Introduction
The retinal pigment epithelium (RPE) is a polarized,
monolayer of pigmented cells that forms the outer retinal layer,
and maintains the integrity of the photoreceptors, primarily by
phagocytosing and recycling the retinal photoreceptor outer
segments (1). RPE cells are a
major source of proinflammatory cytokines, including interleukin
(IL)-6, and chemokines, such as monocyte chemotactic protein
(MCP)-1 and IL-8 (1). RPE cells
also secrete regulated on the activation of normal T-cell expressed
and secreted (RANTES) and interferon (IFN)-γ induced protein
(IP)-10 kDa (IP-10) (1–3). Furthermore, these cytokines and
chemokines secreted by RPE cells play important roles in the
activation of other immune cells under inflammatory conditions of
the posterior segment of the eye (1).
Tumor necrosis factor-α (TNF-α) is an inflammatory
cytokine that contributes to the progression of non-infectious
uveitis, and it has been shown that blocking TNF-α is effective for
treating refractory uveitis (4–8).
Furthermore, TNF-α receptors activate the NF-κB signaling pathway
(9). NF-κB, a member of a family
of ubiquitously expressed proteins, is usually found in an inactive
state in the cytoplasm, except during immune and inflammatory
responses (9,10). The NF-κB family includes REL
proto-oncogene (Rel)A, RelB, c-Rel, p50/p105 and p52/p100, all of
which form homo- or heterodimers with each other (11). Inhibitors of NF-κB (IκB) proteins
are phosphorylated and are degraded by proteasomes (12). Moreover, released NF-κB dimers
translocate into the nucleus and bind to κB sites in the promoter
and enhancer regions of targeted genes of various inflammatory
cytokines and chemokines, including IL-1, IL-2, IL-6, TNF and
macrophage inflammatory protein-1/2, and adhesion molecules, such
as intercellular adhesion molecule-1 (ICAM-1) (11,13).
Dehydroxymethylepoxyquinomicin (DHMEQ) is a low
molecular weight inhibitor of the NF-κB signaling pathway, and its
structure is related to that of epoxyquinomicin C, which is an
antibiotic (14,15). DHMEQ suppresses the TNF-α-induced
nuclear translocation of NF-κB, but it does not prevent the
phosphorylation and degradation of IκB (16). A previous study also revealed that
DHMEQ binds directly to the Rel-family proteins to prevent their
DNA-binding activity (17).
Furthermore, it has been revealed that DHMEQ is able to suppress
inflammation and the progression of cancer in animal models without
obvious adverse effects (18).
The cells of the human RPE cell line, ARPE-19, are
frequently used in in vitro studies to investigate the
mechanisms involved in posterior segment inflammatory disorders,
including uveitis and age-related macular degeneration (19–21).
Therefore, the aim of the present study was to determine whether
DHMEQ has inhibitory effects on the expression of ICAM-1 in
TNF-α-stimulated ARPE-19 cells. In addition, the present study
examined whether DHMEQ can affect the production of
TNF-α-stimulated ARPE-19 cells and the expression of NF-κB
related-genes in TNF-α-stimulated ARPE-19 cells treated with
DHMEQ.
Materials and methods
Materials
DHMEQ was synthesized by Umezawa and Chaicharoenpong
(16), and for the present study
it was dissolved in 100% DMSO at a concentration of 10 mg/ml and
stored at −30°C (14,16). Before use in cell cultures, DHMEQ
was diluted with the culture medium (DMEM/F-12; Invitrogen; Thermo
Fisher Scientific, Inc.) to a final concentration of ≤0.1%.
Dexamethasone was purchased from Sigma-Aldrich (Merck KGaA).
Cell cultures
ARPE-19 cells were purchased from the American Type
Culture Collection and maintained in DMEM/F-12 supplemented with
10% FBS, 100 U/ml penicillin and 0.1 mg/ml streptomycin (all from
Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2 in air. After reaching confluency, ARPE-19 cells
were detached with a trypsin-EDTA solution (0.05%) (Thermo Fisher
Scientific, Inc.) and plated for subcultures. Cells were passaged
every 4–6 days, and those used in each experiment were confluent
and exhibited no visible pigmentation. Moreover, cells were
maintained for 3 weeks before the experimental procedures and were
used at passages 4–6.
Cell viability determined by MTT
assay
The effects of various concentrations of DHMEQ on
ARPE-19 cells were evaluated by MTT. Cells were grown at 37°C in
96-well plates at a density of 2×104 cells/well for 24
h. Upon confluency, the medium was replaced with a serum-free
medium, and ARPE-19 cells were cultured at 37°C for 24 h with 0.1,
1.0, 5.0, 10.0, 50.0 or 100.0 µg/ml DHMEQ or without DHMEQ at 37°C
for 24 h. After 24 h, the assay was performed by adding 10 µl MTT
solution to the wells (Biotium, Inc.). After incubation for 4 h at
37°C, 200 µl DMSO was added to the cells. After incubation at room
temperature for 5 min, the optical density (OD) at 570 nm (signal
absorbance) and 630 nm (background absorbance) was measured using a
microplate reader, and the normalized absorbance values (OD at 570
nm and OD at 630) were determined.
Flow cytometric analyses
ARPE-19 cells were seeded in 6-well plates at
2×105 cells/well and cultured for 24 h. Upon confluency,
the medium was replaced with serum-free medium, and cells were or
were not exposed to 20 ng/ml TNF-α (R&D Systems, Inc.) with
DHMEQ (1.0, 10.0 and 100.0 µg/ml) or without DHMEQ at 37°C for 24
h. After exposure, cells were washed with phosphate-buffered saline
(PBS, pH 7.4), detached by trypsin-EDTA (0.05%) and suspended in
PBS. For staining of prepared cells, Annexin V-FITC solution (5 µl;
Nacalai Tesque, Inc.) was added to 100 µl of cell suspension
(1×106 cells), then <1% propidium iodide (PI)
solution (5 µl; Nacalai Tesque, Inc.) was added to the cell
suspension, according to the manufacturer's instructions (cat. no.
15342; Nacalai Tesque, Inc.). The cells were incubated at room
temperature for 15 min and analyzed by flow cytometry (FACSCalibur)
using CellQuest Pro software version 6.0 (both BD Biosciences).
