Introduction
Shellfish, a major sensitizing component of seafood,
is widely consumed worldwide due to its nutritional value.
Therefore, the increase in immunoglobulin (Ig)E-mediated seafood
allergy reports is particularly related to shellfish (1). The prevalence rates of shellfish
allergy range from 0.5 to 2.5% depending on geographic locations,
dietary habits and age. Unlike most other food allergies, a
shellfish allergy persists for life in up to 90% of patients and is
often associated with severe systemic anaphylactic reactions.
Seafood-associated shellfish includes crustaceans and mollusks. The
majority of species that cause allergic reactions are the
crustaceans, with shrimp being by far the most frequently involved
(1–3).
Tropomyosin, a protein from muscle, was the first
major allergen identified in shrimp (4,5).
Sensitization to the major allergen tropomyosin has been found in
80% of shrimp-allergic patients (6,7).
Tropomyosin is also reported as being a panallergen of numerous
other species such as lobster, crab and mollusks (8). Due to evolutionary lineages, there is
a 78–98% amino acid homology of tropomyosin between crustaceans and
mites, and 80–97% between crustaceans and cockroaches (3). Consequently, the highly conserved
amino acid sequence of tropomyosin is responsible for clinical
cross-reactivity among shellfish species (9). Tropomyosin is also considered to be
responsible for cross-reactivity between food and aeroallergens of
animal origin such as dust mites or cockroaches (10,11).
Tropomyosin is important for the diagnosis of
seafood allergy. A recombinant tropomyosin from Penaeus
aztecus, Pen a 1, has been commercially available for the
component-resolved diagnostics (CRD) of shrimp allergy and could
provide a more accurate diagnosis (12,13).
Clearly, research on food allergens can be useful for the treatment
of food allergy (14,15). Mapping the IgE and IgG4
epitopes of tropomyosin may reveal relevant information about
antigen structure, on the basis of which a safe hypoallergenic
agent can be designed to treat shrimp allergy (16).
However, there are still some problems with the
clinical use of tropomyosin, which requires further study. One
problem is that previous studies demonstrated that sensitization to
allergens and their peptides varies among patients due to
geographical or ethnic differences. First, the tropomyosin sIgE
frequencies vary. Asero et al (17) found that less than half (41.6%) of
the Italian adult patients with shrimp allergy reacted to
tropomyosin (Pen a 1). The variability depends on the route and
dose exposure to allergens and individuals of different ages from
different ethnic backgrounds (18,19).
CRD has revealed that these sensitization profiles might show
geographical differences with clearly distinct clinical outcomes
(20). Second, the major
sequential IgE binding epitopes of tropomyosin (Pen a 1) have been
identified using overlapping peptide mapping by SPOTs
membrane-based immunoassays to elucidate sensitization profiles
(21,22), but previously published results
have demonstrated great heterogeneity in the number of epitopes and
their locations for the same allergens (23,24).
These differences are probably related to the technology used, the
overlapping peptide length and the populations selected (25–27).
Third, the role of IgG4 may be different in different
species and the role of IgG4 in shrimp allergy is not
fully understood. IgG4 epitopes have been reported to be
associated with immunologic tolerance to milk and peanuts (28). On the other hand, IgG4
has also been considered to be associated with atopy and allergic
sensitization (29).
Limited work has been conducted regarding the
potential allergenicity and antigenicity of tropomyosin and its
peptides in patients from coastal areas of northern China (30). The object of the present study was
to determine the frequency of IgE and IgG4 antibodies
reactivity to shrimp tropomyosin (Pen a 1) in the northern Chinese
population. The present study also investigated the IgE and
IgG4 specificity and diversity to sequential epitopes of
Pen a 1 in Pen a 1-positive patients.
Materials and methods
Patients
A total of 92 subjects were consecutively recruited
from Tianjin Port Hospital and Academy of Traditional Chinese
Medicine Affiliated Hospital between January 2018 and November
2018. Patient characteristics are shown in Table I. Upon study entry, all
participants underwent a detailed medical examination and clinical
history review. Clinical allergy was diagnosed by an experienced
allergologist using the following criteria: i) A convincing history
of acute allergic reactions after contact (including urticaria,
abdominal pain and wheezing) and ii) increased sIgE levels [cutoff:
>0.35 kUA/l, measured by fluorescence enzyme
immunoassay (ImmunoCAP, Phadia AB)] as defined by the European
Academy of Allergy and Clinical Immunology guidelines (31). The study protocol was approved by
the Ethics Committees of Tianjin Medical University (grant no.
TMUHMEC2017008) and written informed consent was obtained from the
patients and volunteers prior to study entry.
 | Table I.Demographic and clinical
characterization of subjects. |
Table I.
Demographic and clinical
characterization of subjects.
| Patient
characteristics | Shrimp allergic,
n=35 | HDM/cockroach
allergic, n=29 | Control, n=28 |
|---|
| Sex, no. (%) |
|
|
|
|
Male | 119
(54.3%) | 12
(41.4%) | 16
(57.1%) |
|
Female | 116
(45.7%) | 17
(58.6%) | 12
(42.9%) |
| Age, median with
range (years) | 26 (2–67) | 17 (5–45) | 32 (10–61) |
| sIgE, median with
range (kUA/l) |
|
|
|
|
Shrimp | 6.80
(0.36->100) | <0.35 | <0.35 |
| Dust
mite | 4.3
(<0.35->100) | 10.21
(0.91->100) | <0.35 |
|
Cockroach | 5.7
(<0.35->100) | 2.64
(0.36–3.74) | <0.35 |
| Allergic symptoms
(most frequent only) |
|
|
|
|
Cutaneous | 23 (65.7%) | 2 (6.9%) | 0 |
|
Digestive | 16 (45.7%) | 1 (3.4%) | 0 |
|
Respiratory | 7
(20.0%) | 27 (93.1%) | 0 |
|
Anaphylaxis | 3 (8.6%) | 0 (0%) | 0 |
| Other
reported allergies | 19 (54.3%) | 12 (41.4%) | 0 |
Preparation of recombinant
tropomyosin
The gene coding Pen a 1 protein sequences (GeneBank
NO. DQ151457) was synthesized and cloned into the BamHI and
HindIII sites of pET28a (+) expression vector using the DNA
Ligation kit (cat. no. D6020A; Takara Bio, Inc.), according to the
manufacturer's protocol. The resulting plasmid containing the Pen a
1 coding regions and a poly-histidine affinity purification tag
(6XHis) at the N-terminus was subsequently transformed into E.
