software) was used for data analysis.
Results
Lipin1 is decreased in ahKO mice
Lipin1 is regulated by HIF-1α in cells of
non-adipocyte origin (20).
However, the regulation of Lipin1 by HIF-1α in adipocytes is
unknown. Therefore, we investigated the expression of Lipin1 in
adipose tissue from ahKO mice. Wild type (WT) and ahKO mice were
fed an HFD for 15 weeks and then the epididymal adipose tissues
were resected. Western blots showed that the expression levels of
Lipin1 in the epididymal adipose tissues of ahKO mice significantly
decreased compared with WT mice (Fig.
1). This result indicates that HIF-1α regulates Lipin1 in
adipocytes.
Lipin1 regulation by HIF-1α in
differentiated 3T3-L1 adipocytes
To assess whether HIF-1α regulates Lipin1 in
adipocytes, 3T3-L1 cells were differentiated to adipocytes and
treated with a HIF-1α activator, DMOG, for 4 h. DMOG treatment
significantly increased HIF-1α expression levels in the adipocytes
(Fig. 2A and B). In addition, DMOG
also elevated Lipin1 expression levels in the differentiated
adipocytes under the same conditions (Fig. 2A and C). To confirm the regulation
of Lipin1 by HIF-1α, the effect of YC-1, an inhibitor of HIF-1α, on
the elevation of Lipin1 was studied. The DMOG-induced increases of
HIF-1α and Lipin1 were decreased by YC-1 (Fig. 2A-C), suggesting that HIF-1α
regulates Lipin1 in the differentiated adipocytes.
DMOG suppresses the differentiation of
3T3-L1 pre-adipocytes into adipocytes
