Introduction
Renal tumors are the second most common abdominal
tumor in infants and children. Different types of renal tumor have
distinct histological features, and Wilms tumor (WT) is the most
frequently occurring type, accounting for ~95% of pediatric renal
tumors in the United States (1). A
total of seven in one million American children <15 years of age
are affected by WT annually (2). In
the past few decades, the prognosis of patients with WT has
significantly improved. According to a statistic published in 2018,
the overall 5-year survival rate is >90% (3). Nevertheless, the main therapeutic
regimen for WT is still surgery combined with radiotherapy and
chemotherapy (4). However, children
who receive high-intensity radiotherapy and chemotherapy are more
likely to experience complications, including renal impairment,
cardiotoxicity, hepatotoxicity, delayed growth, infertility and
secondary malignancies (5–9). Surgical resection also has a number of
limitations, such as bilateral tumors, tumor in a solitary kidney
and extensive pulmonary metastases (10). Therefore, understanding the
molecular mechanisms underlying the progression of WT and
developing molecular-targeted therapies are required.
Only 2% of the human genome comprises protein-coding
genes (11), while the majority is
transcribed into non-coding RNAs, including long non-coding RNAs
(lncRNAs) and microRNAs (miRNAs/miRs) (12). lncRNAs range in length from 200
nucleotides to 100 kb (13). An
increasing number of studies have reported that lncRNAs are
involved in the biological behaviors of tumor cells, including
proliferation, apoptosis, the cell cycle, invasion and migration
(14–18). According to the theory of
competitive endogenous RNA (ceRNA), lncRNAs act as a sponge for
miRNAs, weakening the effects of miRNAs on mRNAs (19). Several other studies have confirmed
this hypothesis and constructed ceRNA networks for various types of
cancer (20–22). However, there is currently limited
research on lncRNA-mediated ceRNAs and the role of lncRNAs in the
prognosis of patients with WT (23–26).
Therefore, the construction of a ceRNA network could aid in the
discovery of novel molecular targets and the development of
therapeutic strategies to improve the prognosis of patients with
WT.
The aim of the present study was to construct a
ceRNA network to identify potential lncRNAs involved in WT. The
expression profiles of lncRNAs, miRNAs and mRNAs were obtained from
the Therapeutically Applicable Research to Generate Effective
Treatments (TARGET) database. Furthermore, a lncRNA-miRNA-mRNA
ceRNA network of WT was constructed by integrated analysis.
Additionally, the prognostic value of lncRNAs in the ceRNA network
was analyzed.
Materials and methods
Data collection and processing
RNA sequencing (RNA-Seq) data of 131 samples, miRNA
sequencing (miRNA-Seq) data of 138 samples and clinical prognostic
information of 655 samples were retrieved from the TARGET database
via the Genomic Data Commons Data Portal (portal.gdc.cancer.gov). The inclusion criteria were as
follows: i) Tissue samples with RNA-Seq data, miRNA-Seq data and
clinical prognostic information; and ii) samples from primary
tumors. A total of 120 primary tumor tissues and six adjacent
normal kidney tissues were included in the present study. Perl
(version 5.28.0; http://www.perl.org/) and R software (version 3.6.0;
http://www.r-project.org/) were used for
data processing. Differences in the expression of genes between the
two groups were analyzed using the principal component analysis
(PCA) tool (version 1.2.0; http://github.com/kevinblighe/PCAtools) for R. Data
used for validating the results were downloaded from the Gene
Expression Omnibus (GEO) dataset GSE66405 (27), which consisted of 28 tumor and four
adjacent normal kidney tissues. A flowchart illustrating the
generation of the ceRNA network is presented in Fig. S1.
Identification of differentially
expressed genes (DEGs)
After data normalization, the ‘edgeR’ package
(version 3.24.3) for R (28), with
the limma method (version 3.38.3) (29), were used to identify the
differentially expressed lncRNAs (DElncRNAs), mRNAs (DEmRNAs) and
miRNAs (DEmiRNAs) between the WT and normal tissues. The cut-off
value for DEGs was |log2 fold-change (FC)|>1 with a false
discovery rate (FDR) of <0.05. The DElncRNAs, DEmRNAs and
DEmiRNAs were visualized by volcano maps and heat maps using the
‘ggplot2’ (version 3.2.0; http://ggplot2.tidyverse.org/) and ‘pheatmap’ (version
1.10.1; https://cran.r-project.org/web/ packages
/pheatmap/index.html) R packages.
Functional and pathway enrichment
analyses
To further understand the molecular mechanisms of
WT, the ‘clusterProfiler’ (version 3.10.1) R package (30) was used to analyze the Gene Ontology
(GO) annotation (http://geneontology.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway enrichment (https://www.kegg.jp/) of DEmRNAs in the ceRNA network.
A corrected P-value of <0.01 was considered to indicate a
statistically significant difference. The results are presented as
bubble plots, generated using the ‘ggplot2’ package in R.
Construction of the ceRNA network
The miRcode (version 11; www.mircode.org) database was used to predict
interactions between DElncRNAs and DEmiRNAs. Then, the miRNA target
prediction database miRDB (version 6.0; www.mirdb.org) and TargetScan (release 7.2; www.targetscan.org) and miRTarBase (release 7.0;
mirtarbase.mbc.nctu.edu.tw) databases
were used to predict the target mRNAs of the DEmiRNAs. Only mRNAs
present in all three databases were considered as candidate mRNAs
that interacted with DEmRNAs. The overlapping mRNAs were considered
to be target DEmRNAs of the DEmiRNAs. The analysis was carried out
using Perl. Finally, the DElncRNA-DEmiRNA-DEmRNA network was
constructed and visualized using Cytoscape software (version 3.6.1;
http://cytoscape.org/).