coli BL 21 (DE3) competent cells (Tiangen Biotech Co., Ltd.)
using the heat shock transformation method. Briefly, 5 µl
pET28a-Pen a 1 plasmid was transformed into 100 µl E. coli
BL21 cells (DE3) and incubated on ice for 30 min, prior to being
heated in a water bath at 40°C for 60 sec, followed by an ice bath
for 2 min. The transformants were streaked on LB agar plate
supplemented with kanamycin (50 mg/ml). When cultures reached an
optical density at 600 nm level of 0.4–3.6, the expression of Pen a
1 was induced by adding 1 mM isopropyl-B-D-thiogalactoside
(Invitrogen; Thermo Fisher Scientific, Inc.) and then incubated for
3 h at 37°C and 220 rpm on an orbital shaker. E. coli cells
were harvested by centrifugation at 4,000 × g for 5 min at 4°C,
resuspended in phosphate-buffered saline (PBS, 0.01 M, pH 7.4), and
sonicated at 60 kHz, with 10 sec pulse-on and 10 sec pulse-off for
five cycles on ice. The recombinant protein was purified from the
culture supernatant using Ni+-NTA affinity column
chromatography (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Briefly, prior to the
purification, 6 ml binding buffer (50 mM PBS, 0.3 M NaCl) was used
to prepare the 10 ml purification column. Then, 5 ml lysate was
added into the column and incubated for 30–60 min at 4°C using
gentle agitation to keep the resin suspended in the lysate
solution. The column was washed three times with washing buffer (20
mM imidazole, 50 mM PBS, 0.3 M NaCl) to remove the proteins that
were not bound to the resin. Finally, the target protein was eluted
with elution buffer (250 mM imidazole, 50 mM PBS, 0.3 M NaCl); the
column flow rate was ~1.0 ml/min and all of the obtained eluted
fractions were collected for further analysis. Then the
concentration of purified recombinant tropomyosin was determined
using a bicinchoninic acid assay and further characterized by
SDS-PAGE gel electrophoresis and mass spectrometry. The purity of
the recombinant allergen was determined by ImageJ software version
1.8.0 (National Institutes of Health). SDS-PAGE was performed using
the Bio-Rad Mini Protean II cell (Bio-Rad Laboratories, Inc.). 25
µg recombinant protein was boiled for 5 min in loading buffer
before being separated using 12% polyacrylamide gels. Following the
protein separation, the protein was visualized using Coomassie
Brilliant Blue R-250 staining (Sigma-Aldrich; Merck KGaA).
Based on the results of the SDS-PAGE, the spot
identified in the Coomassie G250-stained gel that matched the
position of the theoretical molecular weight was excised and
submitted for analysis by MS/MS at the proteomic service provided
by Sangon Biotech Co., Ltd. Briefly, prior to MS/MS analysis, the
purified recombinant fusion protein was digested in-gel into
smaller peptides using trypsin (Beijing Solarbio Science &
Technology Co., Ltd). A tandem matrix-assisted laser desorption
ionization time-of-flight (MALDI-TOF/TOF) mass spectrometer (Model
4800; Applied Biosystems; Thermo Fisher Scientific, Inc.) was used
for peptide sequencing and the acquired data was further analyzed
using ABI GPS Explorer and MASCOT software (http://www.matrixscience.com). Databases, including
the NCBI database (http://www.ncbi.nlm.nih.gov), were searched to
characterize the protein.
Preparation of biotinylated
recombinant Pen a 1
EZ-Link Sulfo-NHS-LC-LC-Biotin was purchased from
Thermo Fisher Scientific, Inc. Recombinant Pen a 1 was biotinylated
according to manufacturer's protocol. Briefly, the protein was
dissolved in PBS. The appropriate volume of biotin reagent solution
was added to the protein solution at a molar ratio of 20:1 and
incubated on ice for 2 h. Calculations were performed based on the
product instructions. To remove unreacted biotin, the solution was
desalted or dialyzed against PBS buffer at 4°C. Finally, the
conjugate was stored at 4°C until further use.
Preparation of Pen a 1 peptides
A total of 65 linear epitopes of Penaeus
aztecus tropomyosin (Pen a 1) were be found in the Immune
Epitope Database (http://www.iedb.org). All 9 major
epitopes of Pen a 1 as reported in the literature (21,22)
were chosen for the present study. A ‘major epitope’ of an allergen
is defined as an IgE-Binding region recognized by >50% of
allergic patients (21,32) whereas a ‘minor epitope’ was
recognized by <50% of allergic patients.
The 9 peptides and their corresponding biotinylated
peptides with a purity of >95% by high performance liquid
chromatography, consisting of 15–27 amino acids in length and
corresponding to the primary sequences of tropomyosin, were
commercially synthesized (Sangon Biotech Co., Ltd.). Peptides were
suspended in PBS at 1 mg/ml and stored at −70°C until use. The
sequences of synthetic peptides are shown in Table SI.
A light-initiated chemiluminescent
assay
The allergen specific IgE and IgG4 antibody levels
are usually low in serum and not easy to detect by routine methods
such as ELISA. Sandwich fluoro-immunochemical,
lumino-immunochemical and radioimmunologic are common methods to
increase the detection sensitivity (33).
