Introduction
Atherosclerosis (AS) is a chronic systemic disease
in which the inside of an artery narrows due to the build-up of
plaque, particularly in the brain, heart and lower extremities
(1). Previous studies have
reported that the initial step of AS is intimal injury, followed by
platelet aggression or leukocyte invasion beneath the endothelial
monolayer (2,3). When AS becomes severe, it may result
in coronary artery disease, peripheral artery disease or kidney
issues, depending on which arteries are affected (4).
The aberrantly increased proliferation and migration
of vascular smooth muscle cells (VSMCs) are critical in the
pathogenesis and progression of AS (5,6). In
addition, previous studies have suggested that the proliferation of
VSMCs can be induced or stimulated by cytokines and growth factors
(7,8), such as platelet-derived growth factor
(PDGF). Platelet-derived growth factor type BB (PDGF-BB), a subunit
of PDGF, has been reported to regulate cell growth and division
(9,10). In particular, PDGF-BB plays a
critical role in initiating numerous biological effects by
activating intracellular transduction pathways that are critical in
modulating the proliferation and migration of VSMCs (8,11).
Collectively, preventing PDGF-modulated VSMC proliferation and
migration may contribute to the treatment of AS.
MicroRNAs (miRNAs or miRs) are a class of
non-coding, short (22 nucleotides in length) endogenous RNAs that
post-transcriptionally regulate gene expression by binding to the
3′-untranslated region (UTR) of their target genes (12,13).
miRNAs carry out critical functions in cardiovascular systems
(14–16), including AS (17,18).
Accumulating evidence has demonstrated that miR-142-5p is
upregulated in the plaques of apoE−/− mice with AS
(19) and that the overexpression
of miR-142-5p may induce VSMC proliferation (20). However, the effect of miR-142-5p on
modulating HASMC proliferation and migration remains unclear.
Therefore, this study aimed to explore the roles of miR-142-5p in
the regulation of HASMC physiology and to elucidate the underlying
mechanisms.
Materials and methods
Patient studies
Serum of patients with AS was obtained from 35
patients who had been diagnosed with AS by Doppler ultrasonography
(age, 41.9±7.1 years; sex, 17 males and 18 females), and normal
serum specimens were obtained from 35 healthy volunteers (age,
42.5±6.9 years; sex, 19 males and 16 females). All the specimens
were acquired between January 2016 and June 2017 at the Central
Hospital of Wuhan, Tongji Medical College, Huazhong University of
Science and Technology. The present study was approved by the
Ethics Committee of the Central Hospital of Wuhan, Tongji Medical
College, Huazhong University of Science and Technology and prior
informed consent was obtained from the patients with AS and the
healthy volunteers.
Cells and cell culture
The human artery vascular smooth muscle cell line,
HASMC (BeNa Culture Collection), was purchased and incubated in
DMEM (Thermo Fisher Scientific, Inc.), supplemented with 10% FBS
and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere with 5% CO2 for
24 h.
Cell transfection
Cells were seeded in 96-wells with a density of
1×104 cells/well containing 25 ng/ml of PDGF-BB
(HEGFP-1601; Cyagen) and cultured for 24 h to reach 60% confluence.
Subsequently, the expression levels of miR-142-5p before and after
the addition of PDGF-BB were detected accordingly. The cells were
then transfected with either 50 nM miR-142-5p inhibitor
(5′-CACAAGGUAGAAAGCACUACU-3′) or NC (5′-GUGUAACACGUCUAUACGCCCA-3′;
Biomics Biotechnologies Co. Ltd.) using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as per
the manufacturer's instructions. In order to confirm the
transfection efficiency of miR-142-5p, the cells were divided into
3 groups as follows: i) The control group, which were untransfected
cells; ii) the NC group, in which cells were transfected with NC
(miR-142-5p inhibitor negative control); iii) the inhibitor group,
in which cells were transfected with miR-142-5p inhibitor. After
transfection, the transfected cells were incubated for 24 h prior
to further experimentation.
In addition, in order to confirm the transfection
efficiency of myocardin like 2 (MKL2), the cells were divided into
3 groups as follows: i) The control group, which were untransfected
cells; ii) the small interfering RNA (siRNA) group, in which cells
were transfected with negative control siRNA; iii) the siRNA-MKL2
group, in which cells were transfected with siRNA against MKL2.
