Introduction
Hepatocellular carcinoma (HCC) is one of the most
lethal and prevalent cancers, closely associated with cirrhosis and
fibrosis with a variable etiology (1). Notably, its incidence and mortality
are higher in developing countries, with an increasing burden on
developed countries (2).
Predominantly, HCC is caused by chronic hepatitis B and C viral
infections, alcoholic liver and non-alcoholic fatty liver disease
(3). Chronic inflammation promotes
HCC progression (4). Furthermore,
HCC is closely associated with immune suppression and tolerance
(5). A great number of cytokines,
immune-inhibitory receptors, pro-inflammatory cytokines and their
ligands also prompt immunosuppression to contribute to HCC
progression (6). Following viral
hepatitis or chronic liver insults, elevated interleukin (IL) 6
activates hepatocyte proliferation, which ultimately results in HCC
(7).
As a key cellular compartment, the endoplasmic
reticulum (ER) is indispensable in protein synthesis and protein
maturation, which needs the participation of folding enzymes and
chaperones (8). Protein
homeostasis is disturbed, with ER stress (ERS) occurring, when the
amount of unfolded protein exceeds the ER capacity (9). ERS induction by infection has been
implicated in HCC and disease progression with chronic inflammation
via enhanced inflammation, oxidative stress-mediated DNA damage and
hepatocyte proliferation. Notably, ERS is crucial for cancer cells
to preserve malignancy and maintain resistance to therapy. Indeed,
ERS participates in HCC radiation and chemotherapy resistance
(10,11). Furthermore, the escape of tumor
cells from immunosurveillance has been attributed to ERS (9). ERS results in the gathering of
misfolded or unfolded proteins. Under this situation, the unfolded
protein response (UPR) in cells is activated to alleviate the
superabundant protein load, including transient reduction of
protein translation and misfolded protein degradation (8). In addition, folding enzymes and
molecular chaperones strengthen the ER capability to fold and
degrade more proteins (12).
Continuous UPR activation occurs in various types of
cancer and is believed to facilitate oncogenic processes. The UPR
can be activated by three ER sensors: Activating transcription
factor-6 (ATF6), inositol-requiring enzyme 1 (IRE1) and protein
kinase RNA-like endoplasmic reticulum kinase (PERK). The ER lumen
houses several chaperones, including glucose-regulated protein-78
(GRP78)/binding immunoglobulin protein (BiP) and −94 (GRP94), and
protein disulfide-isomerase A4 (PDIA4) (13). Molecular chaperones play an
essential role in maintaining ER protein homeostasis. Normally,
GRP78 is bound to the three ER sensors, maintaining them in an
inactive form (14,15). Upon dissociation from GRP78, PERK
and IRE1 transform into oligomers or homodimers, activating the
downstream pathways through autophosphorylation. Upon release by
BiP, ATF6 is transformed into a transcription factor, stimulating
the transcription of different ER chaperones (16,17).
The effects of PERK and IRE1 are both proapoptotic and
pro-survival, whereas the effect of ATF6 is principally
cytoprotective (18). In human
HCC, GRP78, ATF6, IRE1 and PERK expressions are higher than the
basal levels and negatively associated with the overall survival
and clinicopathological scores in HCC patients (4,19).
Natural compounds exhibit promising applications in
cancer therapy attributed to their special pharmacological
activities and low toxicity (20).
The roots of Cynanchum auriculatum Royle ex Wight, known as
‘baishouwu’ in China and in other Asian countries, have been
widely used as a tonic supplement for strengthening kidney function
in clinical settings (21).
Caudatin has the highest antitumor capacity among several C-21
steroidal glycosides isolated from baishouwu, exhibiting
selectivity towards hepatoma cell lines compared with other tumor
cell lines (22). Furthermore, the
inhibitory effect of caudatin has been validated in a H22 solid
tumor model in vivo (20).
Caudatin prevents tumor progression by stimulating DNA
damage-mediated cell cycle arrest (23) or apoptosis (24). Previously, the present authors
demonstrated that caudatin effectively inhibited human hepatoma
cell growth and metastasis (25).
However, the in vivo effect of caudatin in the orthotopic
tumor model has not yet been elucidated. Therefore, the present
study used the diethylnitrosamine (DEN)-induced cirrhotic rat model
with HCC to test the safety and antitumor efficacy of caudatin and
explore the mechanism of action.
Materials and methods
Reagents and materials
Caudatin, Tween-20, bovine serum albumin and sodium
dodecyl sulfate were purchased from Sigma-Aldrich Shanghai Trading
Co. Ltd. The various antibodies used were: ATF4 (cat. no.
sc-390063) were obtained from Santa Cruz Biotechnology, Inc. eIF2α
(cat. no. 5324), phosphorylated (p)-eIF2α (cat. no. 3398), GRP78
(cat. no. 3183), IRE1α (cat. no. 3294), p-PERK (cat. no. 3179) and
PERK (cat. no. 3192) were purchased from Cell Signaling Technology,
Inc. Tubulin (cat. no. ab7291), p-IRE1α (cat. no. ab48187), Ki67
(cat. no. ab16667), ATF6 (cat. no. ab203119), GAPDH (cat. no.
ab181602), PDIA4 (cat. no. ab82587) and GRP94 (cat. no. ab2791)
were purchased from Abcam. All other chemicals were of analytical
grade and were obtained commercially.
Diethylnitrosamine-induced HCC rat
model
A total of 18 female Sprague-Dawley rats (age, 2
months; weight, 200±20 g) were obtained from Shanghai Lab Animal
Research Center. Rats were maintained on a standard diet and water
ad libitum (12 h light/dark cycle with humidity of 60±5% and
temperature 22±3°C). Rats were intraperitoneally injected with 70
mg/kg of diethylnitrosamine (DEN; Sigma-Aldrich, Merck KGaA) once
per week for 10 continuous weeks. All animal experiments were
approved by the Institutional Animal Care and Use Committee of
Jiangsu Provincial Academy of Chinese Medicine (approval no.
AEWC-20170727-05). The 18 rats were divided into three groups and
received treatment from week 6–20, with six rats in each group:
DEN-treated control group, and low and high doses of Caudatin
groups (25 or 50 mg/kg, respectively; oral administration), 6 days
oral administration per week. Rats were sacrificed 10 weeks
following the last DEN injection. Normal rats were used as the
blank group.