ARPE-19 cells were seeded in 6-well plates at
2×105 cells/well and cultured for 24 h. Upon confluency,
the medium was replaced with serum-free medium, and cells were or
were not exposed to 20 ng/ml TNF-α (R&D Systems, Inc.) with
DMSO (0.1%) or DHMEQ (1.0 or 10.0 µg/ml) at 37°C for 24 h. After
exposure, cells were washed with PBS (pH 7.4), detached by
trypsin-EDTA (0.05%) and suspended in PBS. The prepared ARPE-19
cells were incubated with phycoerythrin-conjugated monoclonal
antibody to ICAM-1 (1:100; cat. no. 555511; BD Biosciences) at 4°C
for 20 min and analyzed by flow cytometry (FACSCalibur) using
CellQuest Pro software version 6.0 (both BD Biosciences).
Chemokine assay in culture
supernatants
ARPE-19 cells were seeded in 6-well plates at a
density of 2×105 cells/well and cultured for 24 h. Upon
confluency (80-90% confluency), the medium was replaced with
serum-free medium and cells were exposed to 20 ng/ml TNF-α and DMSO
(0.1%), DHMEQ (1.0 µg/ml=4.0 µM, 10 µg/ml=40 µM) or dexamethasone
(40 µM) at 37°C for 24 h. After exposure, the supernatant was
collected from each well, and the levels of IL-8 and MCP-1 in the
supernatant were determined by Quantikine® Colorimetric
Sandwich ELISA kits (R&D Systems, Inc.; IL-8. cat. no. D8000C;
MCP-1, cat. no. DCP00).
In another experiment, ARPE-19 cells were exposed to
20 ng/ml TNF-α and DMSO (0.1%) or DHMEQ (10 µg/ml) at 37°C for 6,
12 and 24 h. After exposure, the supernatant was collected from
each well and the levels of IL-8 and MCP-1 in the supernatant was
determined by the Quantikine® ELISA kits (R&D
Systems, Inc.) as described above.
NF-κB-associated gene expression level
assay
ARPE-19 cells were seeded in 6-well plates at a
density of 2×105 cells/well and cultured for 24 h. Upon
confluency, the medium was replaced with serum-free medium and
cells were exposed to 20 ng/ml TNF-α in the absence or presence of
DHMEQ (10 µg/ml) at 37°C for 24 h. After exposure, cells were
washed with PBS, detached by trypsin-EDTA (0.05%) and suspended in
PBS. Total RNA from the prepared ARPE-19 cells was extracted with
ISOGEN (Nippon Gene Co., Ltd.) according to the manufacturer's
instructions. Briefly, 10 µg total RNA from each sample was
reverse-transcribed using the High Capacity RNA-to-cDNA kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.; reverse
transcription conditions were as follows: Initial incubation at
37°C for 60 min and 95°C for 5 min), then loaded onto Human NFκB
Pathway TaqMan® Array plates (cat. no. 4414095; Applied
Biosystems; Thermo Fisher Scientific, Inc.) for profiling of
NF-κB-associated 92 genes expression levels. PCR was performed on a
QuantStudio™ 12K Flex Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Raw cycle threshold (Cq) values were calculated with
SDS software 1.2.3. (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The sequences of the forward and reverse primers are not
commercially available. Data were analyzed according to the
comparative Cq method, and the global median normalization method
was used (22). The
2−ΔΔCq method was performed to calculate the expression
level of the fold change (23).
The fold change in ARPE-19 cells exposed to 20 ng/ml TNF-α in the
absence or presence of DHMEQ was calculated for each gene; genes
with a 2-fold increase in this ratio were defined arbitrarily as
upregulated in cells exposed to DHMEQ, whereas those with a 2-fold
decrease were defined as downregulated genes.
Quantitative PCR analysis
In order to validate the expression levels of genes
[lymphotoxin β receptor (LTBR), MCP-1, and Toll-like receptor 4
(TLR4)], which were either upregulated or downregulated in ARPE-19
cells treated with DHMEQ, quantitative PCR was carried out in
duplicate using the TaqMan® Universal PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) on a
QuantStudio™ 12K Flex Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.), following the manufacturer's
protocol. ARPE-19 cells were seeded in 6-well plates at a density
of 2×105 cells/well and cultured for 24 h. Upon
confluency, the medium was replaced with serum-free medium and
cells were exposed to 20 ng/ml TNF-α exposed to DMSO (0.1%) or
DHMEQ (10 µg/ml) at 37°C for 24 h. After exposure, cells were
washed with PBS, detached by trypsin-EDTA (0.05%) and suspended in
PBS. Total RNA from the prepared ARPE-19 cells was extracted with
ISOGEN (Nippon Gene Co., Ltd.) as described above and
reverse-transcribed using the High Capacity RNA-to-cDNA kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) PCR conditions
were as follows: Initial incubation at 50°C for 2 min and 95°C for
10 min, followed by 40 cycles two-step cycling (denaturing at 95°C
for 15 sec, annealing/extension at 60°C for 60 sec). The TaqMan
primers/probes pairs were obtained from Applied Biosystems (Thermo
Fisher Scientific, Inc.) using inventoried TaqMan gene expression
assays [LTBR assay ID, Hs01101194_m1; MCP-1 assay ID,
Hs00234140_m1; TLR4 assay ID, Hs00152939_m1]. For an endogenous
control mRNA, the β-actin (assay ID, Hs99999903_m1, Thermo Fisher
Scientific, Inc.) was used for data normalization of the mRNA
expression levels.
Statistical analyses
Data are presented as the mean ± SD. Statistical
significance was evaluated with unpaired t-tests. To compare data
among ≥3 groups, one-way ANOVA using the Bonferroni's multiple
comparison tests was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of DHMEQ on the viability of
ARPE-19 cells
Confluent ARPE-19 cells were exposed to various
concentrations of DHMEQ, and it was revealed that DHMEQ at
concentrations ≤10 µg/ml did not have toxic effects on cells.
However, DHMEQ at concentrations of 50 and 100 µg/ml significantly
inhibited the viability of ARPE-19 cells compared with the
viability of cells cultured without DHMEQ (Fig. 1). Therefore, these results
indicated that higher concentrations of DHMEQ, such as 50 and 100
µg/ml, reduce the viability of ARPE-19 cells.