Lipin1 has two different functions: one is as an
enzyme regulating lipid metabolism (14,15)
and the other is as a mediator of differentiation into adipocytes
(17). Therefore, we studied the
relationship between HIF-1α and Lipin1 along with the effect of
DMOG on the differentiation of 3T3-L1 pre-adipocytes into
adipocytes. we studied the effect of DMOG on differentiation of
3T3-L1 cells. Treatment with DMOG significantly increased HIF-1α
expression levels in 3T3-L1 cells on days 2, 4, and 8 during
differentiation (Fig. 3A and B).
In contrast, the expression levels of Lipin1 significantly
decreased on day 6 and 8. The differentiation efficiency of 3T3-L1
cells was significantly reduced with DMOG (Fig. 3D and E). These results show that
DMOG reduces Lipin1 expression levels and differentiation
efficiency, whereas HIF-1α expression levels increase.
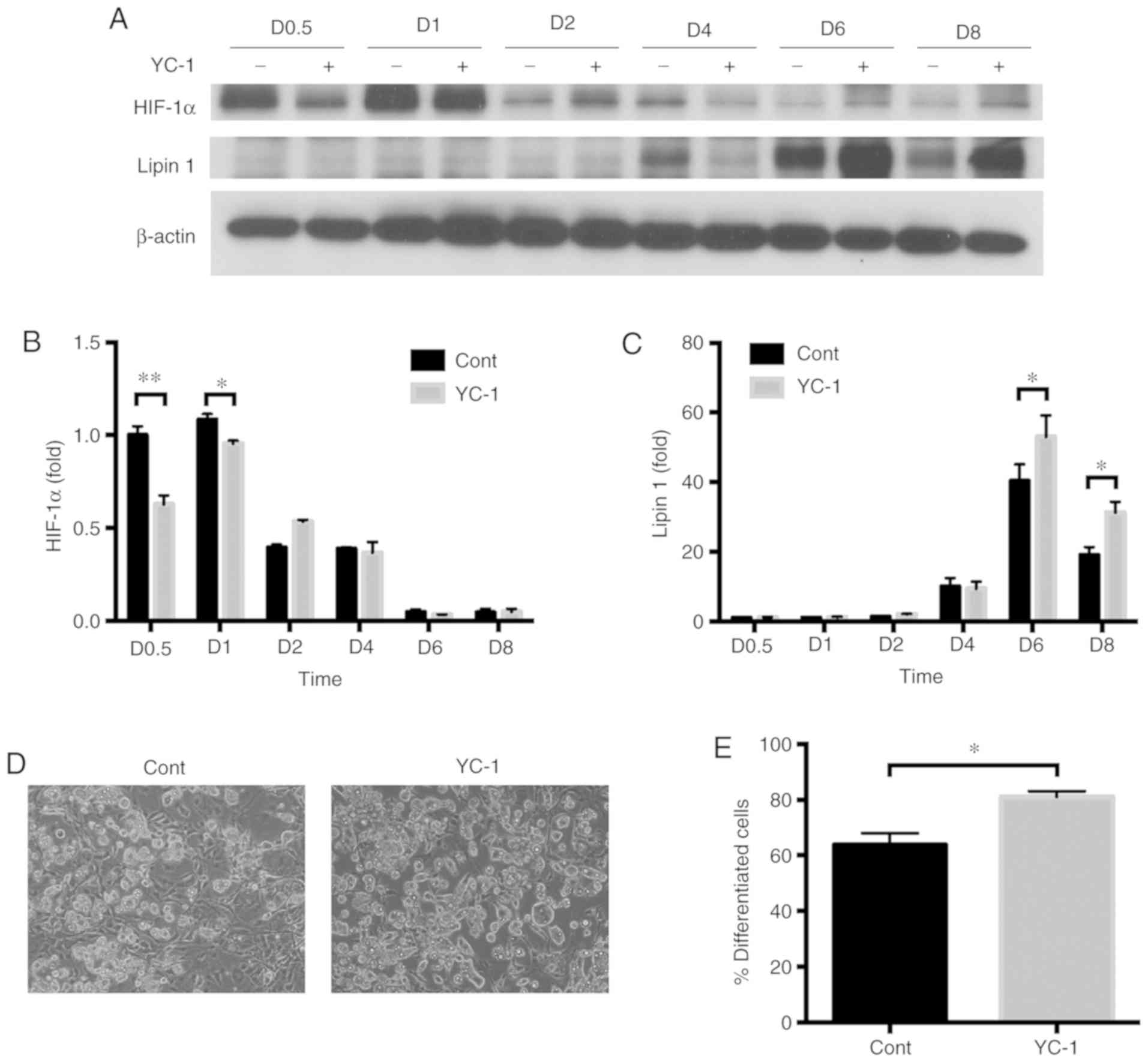
YC-1 accelerates the differentiation
of 3T3-L1 pre-adipocytes into adipocytes
The effect of the HIF-1α inhibitor, YC-1, during
differentiation of 3T3-L1 cells, was investigated. HIF-1α was
induced in the initial 24 h and then gradually decreased under the
normal conditions for differentiation (Fig. 4A and B). In contrast, Lipin1
increased at day 4 after differentiation initiation. Next, the
effect of YC-1 on differentiation of 3T3-L1 pre-adipocytes into
adipocytes was investigated. 3T3-L1 pre-adipocytes were
differentiated in differentiation medium containing YC-1. Addition