Biochemical assays
Prior to sacrifice, the blood of rats was collected
and centrifuged at 1,411 × g for 10 min at 4°C to measure serum
aspartate aminotransferase (AST), alanine transaminase (ALT) and
total bilirubin (TBIL) using an autoanalyzer (Type 7020, Hitachi,
Ltd.). The supernatant of liver homogenates was used for the
measurements of malondialdehyde (MDA). MDA was determined
spectrophotometrically at 535 nm. Intracellular cytokine levels
were monitored in liver whole-cell lysate using IL-6 (cat.
no.431307), IL-1β (cat. no. 437007), monocyte chemoattractant
protein-1 (MCP-1; cat. no. 438807) and tumor necrosis factor (TNF)
α (cat. no. 438207) ELISA kits (Biolegend, Inc.).
MRI
Rats were anesthetized with 2% isoflurane during MRI
observation in a wrist coil. A supine position was scanned using a
1.5 T MRI scanner (Echo speed; GE Healthcare). T1-weighted,
T2-weighted and diffusion-weighted imaging (DWI) sequences were
performed. Rats were injected with Magnevist (Schering; Bayer
HealthCare Pharmaceuticals) through the tail vein and
contrast-enhanced MR scanning (T1CE) was performed following
injection.
Immunohistochemistry and hematoxylin
and eosin (H&E) staining
Livers were excised and, following 48 h fixation in
paraformaldehyde at room temperature, and paraffin sections
(thickness, 4-µm) were prepared. Sections were deparaffinized by
rinsing twice in xylene for 10 min each. The tissue sections were
hydrated with ethanol series (100, 95, 75 and 50%) and washed in
distilled water. Heat-induced epitope retrieval was achieved with
Tris-EDTA (pH 8). Endogenous peroxidase activity was quenched by 3%
H2O2 and incubated with 5% normal goat serum
(cat. no. 7481; Abcam) at room temperature for 30 min, followed by
overnight incubation with anti-Ki-67 (1:500), anti-GRP78 (1:200)
antibody at 4°C. Sections were then washed with PBS and incubated
with goat anti-rabbit IgG H&L (horseradish peroxidase)
antibodies (cat. no. 205718; 1:2,000; Abcam) at 37°C for 30 min.
Immunoreactivity was identified as brown in liver sections
counterstained with hematoxylin.
The deparaffinized sections (thickness, 4-µm) was
also stained with H&E kits (Servicebio, Inc.) according to the
manufacturer's instructions. Sections were stained with hematoxylin
at room temperature for 5 min, followed by 0.5% eosin staining at
room temperature for 3 min. The H&E staining was independently
inspected by a pathologist in a blinded manner. The length of the
scale bar is given in the figure legends.
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
RNA was extracted from liver tumors or normal livers
with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). RNA
extraction, cDNA synthesis and qPCR were performed according to the
manufacturer's protocols. Initially, the RNA samples were screened
based on their purity (260/280 ≥1.8) with a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA was
synthesized via Superscript III, using 5 µg of RNA and oligo-dT as
primer. RT-qPCR was performed on an ABI 7900HT real-time PCR
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with primers as follows: GAPDH (NM_017008.4,
5′-AGTGCCAGCCTCGTCTCATA-3′, 5′-TACGGCCAAATCCGTTCACA-3′), GRP78
(NM_013083.2, 5′-TCGACTTGGGGACCACCTAT-3′,
5′-GCGGCCGTTCTTGAATACAC-3′), GRP94 (NM_001012197.2,
5′-TAAGCTCTATGTGCGCCGAG-3′, 5′-TCACGGGAAACATTGAGGGG-3′), ATF4
(NM_024403.2, 5′-TTAAGCCATGGCGCTCTTCA-3′,
5′-GACATTAAGTCCCCCGCCAA-3′), PDIA4 (NM_053849.1,
5′-AGTGGAGAGGACGTCAATGC-3′, 5′-CCCTGACTGGTCCCTTGTTG-3′), ERDJ4
(NM_012699.2, 5′-AACAGGACGAAGGTTGCTCG-3′,
5′-AACTGACTGTGGAGTTGCCA-3′) and GADD34 (NM_133546.3,
5′-GAGAATGTGGCCCCAGTTGA-3′, 5′-ACAATGCTGGGTACTCTGGC-3′). The
cycling conditions were as follows: Initial denaturation at 95°C
for 30 sec, followed by 40 cycles of primer annealing at 60°C for
30 sec and an extension of amplicon at 72°C for 1 min. The RNA
levels were quantified using the 2−ΔΔCq method (26).
Western blot analysis
Protein extraction was performed by homogenizing the
rat liver in ice-cold hypotonic buffer containing PMSF and Nonidet
P-40. Total protein was quantified, mixed with sample buffer and
boiled at 90°C for 5 min. The protein concentration was determined
with a bicinchoninic acid kit (Beyotime Institute of
Biotechnology). Equal amount of protein (30 µg) was separated by
electrophoresis in 12% SDS-PAGE, transferred to PVDF membranes.
After blocking with 5% non-fat milk in TBST (0.1% Tween-20) for 1 h
at room temperature, the membranes were incubated with antibodies
against ATF4 (1:1,000), eIF2α (1:500), p-eIF2α (1:500), GRP78
(1:1,000), IRE1α (1:1,000), p-PERK (1:500), PERK (1:1,000), Tubulin
(1:2,000), p-IRE1α (1:500), Ki67 (1:2,000), ATF6 (1:2,000), GAPDH
(1:5,000), PDIA4 (1:1,000) or GRP94 (1:1,000) overnight at 4°C. All
the antibodies were diluted in 5% non-fat milk in TBST. Slides were
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG H&L (cat. no. 205718; 1:2,000; Abcam) or goat
anti-rat IgG H&L (cat. no. 97057; 1:2,000; Abcam) secondary
antibodies for 2 h at room temperature. The immune complexes were
visualized with an enhanced chemiluminescence detection kit (GE
Healthcare Life Sciences). The quantification of protein expression
was normalized with control protein expression. Band intensity
quantification was performed using ImageQuant TL software (version
7.0; GE Healthcare Life Sciences).
Statistical analysis
Assays were conducted in three independent
experiments. Statistical comparisons were performed using one-way
analysis of variance followed by Tukey's multiple comparison post
hoc test using GraphPad Prism 5.0 software (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Caudatin inhibited HCC development in
rats
The therapeutic effect of caudatin in DEN-induced
HCC rats was assessed using MRI. The liver tumors appeared
homogeneously hypo- or isointense in T1-weighted MR images and
hyperintense in DWI and T2 sequence images relative to the adjacent
normal liver (Fig. 1). The liver
tumors appeared iso- or slightly hypo-intense on T1CE images, as
indicated by the red asterisk in Fig.