Briefly, the cells were seeded in 96-wells at a density of
1×104 cells/well and grown for 24 h to reach 60%
confluence. The cells were then transfected with either 20 µM siRNA
or MKL2 siRNA using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.,) as per the manufacturer's
instructions. After transfection, the transfected cells were
incubated for 24 h prior to further experimentation. siRNA-MLK2 and
negative control siRNA were designed and purchased from Shanghai
GenePharma Co., Ltd. The sequences were as follows: Negative
control siRNA forward, 5′-CGCCCTCATCAGTGCATACAA-3′ and reverse,
5′-CATAGCAAAGAAAGACTTAAA-3′; siRNA-MKL2 forward,
5′-CGCCATCATCGATGACTACAA-3′ and reverse,
5′-CTACGAGCAGATCAAGATAAA-3′.
Cell proliferation measured by CCK-8
assay
A CCK-8 assay was conducted to measure HASMC
proliferation, according to the manufacturer's protocol. Briefly,
the cells were seeded into 96-well plates at a density of
2×103 cells/well. The cells were then incubated at 37°C
in serum-free DMEM containing 25 ng/ml of PDGF-BB (HEGFP-1601;
Cyagen) for 24 h following transfection. Subsequently, 10 µl CCK-8
solution (Dojindo Molecular Technologies, Inc.) was added to each
well followed by incubation for a further 4 h at room temperature
and the absorbance was measured at 450 nm using Multiscan FC
Microplate Photometer (Thermo Fisher Scientific, Inc.).
Cell migration measured by wound
scratch assay
The HASMCs were seeded into a 12-well plate at a
density of 1×103 cells/well. The cells were then
incubated at 37°C in serum-free DMEM containing the 25 ng/ml of
PDGF-BB (HEGFP-1601; Cyagen) for 24 h following transfection. A
straight scratch wound was then created using a sterilized 10-µl
pipette in each well. Following 24 h of incubation at 37°C in a
humidified atmosphere with 5% CO2, the wound was
visualized and photographed under an inverted microscope at ×200
magnification (BDS500 Trinocular; SCOPE).
Cell migration measured by Transwell
migration assay
A total of 200 µl transfected cells were resuspended
in serum-free DMEM medium and placed in the upper compartment of
8-µm pore size Transwell chambers without Matrigel (Costar Inc.),
while the lower chamber was supplemented with serum-free DMEM
containing 5 ng/ml of PDGF-BB. Following incubation for 24 h at
37°C, 5% CO2, non-migrated HASMCs in the upper
compartment were removed using cotton swabs, while the migrated
cells were stained with 0.1% crystal violet (Sigma-Aldrich; Merck
KGaA) for 30 min at room temperature. The cells on the bottom side
of the membrane were calculated under a microscope (SZX7-1063;
Olympus Corporation) in order to confirm the number of migrated
cells.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the serum of patients
with AS and the healthy volunteers or the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Subsequently,
the RNA was reversed transcribed into cDNA using the TaqMan
MicroRNA Reverse Transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) or TaqMan Gene Expression Assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.), respectively as per
the manufacturer's instructions. The cDNA was used to template with
the SYBR-Green PCR Master Mix kit (Applied Biosystems; Life
Technologies) on the CFX96 Real-Time RCR (Bio-Rad) according to the
manufacturer's instructions. The PCR reactions were described as
follows: An initial denaturation at 95°C for 5 min; 40 thermal
cycles of denaturation at 95°C for 30 sec, annealing at 56°C for 30
sec and extension at 72°C for 30 sec; final extension at 60°C for 5
min. For relative quantification, the levels of individual gene
mRNA transcripts were normalized to GAPDH, while the expression
level of miR-142-5p was normalized to U6. The expression levels of
relative genes were calculated using the 2−∆∆Cq method
(21). All the experiments were
conducted at least in triplicate. The following primers were used:
miR-142-5p forward, 5′-AACTCCAGCTGGTCCTTAG-3′ and reverse,
5′-TCTTGAACCCTCATCCTGT-3′; U6 forward, 5′-CTCGCTTCGGCAGAC-3′ and
reverse, 5′-AACGCTTACGAATTT-3′; MKL2 forward,
5′-AGATCAGAAGGGTGAGAAGAATG-3′ and reverse,
5′-GGATGGTCTGGTAGTTGTAGTG-3′; matrix metalloproteinase (MMP)2
forward, 5′-TGTGTTCTTTGCAGGGAATGAAT-3′ and reverse,
5′-TGTCTTCTTGTTTTTGCTCCAGTTA-3′; MMP9 forward,
5′-CCTCTGGAGGTTCGACGTGA-3′ and reverse,
5′-TAGGCTTTCTCTCGGTACTGGAA-3′; GAPDH forward,
5′-ACTCCACTCACGGCAAATTC-3′ and reverse,
5′-TCTCCATGGTGGTGAAGACA-3′.