The levels of sIgE and sIgG4 to rPen a 1
as well as its peptides were measured using a light-initiated
chemiluminescent assay (LICA) carried out as previously described
(34,35). LICA is based on the formation of
nanoparticle pairs and luminescence oxygen channeling immunoassay
technology (36,37). Studies have shown that this new
method possesses excellent performance characteristics with high
sensitivity, broad analytical range and rapid turnaround cycles and
is suitable for immunodetection of allergen sIgE and
sIgG4 (34,35). Two-step assay procedures were
performed. Briefly, test serum samples (25 µl) diluted 1:20 in PBS
containing 1% human serum albumin (Beijing Solarbio Science &
Technology Co., Ltd.) were added to the 96-well plates. A 50 µl mix
of anti-human IgE (cat. no. ab7382; Abcam) and/or IgG4
antibody (cat. no. ab238320; Abcam)-chemibeads (Beyond Biotech,
Co., Ltd.) (33,34), diluted 1:1,000, and biotinylated
rPen a 1 and/or peptides, diluted 1:200 in PBS/HSA, was then added
to each well and incubated at 37°C for 30 min with mild agitation.
Subsequently, 150 µl streptavidin-coated sensibeads (Beyond
Biotech, Co., Ltd.) were added. The reaction mixture was incubated
at 37°C for 10 min in the dark. Finally, the chemiluminescence (CL)
signal was measured on a LICA instrument (LICA series 500; Beijing
Chemclin Biotech Co., Ltd.) and expressed as Relative Light Units.
This immunoassay was run with samples in duplicate.
The healthy nonatopic sera were processed in
parallel as negative controls. If the CL value of the tested sera
was higher than the cut-off values, determined by adding two
standard deviations to the mean CL value of the negative control,
the reactivity was considered positive.
Peptide inhibition experiment
A peptide inhibition assay for IgE binding was
performed as described above except that the serum pool from 3
patients was preincubated with several peptides unconjugated with
biotin (the first, the third, the fifth, the seventh and the ninth
peptide) at a concentration of 5 µg/ml at 37°C for 30 min with mild
agitation. The same serum pool without peptides addition was
analyzed in parallel and incubated under the same conditions as a
control.
Statistical analysis
Mean values for each serologic parameter and
log-transformed antibody levels (Igs binding to rPen a 1 and its
peptides) were compared among patients and control subjects.
Between the two groups, differences were compared with two-tailed
Student's t-test for parametric data and/or with the Mann-Whitney U
test for non-parametric data. The frequency of Igs responses to Pen
a 1 and its peptides between patients and control subjects were
compared using a χ2 test. Spearman's correlation was
used to evaluate the correlation. P<0.05 was considered to
indicate a statistically significant difference. All statistical
computations were performed using GraphPad Prism Version 7.0
(GraphPad Software, Inc.) and R Version 3.6.1 (R core Team;
http://cran.r-project.org) software.
Results
Patient characteristics
The demographic and clinical characteristics of the
subjects are presented in Table I.
According to their clinical reactivity, 35 individuals were
diagnosed with a shrimp allergy based on a clear-cut clinical
history of allergic reactions immediately following shrimp
ingestion and positive shrimp-sIgE of >0.35 kUA/l.
Furthermore, 29 subjects were allergic to HDM and/or cockroaches
with a convincing history of a recent hypersensitivity reaction,
including allergic rhinitis and/or asthma, to HDM and/or
cockroaches and increased serum sIgE antibodies. As control group,
28 nonatopic healthy subjects (no convincing history of clinical
reaction and negative serum sIgE level) were also included for
providing negative serum. None of the patients received
immunotherapy. Glucocorticoid or antihistamine drug such as
loratadine or were used to alleviate symptoms.
Expression and purification of rPen a
1
The recombinant shrimp tropomyosin, rPen a 1, was
produced in Escherichia coli and purified as described above
(Fig. 1, Tables II and SII). With respect to purified rPen a 1,
a unique band with the expected MW (~37 kDa) was observed in
SDS-PAGE analysis. The 95–99% purity was obtained by further
analyzing SDS-PAGE results using ImageJ software.
 | Table II.Recombinant tropomyosin from
Penaeus aztecus, rPen a 1 identified by Tandem mass
spectrometry and Mascot database searches. |
Table II.
Recombinant tropomyosin from
Penaeus aztecus, rPen a 1 identified by Tandem mass
spectrometry and Mascot database searches.