Effect of DHMEQ on the induction of
apoptosis and necrosis of ARPE-19 cells
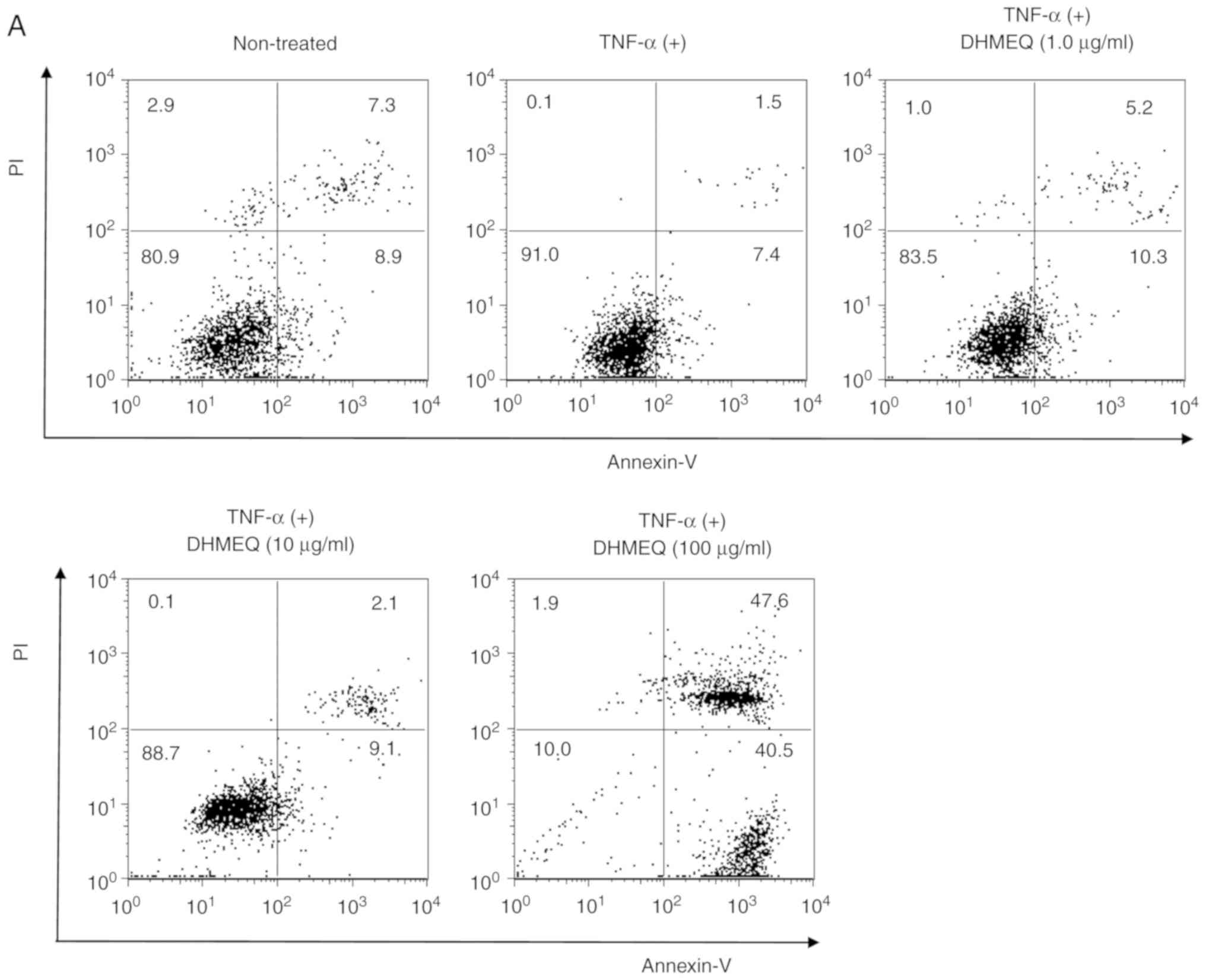
To determine whether DHMEQ induces apoptosis or
necrosis in ARPE-19 cells, Annexin-V and/or PI-positive cells were
analyzed by flow cytometry. It was demonstrated that concentrations
of 1.0 and 10 µg/ml DHMEQ did not alter the percentage of apoptotic
cells (Annexin-V-positive and PI-negative; Fig. 2A and B). However, a dose of 100
µg/ml DHMEQ significantly increased the number of apoptotic cells
compared with cells treated with TNF-α (20 ng/ml) without DHMEQ
(Fig. 2A and B). In addition,
concentrations of 100 µg/ml DHMEQ significantly increased the
number of necrotic cells compared with cells treated with TNF-α (20
ng/ml) without DHMEQ (Annexin-V-positive and PI-positive; Fig. 2A and B). Collectively, the results
indicated that a high concentration of DHMEQ, such as 100 µg/ml,
promoted the induction of apoptosis and necrosis of ARPE-19 cells.
Based on these findings, a concentration of DHMEQ <100 µg/ml was
used in subsequent experiments.