Western blot analysis
Total proteins were lysed with RIPA lysis buffer
(Beyotime Institute of Biotechnology) supplemented with a protease
inhibitor cocktail (K1010; Apexbio) as per the manufacturer's
instructions. The concentration of proteins was measured using a
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). The
proteins (20 µg/lane) were separated by 10% SDS-PAGE (cat. no.
LC26755; Invitrogen; Thermo Fisher Scientific, Inc.) and
transferred onto polyvinylidene fluoride membranes (cat. no.
abs932; Absin). The membranes were blocked with Tris-buffered
saline (TBS; cat. no. SIG-32380-500; Kanglang) containing 5%
non-fat milk at 37°C for 2 h. After blocking, the proteins were
probed with the following primary antibodies overnight at 4°C:
Rabbit anti-MKL2 (cat. no. ab191496; 1:1,000), rabbit anti-MMP2
(cat. no. ab37150; 1:1,000), rabbit anti-MMP9 (cat. no. ab38898;
1:1,000) and rabbit anti-GAPDH (cat. no. ab9485; 1:2,500) (all from
Abcam). Subsequently, the blots were washed with Tris-buffered
saline/Tween-20 (TBST; T1081-500; Salarbio) three times and
incubated with the secondary antibody IgG H&L (cat. no. ab6940;
1:1,000; Abcam) at 37°C for 1 h. After washing, the signals were
detected using chemiluminescence HRP Substrate (Clontech), imaged
using a GE ImageQuant Las 4000 mini phosphorimager (GE Healthcare
Life Sciences) and presented as the density ratio vs. GAPDH. The
quantification was performed using ImageJ software version 1.41
(National Institutes of Health). All the procedures above were
conducted in triplicate.
Luciferase reporter assay
Using the online tool TargetScan, MKL2 was
considered to be a putative target gene of miR-142-5p. In order to
confirm the targeted association between miR-142-5p and MKL2, a
dual-luciferase reporter assay was performed accordingly. In brief,
the MKL2 mRNA 3′-UTR containing the putative or mutated binding
sites for miR-142-5p were amplified by PCR and cloned into the
pMIR-REPORT Luciferase (BR031; Fenghbio). The HASMC cell line (BeNa
Culture Collection) was co-transfected with 20 µM miR-142-5p mimics
or NC using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.,). Luciferase activities were
determined at 48 h using the Dual-luciferase Reporter Gene Assay
kit (Promega Corp.). Luciferase activity was compared to
Renilla luciferase activity as per the manufacturer's
protocol. The miR-NC (5′-GUGUAACACGUCUAUACGCCCA-3′) and miR-142-5p
mimic (5′-CAUAAAGUAGAAAGCACUACU-3′) were designed and synthesized
by Biomics Biotechnologies Co. Ltd.
Statistical analysis
SPSS version 17.0 software (SPSS, Inc.) was used to
analyze the data. The data are presented as the mean ± SD in the
present study. Pearson's correlation analysis was performed to
evaluate the correlation between miR-142-5p and MKL2. The Student's
t-test is applied to distinguish differences between 2 groups,
while one-way analysis of variance (ANOVA) followed by Newman-Keuls
test was used to analyze the data among ≥3 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-142-5p is upregulated in the serum
of patients with AS
As demonstrated in Fig.
1A, the expression level of miR-142-5p was significantly
upregulated in the serum of patients with AS, compared to that of
the healthy volunteers (P<0.01).
MKL2 is downregulated in the serum of
patients with AS
As demonstrated in Fig.
1B, the mRNA expression level of MKL2 was prominently
downregulated in the serum of patients with AS, in contrast with
the serum of healthy volunteers (P<0.01). Moreover, the MKL2
protein expression results shown in Fig. 1D and E presented a similar trend of
variation (P<0.01) as the mRNA results.
MKL2 expression is negatively
regulated by miR-142-5p
As demonstrated in Fig.
1C, the expression of miR-142-5p negatively correlated with the
expression level of MKL2 in the serum of patients with AS
(r=−0.505; P=0.002).