Survival analysis
The ‘survival’ package in R was used to analyze
DElncRNAs, DEmiRNAs and DEmRNAs associated with the prognosis of
WT. All samples were divided into high or low expression groups,
according to the median RNA expression level. The Kaplan-Meier
method and the log-rank test were used to generate and compare
survival curves. P<0.05 was considered to indicate a
statistically significant difference. Furthermore, Pearson
correlation analyses by R software (version 3.6.0; http://www.r-project.org/) were performed to examine
the correlation between the expression of the DElncRNAs and the
corresponding DEmRNAs in the ceRNA network.
Validation of DElncRNAs
The DElncRNAs were compared according to the
National Wilms Tumor Study staging system (4). In addition, the expression profiles of
the DElncRNAs were extracted from the GSE66405 gene expression
matrix (27). The DElncRNA
expression profiles in the tumor and normal groups were compared
using an unpaired Student's t-test. Statistical analysis was
performed using GraphPad Prism software (version 8.0; GraphPad
Software, Inc.).
Results
Identification of the DElncRNAs,
DEmiRNAs and DEmRNAs
The present study included 120 primary tumor tissues
and six adjacent normal kidney tissues. The clinical features of
the patients are presented in Table
I. The RNA-seq (mRNAs and lncRNAs) and miRNAs in the two groups
were expressed in two clear groups in the PCA plot (Fig. 1). Subsequently, 442 DElncRNAs (234
upregulated and 208 downregulated; Fig.
2A and B), 214 DEmiRNAs (120 upregulated and 94 downregulated;
Fig. 2C and D) and 4,912 DEmRNAs
(2056 upregulated and 2856 downregulated; Fig. 2E and F) between the WT and normal
tissues were identified according to the cut-off criteria
(|log2FC|>1; FDR <0.05).
 | Figure 2.Differential expression of lncRNAs,
miRNAs and mRNAs. (A) Volcano plot and (B) heatmap of DElncRNAs.
(C) Volcano plot and (D) heatmap of DEmiRNAs. (E) Volcano plot and
(F) heatmap of DEmRNAs. In the volcano plots and heatmaps, red
represents upregulated genes, green represents downregulated genes
and black represents unchanged genes. A total of 442 DElncRNAs (234
upregulated and 208 downregulated), 214 DEmiRNAs (120 upregulated
and 94 downregulated) and 4,912 DEmRNAs (2,056 upregulated and
2,856 downregulated) were identified according to the cut-off
criteria (|log2 fold-change|>1 and FDR <0.05). DE,
differentially expressed; lncRNA, long non-coding RNA; miRNA,
microRNA; FDR, false discovery rate. |
 | Table I.Clinical characteristics of patients
with Wilms tumor in the Therapeutically Applicable Research to
Generate Effective Treatments database. |
Table I.
Clinical characteristics of patients
with Wilms tumor in the Therapeutically Applicable Research to
Generate Effective Treatments database.
| Characteristic | Number (%) |
|---|
| Sex |
|
Male | 53 (44.2) |
|
Female | 67 (55.8) |
| Ethnicity |
|
Black | 19 (15.8) |
|
White | 89 (74.2) |
|
Other | 5 (4.2) |
| Not
reported | 7 (5.8) |
| Age at
diagnosis |
| <24
months | 16 (13.3) |
| >24
months | 104 (86.7) |
| Histology |
|
DAWT | 40 (33.3) |
|
FHWT | 80 (66.7) |
| NMTS stage |
| I | 15 (12.5) |
| II | 48 (40.0) |
|
III | 44 (36.7) |
| IV | 12 (10.0) |
| V | 1 (0.8) |
| Progression or
relapse |
|
Yes | 95 (79.2) |
| No | 25 (20.8) |
Functional and pathway enrichment
analyses
The functions and pathways of the 4,912 DEmRNAs were
investigated using the ‘clusterProfiler’ R package. The results
suggested that 473 GO terms (355 biological processes, 89 cell
components and 29 molecular functions) and 18 KEGG pathways were
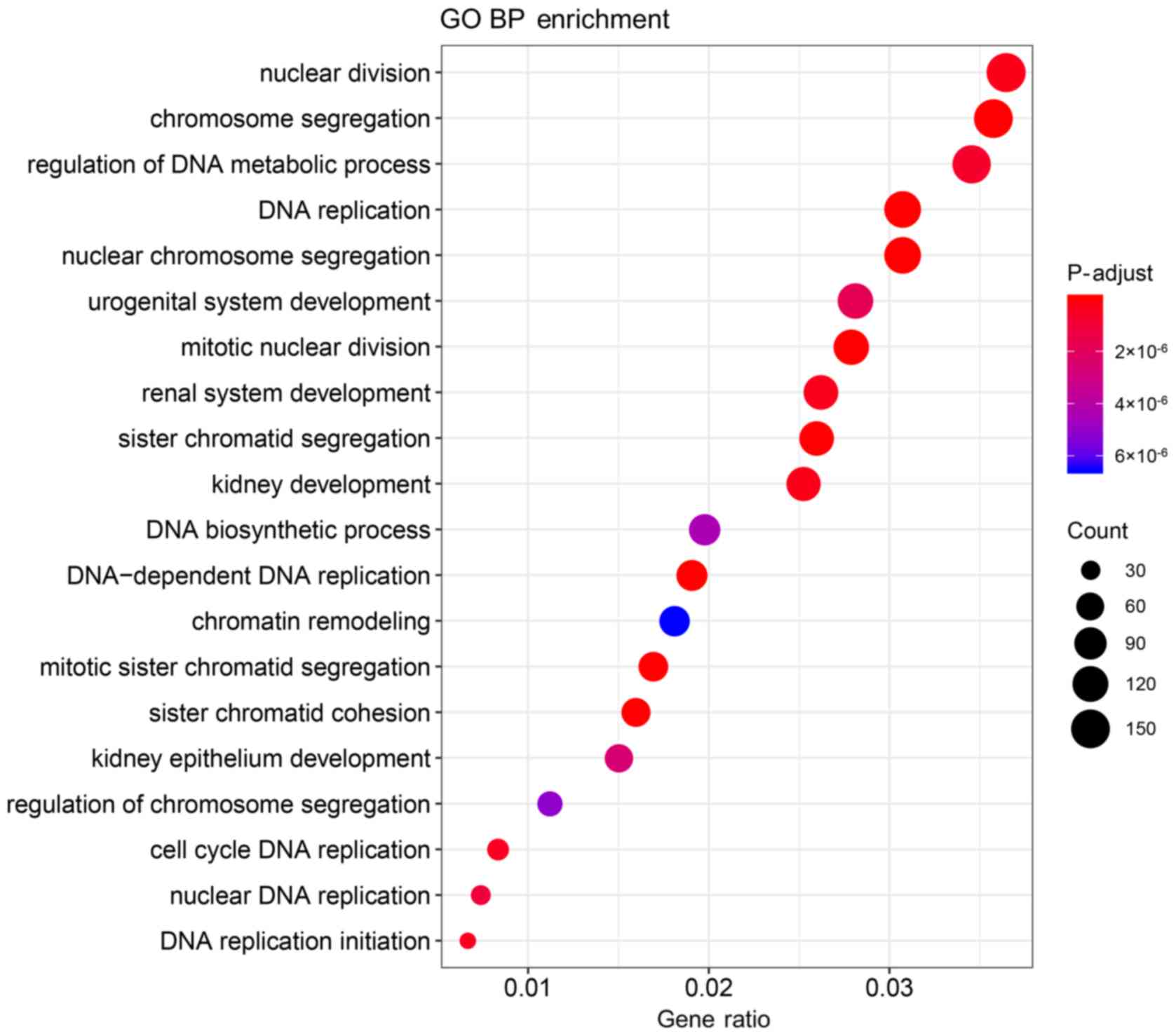
significantly enriched in the DEmRNAs (Tables SI and SII). The top 20 significantly enriched
biological processes of the GO terms are presented in Table II and Fig. 3, and the significantly enriched KEGG
pathways are presented in Table
III and Fig. 4.