1. DEN-induced rats demonstrated a higher number of tumor
nodules in the liver as observed in the MRI analysis. Tumor nodules
were considerably inhibited in the caudatin group compared with the
model rats, particularly in the 50 mg/kg treatment group.
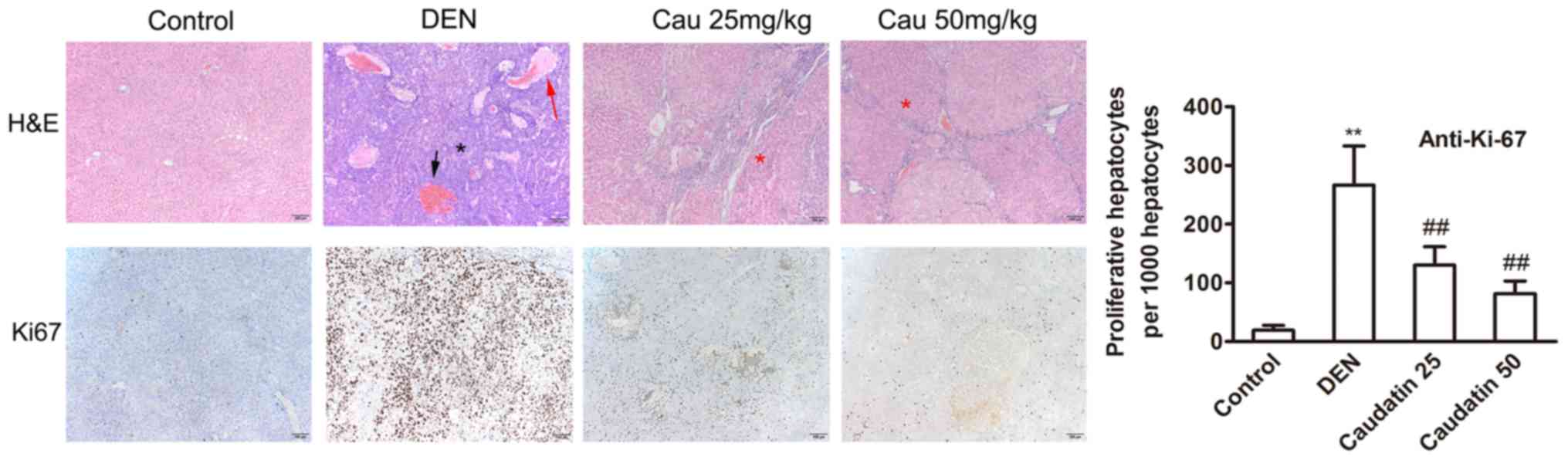
DEN-initiation resulted in multiple and visible
surface liver tumors (data not shown). In histological staining
during end-point necropsy, histopathology examinations further
verified the observations of MR imaging. The DEN-induced tumors
were either well, moderate, or poorly differentiated HCC,
confirming the progress of HCC as a result of the DEN insult
(Figs. 2 and S1). At week 20, tumor nodules were
observed in a background of fibrosis (Figs. 2 and S1). Additionally, hemorrhage, adenoid
structures and necrosis were observed in the DEN group.
Furthermore, pseudoadenoid structures were formed by necrosis of
central tumors (Fig. S1). In the
caudatin group, tumor necrosis was attenuated and the number of
cancerous cells reduced; however, fibrosis, hepatic sinus dilation
and infiltration of inflammatory cells were still obvious (Fig. 2). Caudatin also inhibited the
proliferation marker Ki-67 (Fig.
2). Food intake by weight was similar among the four groups and
caudatin demonstrated no significant effect on body weight
(Table I). Notably, caudatin
substantially reduced HCC incidence, macroscopic nodules and
hepatic preneoplastic lesions that may advance into tumors
(Table I).
 | Table I.Effect of caudatin on the body
weight, food consumption and the number of macroscopic hepatocyte
nodules. |
Table I.
Effect of caudatin on the body
weight, food consumption and the number of macroscopic hepatocyte
nodules.
|
| Groups |
|---|
|
|
|
|---|
| Variable | Control | DEN | Caudatin 25
mg/kg | Caudatin 50
mg/kg |
|---|
| Final body weight,
g | 559.4±19.5 |
400.1±37.2a | 414.2±39.3 | 447.3±49.7 |
| Food consumption,
g/d | 2.9±0.2 | 3.0±0.2 | 2.9±0.1 | 3.0±0.2 |
| Total no. of
nodules | 0 | 78 | 55 | 45 |
| Mean measurement of
nodule | 0 | 13±2a | 9±1b | 7±1b |
| >3 mm | 0 | 39 | 22 | 15 |
| <3 to >1
mm | 0 | 19 | 18 | 12 |
| <1 mm | 0 | 20 | 15 | 18 |
Effect of caudatin on biochemical
markers of hepatic injury
The serum levels of TBIL, AST, ALT and
malondialdehyde MDA were significantly elevated in the liver
homogenates following DEN treatment (Table II; P<0.05). ALT was elevated to
30.65±2.02 in DEN-treated rats and reduced to 18.61±0.85 in
caudatin-treated rats (50 mg/kg) (P<0.05), compared with the
normal group. The base level of ALT in the control group was
10.85±1.01 U/ml. Simultaneously, AST increased in DEN rats
(36.04±2.48) and markedly decreased to 23.10±1.18 U/ml in
caudatin-treated rats (P<0.05). TBIL increased to 1.02±0.18 in
the DEN group (vs. 0.27±0.08, control) but was reduced
substantially (0.77±0.19) in rats administered caudatin following
DEN (P<0.05). In the DEN-induced rats, the MDA level was
528.18±58.42 mol/g in liver tissues and reduced to 370.19±32.57
mol/g in the caudatin-treated group (P<0.05). The prevention of
ALT, AST and TBIL leakage from the liver suggested a potential
protective effect of caudatin in DEN-induced liver damage.
 | Table II.The effect of caudatin on biochemical
markers of hepatic injury in DEN rats. |
Table II.