Suppression of ICAM-1 in ARPE-19 cells
by DHMEQ
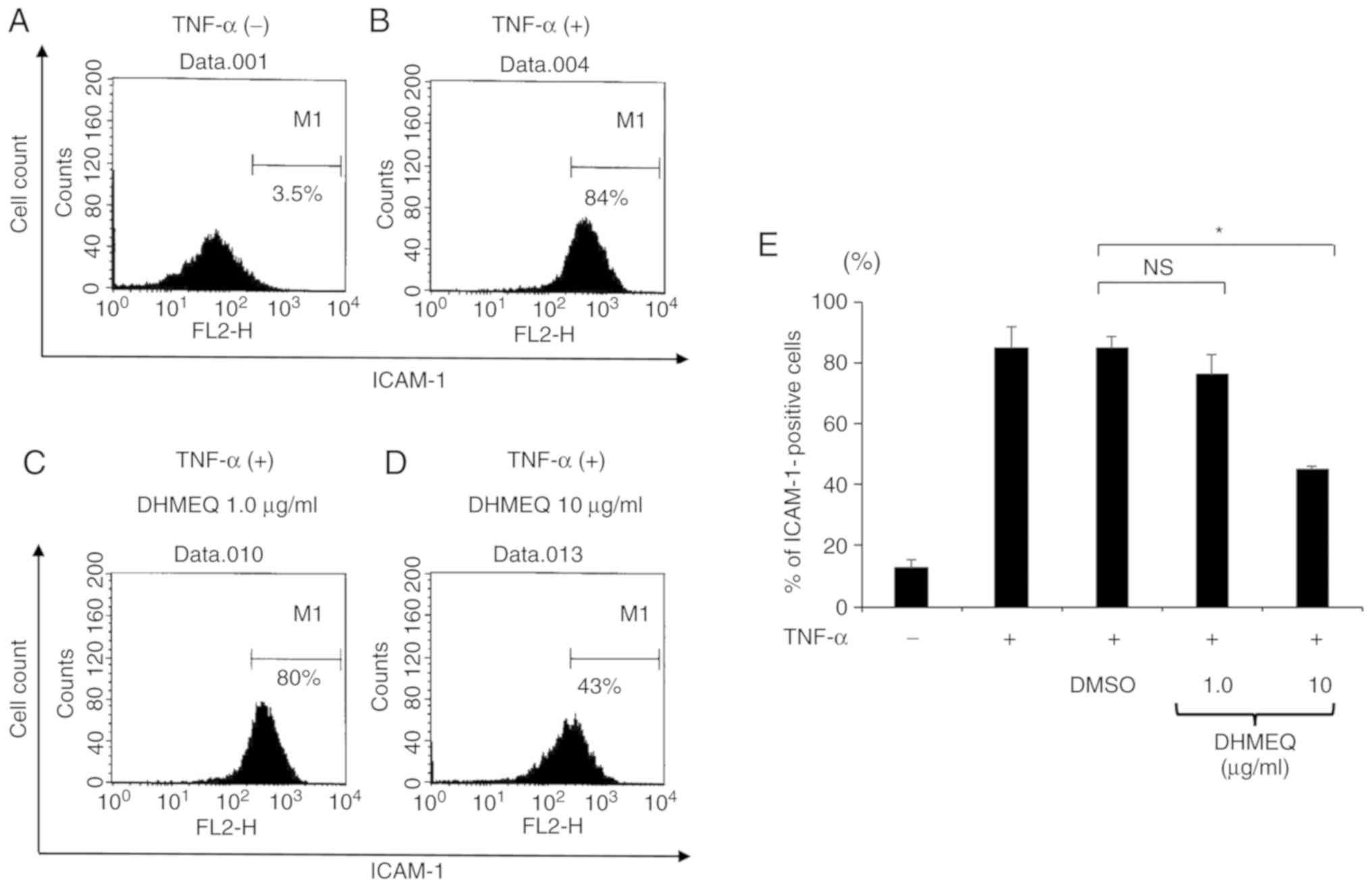
Previous studies have revealed that TNF-α can
enhance the protein expression of ICAM-1 synthesized by ARPE-19
cells (24). To assess these
findings, the present study stimulated ARPE-19 cells with TNF-α (20
ng/ml) for 24 h and evaluated the protein expression of ICAM-1 by
flow cytometry. The results revealed that the expression of ICAM-1
was increased 7-fold in cells stimulated by TNF-α compared with
cells cultured without TNF-α (Fig. 3A
and B).
The effects of DHMEQ on the protein expression level
of ICAM-1 were also examined in ARPE-19 cells stimulated with
TNF-α. It was revealed that DHMEQ (10 µg/ml) significantly reduced
the expression of ICAM-1 in cells by ~50% compared with cells
treated with DMSO (Fig. 3C-E).
Thus, it was speculated that DHMEQ may be able to decrease
TNF-α-induced ICAM-1 expression in ARPE-19 cells.
Suppressive effect of DHMEQ on
chemokine production in TNF-α-stimulated ARPE-19 cells
MCP-1 and IL-8 have been revealed to be the major
chemokines produced by TNF-α-stimulated ARPE-19 cells (25), and dexamethasone has been reported
to have anti-inflammatory effects on ARPE-19 cells (20). Therefore, the present study
investigated whether DHMEQ is able to decrease the protein
expression levels of MCP-1 and IL-8 in ARPE-19 cells stimulated
with TNF-α. Moreover, the anti-inflammatory effect of DHMEQ was
compared with that of dexamethasone in cells. It was demonstrated
that the production of IL-8 and MCP-1 from cells treated with DHMEQ
(40 µM=10 µg/ml) was significantly decreased compared with ARPE-19
cells treated with DMSO. In addition, there was a significant
difference in the production of IL-8 and MCP-1 between cells
treated with DHMEQ (40 µM) and those treated with dexamethasone (40
µM; Fig. 4A and B). These findings
revealed that DHMEQ has strong suppressive effects on the
production of MCP-1 and IL-8 by ARPE-19 cells compared with
dexamethasone. Furthermore, the results indicated that DHMEQ
significantly reduced the protein expression levels of MCP-1 and
IL-8 at 6, 12 and 24 h (Fig. 4C and
D). Therefore, DHMEQ may be able to decrease the TNF-α-induced
chemokine production in ARPE-19 cells at several time-points after
co-culturing.