 | Table II.Top 20 enriched biological processes
of GO analysis in Wilms tumor. |
Table II.
Top 20 enriched biological processes
of GO analysis in Wilms tumor.
| ID | GO term | Count | P-value |
|---|
| GO:0006261 | DNA-dependent DNA
replication | 80 |
3.73×10−13 |
| GO:0006260 | DNA
replication | 129 |
1.55×10−12 |
| GO:0007059 | Chromosome
segregation | 150 |
2.25×10−12 |
| GO:0000819 | Sister chromatid
segregation | 109 |
2.25×10−12 |
| GO:0140014 | Mitotic nuclear
division | 117 |
2.52×10−10 |
| GO:0098813 | Nuclear chromosome
segregation | 129 |
3.17×10−10 |
| GO:0007062 | Sister chromatid
cohesion | 67 |
3.99×10−9 |
| GO:0000070 | Mitotic sister
chromatid segregation | 71 |
7.23×10−9 |
| GO:0000280 | Nuclear
division | 153 |
3.08×10−7 |
| GO:0001822 | Kidney
development | 106 |
3.08×10−7 |
| GO:0072001 | Renal system
development | 110 |
4.20×10−7 |
| GO:0006270 | DNA replication
initiation | 28 |
4.20×10−7 |
| GO:0044786 | Cell cycle DNA
replication | 35 |
4.73×10−7 |
| GO:0051052 | Regulation of DNA
metabolic process | 145 |
7.00×10−7 |
| GO:0033260 | Nuclear DNA
replication | 31 |
1.02×10−6 |
| GO:0001655 | Urogenital system
development | 118 |
1.78×10−6 |
| GO:0072073 | Kidney epithelium
development | 63 |
2.58×10−6 |
| GO:0071897 | DNA biosynthetic
process | 83 |
4.43×10−6 |
| GO:0051983 | Regulation of
chromosome segregation | 47 |
5.12×10−6 |
| GO:0006338 | Chromatin
remodeling | 76 |
6.51×10−6 |
 | Table III.Kyoto Encyclopedia of Genes and
Genomes pathway enrichment analysis of differentially expressed
genes in Wilms tumor. |
Table III.
Kyoto Encyclopedia of Genes and
Genomes pathway enrichment analysis of differentially expressed
genes in Wilms tumor.
| ID | Description | Count | P-value |
|---|
| hsa03030 | DNA
replication | 25 |
5.74×10−6 |
| hsa05166 | Human T-cell
leukemia virus 1 infection | 101 |
9.80×10−6 |
| hsa04110 | Cell cycle | 56 |
4.65×10−5 |
| hsa04142 | Lysosome | 54 |
1.65×10−4 |
| hsa04514 | Cell adhesion
molecules (CAMs) | 59 |
6.57×10−4 |
| hsa04218 | Cellular
senescence | 63 |
1.25×10−3 |
| hsa04115 | p53 signaling
pathway | 33 |
3.19×10−3 |
| hsa04015 | Rap1 signaling
pathway | 75 |
3.63×10−3 |
| hsa04010 | MAPK signaling
pathway | 101 |
3.63×10−3 |
| hsa05145 | Toxoplasmosis | 46 |
3.63×10−3 |
| hsa05120 | Epithelial cell
signaling in Helicobacter pylori infection | 31 |
3.74×10−3 |
| hsa04360 | Axon guidance | 65 |
3.74×10−3 |
| hsa03410 | Base excision
repair | 18 |
5.35×10−3 |
| hsa01040 | Biosynthesis of
unsaturated fatty acids | 14 |
5.40×10−3 |
| hsa04659 | Th17 cell
differentiation | 43 |
5.41×10−3 |
| hsa04714 | Thermogenesis | 80 |
5.41×10−3 |
| hsa04658 | Th1 and Th2 cell
differentiation | 38 |
5.77×10−3 |
| hsa04670 | Leukocyte
transendothelial migration | 44 |
7.20×10−3 |
Construction of the ceRNA network
Using the miRcode database, 206 miRNAs were
identified as the targets of 45 DElncRNAs. The 206 miRNAs were then
intersected with the DEmiRNAs to identify 16 miRNAs that were
regulated in an opposing manner to the 33 corresponding
DElncRNAs.