Transfection efficiency of miR-142-5p
and MKL2
Following treatment with PDGF-BB, the expression
level of miR-142-5p was determined. As demonstrated in Fig. 2A, the expression level of
miR-142-5p significantly increased following treatment with PDGF-BB
(P<0.01). Following transfection, the transfection efficiency of
miR-142-5p and MKL2 was measured, respectively. As shown in
Fig. 2B, the expression level of
miR-142-5p markedly decreased in the inhibitor group, as compared
with the NC group (P<0.01), while there was no variation between
the NC and control group. As demonstrated in Fig. 2C, the mRNA expression level of MKL2
markedly decreased in the siRNA-MKL2 group, as compared with the
siRNA group (P<0.01), whereas there was no variation between the
siRNA and control group. Moreover, the MKL2 protein expression
results shown in Fig. 2D and E
presented a similar trend of variation as the mRNA results
(P<0.01).
 | Figure 2.Transfection efficiency of miR-142-5p
and MKL2. (A) The expression level of miR-142-5p before and after
treatment with PDGF-BB. **P<0.01, PDGF-BB vs. before group. (B)
The relative expression level of miR-142-5p following transfection
with miR-142-5p inhibitor. (C) The relative mRNA expression level
of MKL2 following transfection with siRNA against MKL2
(siRNA-MKL2). (D) The relative protein expression of MKL2 following
transfection with siRNA-MKL2. (E) The protein/GAPDH expression
levels of MKL2 following transfection with siRNA-MKL2 are
presented. **P<0.01, siRNA-MKL2 vs. siRNA group. Before, cells
before treatment with PDGF-BB; PDGF-BB, cells treated with PDGF-BB;
Control, untreated cells; NC, miR-142-5p inhibitor negative
control-transfected cells; inhibitor, miR-142-5p
inhibitor-transfected cells; siRNA, cells transfected with siRNA;
siRNA-MKL2, cells transfected with siRNA-MKL2; PDGF-BB,
platelet-derived growth factor type BB; MKL2, myocardin-like
protein 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Downregulation of miR-142-5p inhibits
PDGF-BB-induced HASMC proliferation
As demonstrated in Fig.
3, the downregulation of miR-142-5p significantly inhibited
HASMC proliferation compared with the NC group (P<0.01).
However, the decreased cell viability induced by transfection with
miR-142-5p inhibitor was partly reversed by co-transfection with
siRNA-MKL2 (P<0.05).
Downregulation of miR-142-5P inhibits
the migration of HASMCs following treatment with PDGF-BB
The wound scratch results shown in Fig. 4 demonstrated that the
downregulation of miR-142-5p prominently inhibited PDGF-BB-induced
HASMC migration in contrast with the NC group (P<0.01). However,
the suppressed cell migratory ability induced by transfection with
miR-142-5p was partly reversed by co-transfection with siRNA-MKL2
(P<0.01). Similar results were obtained in the Transwell
migration assay (P<0.01; Fig.
5).
Effect of miR-142-5p on the expression
levels of MKL2, MMP2 and MMP9
As demonstrated in Fig.
6A-C, as the expression level of miR-142-5p was decreased, the
mRNA expression level of MKL2 was increased, while the mRNA
expression levels of MMP2 and MMP9 were significantly decreased
(P<0.01), compared with the NC group. However, these effects
were partly reversed by co-transfection with siRNA-MKL2 (MLK2 and
MMP2, P<0.01; MMP9, P<0.05). Furthermore, the protein
expression results shown in Fig. 6D
and E presented a similar trend of variation as well
(P<0.01).