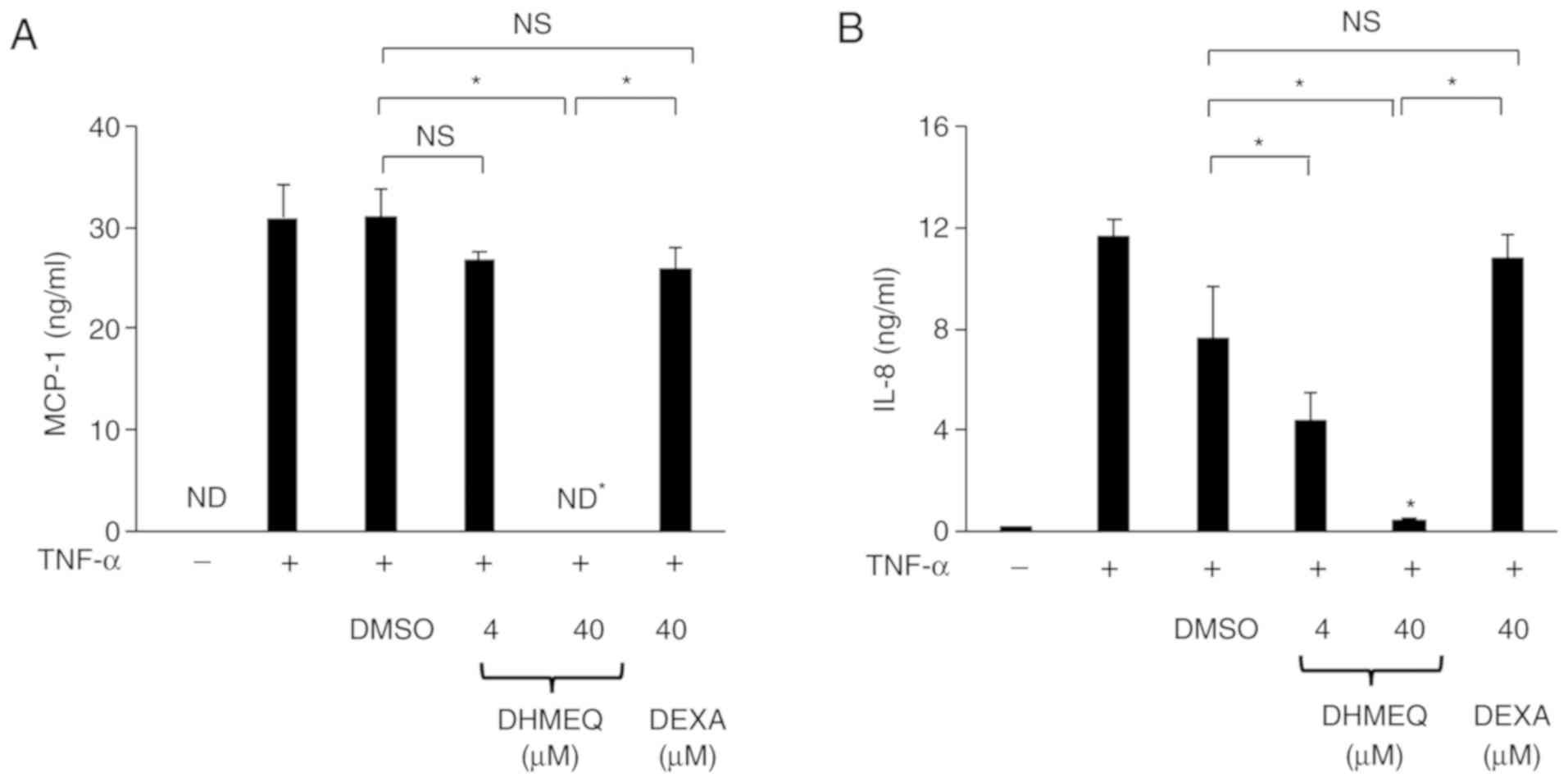 | Figure 4.Effect of DHMEQ and DEXA on the
levels of MCP-1 and IL-8 in ARPE-19 cells stimulated by TNF-α.
Cells were cultured with TNF-α (20 ng/ml) in the presence of DMSO
(0.1%), DHMEQ (1 µg/ml=4 µM, 10 µg/ml=40 µM) or DEXA (40 µM) for 24
h. The effect of DHMEQ on levels of (A) MCP-1 and (B) IL-8 in the
culture supernatants of cells stimulated with TNF-α. ARPE-19 cells
were cultured with TNF-α (20 ng/ml) in the presence of DMSO (0.1%)
or DHMEQ (10 µg/ml) for 6, 12 and 24 h. The effect of DHMEQ on
levels of (C) MCP-1 and (D) IL-8 in the culture supernatants of
cells stimulated with TNF-α, as measured by ELISA. Data are
presented as the mean ± SD, n=3. *P<0.05. ND, not detected;
DEXA, dexamethasone; NS, not significant; TNF-α, tumor necrosis
factor-α; MCP-1, monocyte chemoattractant protein-1; IL,
interleukin; DHMEQ, dehydroxymethylepoxyquinomicin. |
Suppression of NF-κB-related
inflammatory gene expression levels of ARPE-19 cells by DHMEQ
To determine the alterations of the expression
levels of NF-κB-associated inflammatory genes in ARPE-19 cells
exposed to DHMEQ, the present study compared RNA isolated from
TNF-α-stimulated cells in the absence or presence of DHMEQ, using
the Human NF-κB Pathway TaqMan® Array Plates that
analyze 92 NF-κB-associated inflammatory genes. Moreover, summaries
of the differentially expressed genes between the two cell
populations are presented in Table
I. A total of 19 genes were revealed to be upregulated and 25
genes were revealed to be downregulated in cells exposed to DHMEQ
compared with those in the absence of DHMEQ. The differentially
expressed genes are presented in Tables I and II. The gene expression levels of
cytokines and chemokines, including MCP-1, ICAM-1, IL-6 and IL-8,
and TLR2, TLR3 and TLR4, were downregulated in ARPE-19 cells
treated with DHMEQ (Table I). In
addition, DHMEQ suppressed TNF superfamily member 15 (TNFSF15) and
TNF-α-induced protein 3 (TNFAIP3; Table I). However, it was revealed that
DHMEQ increased the expression levels of numerous genes associated
with the NF-κB signaling pathway, including prostaglandin E
synthase (PTGES), mitogen-activated protein kinase 14 (MAP3K14),
LTBR and TNFRSF1A associated via death domain (TRADD).
 | Table I.Summary of downregulated genes in
ARPE-19 cells stimulated with TNF-α in the presence of DHMEQ. |
Table I.
Summary of downregulated genes in
ARPE-19 cells stimulated with TNF-α in the presence of DHMEQ.
| Target gene | Probe ID | Fold change |
|---|
| BIRC5 | Hs00977611_g1 | 0.040 |
| TLR2 | Hs00152932_m1 | 0.053 |
| TRAF5 | Hs00182979_m1 | 0.082 |
| TNFSF15 | Hs00353710_s1 | 0.088 |
| BCL10 | Hs00184839_m1 | 0.127 |
| CHUK | Hs00989507_m1 | 0.130 |
| CSF2 | Hs00171266_m1 | 0.164 |
| BCL2 | Hs00608023_m1 | 0.228 |
| HPRT1 | Hs99999909_m1 | 0.236 |
| MCP-1 | Hs00234140_m1 | 0.238 |
| FADD | Hs00538709_m1 | 0.249 |
| TLR4 | Hs00152939_m1 | 0.274 |
| MALT1 | Hs00198984_m1 | 0.275 |
| EDARADD | Hs00369830_m1 | 0.281 |
| TNFAIP3 | Hs00234713_m1 | 0.388 |
| ICAM1 | Hs00164932_m1 | 0.389 |
| IRAK1BP1 | Hs00418138_m1 | 0.396 |
| CD83 | Hs00188486_m1 | 0.414 |
| TLR3 | Hs00152933_m1 | 0.416 |
| REL | Hs00968436_m1 | 0.437 |
| CSF1 | Hs00174164_m1 | 0.456 |
| CXCL1 | Hs00236937_m1 | 0.456 |
| ZNF675 | Hs00603247_m1 | 0.456 |
| IL6 | Hs00174131_m1 | 0.490 |
| RIPK1 | Hs00169407_m1 | 0.490 |
 | Table II.Summary of upregulated genes in
ARPE-19 cells stimulated with TNF-α in the presence of DHMEQ. |
Table II.