Subsequently, 158 target genes of the
14 miRNAs were predicted using the miRDB, TargetScan and miRTarBase
databases
These 158 target genes were identified as DEmRNAs.
Thus, 32 DElncRNAs, 14 DEmiRNAs and 158 DEmRNAs were included in
the ceRNA network generated by Cytoscape software (Fig. 5).
Survival analysis
To further understand the effect of DElncRNAs,
DEmiRNAs and DEmRNAs on the prognosis of WT, Kaplan-Meier curves of
the 32 DElncRNAs, 14 DEmiRNAs and 158 DEmRNAs were analyzed for
overall survival rate (OS). The results suggested that three
DElncRNAs, three DEmiRNAs and 17 DEmRNAs were significantly
associated with the OS (P<0.05). Of the three DElncRNAs, MYCN
opposite strand (MYCNOS) and deleted in lymphocytic leukemia 2
(DLEU2) were negatively associated with the OS
(P=1.178×10−2, P=1.444×10−2), while
chromosome 8 open reading frame 31 (C8orf31) was positively
associated (P=2.357×10−2) with the OS (Fig. 6A-C). All three of the DEmiRNAs
(hsa-miR-135a-5p, hsa-miR-363-3p and hsa-miR-125b-5p) were
positively associated (P=2.903×10−3,
P=1.097×10−2, P=3.008×10−2, respectively)
with the OS (Fig. 6D-F). The
expression profile of the three DElncRNAs and the clinical features
of the 120 patients with WT are presented in Fig. 6G. Among the 17 significant DEmRNAs
(data not presented), five mRNAs [family with sequence similarity
102 member A (FAM102A), forkhead box P4 (FOXP4), WT1 transcription
factor (WT1), tripartite motif (TRIM) containing TRIM36 and TRIM71]
were negatively associated (P=2.453×10−2,
P=4.4968×10−2, P=3.671×10−2,
P=4.271×10−2, P=3.403×10−2) with the OS,
whereas the other 12 mRNAs [fibrillin 2 (FBN2), glucosamine
(N-acetyl)-6-sulfatase (GNS), Kruppel like factor 10 (KLF10), LIM
domain and actin binding 1 (LIMA1), protein phosphatase 1
regulatory subunit 3B (PPP1R3B), pleckstrin and Sec7 domain
containing 3 (PSD3), STAT3, STAT6, transmembrane protein (TMEM)127,
UDP-glucuronate decarboxylase 1 (UXS1), very low density
lipoprotein receptor (VLDLR) and zinc finger and BTB domain
containing 4 (ZBTB4) were positively associated
(P=2.287×10−2, P=3.143×10−2,
P=1.635×10−2, P=4.366×10−3,
P=3.102×10−2, P=1.148×10−2,
P=4.057×10−2, P=3.406×10−2,
P=2.462×10−2, P=2.985×10−2,
P=4.977×10−2, P=4.012×10−2) with the OS.
Moreover, a correlation analysis was performed between the three
DElncRNAs and the 17 DEmRNAs associated with OS. The significantly
correlated DElncRNAs and DEmRNAs are presented in Fig. 7. Considering that the three
DElncRNAs displayed an association with the survival of patients
with WT, univariate and multivariate Cox regression analyses of OS
were performed with the three DElncRNAs and the clinical features
(Table IV). The univariate
analysis suggested that ethnicity, stage and MYCNOS expression
significantly affected the OS of patients with WT (P<0.05;
Table IV). The multivariate
analysis suggested that stage and MYCNOS expression were
independent prognostic indicators in patients with WT (P<0.05;
Table IV).
 | Figure 6.Survival analysis and expression
profiles of the three DElncRNAs. Kaplan-Meier survival curves of
the three DElncRNAs: (A) MYCNOS, (B) DLEU2 and (C) C8orf31, and the
three DEmiRNAs: (D) hsa-miR-125b-5p, (E) hsa-miR-135a-5p and (F)
hsa-miR-363-3p, associated with overall survival in WT. (G)
Expression profile of the three DElncRNAs and the clinical features
of the 120 patients with WT. DE, differentially expressed; lncRNA,
long non-coding RNA; MYCNOS, MYCN opposite strand; DLEU2, deleted
in lymphocytic leukemia 2; C8orf31, chromosome 8 open reading frame
31; miRNA, microRNA; miR, microRNA; WT, Wilms tumor; DAWT, diffuse
anaplastic histology WT; FHWT, favorable histology WT. |
 | Table IV.Univariate and multivariate Cox
regression analysis of overall survival in the TARGET. |
Table IV.
Univariate and multivariate Cox
regression analysis of overall survival in the TARGET.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Sex (male vs.
female) | 0.0550 | 1.7240 | 0.9880–3.0090 | 0.0710 | 1.7490 | 0.9530–3.2090 |
| Ethnicity (black
vs. white) | 0.0380 | 2.0110 | 1.0410–3.8870 | 0.1590 | 1.6450 | 0.8230–3.2890 |
| Age (>2 vs.
<2 years) | 0.2070 | 1.8140 | 0.7200–3.5760 | 0.7330 | 1.2050 | 0.4130–3.5130 |
| NMTS Stage (III–V
vs. I–II) | <0.0001 | 3.3020 | 1.8190–3.9940 | 0.0030 | 2.8570 | 1.4370–3.6840 |
| Histology (DAWT vs.