The effect of caudatin on biochemical
markers of hepatic injury in DEN rats.
| Treatment | ALT, U/ml | AST, U/ml | TBIL, mg/dl | MDA, mol/g
tissue |
|---|
| Control | 10.85±1.01 | 14.75±0.71 | 0.27±0.08 | 336.70±23.98 |
| DEN |
30.65±2.02a |
36.04±2.48a |
1.02±0.18a |
528.18±58.42a |
| Caudatin 25
mg/kg |
27.29±1.60b |
28.35±1.18b | 0.83±0.11 |
436.79±22.97b |
| Caudatin 50
mg/kg |
18.61±0.85b |
23.10±1.18b |
0.77±0.19b |
370.19±32.57b |
Caudatin-mediated repression in
hepatic tumorigenesis is associated with reduced hepatic
pro-inflammatory cytokines
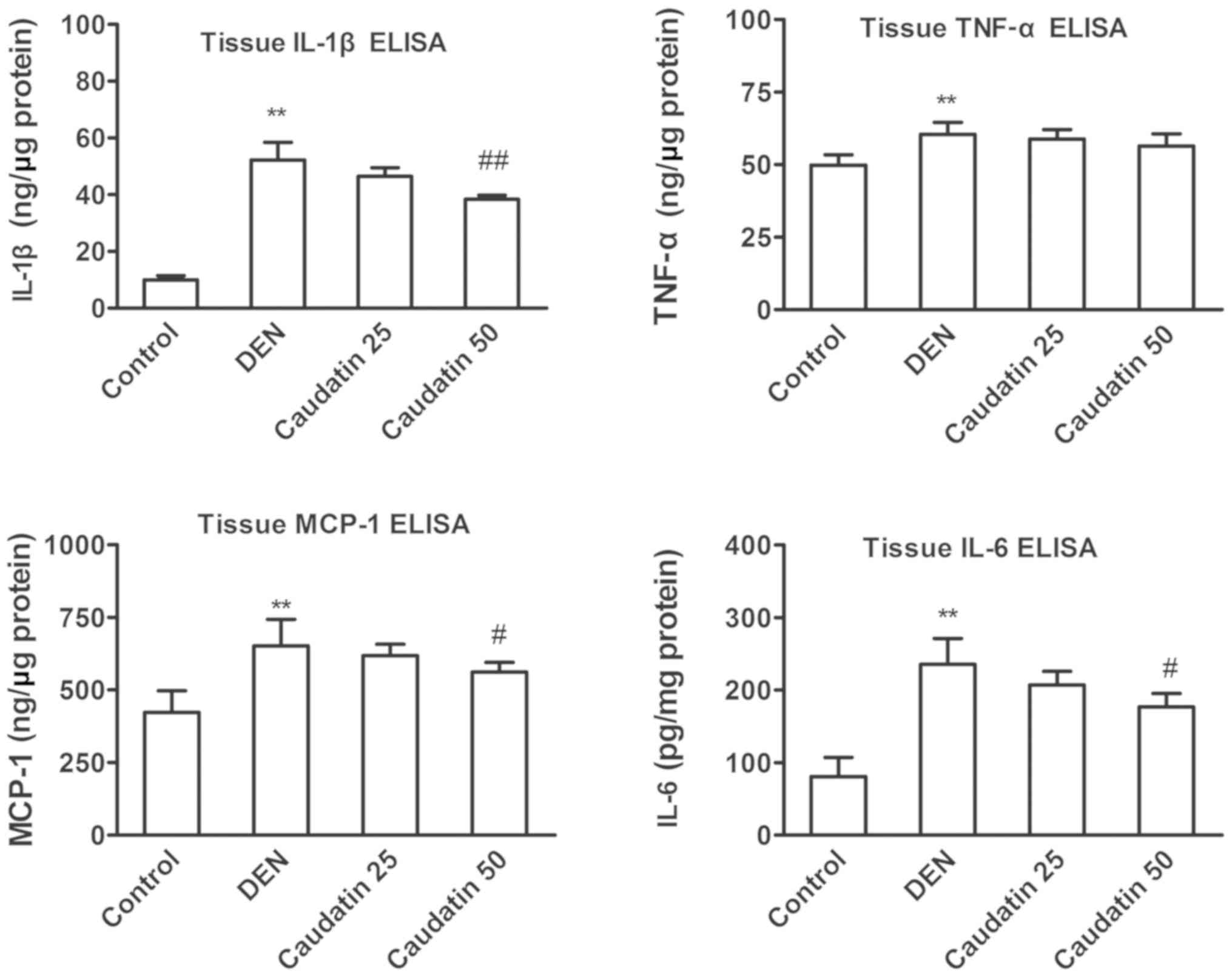
Numerous animal studies have suggested that
DEN-promoted liver tumorigenesis is associated with an amplified
pro-inflammatory response (27–29).
In the present study, the infiltration of inflammatory cells was
demonstrated in the H&E staining of the liver (Fig. 2). Consistently, the tissue protein
levels of pro-inflammatory cytokines, including IL-6, TNF-α, MCP-1
and IL-1β, were elevated in the DEN group compared with the control
group (Fig. 3). IL-6, MCP-1 and
IL-1β were reduced in caudatin-treated rats (P<0.05).
Effect of caudatin on chaperone
expression in the HCC model
Reportedly, DEN-induced hepatocarcinogenesis induces
ERS (13). Therefore, the
expression of the (co-)chaperones GRP94, GRP78 and PDIA4 in liver
nodules was examined. Based on immunohistochemical analysis, it was
observed that the basal level of pro-survival GRP78 in non-HCC
livers was markedly low. Notably, a considerable amount of GRP78
was observed in the liver nodules of the DEN group (Fig. 4). However, an inhomogeneous pattern
of GRP78-positive HCC cells was observed within the nodules, with
only a few GRP78-positive cells in the surrounding tissue. The
protein and mRNA level of GRP78 was reduced following caudatin
treatment (Fig. 4). Additionally,
both mRNA and protein levels of GRP94 and PDIA3 followed a similar
pattern.
Effect of caudatin on the expression
of ER sensors and UPR targets in DEN-induced liver tumors
Next, the effects of caudatin on the pattern of UPR
signaling in liver nodules was evaluated. First, the deactivation
of UPR targets was examined at the protein level. Reportedly, the
PERK-eIF2α-ATF4 pathway is activated during hepatocarcinogenesis
(30). p-PERK mediates the
phosphorylation of the eIF2a, p-eIF2a subsequently activates ATF4
and ATF4 upregulates the transcription of growth arrest and
DNA-damage-inducible protein 34 (GADD34) (31). In the present study, DEN markedly
activated the PERK-eIF2α-ATF4 pathway, as indicated by the
significantly enhanced expression of p-PERK and p-eIF2α and
downstream signaling molecules (ATF4 and GADD34). Consistent with
the transcriptional suppression, caudatin inhibited the ATF4
protein levels (Fig. 5A).
Furthermore, caudatin repressed ATF4 and GADD34 transcription
(Fig. 5B). In addition, the
phosphorylation of PERK and eIF2a was inhibited following caudatin
treatment.
 | Figure 5.Effect of caudatin on the expression
of IRE1α and ATF6 and activation of the PERK/eIF2α/ATF4 pathway in
the DEN-induced HCC model. (A) Representative western blot analysis
of ERS protein expression. (B) Reverse transcription-quantitative
PCR of ERDJ4, ATF4, GADD34 following caudatin (50 mg/kg) treatment.