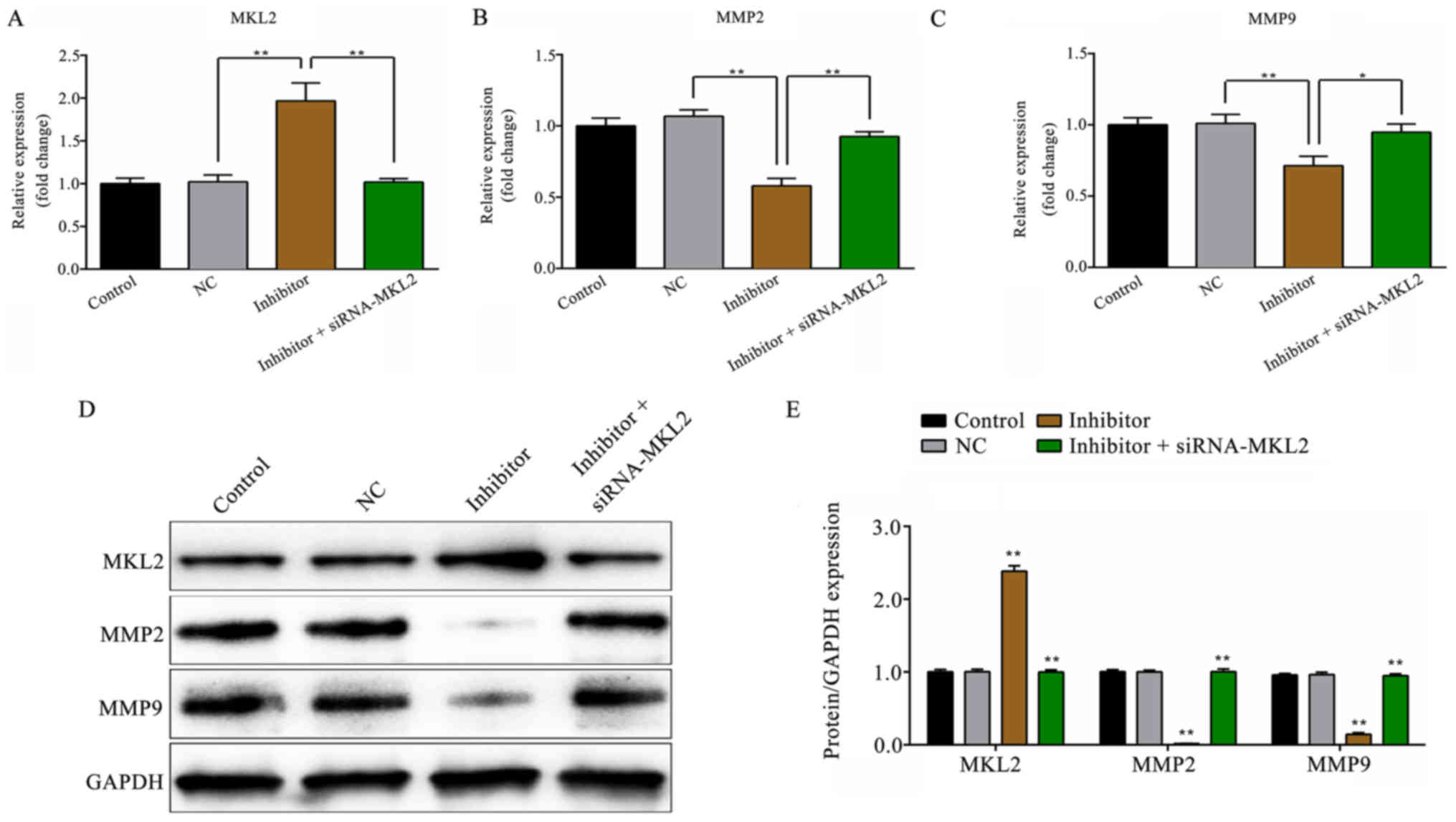 | Figure 6.Effect of miR-142-5p on the mRNA and
protein expression levels of MKL2, MMP2 and MMP9. (A) The relative
mRNA expression level of MKL2. (B) The relative mRNA expression
level of MMP2. (C) The relative expression level of MMP9. (D)
Western blot analysis was employed to measure the protein
expression levels of MKK2, MMP2 and MMP9. The quantified result of
(E) is presented. *P<0.05, inhibitor + siRNA-MKL2 vs. inhibitor
group; **P<0.01, inhibitor vs. NC group and inhibitor +
siRNA-MKL2 vs. inhibitor group. Control, untransfected cells; NC,
miR-142-5p inhibitor negative control-transfected cells; inhibitor,
miR-142-5p inhibitor-transfected cells; siRNA-MKL2, cells were
transfected with siRNA-MKL2; MKL2, myocardin-like protein 2; MMP2,
matrix metalloproteinase-2; MMP9, matrix metalloproteinase-9;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
MKL2 is a target gene of
miR-142-5p
The putative seed sequences for miR-142-5p at the
3′-UTR of MKL2 are presented in Fig.
7A. As demonstrated in Fig.
7B, transfection with miR-142-5p mimics significantly decreased
the luciferase activity in the WT group, compared with the results
in the NC group (P<0.01). However, there was no significant
difference observed in the mutant groups.
Discussion
miRNAs haven been reported to play critical roles in
cardiovascular diseases, including AS (22,23).