FHWT) | 0.8380 | 1.0640 | 0.5870–3.9280 | 0.9350 | 0.9680 | 0.4470–3.0990 |
| MYCNOS | 0.0030 | 1.4510 | 1.1330–3.8570 | 0.0420 | 1.3550 | 1.1060–3.8150 |
| DLEU2 | 0.0550 | 1.3500 | 0.9940–3.8360 | 0.8560 | 1.0600 | 0.7220–3.4840 |
| C8orf31 | 0.0500 | 0.7580 | 0.5740–3.0010 | 0.7590 | 0.9530 | 0.6980–3.2990 |
Validation of DElncRNAs
The favorable histology WTs in the TARGET database
cohort (Table I) of the present
study were obtained from patients with relapse, and therefore, only
the differences in the expression of the three lncRNAs in the late
and early stages of WT were compared. The results suggested that
MYCNOS and DELU2 were highly expressed (P=2.98×10−2,
P=9.0×10−4) in the late stages of WT, whereas C8orf31
was highly expressed (P=4.9×10−3) in the early stages of
WT (Fig. 8A-C). To validate that
the three lncRNAs were also differentially expressed in other
datasets, the GSE66405 dataset from GEO was investigated. DLEU2 and
C8orf31 were differentially expressed in tumor and normal tissues
in the GSE66405 dataset (P=1.25×10−2,
P<1.0×10−4; Fig. 8E and
F). Additionally, the expression of MYCNOS in the tumor tissues
was higher than that in the normal tissues, but this difference was
not statistically significant (P=1.71×10−1; Fig. 8D).
Discussion
WT presents high morbidity and mortality in
children; although the overall 5-year survival rate for patients
with WT has improved from 20% in the 1960s to >90% in the 2000s
(3,4), the current treatments for WT,
including surgery, chemotherapy and radiation, still have
limitations and complications, including death (31,32). A
study involving 6,185 patients with WT reported that the risk of
death in WT survivors remained high several years after the
original diagnosis (33).
Therefore, the identification of novel key molecules as targets for
biotherapy would be beneficial for improving the current status of
WT treatment.
Recently, lncRNAs have gained increasing attention
(14). Different lncRNAs influence
several cellular processes, such as development and development,
antiviral response and gene imprinting, but the specific function
of the majority of lncRNAs is still unknown (34,35).
Numerous theories and hypotheses have been proposed to explain how
lncRNAs regulate the biological behavior of tumor cells (34,36).
The ceRNA hypothesis (19), which
states that lncRNAs regulate mRNAs by competing for miRNAs, has
been proven (20,37–39). A
number of previous studies have investigated ceRNAs in other
pediatric malignant tumors (40,41),
but there are only a few studies on the role of ceRNAs in WT
(23–26). To further understand how
lncRNA-related ceRNA networks affect WT, the present study analyzed
large-scale sequencing data of WT tissues from the TARGET
database.
In the present study, 442 lncRNAs, 214 miRNAs and
4,912 mRNAs were identified as DEGs between normal and WT tissues.
Moreover, functional analysis of the DEmRNAs was performed to
determine their potential biological mechanisms. The GO enrichment
analysis indicated that ‘DNA-dependent DNA replication’, ‘DNA
replication’, ‘chromosome segregation’, ‘sister chromatid
segregation’, ‘mitotic nuclear division’, ‘nuclear chromosome
segregation’, ‘sister chromatid cohesion’, ‘mitotic sister
chromatid segregation’, ‘nuclear division’ and ‘kidney development’
were the main biological processes of the DEGs associated with WT.
The majority of the identified biological processes were associated
with cell mitosis and kidney development, which are closely
associated with the occurrence and development of renal tumors
(42,43). The KEGG pathway enrichment analysis
indicated that the majority of DEmRNAs were enriched in ‘DNA
replication’, ‘human T-cell leukemia virus 1 infection’, ‘cell
cycle’, ‘lysosome’, ‘cell adhesion molecules’, ‘cellular
senescence’, ‘p53 signaling pathway’, ‘Rap1 signaling pathway’,
‘MAPK signaling pathway’ and ‘toxoplasmosis’, with ‘MAPK signaling
pathway’ displaying the highest level of significance. A recent
study suggested that the mitogen-activated protein kinase/ERK
signaling pathway plays a role in WT (44). Among the DEGs, 32 lncRNAs, 14 miRNAs
and 158 mRNAs were included in the ceRNA network. Su et al
(11) reported that small nucleolar
RNA host gene (SNHG)6 decreases the proliferation, migration and
invasion of WT cell lines by regulating miR-15a. The SNHG family
genes, including SNHG1, SNHG5 and SNHG6, were also present in the
ceRNA network of the present study. Their regulatory network with
miRNAs may be even more complex (45,46).
Furthermore, three DElncRNAs (MYCNOS, DLEU2 and C8orf31), three
DEmiRNAs (hsa-miR-135a-3p, hsa-miR-363-3p and hsa-miR-125b-5p) and
17 DEmRNAs (WT1, FAM102A, FOXP4, TRIM36, TRIM71, FBN2, GNS, KLF10,
LIMA1, PPP1R3B, PSD3, STAT3, STAT6, TMEM127, UXS1, VLDLR and ZBTB4)
were significantly associated with OS. MYCNOS is transcribed in the
anti-sense strand across exon1 and intron1 from MYCN (47). The gene is highly expressed in small
cell lung cancer, MYCN-amplified rhabdomyosarcoma and neuroblastoma
(48,49). Zhao et al (50) reported that MYCNOS cooperated with
CCCTC-binding factor to promote the proliferation, invasion and
metastasis of neuroblastoma cells in vitro and in
vivo. In the present study, MYCNOS was upregulated and
associated with poor OS in the TARGET dataset. However, in the
GSE66405 dataset, the difference in MYCNOS expression was not
statistically significant between the tumor and normal tissues.
This difference between results might be due to the small sample
size of the present study. DLEU2 has been reported to affect the
biological behaviors of multiple types of cancer, such as laryngeal
carcinoma and leukemia (51,52).
Xie et al (51) reported
that DLEU2 can induce the proliferation, migration and invasion of
laryngeal carcinoma cells via miR-16-1. The present study also
suggested that C8orf31, in the ceRNA network, was highly expressed
in WT tissues. However, the high expression of C8orf31 was detected
only in early stage tumors and observed in patients with improved
survival. This suggested that the upregulation of C8orf31 may be a
protective mechanism in WT. As the malignancy of the tumor
increased, the expression of C8orf31 decreased. However, based on
the present results also a correlation between these factors can be
determined, and not a causative effect. Thus, further investigation
is required to identify the function of C8orf31 in WT and other
tumors.
miRNAs can regulate various target genes, playing an
important role in the development of cancer (53,54).