| GenBank
protein | Protein
description | Theoretical
molecular weight/D | Score | Matching peptide
number |
|---|
| AAZ76743 | Penaeus
monodon, tropomyosin | 32830 | 292 | 4 |
| CDW59661 | Trichuris
trichiura, elongation factor tu | 24465 | 171 | 2 |
| KZC10872 | Dufourea
novaeangliae tropomyosin-1 | 35055 | 108 | 2 |
rPen a 1-sIgE and sIgG4
responses
The IgE and IgG4 binding ability of rPen
a 1 was evaluated by LICA. The comparison of IgE and
IgG4 binding between patients and control subjects is
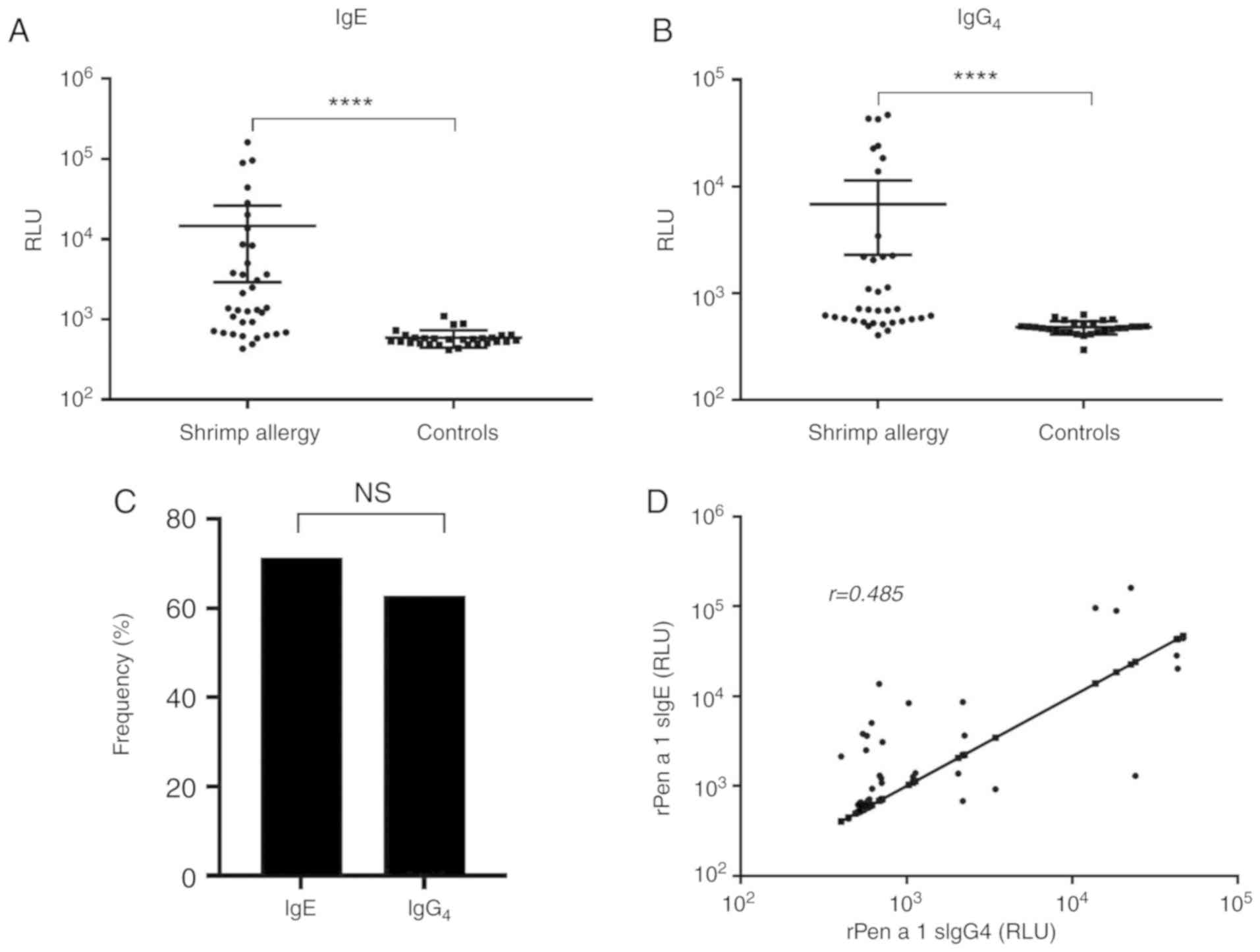
shown in Fig. 2A and B. The shrimp
allergic and nonatopic groups demonstrated significant differences
regarding rPen a 1-sIgE and sIgG4 levels. rPen a 1-sIgE
and sIgG4 levels were significantly increased in shrimp
allergic patients than in controls (P<0.0001 for each). As shown
in Fig. 2C, 25 (71.4%) shrimp
allergic patients were sensitized to rPen a 1 and 22 (62.9%)
contained sIgG4 to rPen a 1. In addition, sIgE to rPen a
1 was significantly correlated with sIgE to shrimp extract
(r=0.668, P<0.0001) and sIgG4 to rPen a 1 (r=0.484,
P=0.003, Fig. 2D).
Cross-reactivity study
Cross-reactive responses to tropomyosin between
crustaceans and HDM and/or cockroaches were frequently observed.
However, in the present clinical and serological study, the HDM
and/or cockroach allergic patients and controls did not show
significant differences in rPen a 1 sIgE levels (P=0.196),
which indicated that no IgE to rPen a 1 was detected among patients
with HDM and/or cockroach allergy (Fig. 3).
IgE and IgG4 binding
regions of rPen a 1
Individual sera from 25 patients with positive rPen
a 1 sIgE levels and 22 patients with positive sIgG4
levels together with 4 healthy volunteers were used to identify IgE
and IgG4 binding areas on Pen a 1. The frequency of
positive IgE and IgG4 binding to each peptide of Pen a 1
is shown in Fig. 4. All synthetic
peptides were recognized by between 28–48% of the sera tested for
IgE binding. None of the peptides were major epitopes in this
population and 9 peptides were identified as IgG4
epitopes because the majority of patients (>50%) had positive
IgG4 antibodies to all synthetic peptides. The most
frequent IgG4 recognized antigenic areas were the second
and the eighth peptides (AA 85–105 and AA 259–273), both of which
were recognized by 90.9% of the patients. In addition, IgE and
IgG4 binding regions of Pen a 1 largely overlapped.
Epitope profiles of individual
patients
As demonstrated in Fig.
5, the number of IgE and IgG4 positive peptides per
subject and recognition patterns varied considerably among 25
shrimp-allergic, rPen a 1-reactive individuals. A total of 28%
(7/25) of the sera demonstrated no peptide sIgE binding reactivity,
while 16% (4/25) sera exhibited positive IgE binding to all
peptides. The mean number of IgE binding peptides recognized per
individual was 3.2 (range, 0–9). Serum sIgE levels to rPen a 1 was
positively correlated with the number of IgE positive peptides
(r=0.680, P<0.001). The number of sera which recognized
IgG4 binding peptides varied from 0 (0%) for 2 peptides
to 8 (67%) for 9 peptides.
The specificity of IgE binding was assessed by a
peptide inhibition assay. As shown in Fig. 6, peptides preincubated with the
serum pool partially inhibited the IgE binding to the same peptide
or neighboring peptide, indicating that the detected binding was
due to epitope-sIgE antibodies.