**P<0.01 vs. control, ##P<0.01 vs. DEN group.
IRE1, inositol requiring enzyme 1; ATF, activating transcription
factor; PERK, PKR-like endoplasmic reticulum kinase; eIF2α,
eukaryotic initiation factor 2α; DEN, diethylnitrosamine; HCC,
hepatocellular carcinoma; ERDJ4, endoplasmic reticulum DnaJ homolog
4; GADD34, growth arrest and DNA damage-inducible protein; p-,
phosphorylated. |
Western blotting revealed a robust cleavage of ATF6
in liver nodules compared with the mild expression of cleaved-ATF6
observed in saline-treated rat livers, indicating that the ATF6
pathway was activated at week 20 (Fig.
5A). Caudatin treatment reduced ATF6 cleavage. The reduced
ratio of full ATF6/cleaved ATF6 protein expression was clearly
upregulated following caudatin treatment.
Next, the IRE1 pathway was investigated. IRE1
activation promotes X-box binding protein 1 (XBP1) mRNA splicing to
generate a more active spliced XBP1 (XBP1s), inducing genes
involved in protein folding, such as endoplasmic reticulum DnaJ
homolog 4 (ERDJ4) (32). Notably,
the ratios of phosphorylated IRE1 to total IRE1 levels were higher
in liver nodules than normal liver tissues. However, caudatin
failed to impact the expression of phosphorylated IRE1.
Consistently, the RT-qPCR analyses revealed a non-significant
decrease in the mRNA level of the XBP-1 specific target gene, ERDJ4
(33) (Fig. 5B). In summary, these results
indicated that the DEN-triggered excessive UPR in rats was
suppressed by caudatin, probably through inhibition of the ATF6 and
PERK-eIF2α-ATF4 pathways, but not the IRE1 pathway.
Discussion
In the present study, caudatin markedly decreased
the incidence of HCC, and reduced macroscopic nodules and hepatic
preneoplastic lesions in the DEN-induced HCC model. DEN, a widely
used hepatocarcinogen, affects cancer initiation by inducing the
formation of DNA-strand breaks and carcinogen adducts, thereby
resulting in hepatocarcinogenesis (34). Reactive oxygen species (ROS) and
ERS have been demonstrated to play a role in DEN-induced
hepatocellular carcinogenesis (35). Oxidative stress can be enforced by
ERS (36). Furthermore,
nonalcoholic steatohepatitis, hepatosteatosis and viral hepatitis
also increase the risk of HCC. ERS is involved in the pathogenesis
of these disorders (37,38). MDA, ALT, AST and TBIL are valuable
markers, widely used in animal studies to diagnose and observe the
progress of hepatocarcinogenesis (39,40).
Consistently, the values of previously mentioned parameters
increased sharply in the DEN-group as compared with the normal
control group (Table II) in the
present study. Normalization of elevated TBIL, MDA, AST and ALT
release from the liver proposes a potent protective effect of
caudatin against DEN-induced liver damage.
ER provides a quality control system, regulating the
modification and folding of membrane and secretory proteins and
eliminating misfolded polypeptides through autophagic degradation
or ER-associated degradation (41). A variety of toxic insults including
Ca2+ overload, failure of protein folding, synthesis,
degradation or transport and hypoxia can interrupt the ER function
and give rise to ERS (42). The
accumulation of misfolded and unfolded proteins in the ER is
denoted as ERS (43,44). Tumors frequently yield increased
mutant proteins, beyond the normal ER capacity, with the vascular
supply ultimately failing to meet the nutrient demands (45). The UPR reinstates protein folding
homeostasis and alleviates ERS by decreasing protein synthesis,
facilitating protein degradation, augmenting protein folding and
enhancing lipid synthesis (46).
Additionally, the UPR exerts an essential role in cancer cells
maintaining malignancy and therapy resistance (47). Compared with normal tissues,
sustained UPR activation has been described in numerous solid tumor
types, including HCC liver tissues (48).
The major chaperone, GRP78, is a dominant supervisor
of the UPR (49). Generally, GRP78
is associated with a greater risk of cancer recurrence and poor
outcomes. GRP78 can impede pro-apoptotic and caspase-4 pathways,
stimulating metastasis and therefore yielding a worse prognosis
(50). In some
toxicology/pharmacokinetic studies in patients and monkeys,
anti-GRP78 antibodies were well tolerated (51,52).
Furthermore, despite the partial GRP78 levels following treatment
with anti-GRP78 agents, the adult liver can function normally,
indicating that the anti-GRP78 damage to the normal liver may be
limited (51). In the present
study, the base liver levels of pro-survival GRP78 were relatively
low and DEN treatment induced a marked amount of GRP78 in the liver
tumors. In immunohistochemical staining, the number of
GRP78-positive cells were considerably reduced by caudatin
treatment. This result was further confirmed by RT-qPCR and western
blot analysis. A similar tendency was observed with other
chaperones including GRP94 and PDIA4.
ERS stimulates exosome release in PERK- and
IRE1α-dependent manners (53). A
rapid increase in ER-related proteins, such as ATF6 and spliced
XBP1, is observed in patients with severe liver fibrosis or with
HCC, as well as in CCl4-induced fibrotic mouse liver
tissues (19,54,55).
Consistently, both IRE1 and ATF6, as well as the PERK arms of the
UPR, exhibited notable tumor-specific activation in the present
study. ATF6 cleavage was elevated in the DEN-induced rat liver
nodules compared with the saline group at week 20. The DEN-induced
activation of ERS sensors, the PERK pathway and ATF6 cleavage, were
evidently reduced by caudatin treatment. Nevertheless, the effect
of caudatin on the ratio of phosphorylated IRE1α/total IRE1α and on
the transcription of the downstream target, ERDJ4, was minimal.
IRE1 is the most conserved transducer of the UPR, a surveillance
mechanism that guarantees homeostasis in the eukaryotic ER.
Notably, PERK mediates the cell cycle exit during the mammalian UPR
(56). IRE1 is activated by the
binding to unfolded proteins or separation from the suppressive
interaction with chaperone GRP78; ultimately, IRE1 catalyzes the
XBP1 transcript (57). XBP1 mRNA
further splices into XBP1s, which participates in the protein
folding of ERDJ4. In addition, GRP78 overexpression coupled with
ERDJ4 shrinks the induction of CHOP in ERS and decreases
ERS-induced apoptosis (58).