In the present study, three miRNAs (hsa-miR-135a-3p, hsa-miR-363-3p
and hsa-miR-125b-5p) were identified in the ceRNA network and were
positively associated with OS. Previous studies have reported that
the three miRNAs act as tumor suppressors in a number of types of
cancer (55–61). Fukagawa et al (56) reported that the overexpression of
miR-135a-3p enhanced ovarian cancer cell sensitivity to
chemotherapy drugs and suppressed proliferation. Li et al
(61) reported that miR-363-3p was
downregulated in renal cell carcinoma and reduced the inhibition of
the oncogenic cyclic adenosine monophosphate responsive element
binding protein 1. miR-125b-5p can inhibit γδ T cell activity,
promote γδ T cell apoptosis and subsequently suppress the immune
response to infections and tumorigenesis (62). Additionally, 17 DEmRNAs in the ceRNA
network were identified and were significantly related to the OS
rate, including WT1, which is closely related to the development of
WT (63). Certain well-known genes,
including FOXP4 and STAT3/6, play a role in tumorigenesis but not
in WT, and certain less reported genes, including FAM102A and FBN2,
require further investigation (64,65).
In the present study, MYCNOS and TRIM71 displayed the highest
linear correlation. A number of previous studies have demonstrated
that high levels of TRIM71, also known as LIN41, are associated
with hepatocellular carcinoma (66)
and myxoid liposarcoma (67), while
another study reported that TRIM71 inhibited tumorigenesis via the
regulation of the Lin28B-let-7-high mobility group AT-hook 2
signaling pathway in colorectal carcinoma cells and non-small cell
lung cancer cells (68). Therefore,
further investigation is required to identify the roles of various
genes and molecular mechanisms underlying WT. Although the results
of the present study were validated using another dataset, a
potential limitation of the present study was the small sample
size, therefore further investigation using larger sample sizes is
required.
To conclude, the present study investigated the
expression of lncRNAs, miRNAs and mRNAs specific to WT and their
potential functions. A ceRNA network was constructed to provide a
novel insight into the role of lncRNAs in the development of WT.
The three lncRNAs identified in the ceRNA network may aid in the
prognosis of WT and serve as potential targets for clinical
therapy.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the TARGET (https://ocg.cancer.gov/programs/target/data-matrix)
and GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE66405)
repository.
Authors' contributions
DS was involved in the conception of the study. ZW
designed and drafted the manuscript. ZW, LQ, HC and DS collected
and analyzed the data. HC was involved in interpretation of data.
ZW, HC, LQ and DS revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bernstein L, Linet M and Smith MA: Cancer
Incidence and Survival Among Children and Adolescents: United
States SEER Program, 1975-1995. National Cancer Institute;
Bethesda, MD: pp. p791999
|
|
2
|
Grovas A, Fremgen A, Rauck A, Ruymann FB,
Hutchinson CL, Winchester DP and Menck HR: The National Cancer Data
Base report on patterns of childhood cancers in the United States.
Cancer. 80:2321–2332. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koshinaga T, Takimoto T, Oue T, Okita H,
Tanaka Y, Nozaki M, Tsuchiya K, Inoue E, Haruta M, Kaneko Y, et al:
Outcome of renal tumors registered in Japan Wilms Tumor Study-2
(JWiTS-2): A report from the Japan Children's Cancer Group (JCCG).
Pediatr Blood Cancer. 65:e270562018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Metzger ML and Dome JS: Current therapy
for Wilms' tumor. Oncologist. 10:815–826. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith GR, Thomas PR, Ritchey M and Norkool
P: Long-term renal function in patients with irradiated bilateral
Wilms tumor. National Wilms' Tumor Study Group. Am J Clin Oncol.
21:58–63. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Green DM, Grigoriev YA, Nan B, Takashima
JR, Norkool PA, D'Angio GJ and Breslow NE: Congestive heart failure
after treatment for Wilms' tumor: A report from the National Wilms'
Tumor Study group. J Clin Oncol. 19:1926–1934. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green DM, Norkool P, Breslow NE,
Finklestein JZ and D'Angio GJ: Severe hepatic toxicity after
treatment with vincristine and dactinomycin using single-dose or
divided-dose schedules: A report from the National Wilms' Tumor
Study. J Clin Oncol. 8:1525–1530. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Green DM, Peabody EM, Nan B, Peterson S,
Kalapurakal JA and Breslow NE: Pregnancy outcome after treatment
for Wilms tumor: A report from the National Wilms Tumor Study
Group. J Clin Oncol. 20:2506–2513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Dijk IW, Oldenburger F, Cardous-Ubbink
MC, Geenen MM, Heinen RC, de Kraker J, van Leeuwen FE, van der Pal
HJ, Caron HN, Koning CC, et al: Evaluation of late adverse events
in long-term Wilms' tumor survivors. Int J Radiat Oncol Biol Phys.
78:370–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, et al:
SEER Cancer Statistics Review, 1975–2014. NIH publication National
Cancer Institute. (Bethesda, MD). p9962017.
|
|
11
|
International Human Genome Sequencing
Consortium, . Finishing the euchromatic sequence of the human
genome. Nature. 431:931–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang K, Lu C, Huang X, Cui J, Li J, Gao
Y, Liang W, Liu Y, Sun Y, Liu H, et al: Long noncoding RNA AOC4P
regulates tumor cell proliferation and invasion by
epithelial-mesenchymal transition in gastric cancer. Therap Adv
Gastroenterol. 12:17562848198276972019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tran DDH, Kessler C, Niehus SE, Mahnkopf
M, Koch A and Tamura T: Myc target gene, long intergenic noncoding
RNA, Linc00176 in hepatocellular carcinoma regulates cell cycle and
cell survival by titrating tumor suppressor microRNAs. Oncogene.