Discussion
The present study analyzed profiles of IgE and
IgG4 against shrimp tropomyosin, Pen a 1 and its
epitopes among patients from coastal areas of northern China using
LICA. It was found that Pen a 1 is a major allergen recognized by
the majority of shrimp allergic patients. The data from the present
study demonstrated that 71.4% of shrimp-allergic patients in this
population exhibited IgE reactivity to shrimp tropomyosin. The
current study demonstrated that Pen a 1 was the major shrimp
allergen, which was consistent with previous studies (6,7) and
confirmed its clinical diagnostic value in patients from coastal
areas of northern China. However, none of the 9 epitopes are major
(reaction frequency >50%) IgE-binding regions, indicating that
the epitope profile may be different for other regions.
In particular, 62.9% of allergic sera had increased
IgG4 antibodies to Pen a 1 compared with the non-atopic
healthy group. A strong IgG4 response accompanied the
presence of IgE to Pen a 1. This was consistent with previous
studies which revealed that levels of sIgG and IgG subclasses were
significantly higher in individuals with allergic sensitization and
could develop in parallel with or invariably preceding IgE
responses (38,39). However, the role played by allergen
sIgG and sIgG4 antibodies in allergic reactions, unlike
that of IgE, remains controversial. On one hand, allergen-related
IgG4 antibodies have been proposed to be associated with
the development of clinical tolerance due to their induction by
allergen-specific immunotherapy (40,41).
On the other hand, a previous study reported peanut-specific
IgG4 is significantly higher in the avoidance group
compared with the peanut-eating group in peanut-sensitized children
and supposes that IgG4 may not indicate tolerance from
sensitization (38). There is also
a suggestion that allergen-related IgG antibodies are an
epiphenomenon with no functional relevance (42). From the point of view of the
present study, it is the difference between natural- and
therapy-induced tolerance that causes these contradictions. In
therapy-induced tolerance, allergen-specific IgG4 should
be considered as a protective factor rather than the cause of
sensitization (28). In natural
tolerance, there may be two mechanisms: One is
IgG4-associated tolerance (43) and the other is
IgG4-independent tolerance (38).
In the present study, the IgE reactivity to rPen a 1
was not observed in HDM and/or cockroach allergic patients with no
sensitization to shrimp. This meant tropomyosin did not appear to
be the markers of cross-reactivity with other arthropods in this
population, which appears to conflict with previous studies
(8,10,11,44).
One possible explanation could be the difference between the
recombinant allergens and their native counterparts due to lack of
posttranscriptional modifications (27). Conformational epitopes play an
important role in allergenicity, particularly for globular inhaled
allergens (45,46). However, this was unconvincing as
Reese et al (6) found the
recombinant tropomyosin (Pen a 1) behaved similarly to its native
counterpart. Another possibility could be that tropomyosin was a
minor allergen in HDM and/or cockroaches since Hu et al
(47) reported the frequency of
Der p 10 was only 10% in mite allergic patients in China, so
tropomyosin may not be the major cause of cross-reactivity between
shrimp and other arthropods. Similarly, Pascal et al
(48) recently described that
individuals allergic to HDM and/or cockroaches, with no
sensitization to shrimp, recognized arginine kinase and hemocyanin.
Immunoblotting using HDM extract should be included in future
studies to confirm a presence of sIgE against HDM tropomyosin in
HDM and/or cockroach allergic patients. Despite the above, the
detection of specific IgE to allergenic components could provide
higher specificity for allergy diagnosis (48); accurate allergy diagnosis without
oral food challenge test remains a challenge (31,49)
because it is heavily influenced by cross-reaction. When
considering cross-reactions, it is important to distinguish whether
a specific allergen is the cause of allergic symptoms (50). For instance, tropomyosin has been
proved to be non-allergenic in the majority of vertebrate foods
although ~1/2 of the amino acid sequences between invertebrate and
vertebrate tropomyosin are similar (3). That is, the biologic activity of
cross-reactive allergens is often relatively low and analysis of
recognition profiles of specific epitopes on allergen could better
diagnose allergies (32).
The peptides of Pen a 1 were further evaluated by
LICA to identify IgE and IgG4 binding regions. It was
worth noting the following points: First, the IgE and IgG4 epitope
mapping of Pen a 1 are fundamental for designing safe
hypoallergenic agents for the treatment of shrimp allergy.
Effective immunotherapy for food allergy with IgE binding epitope
modified hypoallergens has been reported (16,51):
28% (7/25) of patients were sensitized to tropomyosin but did not
recognize any major epitope of Pen a 1. In addition, none of the 9
peptides were the major epitopes recognized by the north Chinese
population. This indicated that there may be geographical or ethnic
differences with regard to the epitopes profiles of tropomyosin.
The major epitope of a certain allergen in one population may be a
minor epitope in another population. This means an immunotherapy
product designed for one region may have no effect in another.
Individual allergen specific immunotherapy should be recommended as
a plan. Secondly, it was found that the number of peptides
recognized by IgE was positively correlated to level of allergen
sIgE (r=0.680, P<0.001), which was similar to the result
of a previous study (25). This
indicated that the diversity of IgE binding epitopes is positively
correlated with rPen a 1 IgE levels. However, according to the
present study, there was no association between the number of IgE
epitopes of Pen a 1 and shrimp-allergic symptoms. Other studies
have demonstrated that identification of allergen epitopes is of
great value in the diagnosis of food allergies (52–55).
IgE-recognizing certain sequential immunodominant regions as well
as broad IgE epitope diversity correlate with the severity of an
allergic reaction (48,56). One possible explanation as to why
the present study arrived at a different conclusion could be that
the number of tropomyosin peptides synthesized was insufficient and
the epitopes of other major allergens in shrimp were not tested.
Another explanation could be that IgG4-related tolerance
may alleviate the symptoms. On the last point, the epitope
recognition regions of IgE and IgG4 largely overlapped,
which was in agreement with previous studies (48,57).