Moreover, the dysregulation of miRNAs may regulate the
proliferation and migration of VSMCs (24,25),
which may lead to the development of AS through different stimuli
(26,27). miR-142-5p is a member of the
miR-142 family, playing a critical role in regulating tumorigenesis
(28,29) and immune diseases (30). A previous study demonstrated that
miR-142-5p was prominently increased in the plaques of
apoE−/− mice with AS (19). Consistent with the findings of this
previous study, in the present study, miR-142-5p was significantly
upregulated in the serum of patients with AS compared with that of
healthy volunteers, as determined by RT-qPCR. To further
investigate the detailed role of miR-142-5p in HASMCs in AS, CCK-8,
Transwell migration and wound scratch assays were conducted to
examine cell proliferation and migration, respectively. According
to the results, the downregulation of miR-142-5p was demonstrated
to inhibit the proliferation and migration of PDGF-BB-treated
HASMCs, which was consistent with the findings of previous study by
Kee et al (20), who
demonstrated that miR-142-5p promoted VSMC proliferation by
targeting B cell translocation gene 3 (BTG3).
As is known, miRNAs negatively regulate the
expression of their target genes. After confirming that MKL2 is a
likely target gene of miR-142-5p by a dual-luciferase reporter
assay, correlation analysis further demonstrated that miR-142-5p
negatively regulated MKL2 in AS. MKL2 acts as co-activator,
controlling genes of relevance for myogenic differentiation and
motile function (31), carrying
out critical functions in regulating cell proliferation and
migration (32,33). Furthermore, another study revealed
that MKL2 functioned as a pro-migratory gene in VSMCs (34). In the present study, MKL2 was found
to be underexpressed in the serum of patients with AS compared with
that of healthy volunteers, which was consistent with the findings
of a previous study (35). In
addition, via gain-of-function and loss-of-function approaches,
transfection with miR-142-5p inhibitor significantly increased the
mRNA and protein expression level of MKL2 in HASMCs; however,
transfection with MKL2-siRNA resulted in a decreased MKL2
expression. Moreover, the inhibition of HASMC proliferation and
migration induced by transfection with miR-142-5p inhibitor was
partly reversed by co-transfection with MKL2-siRNA. Collectively,
the results of this study suggested that the downregulation of
miR-142-5p participated in the inhibition of HASMC proliferation
and migration, which is, at least in part, dependent on targeting
MKL2.
MMP2 and MMP9 are crucial enzymes of the MMP family
members involved in extracellular matrix remodeling and cell
migration (36). Moreover, MMP2
and MMP9 expression levels in VSMCs have been reported to be
associated with AS (37–39) by regulating the proliferation and
migration of VSMCs (40,41), indicating a pathogenic role for
MMP2 and MMP9 in regulating the progression of AS. Furthermore,
previous studies have demonstrated that the downregulation of MMP2
and MMP9 inhibit cell migration (42–44).
Consistent with previous studies, in the present study, the
suppression of MMP2 and MMP9 by transfection with miR-142-5p
inhibitor contributed to the decreased migration of HASMCs.
In conclusion, this study found that miR-142-5p
expression was markedly upregulated in the serum of patients with
AS. Moreover, the downregulation of miR-142-5p may inhibit HASMC
proliferation and migration partly by targeting MKL2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW performed the statistical analysis and wrote the
manuscript. YS collected the patient data and performed the
statistical analysis. SD was involved in the study design, data
acquisition and manuscript revision. CY and JW were involved in the
study design, discussion and completion of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Central Hospital of Wuhan, Tongji Medical College,
Huazhong University of Science and Technology and prior informed
consent was obtained from the patients with AS and the healthy
volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gan WQ, Man SF, Senthilselvan A and Sin
DD: Association between chronic obstructive pulmonary disease and
systemic inflammation: A systematic review and a meta-analysis.