37:75–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Guo C, Wang L, Luo G, Huang C, Li
Y, Liu D, Zeng F, Jiang G and Xiao X: Long noncoding RNA GAS5
promotes bladder cancer cells apoptosis through inhibiting EZH2
transcription. Cell Death Dis. 9:2382018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu H, Zheng JF, Hou CZ, Li Y and Liu PS:
Up-regulation of long intergenic noncoding RNA 01296 in ovarian
cancer impacts invasion, apoptosis and cell cycle distribution via
regulating EMT. Cell Signal. 62:1093412019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong X, Jin Z, Chen Y, Xu H, Ma C, Hong X,
Li Y and Zhao G: Knockdown of long non-coding RNA ANRIL inhibits
proliferation, migration, and invasion but promotes apoptosis of
human glioma cells by upregulation of miR-34a. J Cell Biochem.
119:2708–2718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu Z and Li S: Long noncoding RNA
LINC01278 favors the progression of osteosarcoma via modulating
miR-133a-3p/PTHR1 signaling. J Cell Physiol. Jan 29–2020.(Epub
ahead of print). doi: 10.1002/jcp.29582. View Article : Google Scholar
|
|
22
|
Xu W, Yu S, Xiong J, Long J, Zheng Y and
Sang X: CeRNA regulatory network-based analysis to study the roles
of noncoding RNAs in the pathogenesis of intrahepatic
cholangiocellular carcinoma. Aging (Albany NY). 12:1047–1086. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su L, Wu A, Zhang W and Kong X: Silencing
long non-coding RNA SNHG6 restrains proliferation, migration and
invasion of Wilms' tumour cell lines by regulating miR-15a. Artif
Cells Nanomed Biotechnol. 47:2670–2677. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu KR, Sun QF and Zhang YQ: Long
non-coding RNA LINP1 induces tumorigenesis of Wilms' tumor by
affecting Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol
Sci. 23:5691–5698. 2019.PubMed/NCBI
|
|
25
|
Liu Z, He F, OuYang S, Li Y, Ma F, Chang
H, Cao D and Wu J: miR-140-5p could suppress tumor proliferation
and progression by targeting TGFBRI/SMAD2/3 and IGF-1R/AKT
signaling pathways in Wilms' tumor. BMC Cancer. 19:4052019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu S, Fu W, Zhang L, Fu K, Hu J, Jia W
and Liu G: LINC00473 antagonizes the tumour suppressor miR-195 to
mediate the pathogenesis of Wilms tumour via IKKalpha. Cell Prolif.
Feb 51–2018.(Epub ahead of print). doi: 10.1111/cpr.12416.
View Article : Google Scholar
|
|
27
|
Ludwig N, Werner TV, Backes C, Trampert P,
Gessler M, Keller A, Lenhof HP, Graf N and Meese E: Combining miRNA
and mRNA expression profiles in Wilms tumor subtypes. Int J Mol
Sci. 17:4752016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ritchey ML, Shamberger RC, Haase G,
Horwitz J, Bergemann T and Breslow NE: Surgical complications after
primary nephrectomy for Wilms' tumor: Report from the National
Wilms' Tumor Study Group. J Am Coll Surg. 192:63–68, quiz 146.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Termuhlen AM, Tersak JM, Liu Q, Yasui Y,
Stovall M, Weathers R, Deutsch M, Sklar CA, Oeffinger KC, Armstrong
G, et al: Twenty-five year follow-up of childhood Wilms tumor: A
report from the Childhood Cancer Survivor Study. Pediatr Blood
Cancer. 57:1210–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cotton CA, Peterson S, Norkool PA,
Takashima J, Grigoriev Y, Green DM and Breslow NE: Early and late
mortality after diagnosis of wilms tumor. J Clin Oncol.
27:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hon CC, Ramilowski JA, Harshbarger J,
Bertin N, Rackham OJ, Gough J, Denisenko E, Schmeier S, Poulsen TM,
Severin J, et al: An atlas of human long non-coding RNAs with
accurate 5 ends. Nature. 543:199–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Wang C, Wu T and Sun F: Long
non-coding RNA TP73-AS1 promotes TFAP2B-mediated proliferation,
metastasis and invasion in retinoblastoma via decoying of
miRNA-874-3p. J Cell Commun Signal. Feb 18–2020.(Epub ahead of
print). doi: 10.1007/s12079-020-00550-x. View Article : Google Scholar
|
|
38
|
Wang J, Cao Y, Lu X, Wang X, Kong X, Bo C,
Li S, Bai M, Jiao Y, Gao H, et al: Identification of the regulatory
role of lncRNA SNHG16 in myasthenia gravis by constructing a
competing endogenous RNA Network. Mol Ther Nucleic Acids.
19:1123–1133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu J, Zhang J, Shan F, Wen J and Wang Y:
SSTR5 AS1 functions as a ceRNA to regulate CA2 by sponging miR 15b
5p for the development and prognosis of HBV related hepatocellular
carcinoma. Mol Med Rep. 20:5021–5031. 2019.PubMed/NCBI
|
|
40
|
Chi R, Chen X, Liu M, Zhang H, Li F, Fan
X, Wang W and Lu H: Role of SNHG7-miR-653-5p-STAT2 feedback loop in
regulating neuroblastoma progression. J Cell Physiol.
234:13403–13412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fei D, Zhang X, Liu J, Tan L, Xing J, Zhao
D and Zhang Y: Long noncoding RNA FER1L4 suppresses tumorigenesis
by regulating the expression of PTEN targeting miR-18a-5p in
osteosarcoma. Cell Physiol Biochem. 51:1364–1375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arai E, Gotoh M, Tian Y, Sakamoto H, Ono
M, Matsuda A, Takahashi Y, Miyata S, Totsuka H, Chiku S, et al:
Alterations of the spindle checkpoint pathway in
clinicopathologically aggressive CpG island methylator phenotype
clear cell renal cell carcinomas. Int J Cancer. 137:2589–2606.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hohenstein P, Pritchard-Jones K and
Charlton J: The yin and yang of kidney development and Wilms'
tumors. Genes Dev. 29:467–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kurtzeborn K, Kwon HN and Kuure S:
MAPK/ERK signaling in regulation of renal differentiation. Int J
Mol Sci. 20:E17792019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie M, Zhang Z and Cui Y: Long Noncoding
RNA SNHG1 contributes to the promotion of prostate cancer cells
through regulating miR-377-3p/AKT2 axis. Cancer Biother Radiopharm.