It has been reported that excessive or sustained
activation of the UPR results in chronic inflammation (59). The transmission of ERS to
macrophages promotes the inflammatory response in the HCC
microenvironment (60).
Additionally, cytokines can augment ERS in a positive feedback
manner and encourage tumor growth (61). IL-6 is one of the best
characterized tumorigenic, inflammatory cytokines, especially
stimulating the development of HCC (62,63).
Elevated IL-6 levels have been observed in both HCC and liver
cirrhosis (64). Even though IL-6
is predominantly secreted by resident immune cells, hepatocytes
contribute to the total IL-6 expression in the liver
microenvironment (7). In turn,
IL-6 hastens compensatory hepatocyte proliferation, principally
through tumor progression (65).
In the present study, liver inflammatory foci and hepatic
pro-inflammatory biomarkers, including IL-6, MCP-1 and IL-1β, were
significantly reduced by caudatin. The levels of Ki67, a mitotic
marker expressed from the mid-G1 phase to the end of mitosis, were
also substantially reduced by caudatin. Notably, accumulating data
has indicated that Ki67 is involved in the regulation of mitotic
progression, including chromatin organization, DNA replication and
interactions with motor proteins to control centrosome separation
(66). A fraction of
Ki-67-positive tumor cells is often correlated with the clinical
course of cancer and the tumor grade (67). A previous study observed a positive
correlation between the Ki67 expression levels and the risk of
local recurrence (68).
In summary, the present study demonstrated caudatin
as an effective anti-hepatocarcinogenesis compound, suggesting that
the anticancer effects are probably mediated by regulating the
PERK-ATF4-eIF2α pathway and ATF6 cleavage. Concerning the decline
of GRP78 by caudatin in liver nodules, further studies are required
to identify the potential of caudatin in ameliorating metastasis
and chemoresistance, advancing the prognosis in HCC patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81773947, 81873055,
81303275 and 81603382) and Foundation for High-Level Talent in Six
Areas of Jiangsu Province (grant no. WSN-042), ‘Double First-Class’
University project of China Pharmaceutical University (grant nos.
CPU2018GF07 and CPU2018PZQ19), Key research projects on
modernization of traditional Chinese medicine (grant no.
2018YFC1706900), Medical innovation team of Jiangsu province (grant
no. CXTDB2017003), Research Plan for Chinese Medicine Bureau of
Jiangsu province (grant no. YB2017034). Scientific Research Project
of State Traditional Chinese Medicine Clinical Base Construction
(grant no. JDZX2015072), Program for Innovative Research Team of
Six Talent Peaks Project in Jiangsu Province (grant no.
SWYY-CXTD-004).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
YL and LF conceived and designed the experiments.
JS, WD and XJ carried out the experiments. JS and WD drafted the
manuscript. ZX and WD performed the MRI. BL and DL helped with the
statistical analysis. LZ and LC helped with the ELISA analysis. JS
and XJ revised the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All of the animal procedures, including housing,
care and experimental protocols, were approved by the Animal Care
and Use Committee of Affiliated Hospital of Integrated Traditional
Chinese and Western Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATF
|
activating transcription factor
|
|
CHOP
|
CCAAT/enhancer-binding protein
homologous protein
|
|
DEN
|
diethylnitrosamine
|
|
eIF2α
|
eukaryotic initiation factor 2α
|
|
ER
|
endoplasmic reticulum
|
|
ERDJ4
|
endoplasmic reticulum DnaJ homolog
4
|
|
GADD34
|
growth arrest and DNA damage-inducible
protein
|
|
GRP78
|
glucose-regulated protein, 78 kDa
|
|
GRP94
|
glucose-regulated protein, 94 kDa
|
|
HCC
|
hepatocellular carcinoma
|
|
IRE1
|
inositol requiring enzyme 1
|
|
PERK
|
PKR-like endoplasmic reticulum
kinase
|
|
PDIA4
|
protein disulfide-isomerase A4
|
|
UPR
|
unfolded protein response
|
|
XBP1s
|
spliced X-box-binding protein 1
|
References
|
1
|
Wang Y, Yang Z, Wang L, Sun L, Liu Z, Li
Q, Yao B, Chen T, Wang C, Yang W, et al: miR-532-3p promotes
hepatocellular carcinoma progression by targeting PTPRT. Biomed
Pharmacother. 109:991–999. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang D, Xiong Z, Xu J, Yin J and Luo R:
Chemopreventive mechanisms of galangin against hepatocellular
carcinoma: A review. Biomed Pharmacother. 109:2054–2061. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shlomai A, de Jong YP and Rice CM: Virus
associated malignancies: The role of viral hepatitis in
hepatocellular carcinoma. Semin Cancer Biol. 26:78–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Fan L, Yu H, Zhang J, He Y, Feng D,
Wang F, Li X, Liu Q, Li Y, et al: Endoplasmic reticulum stress
causes liver cancer cells to release Exosomal miR-23a-3p and
Up-regulate programmed death Ligand 1 expression in macrophages.
Hepatology. 70:241–258. 2019.PubMed/NCBI
|
|
5
|
Li G, Liu D, Cooper TK, Kimchi ET, Qi X,
Avella DM, Li N, Yang QX, Kester M, Rountree CB, et al: Successful
chemoimmunotherapy against hepatocellular cancer in a novel murine
model. J Hepatol. 66:75–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu K, Kryczek I, Chen L, Zou W and Welling
TH: Kupffer cell suppression of CD8+ T cells in human
hepatocellular carcinoma is mediated by B7-H1/programmed death-1
interactions. Cancer Res. 69:8067–8075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park EJ, Lee JH, Yu GY, He G, Ali SR,
Holzer RG, Osterreicher CH, Takahashi H and Karin M: Dietary and
genetic obesity promote liver inflammation and tumorigenesis by
enhancing IL-6 and TNF expression. Cell. 140:197–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clarke HJ, Chambers JE, Liniker E and
Marciniak SJ: Endoplasmic reticulum stress in malignancy. Cancer
Cell. 25:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peñaranda Fajardo NM, Meijer C and Kruyt
FA: The endoplasmic reticulum stress/unfolded protein response in
gliomagenesis, tumor progression and as a therapeutic target in
glioblastoma. Biochem Pharmacol. 118:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan L, Sun G, Ma T, Zhong F, Lei Y, Li X
and Wei W: Melatonin reverses tunicamycin-induced endoplasmic
reticulum stress in human hepatocellular carcinoma cells and
improves cytotoxic response to doxorubicin by increasing CHOP and
decreasing survivin. J Pineal Res. 55:184–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F
and Wei W: Melatonin sensitizes human hepatoma cells to endoplasmic
reticulum stress-induced apoptosis. J Pineal Res. 52:322–331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Tsai YH and Tseng SH: HDAC
Inhibitors and RECK modulate endoplasmic reticulum stress in tumor
cells. Int J Mol Sci. 18(pii): E2582017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vandewynckel YP, Laukens D, Bogaerts E,
Paridaens A, Van den Bussche A, Verhelst X, Van Steenkiste C,
Descamps B, Vanhove C, Libbrecht L, et al: Modulation of the
unfolded protein response impedes tumor cell adaptation to
proteotoxic stress: A PERK for hepatocellular carcinoma therapy.