Summary of upregulated genes in
ARPE-19 cells stimulated with TNF-α in the presence of DHMEQ.
| Target gene | Probe ID | Fold change |
|---|
| PTGES | Hs00610420_m1 | 18.850 |
| MAP3K14 | Hs00177695_m1 | 6.446 |
| LTBR | Hs00158922_m1 | 5.488 |
| TRADD | Hs00182558_m1 | 5.024 |
| BCL3 | Hs00180403_m1 | 4.300 |
| NKIRAS2 | Hs00383387_m1 | 3.855 |
| TNFRSF10A | Hs00269492_m1 | 3.773 |
| IKBKG | Hs00415849_m1 | 3.609 |
| MAP3K7IP1 | Hs00196143_m1 | 3.600 |
| MYC | Hs00153408_m1 | 3.194 |
| ZFP36 | Hs00185658_m1 | 3.091 |
| IRAK1 | Hs00155570_m1 | 3.065 |
| ENPP2 | Hs00196470_m1 | 2.653 |
| TRAF1 | Hs00194638_m1 | 2.614 |
| CARD10 | Hs00367225_m1 | 2.574 |
| NFKBIB | Hs00182115_m1 | 2.570 |
| IRAK2 | Hs00176394_m1 | 2.569 |
| RELA | Hs00153294_m1 | 2.446 |
| NFKB2 | Hs00174517_m1 | 2.341 |
In addition, representative genes, LTBR, MCP-1 and
TLR4, which were identified by NF-κB Pathway Array, were assessed
by quantitative PCR analysis. Although the fold changes were not
exactly the same between the two methods, the gene expression level
of LTBR was significantly increased in the presence of DHMEQ
compared to that in the presence of DMSO, whereas the gene
expression levels of MCP-1 and TLR4 were significantly decreased in
the presence of DHMEQ compared to that in the presence of DMSO
(Fig. 5).
Discussion
The present results indicated that DHMEQ
significantly decreased the protein expression of TNF-α-induced
ICAM-1 in ARPE-19 cells, and also decreased the production of IL-8
and MCP-1 by cells stimulated with TNF-α. In addition, it was
determined that DHMEQ at higher concentrations had increased
anti-inflammatory effects on ARPE-19 cells compared with
dexamethasone. The results also indicated that exposure to DHMEQ
decreased the expression level of ICAM-1 in ARPE-19 cells.
Moreover, the present results are consistent with those from a
previous study, which reported that DHMEQ decreases the expression
level of ICAM-1 in the retina of diabetic mice and that it reduces
the number of retinal-adherent leukocytes (26). Furthermore, the expression of
ICAM-1 in RPE cells has been revealed to be elevated under
inflammatory conditions, leading to the enhancement of
leukocyte-RPE cell interactions (27,28).
Previous studies have also identified increased ICAM-1 expression
levels in ocular tissues of patients with uveitis and revealed that
antibody-based blockage of ICAM-1 led to a suppression of
experimental autoimmune uveoretinitis (29–31).
Collectively, both the present findings and previous results
indicated that DHMEQ may be a potential anti-inflammatory compound
for RPE cells due to its ability to reduce the expression of
ICAM-1.
Elner et al (25) revealed that RPE cells produce
several chemokines, including IL-8 and MCP-1. The present results
revealed that DHMEQ inhibited the production of IL-8 and MCP-1 in
TNF-α-stimulated ARPE-19 cells, although the effect of treatment
with IL-8 on ARPE-19 cells in the presence or absence of DHMEQ was
not examined in the present study. It has been demonstrated that
MCP-1, IL-8 and RANTES are elevated in the ocular tissues of
experimental autoimmune uveitis, which suggests that these
upregulated chemokines are potent chemoattractants in the
pathogenesis of uveitis (32–34).
Wakamatsu et al (35)
revealed that DHMEQ had a positive therapeutic effect on
established murine arthritis, and Iwata et al (36) revealed that DHMEQ was able to
ameliorate experimental autoimmune uveoretinitis. Our previous
study revealed that DHMEQ has anti-inflammatory effects on ocular
inflammation induced by lipopolysaccharide via the inhibition of
TNF-α and IL-6 expression levels in the aqueous humor, which
indicated that DHMEQ may be a potential candidate to treat
intraocular inflammatory diseases (37).
Local and systemic corticosteroids have been used to
control ocular inflammation in patients with uveitis; however,
long-term corticosteroid treatment can lead to adverse local and
systemic side effects (38). The
present results indicated that TNF-α-stimulated ARPE-19 cells were
resistant to dexamethasone in relation to the protein expression
levels of IL-8 and MCP-1, but DHMEQ significantly decreased the
production of IL-8 and MCP-1 of TNF-α-stimulated cells treated with
DHMEQ. Furthermore, previous studies have revealed that TNF
signaling can suppress the action of the glucocorticoid receptor by
interfering with the transactivation function of glucocorticoid
(GC) (39), which contributes to
tissue resistance to GCs in several pathologic inflammatory states
(39). These findings indicate the
possibility that DHMEQ may have anti-inflammatory properties, even
in inflammatory conditions with glucocorticoid resistance.
In the present study, NF-κB-associated gene array
analysis identified that the gene expression levels of cytokines
and chemokines, including MCP-1, ICAM-1, IL-6, TNFSF15 and TNFAIP3,
and TLR2, TLR3 and TLR4 were downregulated in ARPE-19 cells treated
with DHMEQ, which was further demonstrated by quantitative PCR
analysis. Moreover, it was revealed that DHMEQ increased the
expression levels of several genes related to the NF-κB signaling
pathway, including PTGES, MAP3K14, LTBR and TRADD. However, the
present study did not examine the protein expression levels of the
gene products either upregulated or downregulated in ARPE-19 cells
by DHMEQ. In addition, the translocation of p65-NF-κB into the
nucleus in the presence of DHMEQ in TNF-α-stimulated cells was not
investigated. Therefore, future studies are required to assess
post-transcriptional regulation by DHMEQ with western blotting and
to examine the translocation of p65-NF-κB in the presence of DHMEQ
with electrophoretic mobility shift assay.