Coincident shared IgE and IgG4 binding epitopes could be
critical for the development of tolerance by blocking of the sIgE
antibodies to the same allergen by sIgG4 antibodies
(58).
There were some limitations to the present study.
For financial reasons, not all the 65 epitopes of Pen a 1 were
analyzed. Although double blind, placebo-controlled food challenge
is considered the gold standard for diagnosis of food allergy, the
present study did not conduct provocative studies because it is an
in vivo test involving a relatively complex procedure
(59).
In conclusion, the present study used, for the first
time to the best of our knowledge, LICA technology to determine the
IgE and IgG4 responses to Pen a 1 and its peptides among
shrimp allergic patients from coastal areas of northern China. It
was identified that 71.4% of patients with shrimp allergy were
sensitized to Pen a 1 and 62.9% had increased IgG4
antibodies to Pen a 1. Pen a 1 might not be responsible for
cross-reactivity between shrimp and other arthropods. The results
of the present study indicated that a strong IgG4
response accompanied the presence of IgE to Pen a 1. It was also
found that there may be geographical or ethnic differences with
respect to the IgE and IgG4 recognition patterns of the
Pen a 1 peptides. The present study suggested that understanding
the molecular basis of clinical reactivity will be useful for the
accurate diagnose of shrimp allergy and to further implement better
immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81772259).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL participated in all stages of the study,
including preparing and performing the experiments, creating the
figures and analyzing the data. ZL performed the recombinant
protein expression and LICA analysis. YY performed the
cross-reactive experiments, participated in the drafting of the
manuscript and made critical revisions. DK and SL collected serum
and patient information and performed the peptide inhibition assay.
HL conceived and designed the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committees of Tianjin Medical University (approval no.
TMUHMEC2017008) and written informed consent was obtained from the
patients and volunteers prior to study entry.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pedrosa M, Boyano-Martinez T, Garcia-Ara C
and Quirce S: Shellfish allergy: A comprehensive review. Clin Rev
Allergy Immunol. 49:203–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khora SS: Seafood-associated shellfish
allergy: A comprehensive review. Immunol Invest. 45:504–530. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruethers T, Taki AC, Johnston EB, Nugraha
R, Le TTK, Kalic T, McLean TR, Kamath SD and Lopata AL: Seafood
allergy: A comprehensive review of fish and shellfish allergens.
Mol Immunol. 100:28–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naqpal S, Rajappa L, Metcalfe DD and Rao
PV: Isolation and characterization of heat-stable allergens from
shrimp (Penaeus indicus). J Allergy Clin Immunol. 83:26–36. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daul C, Slattery M, Reese G and Lehrer SB:
Identification of the major brown shrimp (Penaeus aztecus) allergen
as the muscle protein tropomyosin. Int Arch Allergy Immunol.
105:49–55. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reese G, Schicktanz S, Lauer I, Randow S,
Lüttkopf D, Vogel L, Lehrer SB and Vieths S: Structural,
immunological and functional properties of natural recombinant Pen
a 1, the major allergen of Brown Shrimp, Penaeus aztecus. Clin Exp
Allergy. 36:517–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Albrecht M, Alessandri S, Conti A, Reuter
A, Lauer I, Vieths S and Reese G: High level expression,
purification and physico- and immunochemical characterisation of
recombinant Pen a 1: A major allergen of shrimp. Mol Nutr Food Res.
52 (Suppl 2):S186–S195. 2008.PubMed/NCBI
|
|
8
|
Faber MA, Pascal M, El Kharbouchi O,
Sabato V, Hagendorens MM, Decuyper II, Bridts CH and Ebo DG:
Shellfish allergens: Tropomyosin and beyond. Allergy. 72:842–848.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leung PS, Chow WK, Duffey S, Kwan HS,
Gershwin ME and Chu KH: IgE reactivity against a cross-reactive
allergen in crustacea and mollusca: Evidence for tropomyosin as the
common allergen. J Allergy Clin Immunol. 98:954–961. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ayuso R, Reese G, Leong-Kee S, Plante M
and Lehrer SB: Molecular basis of arthropod cross-reactivity:
IgE-binding cross-reactive epitopes of shrimp, house dust mite and
cockroach tropomyosins. Int Arch Allergy Immunol. 129:38–48. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeWitt AM, Mattsson L, Lauer I, Reese G
and Lidholm J: Recombinant tropomyosin from Penaeus aztecus (rPen a
1) for measurement of specific immunoglobulin E antibodies relevant
in food allergy to crustaceans and other invertebrates. Mol Nutr
Food Res. 48:370–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang AC, Arruda LK, Santos AB, Barbosa MC,
Chapman MD, Galvão CE, Kalil J and Morato-Castro FF: Measurement of
IgE antibodies to shrimp tropomyosin is superior to skin prick
testing with commercial extract and measurement of IgE to shrimp
for predicting clinically relevant allergic reactions after shrimp
ingestion. J Allergy Clin Immunol. 125:872–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gámez C, Sánchez-García S, Ibáñez MD,
López R, Aguado E, López E, Sastre B, Sastre J and del Pozo V:
Tropomyosin IgE-positive results are a good predictor of shrimp
allergy. Allergy. 66:1375–1383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matricardi PM, Dramburg S, Potapova E,
Skevaki C and Renz H: Molecular diagnosis for allergen
immunotherapy. J Allergy Clin Immunol. 143:831–843. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuo H, Yokooji T and Taogoshi T: Common
food allergens and their IgE-binding epitopes. Allergol Int.