Hepatol Int. 9:93–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J and Lee AS: Stress induction of
GRP78/BiP and its role in cancer. Curr Mol Med. 6:45–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lebeau P, Byun JH, Yousof T and Austin RC:
Pharmacologic inhibition of S1P attenuates ATF6 expression, causes
ER stress and contributes to apoptotic cell death. Toxicol Appl
Pharmacol. 349:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spaan CN, Smit WL, van Lidth de Jeude JF,
Meijer BJ, Muncan V, van den Brink GR and Heijmans J: Expression of
UPR effector proteins ATF6 and XBP1 reduce colorectal cancer cell
proliferation and stemness by activating PERK signaling. Cell Death
Dis. 10:4902019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scaiewicz V, Nahmias A, Chung RT, Mueller
T, Tirosh B and Shibolet O: CCAAT/enhancer-binding protein
homologous (CHOP) protein promotes carcinogenesis in the
DEN-induced hepatocellular carcinoma model. PLoS One. 8:e810652013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shuda M, Kondoh N, Imazeki N, Tanaka K,
Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, et al:
Activation of the ATF6, XBP1 and grp78 genes in human
hepatocellular carcinoma: A possible involvement of the ER stress
pathway in hepatocarcinogenesis. J Hepatol. 38:605–614. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Fu X, Zhao S, Fu X, Zhang H, Shao
L, Li G and Fan C: Antiangiogenic properties of caudatin in
vitro and in vivo by suppression of VEGF-VEGFR2-AKT/FAK
signal axis. Mol Med Rep. 16:8937–8943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan ZW, Xie S, Hu SY, Liao T, Liu P, Peng
KH, Yang XZ, He ZL, Tang HY, Cui Y, et al: Caudatin targets
TNFAIP1/NF-κB and cytochrome c/caspase signaling to suppress tumor
progression in human uterine cancer. Int J Oncol. 49:1638–1650.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng Y and Ding Y: Pharmacokinetics and
tissue distribution study of caudatin in normal and
diethylnitrosamine-induced hepatocellular carcinoma model rats.
Molecules. 20:4225–4237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu XY, Zhang S, Wang K, Yang MF, Fan CD
and Sun BL: Caudatin inhibits human Glioma cells growth through
triggering DNA damage-mediated cell cycle arrest. Cell Mol
Neurobiol. 35:953–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Zhang X, Liu X, Tan Z, Yang C, Ding
X, Hu X, Zhou J, Xiang S, Zhou C and Zhang J: Caudatin induces cell
apoptosis in gastric cancer cells through modulation of
Wnt/beta-catenin signaling. Oncol Rep. 30:677–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo Y, Sun Z, Li Y, Liu L, Cai X and Li Z:
Caudatin inhibits human hepatoma cell growth and metastasis through
modulation of the Wnt/β-catenin pathway. Oncol Rep. 30:2923–2928.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bakiri L, Hamacher R, Graña O,
Guío-Carrión A, Campos-Olivas R, Martinez L, Dienes HP, Thomsen MK,
Hasenfuss SC and Wagner EF: Liver carcinogenesis by FOS-dependent
inflammation and cholesterol dysregulation. J Exp Med.
214:1387–1409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang S, Ma HY, Zhong Z, Dhar D, Liu X, Xu
J, Koyama Y, Nishio T, Karin D, Karin G, et al: NADPH oxidase 1 in
liver macrophages promotes inflammation and tumor development in
mice. Gastroenterology. 156:1156–1172.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mahmoud AM, Mohammed HM, Khadrawy SM and
Galaly SR: Hesperidin protects against chemically induced
hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and
TGF-β1/Smad3 signaling, and amelioration of oxidative stress and
inflammation. Chem Biol Interact. 277:146–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu JW, Liao CY, Yang WY, Lin YM, Jin SL,
Wang HD and Yuh CH: Overexpression of endothelin 1 triggers
hepatocarcinogenesis in zebrafish and promotes cell proliferation
and migration through the AKT pathway. PLoS One. 9:e853182014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobylewski SE, Henderson KA, Yamada KE and
Eckhert CD: Activation of the EIF2α/ATF4 and ATF6 Pathways in
DU-145 Cells by boric acid at the concentration reported in men at
the US mean boron intake. Biol Trace Elem Res. 176:278–293. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shoulders MD, Ryno LM, Genereux JC,
Moresco JJ, Tu PG, Wu C, Yates JR III, Su AI, Kelly JW and Wiseman
RL: Stress-independent activation of XBP1s and/or ATF6 reveals
three functionally diverse ER proteostasis environments. Cell Rep.
3:1279–1292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee AH, Iwakoshi NN and Glimcher LH: XBP-1
regulates a subset of endoplasmic reticulum resident chaperone
genes in the unfolded protein response. Mol Cell Biol.
23:7448–7459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tolba R, Kraus T, Liedtke C, Schwarz M and
Weiskirchen R: Diethylnitrosamine (DEN)-induced carcinogenic liver
injury in mice. Lab Anim. 49 (Suppl 1):S59–S69. 2015. View Article : Google Scholar
|
|
35
|
Lin H, Liu XB, Yu JJ, Hua F and Hu ZW:
Antioxidant N-acetylcysteine attenuates hepatocarcinogenesis by
inhibiting ROS/ER stress in TLR2 deficient mouse. PLoS One.
8:e741302013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Zhang X, Zhang S, Huang H, Wu J,
Wang Y, Yuan L, Liu C, Zeng X, Cheng X, et al: Oxidative stress
mediates Microcystin-LR-induced endoplasmic reticulum stress and
autophagy in KK-1 cells and C57BL/6 mice ovaries. Front Physiol.
9:10582018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu M, Du L, He Z, Yan L, Shi Y, Shang J
and Tang H: Increased ERp57 Expression in HBV-related
hepatocellular carcinoma: Possible correlation and prognosis.