Thorax. 59:574–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ross R and Agius L: The process of
atherogenesis-cellular and molecular interaction: From experimental
animal models to humans. Diabetologia. 35 (Suppl 2):S34–S40. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seely S: Atherosclerosis or hardening of
the arteries? Int J Cardiol. 22:5–12. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owens GK: Regulation of differentiation of
vascular smooth muscle cells. Physiol Rev. 75:487–517. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ross R: Atherosclerosis-an inflammatory
disease. New Engl J Med. 340:115–126. 1996. View Article : Google Scholar
|
|
7
|
Kawai-Kowase K and Owens GK: Multiple
repressor pathways contribute to phenotypic switching of vascular
smooth muscle cells. Am J Physiol Cell Physiol. 292:C59–C69. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ha JM, Yun SJ, Kim KW, Jin SY, Lee HS,
Song SH, Shin HK and Bae SS: Platelet-derived growth factor
regulates vascular smooth muscle phenotype via mammalian target of
rapamycin complex 1. Biochem Biophys Res Commun. 464:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shawky NM and Segar L: Sulforaphane
inhibits platelet-derived growth factor-induced vascular smooth
muscle cell proliferation by targeting Mtor/P70S6kinase signaling
independent of Nrf2 activation. Pharmacol Res. 119:251–264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dzau VJ, Braun-Dullaeus RC and Sedding DG:
Vascular proliferation and atherosclerosis: New perspectives and
therapeutic strategies. Nat Med. 8:1249–1256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding S, Huang H, Xu Y, Zhu H and Zhong C:
miR-222 in cardiovascular diseases: Physiology and pathology.
Biomed Res Int. 2017:49624262017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu H, Liu Z and Zhou L: Roles of miR-17-92
cluster cardiovascular development and common diseases. Biomed Res
Int. 2017:91029092017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seeger T and Boon RA: MicroRNAs in
cardiovascular aging. J Physiol. 594:2085–2094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Price NL, Rotllan N, Canfrán-Duque A,
Zhang X, Pati P, Arias N, Moen J, Mayr M, Ford DA, Baldán Á, et al:
Genetic dissection of the impact of miR-33a and miR-33b during the
progression of atherosclerosis. Cell Rep. 21:1317–1330. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ouimet M, Ediriweera H, Afonso MS,
Ramkhelawon B, Singaravelu R, Liao X, Bandler RC, Rahman K, Fisher
EA, Rayner KJ, et al: microRNA-33 regulates macrophage autophagy in
atherosclerosis. Arterioscler Thromb Vasc Biol. 37:1058–1067. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu RJ, Bi CL, Song JT, Wang L, Ge C, Liu
XX and Zhang M: Upregulation of miR-142-5p in atherosclerotic
plagues and regulation of oxidized low-density lipoprotein-induced
apoptosis in macrophages. Mol Med Rep. 11:3229–3234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kee HJ, Park S, Kwon JS, Choe N, Ahn Y,
Kook H and Jeong MH: B cell translocation gene, a direct target of
miR-142-5p, inhibits vascular smooth muscle cell proliferation by
down-regulating cell cycle progression. FEBS Lett. 587:2385–2392.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Han Z, Fan Y, Zhang J, Chen K, Gao
L, Zeng H, Cao J and Wang C: MicroRNA-9 inhibits NLRP3 inflammasome
activation in human atherosclerosis inflammation cell models
through the JAK2/STAT signaling pathway. Cell Physiol Biochem.
41:1555–1571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang X, Xu Z, Yuan M, Zhang Y, Zhao B,
Wang J, Zhang A and Li G: MicroRNA-16 suppresses the activation of
inflammation microphages in atherosclerosis by targeting PDCD4. Int
J Mol Med. 37:967–975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Cui L, Yuan J, Zhang Y and Sang H:
Circular RNA WDR77 target FGF-2 to regulate vascular smooth muscle
cells proliferation and migration by sponging miR-124. Biochem
Biophys Res Commun. 494:126–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie B, Zhang C, Kang K and Jiang S:
miR-559 inhibits vascular smooth muscle cells proliferation and
migration by targeting TGFB2. PLoS One. 10:e01415122015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bi R, Ding F, He Y, Jiang L, Jiang Z, Mei
J and Liu H: miR-503 inhibits platelet-derived growth
factor-induced human aortic vascular smooth muscle cell
proliferation and migration through targeting the insulin receptor.
Biomed Pharmacother. 84:1711–1716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sudo R, Sato F, Azechi T and Wachi H:
miR-29-mediated elastin down-regulation contributes to inorganic
phosphorus-induced osteoblastic differentiation in vascular smooth
muscle cells. Genes Cells. 20:1077–1087. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Islam F, Gopalan V, Vider J, Lu CT and Lam
AK: MiR-142-5p act as an oncogenenic microRNA in colorectal cancer:
Clinicopathological and functional insights. Exp Mol Pathol.