of YC-1 during differentiation of 3T3-L1 cells reduced HIF-1α
expression levels in the initial 24 h of the differentiation. Under
the same conditions, Lipin1 increased at day 6 and day 8 after
differentiation initiation. YC-1 treatment significantly increased
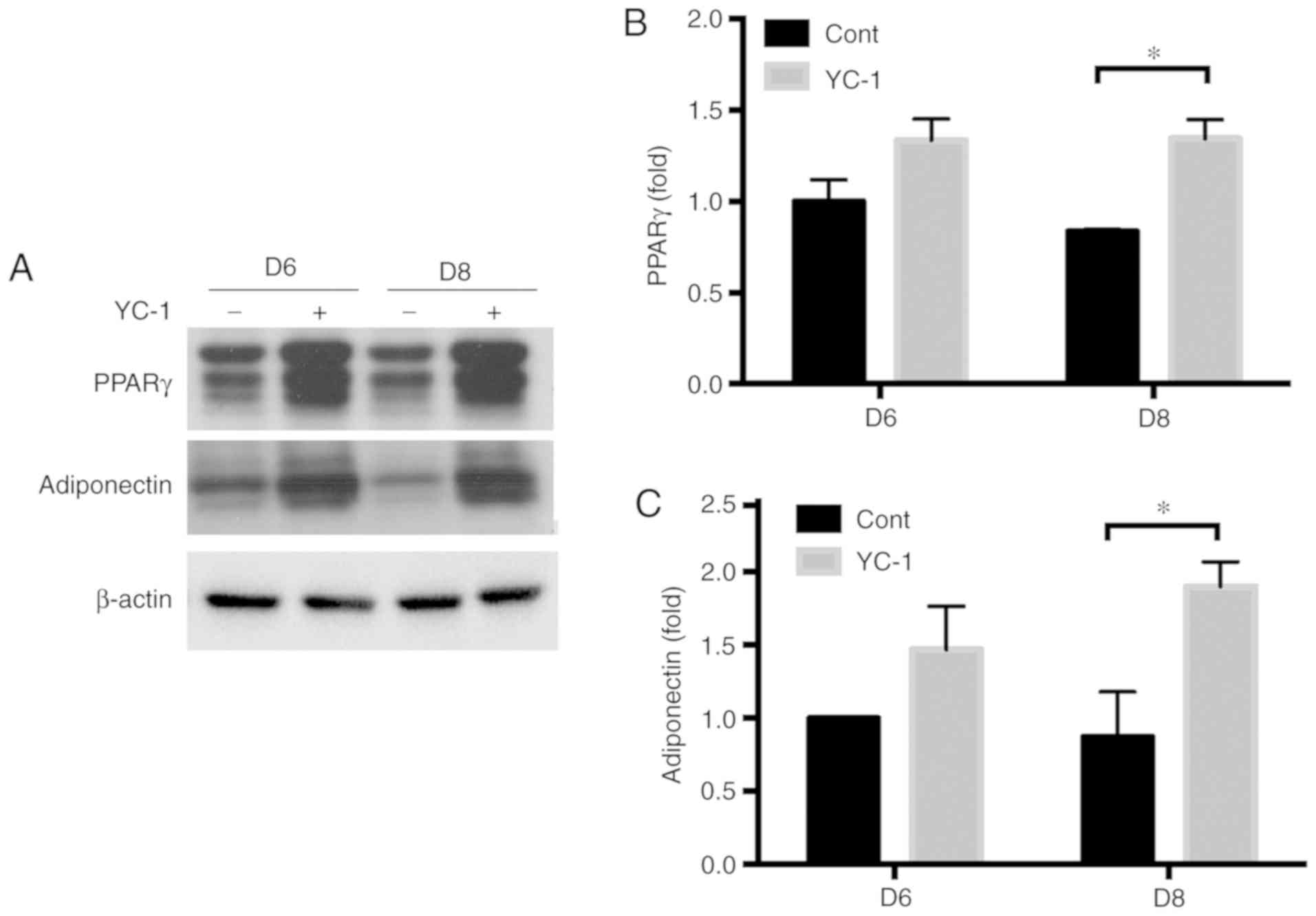
the differentiation efficiency of 3T3-L1 cells (Fig. 4D and E). The expression of PPARγ
(an adipogenic transcription factor) and adiponectin (an
adipose-secreted protein) was significantly increased in YC-1
treated adipocytes at day 8 (Fig.
5).
Discussion
Lipin1 is regulated by HIF-1α in cells of
non-adipocyte origin (20).
However, the regulation of Lipin1 by HIF-1α in adipocytes is
unknown. Therefore, in the present study, we focused on the
regulation of Lipin1 by HIF-1α. We found that Lipin1 was
significantly decreased in epididymal adipose tissues of ahKO mice
(Fig. 1). This result indicates
that HIF-1α regulates Lipin1 in adipocytes in the adipose tissue of
mice. In addition, HIF-1α upregulates Lipin1 in mature adipocytes
but downregulates Lipin1 in pre-adipocytes.
We found that DMOG, a HIF-1α activator, induced
Lipin1 in differentiated adipocytes (Fig. 2). In addition, YC-1, a HIF-1α
inhibitor, canceled the induction of Lipin1 with DMOG (Fig. 2). These results indicate that
HIF-1α regulates Lipin1 in differentiated adipocytes. Previously,
it was shown that Lipin1 is regulated by HIF-1α in cells of
non-adipocyte origin (20,22,23).