The present results demonstrated that 50 and 100
µg/ml DHMEQ had severe cytotoxic effects on cultured ARPE-19 cells,
and that high concentrations of DHMEQ (100 µg/ml) induced apoptosis
and necrosis in TNF-α-stimulated cells. NF-κB is known to play
important roles in protecting cells from apoptosis (40,41).
Furthermore, previous studies have revealed that DHMEQ is able to
induce apoptosis of cancer cells (42,43).
Although the present study used an RPE cell line, it remains to be
determined whether high concentrations of DHMEQ have cytotoxic or
apoptotic effects on primary cultured RPE cells, and healthy and
inflamed RPE cells in vivo. Thus, the relationship between
the anti-inflammatory potential of DHMEQ and the induction of
apoptosis by DHMEQ in healthy or inflamed RPE cells requires
further examination.
In conclusion, the present results indicated that
DHMEQ may have an anti-inflammatory effect on TNF-α-stimulated
ARPE-19 cells. However, it is not fully understood whether DHMEQ
has a suppressive effect on the expression of ICAM-1 and chemokine
production in primary cultured human RPE cells and in vivo
RPE monolayers, thus further studies are required to assess the
anti-inflammatory effects and safety of DHMEQ on human RPE
cells.
Acknowledgements
The authors would like to thank Ms Mirai Kano
(Department of Ophthalmology, Kyorin University, School of
Medicine) for technical assistance and Professor Emeritus Duco
Hamasaki (Bascom Palmer Eye Institute, University of Miami, Miami,
Florida, USA) for editing the manuscript.
Funding
The present study was supported by Grant-in-Aid for
Scientific Research (grant. no. 15K10901) from the Ministry of
Education, Culture, Sports, Science and Technology, Japan and
Research Grant from Kyorin University, Tokyo, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA, HK and YS performed the experiments. YA and HK
designed the experiments. AK contributed to the design of the
methodology. YA, HK, TW, AH and AAO analyzed the results. YA and HK
wrote the paper. KU prepared DHMEQ and analyzed the results. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Holtkamp GM, Kijlstra A, Peek R and de Vos
AF: Retinal pigment epithelium-immune system interactions: Cytokine
production and cytokine-induced changes. Prog Retin Eye Res.
20:29–48. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Momma Y, Nagineni CN, Chin MS, Srinivasan
K, Detrick B and Hooks JJ: Differential expression of chemokines by
human retinal pigment epithelial cells infected with
cytomegalovirus. Invest Ophthalmol Vis Sci. 44:2026–2033. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elner SG, Delmonte D, Bian ZM, Lukacs NW
and Elner VM: Differential expression of retinal pigment epithelium
(RPE) IP-10 and interleukin-8. Exp Eye Res. 83:374–379. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dick AD: Doyne lecture 2016: Intraocular
health and the many faces of inflammation. Eye (Lond). 31:87–96.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugita S, Kawazoe Y, Imai A, Yamada Y,
Horie S and Mochizuki M: Inhibition of Th17 differentiation by
anti-TNF-alpha therapy in uveitis patients with Behcet's disease.
Arthritis Res Ther. 14:R992012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okada AA, Goto H, Ohno S and Mochizuki M;
Ocular Behçet's Disease Research Group Of Japan, : Multicenter
study of infliximab for refractory uveoretinitis in Behcet disease.
Arch Ophthalmol. 130:592–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeuchi M, Kezuka T, Sugita S, Keino H,
Namba K, Kaburaki T, Maruyama K, Nakai K, Hijioka K, Shibuya E, et
al: Evaluation of the long-term efficacy and safety of infliximab
treatment for uveitis in Behcet's disease: A multicenter study.
Ophthalmology. 121:1877–1884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaffe GJ, Dick AD, Brezin AP, Nguyen QD,
Thorne JE, Kestelyn P, Barisani-Asenbauer T, Franco P, Heiligenhaus
A, Scales D, et al: Adalimumab in patients with active
noninfectious uveitis. N Engl J Med. 375:932–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brenner D, Blaser H and Mak TW: Regulation
of tumour necrosis factor signalling: Live or let die. Nat Rev
Immunol. 15:362–374. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayden MS and Ghosh S: NF-kB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamoto H, Cujec TP, Yamanaka H and
Kamatani N: Molecular aspects of rheumatoid arthritis: Role of
transcription factors. FEBS J. 275:4463–4470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Ding J, Yang J, Guo X and Zheng Y:
MicroRNA roles in the nuclear factor kappa B signaling pathway in
cancer. Front Immunol. 9:5462018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ariga A, Namekawa J, Matsumoto N, Inoue J
and Umezawa K: Inhibition of tumor necrosis factor-alpha-induced
nuclear translocation and activation of NF-kappa B by
dehydroxymethylepoxyquinomicin. J Biol Chem. 277:24625–24630. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Umezawa K: Inhibition of tumor growth by
NF-kappaB inhibitors. Cancer Sci. 97:990–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Umezawa K and Chaicharoenpong C: Molecular
design and biological activities of NF-kappaB inhibitors. Mol
Cells. 14:163–167. 2002.PubMed/NCBI
|
|
17
|
Yamamoto M, Horie R, Takeiri M, Kozawa I
and Umezawa K: Inactivation of NF-kappaB components by covalent
binding of (−)-dehydroxymethylepoxyquinomicin to specific cysteine
residues. J Med Chem. 51:5780–5788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin Y, Ukaji T, Koide N and Umezawa K:
Inhibition of late and early phases of cancer metastasis by the
NF-kB inhibitor DHMEQ derived from microbial bioactive metabolite
epoxyquinomicin: A review. Int J Mol Sci. 19:E7292018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juel HB, Faber C, Udsen MS, Folkersen L
and Nissen MH: Chemokine expression in retinal pigment epithelial
ARPE-19 cells in response to coculture with activated T cells.
Invest Ophthalmol Vis Sci. 53:8472–8480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zanon Cde F, Sonehara NM, Girol AP, Gil CD
and Oliani SM: Protective effects of the galectin-1 protein on in
vivo and in vitro models of ocular inflammation. Mol Vis.