104:98–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Liu Z, Fang X and Yang H:
MiR-142-5p suppresses tumorigenesis by targeting PIK3CA in
non-small cell lung cancer. Cell Physiol Biochem. 43:2505–2515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwanhian W, Lenze D, Alles J, Motsch N,
Barth S, Döll C, Imig J, Hummel M, Tinguely M, Trivedi P, et al:
microRNA-142 is mutated in about 20% of diffuse large B-cell
lymphoma. Cancer Med. 1:141–155. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Swärd K, Stenkula KG, Rippe C, Alajbegovic
A, Gomez MF and Albinsson S: Emerging roles of the myocardin family
of proteins in lipid and glucose metabolism. J Physiol.
594:4741–4752. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hampl V, Martin C, Aigner A, Hoebel S,
Singer S, Frank N, Sarikas A, Ebert O, Prywes R, Gudermann T and
Muehlich S: Depletion of the transcriptional coactivators
megakaryoblastic leukemia 1 and 2 abolishes hepatocellular
carcinoma xenograft growth by inducing oncogene-induced senescence.
EMBO Mol Med. 5:1367–1387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pipes GC, Creemers EE and Olson EN: The
myocardin family of transcriptional coactivators: Versatile
regulators of cell growth, migration and myogenesis. Genes Dev.
20:1545–1556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith MC, Hudson CA, Kimura TE, White SJ,
Sala-Newby GB, Newby AC and Bond M: Divergent regulation of actin
dynamics and megakaryoblastic leukemia-1 and −2 (Mkl1/2) by cAMP in
endothelial and smooth muscle cells. Sci Rep. 7:36812017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goikuria H, Freijo MDM, Vega Manrigue R,
Sastre M, Elizagaray E, Lorenzo A, Vandenbroeck K and Alloza I:
Characterization of carotid smooth muscle cells during phenotypic
transition. Cells. 7:E232018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seo KW, Lee SJ, Ye BH, Kim YW, Bae SS and
Kim CD: Mechanical stretch enhances the expression and activity of
osteopointin and MMP2 via the Akt1/AP-1 pathways in VSMC. J Mol
Cell Cardiol. 85:13–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Volcik KA, Campbell S, Chambless LE,
Coresh J, Folsom AR, Mosley TH, Ni HY, Wagenknecht LE, Wasserman BA
and Boerwinkle E: MMP2 genetic variation is associated with
measures of fibrous cap thickness: The atherosclerosis risk in
communities carotid MRI study. Atherosclerosis. 210:188–193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim J and Ko J: Human sLZIP promotes
atherosclerosis via MMP-9 transcription and vascular smooth muscle
cell migration. FASEB J. 28:5010–5021. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eilenberg W, Stojkovic S, Kaider A,
Kozakowski N, Domenig CM, Burghuber C, Nanobachvili J, Huber K,
Klinger M, Neumayer C, et al: NGAL and MMP-9/NGAL as biomarkers of
plaque vulnerability and targets of statins in patiensts with
carotid atherosclerosis. Clin Chem Lab Med. 56:147–156. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma L and Zhang L, Wang B, Wei J, Liu J and
Zhang L: Berberine inhibts Chlamydia pneumoniae infection-induced
vascular smooth muscle cell migration through downregulating MMP3
and MMP9 via PI3K. Eur J Pharmacol. 755:102–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gan J, Li P, Wang Z, Chen J, Liang X, Liu
M, Xie W, Yin R and Huang F: Posuvastatin suppresses
platelet-derived growth factor-BB-induced vascular smooth muscle
cell proliferation and migration via the MAPK signaling pathway.
Exp Ther Med. 6:899–903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Byun HJ, Darvin P, Kang DY, Sp N, Joung
YH, Park JH, Kim SJ and Yang YM: Silibinin downregulates MMP2
expression via Jak2/STAT3 pathway and inhibits the migration and
invasive potential in MDA-MB-231 cells. Oncol Rep. 37:3270–3278.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhen Y, Liu J, Huang Y, Wang Y, Li W and
Wu J: miR-133b inhibits cell growth, migration, and invasion by
targeting MMP9 in non-small cell lung cancer. Oncol Rep.
25:1109–1116. 2017. View Article : Google Scholar
|
|
44
|
Zhang C, Wang L, Chen J, Liang J, Xu Y, Li
Z, Chen F and Du D: Knockdown of Diaph1 expression inhibits
migration and decreases the expression of MMP2 and MMP9 in human
glioma cells. Biomed Pharmacother. 96:596–602. 2017. View Article : Google Scholar : PubMed/NCBI
|





