Our results show that Lipin1 is regulated by HIF-1α in
differentiated adipocytes as well. Previously, it has been shown
that HIF-1α-induced Lipin1 causes the accumulation of lipids in
hepatocytes (20). Therefore,
HIF-1α activation in adipocytes can also cause excess lipid
accumulation, leading to metabolic disorders.
During differentiation of pre-adipocytes into
adipocytes, HIF-1α levels gradually decreased (Figs. 3 and 4). However, although HIF-1α expression
had gradually decreased by day 6 in the DMOG-treated cells its
expression increased at day 8 (Fig.
3). HIF-1α is constantly synthesized and degraded (6). Therefore, the balance between
synthesis and degradation of HIF-1α changes to synthesis dominant
around day 8 after increased differentiation, suggesting that the
effects of DMOG is increased. In addition, because HIF-1α mRNA
levels increase in the initial 3 to 6 h after differentiation
(24), mRNA regulation partly
contributed to the initial increased expression of HIF-1α protein
after differentiation.
During differentiation, YC-1, an inhibitor of
HIF-1α, reduced HIF-1α expression levels in the pre-adipocytes.
However, regulation of Lipin1 by HIF-1α was opposite in
differentiating pre-adipocytes compared with differentiated
adipocytes. YC-1 reduced HIF-1α expression levels during the
initial 24 h, whereas it increased Lipin1 expression levels 6 to 8
days after differentiation initiation (Fig. 4). This result indicates that HIF-1α
inhibition indirectly participated in the upregulation of Lipin1.
To activate Lipin1 during differentiation of adipocytes, the
adipogenic transcription factors PPARγ and C/EBPα are required
(17). In the study, PPARγ was
increased in YC-1 treated adipocytes (Fig. 5). Further, it was reported that
HIF-1α indirectly downregulates PPARγ (25). Therefore, the inhibition of HIF-1α
probably contributes to upregulation of PPARγ, leading to Lipin1
induction during YC-1-affected differentiation of adipocytes.
Adiponectin is an adipokine derived from the adipocytes and has
anti-inflammatory and insulin-sensitizing effects (26). In obese adipose tissues, the
secretion of adiponectin is decreased (27–30).
YC-1-affected differentiation increased adiponectin expression,
indicating that the differentiated adipocytes are healthy. In
addition, DMOG increased HIF-1α expression levels 2, 4, and 8 days
after starting differentiation. On the contrary, Lipin1 was
decreased 6 and 8 days after differentiation initiation. In this
condition, differentiation efficiency was quite low (Fig. 3). Previous reports showed that
hypoxia inhibits adipogenesis of 3T3-L1 cells through HIF-1α
(25,31,32).
Our results suggest that the hypoxic inhibition of adipogenesis may
participate in Lipin1 downregulation.
In in vivo studies, adipocyte-specific
knockout of HIF-1α protects obese mice from insulin resistance and
inflammation (11,12), whereas transgenic mice expressing a
constitutively active form of HIF-1α have insulin resistance and
tissue fibrosis (5). In the
studies, the size of adipocytes were smaller in the
adipocyte-specific HIF-1α knockout mice than in WT mice (11,12).
In the overexpression study of a constitutively active form of
HIF-1α, the transgenic mice showed increased adipocyte size in
subcutaneous white adipose tissues (5). In addition, HIF overexpression with
PHD2 deletion in mice reduces lipolysis and increases lipid storage
(33). Our results suggest that
lipid accumulation in the knockout mice might be decreased by
reduced Lipin1 expression in differentiated adipocytes.
Some limitations exist in the present study. Our
study showed that HIF-1α upregulates Lipin1 in mature adipocytes
but downregulates it in pre-adipocytes. However, the regulation of
Lipin1 by HIF-1α in ahKO mice was not clear. To further study this,
the effects of YC-1 on the adipose tissue of ahKO mice, or HIF-1α
knockdown as reported in the previous study (34), should be observed.
It is possible that regulation mechanisms of Lipin1
by HIF-1α are different between pre-adipocytes and adipocytes.
HIF-1α reduces Lipin1 during differentiation of pre-adipocytes and
reduces differentiation efficiency. HIF-1α directly increases
Lipin1 in differentiated adipocytes and regulates lipid metabolism.