35:109–119. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meng X, Deng Y, Lv Z, Liu C, Guo Z, Li Y,
Liu H, Xie B, Jin Z, Lin F, et al: LncRNA SNHG5 promotes
proliferation of glioma by regulating miR-205-5p/ZEB2 axis.
OncoTargets Ther. 12:11487–11496. 2019. View Article : Google Scholar
|
|
47
|
Krystal GW, Armstrong BC and Battey JF:
N-myc mRNA forms an RNA-RNA duplex with endogenous antisense
transcripts. Mol Cell Biol. 10:4180–4191. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Armstrong BC and Krystal GW: Isolation and
characterization of complementary DNA for N-cym, a gene encoded by
the DNA strand opposite to N-myc. Cell Growth Differ. 3:385–390.
1992.PubMed/NCBI
|
|
49
|
O'Brien EM, Selfe JL, Martins AS, Walters
ZS and Shipley JM: The long non-coding RNA MYCNOS-01 regulates MYCN
protein levels and affects growth of MYCN-amplified
rhabdomyosarcoma and neuroblastoma cells. BMC Cancer. 18:2172018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao X, Li D, Pu J, Mei H, Yang D, Xiang
X, Qu H, Huang K, Zheng L and Tong Q: CTCF cooperates with
noncoding RNA MYCNOS to promote neuroblastoma progression through
facilitating MYCN expression. Oncogene. 35:3565–3576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie ZZ, Xiao ZC, Song YX, Li W and Tan GL:
Long non-coding RNA Dleu2 affects proliferation, migration and
invasion ability of laryngeal carcinoma cells through triggering
miR-16-1 pathway. Eur Rev Med Pharmacol Sci. 22:1963–1970.
2018.PubMed/NCBI
|
|
52
|
Lerner M, Harada M, Lovén J, Castro J,
Davis Z, Oscier D, Henriksson M, Sangfelt O, Grandér D and Corcoran
MM: DLEU2, frequently deleted in malignancy, functions as a
critical host gene of the cell cycle inhibitory microRNAs miR-15a
and miR-16-1. Exp Cell Res. 315:2941–2952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Duan S, Dong X, Hai J, Jiang J, Wang W,
Yang J, Zhang W and Chen C: MicroRNA-135a-3p is downregulated and
serves as a tumour suppressor in ovarian cancer by targeting CCR2.
Biomed Pharmacother. 107:712–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fukagawa S, Miyata K, Yotsumoto F,
Kiyoshima C, Nam SO, Anan H, Katsuda T, Miyahara D, Murata M, Yagi
H, et al: MicroRNA-135a-3p as a promising biomarker and nucleic
acid therapeutic agent for ovarian cancer. Cancer Sci. 108:886–896.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang SH, Zhang WJ, Wu XC, Weng MZ, Zhang
MD, Cai Q, Zhou D, Wang JD and Quan ZW: The lncRNA MALAT1 functions
as a competing endogenous RNA to regulate MCL-1 expression by
sponging miR-363-3p in gallbladder cancer. J Cell Mol Med.
20:2299–2308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dong J, Geng J and Tan W: MiR-363-3p
suppresses tumor growth and metastasis of colorectal cancer via
targeting SphK2. Biomed Pharmacother. 105:922–931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Y, Chen T, Huang H, Jiang Y, Yang L,
Lin Z, He H, Liu T, Wu B, Chen J, et al: miR-363-3p inhibits tumor
growth by targeting PCNA in lung adenocarcinoma. Oncotarget.
8:20133–20144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu J, Li Q, Li R, Ren P and Dong S:
MicroRNA-363-3p inhibits papillary thyroid carcinoma progression by
targeting PIK3CA. Am J Cancer Res. 7:148–158. 2017.PubMed/NCBI
|
|
61
|
Li Y, Wang Y, Fan H, Zhang Z and Li N:
miR-125b-5p inhibits breast cancer cell proliferation, migration
and invasion by targeting KIAA1522. Biochem Biophys Res Commun.
504:277–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu Y, Zhang S, Li Z, Wang H, Li Z, Hu Y,
Chen H, Zhang X, Cui L, Zhang J, et al: miR-125b-5p and miR-99a-5p
downregulate human γδ T-cell activation and cytotoxicity. Cell Mol
Immunol. 16:112–125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee SB and Haber DA: Wilms tumor and the
WT1 gene. Exp Cell Res. 264:74–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim JH, Hwang J, Jung JH, Lee HJ, Lee DY
and Kim SH: Molecular networks of FOXP family: Dual biologic
functions, interplay with other molecules and clinical implications
in cancer progression. Mol Cancer. 18:1802019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Verhoeven Y, Tilborghs S, Jacobs J, De
Waele J, Quatannens D, Deben C, Prenen H, Pauwels P, Trinh XB,
Wouters A, et al: The potential and controversy of targeting STAT
family members in cancer. Semin Cancer Biol. 60:41–56. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen YL, Yuan RH, Yang WC, Hsu HC and Jeng
YM: The stem cell E3-ligase Lin-41 promotes liver cancer
progression through inhibition of microRNA-mediated gene silencing.
J Pathol. 229:486–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
De Cecco L, Negri T, Brich S, Mauro V,
Bozzi F, Dagrada G, Disciglio V, Sanfilippo R, Gronchi A, D'Incalci
M, et al: Identification of a gene expression driven progression
pathway in myxoid liposarcoma. Oncotarget. 5:5965–5977. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yin J, Kim TH, Park N, Shin D, Choi HI,
Cho S, Park JB and Kim JH: TRIM71 suppresses tumorigenesis via
modulation of Lin28B-let-7-HMGA2 signaling. Oncotarget.
7:79854–79868. 2016. View Article : Google Scholar : PubMed/NCBI
|