21:1036–1050. 2015.PubMed/NCBI
|
|
21
|
Yang PM, Wu ZZ, Zhang YQ and Wung BS:
Lycopen inhibits ICAM-1 expression and NF-kB activation by
Nrf2-regulated cell redox state in human retinal pigment epithelial
cells. Life Sci. 155:94–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mestdagh P, Van Vlierberghe P, De Weer A,
Muth D, Westermann F, Speleman F and Vandesompele J: A novel and
universal method for microRNA RT-qPCR data normalization. Genome
Biol. 10:R642009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YH, Chen CL, Liang CM, Liang JB, Tai
MC, Chang YH, Lu DW and Chen JT: Silibinin inhibits ICAM-1
expression via regulation of N-linked and O-linked glycosylation in
ARPE-19 cells. Biomed Res Int. 2014:7013952014.PubMed/NCBI
|
|
25
|
Elner VM, Burnstine MA, Strieter RM,
Kunkel SL and Elner SG: Cell-associated human retinal pigment
epithelium interleukin-8 and monocyte chemotactic protein-1:
Immunochemical and in-situ hybridization analyses. Exp Eye Res.
65:781–789. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagai N, Izumi-Nagai K, Oike Y, Koto T,
Satofuka S, Ozawa Y, Yamashiro K, Inoue M, Tsubota K, Umezawa K and
Ishida S: Suppression of diabetes-induced retinal inflammation by
blocking the angiotensin II type 1 receptor or its downstream
nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci.
48:4342–4350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen JT, Liang JB, Chou CL, Chien MW, Shyu
RC, Chou PI and Lu DW: Glucosamine sulfate inhibits TNF-alpha and
IFN-gamma-induced production of ICAM-1 in human retinal pigment
epithelial cells in vitro. Invest Ophthalmol Vis Sci. 47:664–672.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JT, Chen PL, Chang YH, Chien MW, Chen
YH and Lu DW: Glucosamine sulfate inhibits leukocyte adhesion in
response to cytokine stimulation of retinal pigment epithelial
cells in vitro. Exp Eye Res. 83:1052–1062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Whitcup SM, Chan CC, Li Q and Nussenblatt
RB: Expression of cell adhesion molecules in posterior uveitis.
Arch Ophthalmol. 110:662–666. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Whitcup SM, DeBarge LR, Caspi RR, Harning
R, Nussenblatt RB and Chan CC: Monoclonal antibodies against ICAM-1
(CD54) and LFA-1 (CD11a/CD18) inhibit experimental autoimmune
uveitis. Clin Immunol Immunopathol. 67:143–150. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uchio E, Kijima M, Tanaka S and Ohno S:
Suppression of experimental uveitis with monoclonal antibodies to
ICAM-1 and LFA-1. Invest Ophthalmol Vis Sci. 35:2626–2631.
1994.PubMed/NCBI
|
|
32
|
Crane IJ, McKillop-Smith S, Wallace CA,
Lamont GR and Forrester JV: Expression of the chemokines
MIP-1alpha, MCP-1, and RANTES in experimental autoimmune uveitis.
Invest Ophthalmol Vis Sci. 42:1547–1552. 2001.PubMed/NCBI
|
|
33
|
Foxman EF, Zhang M, Hurst SD, Muchamuel T,
Shen D, Wawrousek EF, Chan CC and Gery I: Inflammatory mediators in
uveitis: Differential induction of cytokines and chemokines in Th1-
versus Th2-mediated ocular inflammation. J Immunol. 168:2483–2492.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Keino H, Takeuchi M, Kezuka T, Yamakawa N,
Tsukahara R and Usui M: Chemokine and chemokine receptor expression
during experimental autoimmune uveoretinitis in mice. Graefes Arch
Clin Exp Ophthalmol. 241:111–115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wakamatsu K, Nanki T, Miyasaka N, Umezawa
K and Kubota T: Effect of a small molecule inhibitor of nuclear
factor-kappaB nuclear translocation in a murine model of arthritis
and cultured human synovial cells. Arthritis Res Ther.
7:R1348–R1359. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwata D, Kitaichi N, Miyazaki A, Iwabuchi
K, Yoshida K, Namba K, Ozaki M, Ohno S, Umezawa K, Yamashita K, et
al: Amelioration of experimental autoimmune uveoretinitis with
nuclear factor-{kappa}B Inhibitor dehydroxy methyl epoxyquinomicin
in mice. Invest Ophthalmol Vis Sci. 51:2077–2084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ando Y, Keino H, Kudo A, Hirakata A, Okada
AA and Umezawa K: Anti-inflammatory effect of
dehydroxymethylepoxyquinomicin, a nuclear factor-kB inhibitor, on
endotoxin-induced uveitis in rats in vivo and in vitro. Ocul
Immunol Inflamm. 28:240–248. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gaudio PA: A review of evidence guiding
the use of corticosteroids in the treatment of intraocular
inflammation. Ocul Immunol Inflamm. 12:169–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Van Bogaert T, De Bosscher K and Libert C:
Crosstalk between TNF and glucocorticoid receptor signaling
pathways. Cytokine Growth Factor Rev. 21:275–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ukaji T and Umezawa K: Novel approaches to
target NF-kB and other signaling pathways in cancer stem cells. Adv
Biol Regul. 56:108–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Castro Barbosa ML, da Conceicao RA,
Fraga AGM, Camarinha BD, de Carvalho Silva GC, Lima AGF, Cardoso EA
and de Oliveira Freitas Lione V: NF-kB signaling pathway inhibitors
as anticancer drug candidates. Anticancer Agents Med Chem.
17:483–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyake A, Dewan MZ, Ishida T, Watanabe M,
Honda M, Sata T, Yamamoto N, Umezawa K, Watanabe T and Horie R:
Induction of apoptosis in Epstein-Barr virus-infected B-lymphocytes
by the NF-kappaB inhibitor DHMEQ. Microbes Infect. 10:748–756.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fukushima T, Kawaguchi M, Yorita K, Tanaka
H, Takeshima H, Umezawa K and Kataoka H: Antitumor effect of
dehydroxymethylepoxyquinomicin, a small molecule inhibitor of
nuclear factor-kB, on glioblastoma. Neuro Oncol. 14:19–28. 2012.
View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|