Regulation of the HIF-1α - Lipin1 system may be a potential
therapeutic target for the treatment of obesity and type 2
diabetes.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by KAKENHI
(grant no. 16K08272).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YK and ST contributed to the conception and design
of the study, acquired and analyzed the data and drafted the
manuscript. YF contributed to acquiring and analyzing the data. TT
and ES contributed to the design of the study, revised the
manuscript and approved the final manuscript.
Ethics approval and consent to
participate
All the experimental procedures were performed in
accordance with the guidelines of the Animal Research Committee,
Tokushima University. The protocol was approved by the Animal
Research Committee, Tokushima University (approval no. 14129).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hosogai N, Fukuhara A, Oshima K, Miyata Y,
Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, et
al: Adipose tissue hypoxia in obesity and its impact on
adipocytokine dysregulation. Diabetes. 56:901–911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rausch ME, Weisberg S, Vardhana P and
Tortoriello DV: Obesity in C57BL/6J mice is characterized by
adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J
Obes. 32:451–463. 2008. View Article : Google Scholar
|
|
3
|
Wang B, Wood IS and Trayhurn P:
Dysregulation of the expression and secretion of
inflammation-related adipokines by hypoxia in human adipocytes.
Pflugers Arch. 455:479–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye J, Gao Z, Yin J and He Q: Hypoxia is a
potential risk factor for chronic inflammation and adiponectin
reduction in adipose tissue of ob/ob and dietary obese mice. Am J
Physiol Endocrinol Metab. 293:E1118–E1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halberg N, Khan T, Trujillo ME,
Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV,
Landskroner-Eiger S, Dineen S, Magalang UJ, et al: HIF 1 alpha
induces fibrosis and insulin resistance in white adipose tissue.
Mol Cell Biol. 29:4467–4483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahimi-Horn MC and Pouysségur J: HIF at a
glance. J Cell Sci. 122:1055–1057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang LE, Arany Z, Livingston DM and Bunn
HF: Activation of hypoxia-inducible transcription factor depends
primarily upon redox-sensitive stabilization of its alpha subunit.
J Biol Chem. 271:32253–32259. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kihira Y, Miyake M, Hirata M, Hoshina Y,
Kato K, Shirakawa H, Sakaue H, Yamano N, Izawa-Ishizawa Y, Ishizawa
K, et al: Deletion of hypoxia-inducible factor-1α in adipocytes
enhances glucagon-like peptide-1 secretion and reduces adipose
tissue inflammation. PLoS One. 9:e938562014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang C, Kim JH, Li F, Qu A, Gavrilova O,
Shah YM and Gonzalez FJ: Hypoxia-inducible factor 1α regulates a
SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes.
J Biol Chem. 288:3844–3857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang C, Qu A, Matsubara T, Chanturiya T,
Jou W, Gavrilova O, Shah YM and Gonzalez FJ: Disruption of
hypoxia-inducible factor 1 in adipocytes improves insulin
sensitivity and decreases adiposity in high-fat diet-fed mice.
Diabetes. 60:2484–2495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reue K and Dwyer JR: Lipin proteins and
metabolic homeostasis. J Lipid Res. 50 (Suppl):S109–S114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phan J and Reue K: Lipin, a lipodystrophy
and obesity gene. Cell Metab. 1:73–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langner CA, Birkenmeier EH, Ben-Zeev O,
Schotz MC, Sweet HO, Davisson MT and Gordon JI: The fatty liver
dystrophy (fld) mutation. A new mutant mouse with a developmental
abnormality in triglyceride metabolism and associated
tissue-specific defects in lipoprotein lipase and hepatic lipase
activities. J Biol Chem. 264:7994–8003. 1989.PubMed/NCBI
|
|
16
|
Finck BN, Gropler MC, Chen Z, Leone TC,
Croce MA, Harris TE, Lawrence JC Jr and Kelly DP: Lipin 1 is an
inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory
pathway. Cell Metab. 4:199–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phan J, Péterfy M and Reue K: Lipin
expression preceding peroxisome proliferator-activated
receptor-gamma is critical for adipogenesis in vivo and in vitro. J
Biol Chem. 279:29558–29564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Péterfy M, Phan J, Xu P and Reue K:
Lipodystrophy in the fld mouse results from mutation of a new gene
encoding a nuclear protein, lipin. Nat Genet. 27:121–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koh YK, Lee MY, Kim JW, Kim M, Moon JS,
Lee YJ, Ahn YH and Kim KS: Lipin1 is a key factor for the
maturation and maintenance of adipocytes in the regulatory network
with CCAAT/enhancer-binding protein alpha and peroxisome
proliferator-activated receptor gamma 2. J Biol Chem.