64:332–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wai CY, Leung NY, Ho MH, Gershwin LJ, Shu
SA, Leung PS and Chu KH: Immunization with Hypoallergens of shrimp
allergen tropomyosin inhibits shrimp tropomyosin specific IgE
reactivity. PLoS One. 9:e1116492014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asero R, Mistrello G, Amato S, Ariano R,
Colombo G, Conte ME, Crivellaro M, De Carli M, Della Torre F,
Emiliani F, et al: Shrimp allergy in Italian adults: A multicenter
study showing a high prevalence of sensitivity to novel high
molecular weight allergens. Int Arch Allergy Immunol. 157:3–10.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roberts G, Ollert M, Aalberse R, Austin M,
Custovic A, DunnGalvin A, Eigenmann PA, Fassio F, Grattan C,
Hellings P, et al: A new framework for the interpretation of IgE
sensitization tests. Allergy. 71:1540–1551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siroux V, Lupinek C, Resch Y, Curin M,
Just J, Keil T, Kiss R, Lødrup Carlsen K, Melén E, Nadif R, et al:
Specific IgE and IgG measured by the MeDALL allergen-chip depend on
allergen and route of exposure: The EGEA study. J Allergy Clin
Immunol. 139:643–654.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hansen KS, Ballmer-Weber BK, Sastre J,
Lidholm J, Andersson K, Oberhofer H, Lluch-Bernal M, Ostling J,
Mattsson L, Schocker F, et al: Component-resolved in vitro
diagnosis of hazelnut allergy in Europe. J Allergy Clin Immunol.
123:1134–1141.e1-e3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ayuso R, Lehrer S and Reese G:
Identification of continuous, allergenic regions of the major
shrimp allergen Pen a 1 (tropomyosin). Int Arch Allergy Immunol.
127:27–37. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reese G, Ayuso R, Carle T and Lehrer SB:
IgE binding epitopes of shrimp tropomyosin, the major allergen Pen
a 1. Int Arch Allergy Immunol. 118:300–301. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mine Y and Wei Zhang J: Identification and
fine mapping of IgG and IgE epitopes in ovomucoid. Biochem Biophys
Res Commun. 292:1070–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jarvinen KM, Beyer K, Vila L, Bardina L,
Mishoe M and Sampson HA: Specificity of IgE antibodies to
sequential epitopes of hen's egg ovomucoid as a marker for
persistence of egg allergy. Allergy. 62:758–765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinez-Botas J, Cerecedo I, Zamora J,
Vlaicu C, Dieguez MC, Gómez-Coronado D, de Dios V, Terrados S and
de la Hoz B: Mapping of the IgE and IgG4 sequential epitopes of
ovomucoid with a peptide microarray immunoassay. Int Arch Allergy
Immunol. 161:11–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerecedo I, Zamora J, Shreffler WG, Lin J,
Bardina L, Dieguez MC, Wang J, Muriel A, de la Hoz B and Sampson
HA: Mapping of the IgE and IgG4 sequential epitopes of milk
allergens with a peptide microarray-based immunoassay. J Allergy
Clin Immunol. 122:589–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steckelbroeck S, Ballmer-Weber BK and
Vieths S: Potential, pitfalls, and prospects of food allergy
diagnostics with recombinant allergens or synthetic sequential
epitopes. J Allergy Clin Immunol. 121:1323–1330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suarez-Farinas M, Suprun M, Chang HL,
Gimenez G, Grishina G, Getts R, Nadeau K, Wood RA and Sampson HA:
Predicting development of sustained unresponsiveness to milk oral
immunotherapy using epitope-specific antibody binding profiles. J
Allergy Clin Immunol. 143:1038–1046. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jenmalm MC and Bjorksten B: Development of
immunoglobulin G subclass antibodies to ovalbumin, birch and cat
during the first eight years of life in atopic and non-atopic
children. Pediatr Allergy Immunol. 10:112–121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Zhao J, Wei N, Feng M, Xian M, Shi
X, Zheng Z, Su Q, Wong GWK and Li J: Cockroach is a major
cross-reactive allergen source in shrimp-sensitized rural children
in southern China. Allergy. 73:585–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muraro A, Werfel T, Hoffmann-Sommergruber
K, Roberts G, Beyer K, Bindslev-Jensen C, Cardona V, Dubois A,
duToit G, Eigenmann P, et al: EAACI food allergy and anaphylaxis
guidelines: Diagnosis and management of food allergy. Allergy.
69:1008–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matricardi PM, Kleine-Tebbe J, Hoffmann
HJ, Valenta R, Hilger C, Hofmaier S, Aalberse RC, Agache I, Asero
R, Ballmer-Weber B, et al: EAACI molecular allergology user's
guide. Pediatr Allergy Immunol. 27 (Suppl 23):S1–S250. 2016.
View Article : Google Scholar
|
|
33
|
Dodig S and Cepelak I: The potential of
component-resolved diagnosis in laboratory diagnostics of allergy.
Biochem Med (Zagreb). 28:0205012018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bian Y, Liu C, She T, Wang M, Yan J, Wei D
and Li H: Development of a light-initiated chemiluminescent assay
for the quantitation of sIgE against egg white allergens based on
component-resolved diagnosis. Anal Bioanal Chem. 410:1501–1510.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Li S, Huang L, Cui Y, She T, Bian Y
and Li H: A light-initiated chemiluminescent assay for rapid
quantitation of allergen-specific IgG4 in clinical samples. Clin
Chim Acta. 489:83–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ullman E, Kirakossian H, Switchenko AC,
Ishkanian J, Ericson M, Wartchow CA, Pirio M, Pease J, Irvin BR,
Singh S, et al: Luminescent oxygen channeling assay (LOCI):
Sensitive, broadly applicable homogeneous immunoassay method. Clin
Chem. 42:1518–1526. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ullman E, Kirakossian H, Singh S, Wu ZP,
Irvin BR, Pease JS, Switchenko AC, Irvine JD, Dafforn A, Skold CN,
et al: Luminescent oxygen channeling immunoassay: Measurement of
particle binding kinetics by chemiluminescence. Proc Natl Acad Sci
USA. 91:5426–5430. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sverremark-Ekstrom E, Hultgren EH, Borres
MP and Nilsson C: Peanut sensitization during the first 5 yr of
life is associated with elevated levels of peanut-specific IgG.