Biomed Res Int. 2017:12526472017.PubMed/NCBI
|
|
38
|
Zhou X, Han D, Yang X, Wang X and Qiao A:
Glucose regulated protein 78 is potentially an important player in
the development of nonalcoholic steatohepatitis. Gene. 637:138–144.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nermin AHS, EL-Maraghy SA and Manal FI:
Diethylnitrosamine- induced hepatocarcinogenesis in rats: Possible
chemoprevention by blueberries. African J Bioch Res. 2:81–87.
2008.
|
|
40
|
Wang P, Ji R, Ji J and Chen F: Changes of
metabolites of acrylamide and glycidamide in acrylamide-exposed
rats pretreated with blueberry anthocyanins extract. Food Chem.
274:611–619. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ghosh S, Nandi S, Ghosh C and
Bhattacharyya K: Fluorescence dynamics in the endoplasmic reticulum
of a live cell: Time-resolved confocal microscopy. Chemphyschem.
17:2818–2823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang RZ, Huang XC, Zhang B, Jia HY, Liao
ZX and Wang HS: 16-O-caffeoyl-16-hydroxylhexadecanoic acid, a
medicinal plant-derived phenylpropanoid, induces apoptosis in human
hepatocarcinoma cells through ROS-dependent endoplasmic reticulum
stress. Phytomedicine. 41:33–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wakai T, Zhang N, Vangheluwe P and Fissore
RA: Regulation of endoplasmic reticulum Ca(2+) oscillations in
mammalian eggs. J Cell Sci. 126:5714–5724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang H, Bao X, Shen Y, Song M, Wang S,
Wang C and Hou J: Engineering protein folding and translocation
improves heterologous protein secretion in Saccharomyces
cerevisiae. Biotechnol Bioeng. 112:1872–1882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li X, Zhang K and Li Z: Unfolded protein
response in cancer: The physician's perspective. J Hematol Oncol.
4:82011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jiang CC, Yang F, Thorne RF, Zhu BK,
Hersey P and Zhang XD: Human melanoma cells under endoplasmic
reticulum stress acquire resistance to microtubule-targeting drugs
through XBP-1-mediated activation of Akt. Neoplasia. 11:436–447.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pfaffenbach KT and Lee AS: The critical
role of GRP78 in physiologic and pathologic stress. Curr Opin Cell
Biol. 23:150–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kao RH, Lai GM, Chow JM, Liao CH, Zheng
YM, Tsai WL, Hsia S, Lai IC, Lee HL, Chuang SE, et al: Opposite
regulation of CHOP and GRP78 and synergistic apoptosis induction by
selenium Yeast and Fish Oil via AMPK activation in lung
adenocarcinoma cells. Nutrients. 10(pii): E14582018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra
SK, Krasnoperov V, Dong D, Liu S, Li D, et al: Monoclonal antibody
against cell surface GRP78 as a novel agent in suppressing PI3K/AKT
signaling, tumor growth, and metastasis. Clin Cancer Res.
19:6802–6811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hensel F, Eckstein M, Rosenwald A and
Brandlein S: Early development of PAT-SM6 for the treatment of
melanoma. Melanoma Res. 23:264–275. 2013.PubMed/NCBI
|
|
53
|
Kanemoto S, Nitani R, Murakami T, Kaneko
M, Asada R, Matsuhisa K, Saito A and Imaizumi K: Multivesicular
body formation enhancement and exosome release during endoplasmic
reticulum stress. Biochem Biophys Res Commun. 480:166–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zuo L, Zhu Y, Hu L, Liu Y, Wang Y, Hu Y,
Wang H, Pan X, Li K, Du N and Huang Y: PI3-kinase/Akt
pathway-regulated membrane transportation of acid-sensing ion
channel 1a/Calcium ion influx/endoplasmic reticulum stress
activation on PDGF-induced HSC Activation. J Cell Mol Med.
23:3940–3950. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schonthal AH: Pharmacological targeting of
endoplasmic reticulum stress signaling in cancer. Biochem
Pharmacol. 85:653–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hamanaka RB, Bennett BS, Cullinan SB and
Diehl JA: PERK and GCN2 contribute to eIF2alpha phosphorylation and
cell cycle arrest after activation of the unfolded protein response
pathway. Mol Biol Cell. 16:5493–5501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sanchez-Alvarez M, Del Pozo MA and Bakal
C: Publisher correction: AKT-mTOR signaling modulates the dynamics
of IRE1 RNAse activity by regulating ER-mitochondria contacts. Sci
Rep. 8:64762018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Z, Chen J, Chen F, Yu D, Li R, Lv C,
Wang H, Li H, Li J and Cai Y: Tauroursodeoxycholic acid alleviates
secondary injury in the spinal cord via up-regulation of CIBZ gene.
Cell Stress Chaperones. 23:551–560. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bettigole SE and Glimcher LH: Endoplasmic
reticulum stress in immunity. Ann Rev Immunol. 33:107–138. 2015.
View Article : Google Scholar
|
|
60
|
Verfaillie T, Garg AD and Agostinis P:
Targeting ER stress induced apoptosis and inflammation in cancer.
Cancer Lett. 332:249–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Taniguchi K and Karin M: IL-6 and related
cytokines as the critical lynchpins between inflammation and
cancer. Semin Immunol. 26:54–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nakagawa H, Umemura A, Taniguchi K,
Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E,
Hidalgo J, et al: ER stress cooperates with hypernutrition to
trigger TNF-dependent spontaneous HCC development. Cancer Cell.
26:331–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xiang DM, Sun W, Ning BF, Zhou TF, Li XF,
Zhong W, Cheng Z, Xia MY, Wang X, Deng X, et al: The HLF/IL-6/STAT3
feedforward circuit drives hepatic stellate cell activation to
promote liver fibrosis. Gut. 67:1704–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gosain R, Anwar S, Miller A, Iyer R and
Mukherjee S: Interleukin-6 as a biomarker in patients with
hepatobiliary cancers. J Gastrointest Oncol. 10:537–545. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cuylen S, Blaukopf C, Politi AZ,
Müller-Reichert T, Neumann B, Poser I, Ellenberg J, Hyman AA and
Gerlich DW: Ki-67 acts as a biological surfactant to disperse
mitotic chromosomes. Nature. 535:308–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Basturk O, Yang Z, Tang LH, Hruban RH,
Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, et
al: The high-grade (WHO G3) pancreatic neuroendocrine tumor
category is morphologically and biologically heterogenous and
includes both well differentiated and poorly differentiated
neoplasms. Am J Surg Pathol. 39:683–690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Inwald EC, Klinkhammer-Schalke M,
Hofstadter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|