283:34896–34906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mylonis I, Sembongi H, Befani C, Liakos P,
Siniossoglou S and Simos G: Hypoxia causes triglyceride
accumulation by HIF-1-mediated stimulation of lipin 1 expression. J
Cell Sci. 125:3485–3493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomita S, Ueno M, Sakamoto M, Kitahama Y,
Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, et
al: Defective brain development in mice lacking the Hif-1alpha gene
in neural cells. Mol Cell Biol. 23:6739–6749. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arai T, Tanaka M and Goda N:
HIF-1-dependent lipin1 induction prevents excessive lipid
accumulation in choline-deficient diet-induced fatty liver. Sci
Rep. 8:142302018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kourti M, Ikonomou G, Giakoumakis NN,
Rapsomaniki MA, Landegren U, Siniossoglou S, Lygerou Z, Simos G and
Mylonis I: CK1δ restrains lipin-1 induction, lipid droplet
formation and cell proliferation under hypoxia by reducing
HIF-1α/ARNT complex formation. Cell Signal. 27:1129–1140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imagawa M, Tsuchiya T and Nishihara T:
Identification of inducible genes at the early stage of adipocyte
differentiation of 3T3-L1 cells. Biochem Biophys Res Commun.
254:299–305. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin Q, Lee YJ and Yun Z: Differentiation
arrest by hypoxia. J Biol Chem. 281:30678–30683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yun Z, Maecker HL, Johnson RS and Giaccia
AJ: Inhibition of PPAR gamma 2 gene expression by the
HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of
adipogenesis by hypoxia. Dev Cell. 2:331–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou S, Lechpammer S, Greenberger JS and
Glowacki J: Hypoxia inhibition of adipocytogenesis in human bone
marrow stromal cells requires transforming growth factor-beta/Smad3
signaling. J Biol Chem. 280:22688–22696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ouchi N and Walsh K: Adiponectin as an
anti-inflammatory factor. Clin Chim Acta. 380:24–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamauchi T, Kamon J, Waki H, Terauchi Y,
Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N,
et al: The fat-derived hormone adiponectin reverses insulin
resistance associated with both lipoatrophy and obesity. Nat Med.
7:941–946. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fruebis J, Tsao TS, Javorschi S,
Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE and Lodish HF:
Proteolytic cleavage product of 30-kDa adipocyte complement-related
protein increases fatty acid oxidation in muscle and causes weight
loss in mice. Proc Natl Acad Sci USA. 98:2005–2010. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berg AH, Combs TP, Du X, Brownlee M and
Scherer PE: The adipocyte-secreted protein Acrp30 enhances hepatic
insulin action. Nat Med. 7:947–953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kern PA, Di Gregorio GB, Lu T, Rassouli N
and Ranganathan G: Adiponectin expression from human adipose
tissue: Relation to obesity, insulin resistance, and tumor necrosis
factor-α expression. Diabetes. 52:1779–1785. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Michailidou Z, Morton NM, Moreno Navarrete
JM, West CC, Stewart KJ, Fernández-Real JM, Schofield CJ, Seckl JR
and Ratcliffe PJ: Adipocyte pseudohypoxia suppresses lipolysis and
facilitates benign adipose tissue expansion. Diabetes. 64:733–745.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang F, Zhang G, Xing T, Lu Z, Li J, Peng
C, Liu G and Wang N: Renalase contributes to the renal protection
of delayed ischaemic preconditioning via the regulation of
hypoxia-inducible factor-1α. J Cell Mol Med. 19:1400–1409. 2015.
View Article : Google Scholar : PubMed/NCBI
|