Pediatr Allergy Immunol. 23:224–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang X, Tsilochristou O, Perna S,
Hofmaier S, Cappella A, Bauer CP, Hoffman U, Forster J, Zepp F,
Schuster A, et al: Evolution of the IgE and IgG repertoire to a
comprehensive array of allergen molecules in the first decade of
life. Allergy. 73:421–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hofmaier S, Comberiati P and Matricardi
PM: Immunoglobulin G in IgE-mediated allergy and allergen-specific
immunotherapy. Eur Ann Allergy Clin Immunol. 46:6–11.
2014.PubMed/NCBI
|
|
41
|
Akdis M and Akdis CA: Mechanisms of
allergen-specific immunotherapy: Multiple suppressor factors at
work in immune tolerance to allergens. J Allergy Clin Immunol.
133:621–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stapel SO, Asero R, Ballmer-Weber BK, Knol
EF, Strobel S, Vieths S and Kleine-Tebbe J; EAACI Task Force, :
Testing for IgG4 against foods is not recommended as a diagnostic
tool: EAACI Task Force Report. Allergy. 63:793–796. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Caubet JC, Lin J, Ahrens B, Gimenez G,
Bardina L, Niggemann B, Sampson HA and Beyer K: Natural tolerance
development in cow's milk allergic children: IgE and IgG4 epitope
binding. Allergy. 72:1677–1685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Farioli L, Losappio LM, Giuffrida MG,
Pravettoni V, Micarelli G, Nichelatti M, Scibilia J, Mirone C,
Cavallarin L, Lamberti C, et al: Mite-induced asthma and IgE levels
to shrimp, mite, tropomyosin, arginine kinase, and Der p 10 Are the
most relevant risk factors for challenge-proven shrimp allergy. Int
Arch Allergy Immunol. 174:133–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Varshney S, Goldblum RM, Kearney C,
Watanabe M and Midoro-Horiuti T: Major mountain cedar allergen, Jun
a 1, contains conformational as well as linear IgE epitopes. Mol
Immunol. 44:2781–2785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lombardero M, Heymann PW, Platts-Mills TA,
Fox JW and Chapman MD: Conformational stability of B cell epitopes
on group I and group II Dermatophagoides spp. allergens. Effect of
thermal and chemical denaturation on the binding of murine IgG and
human IgE antibodies. J Immunol. 144:1353–1360. 1990.PubMed/NCBI
|
|
47
|
Hu H, Luo W, Wu Z, Cai C, Huang H and Sun
B: A pilot study on the allergen-specific IgE to molecular
components on polysensitized mite allergic asthmatic patients in
Guangzhou, China. Mol Immunol. 105:38–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pascal M, Grishina G, Yang AC,
Sánchez-García S, Lin J, Towle D, Ibañez MD, Sastre J, Sampson HA
and Ayuso R: Molecular diagnosis of shrimp allergy: Efficiency of
several allergens to predict clinical reactivity. J Allergy Clin
Immunol Pract. 3:521–529. e10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koplin JJ, Perrett KP and Sampson HA:
Diagnosing peanut allergy with fewer oral food challenges. J
Allergy Clin Immunol Pract. 7:375–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Aalberse RC: Shrimp Serology: We need
tests with more and less cross-reactivity. J Allergy Clin Immunol
Pract. 3:530–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
King N, Helm R, Stanley JS, Vieths S,
Lüttkopf D, Hatahet L, Sampson H, Pons L, Burks W and Bannon GA:
Allergenic characteristics of a modified peanut allergen. Mol Nutr
Food Res. 49:963–971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Beyer K, Jarvinen KM, Bardina L, Mishoe M,
Turjanmaa K, Niggemann B, Ahlstedt S, Venemalm L and Sampson HA:
IgE-binding peptides coupled to a commercial matrix as a diagnostic
instrument for persistent cow's milk allergy. J Allergy Clin
Immunol. 116:704–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shreffler WG, Beyer K, Chu TH, Burks AW
and Sampson HA: Microarray immunoassay: Association of clinical
history, in vitro IgE function, and heterogeneity of allergenic
peanut epitopes. J Allergy Clin Immunol. 113:776–782. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lin J and Sampson HA: The role of
immunoglobulin E-binding epitopes in the characterization of food
allergy. Curr Opin Allergy Clin Immunol. 9:357–363. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matsuo H, Kohno K, Niihara H and Morita E:
Specific IgE determination to epitope peptides of omega-5 gliadin
and high molecular weight glutenin subunit is a useful tool for
diagnosis of wheat-dependent exercise-induced anaphylaxis. J
Immunol. 175:8116–8122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin J, Bruni FM, Fu Z, Maloney J, Bardina
L, Boner AL, Gimenez G and Sampson HA: A bioinformatics approach to
identify patients with symptomatic peanut allergy using peptide
microarray immunoassay. J Allergy Clin Immunol. 129:1321–1328 e5.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang J, Lin J, Bardina L, Goldis M,
Nowak-Wegrzyn A, Shreffler WG and Sampson HA: Correlation of
IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies
with different phenotypes of clinical milk allergy. J Allergy Clin
Immunol. 125:695–702, 702. e1-702.6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vickery BP, Lin J, Kulis M, Fu Z, Steele
PH, Jones SM, Scurlock AM, Gimenez G, Bardina L, Sampson HA and
Burks AW: Peanut oral immunotherapy modifies IgE and IgG4 responses
to major peanut allergens. J Allergy Clin Immunol. 131:128–134
e1-3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sicherer SH and Sampson HA: Food allergy:
A review and update on epidemiology, pathogenesis, diagnosis,
prevention, and management. J Allergy Clin Immunol. 141:41–58.
2018. View Article : Google Scholar : PubMed/NCBI
|