Introduction
Ischemic stroke is one of the most frequently
occurring conditions among older populations, accounting for a
large proportion of the morbidity and mortality rates worldwide
(1,2). Smoking habits, hypertension and
diabetes have all been listed as risk factors of ischemic stroke
(3,4). Ischemic stroke occurs following a
blockage in an artery leading to the brain; thus, an insufficient
supply of oxygen and glucose reaches the brain that is required for
cellular energy, which culminates in irreparable damage (5,6).
Currently, there are no effective approved treatments for ischemic
stroke (7). Patients are normally
treated with recombinant tissue plasminogen activator, undergo
surgical excision of the obstruction in the blood vessel or are
prescribed protective treatments following stroke, such as
fire-needle acupuncture (8).
During treatment, the limited reperfusion time window and rapid
blood reperfusion cause secondary injuries, namely reperfusion
injury, including hemorrhagic transformation and reactive oxygen
species (ROS)-induced injuries (9); thus, current treatment regimens are
not ideal. Despite significant research being conducted on
ischemia-reperfusion injury, little progress has been made.
Therefore, it remains an urgent requirement to investigate
effective treatment targets and determine the mechanism of action
of ischemia-reperfusion injury.
MicroRNAs (miRNAs/miRs) are single-stranded,
non-coding RNAs 22 nucleotides in length, which can silence gene
expression through transiently promoting translational arrest or
inducing the degradation of mRNA (10). Previous studies have reported that
miRNAs are involved in the pathophysiology of ischemic stroke
through suppressing post-stroke angiogenesis, inhibiting oxidative
stress, reducing neuronal loss, suppressing inflammation and
preventing excitotoxic injury (11–14).
Notably, miR-340-5p has been observed to relieve
chronic constriction injury-induced neuropathic pain and decrease
the inflammatory response (15).
miR-340-5p has also been demonstrated to suppress
hypoxia/reoxygenation-induced apoptosis and oxidative stress in
cardiomyocytes (16). Oxidative
stress and inflammation are the main causes of cerebral
ischemia-reperfusion injury, which has been established through
numerous previous studies (17–21).
For example, in one previous study, miR-340-5p was found to inhibit
inflammation and oxidative stress in cardiomyocytes and serve a
role in cerebral ischemia-reperfusion injury (16). Furthermore, it was reported that
miR-340-5p expression levels were decreased in the peripheral blood
following acute ischemic stroke (22), which suggested that miR-340-5p may
serve a vital role in ischemic stroke treatment. Based on these
previous studies, the present study aimed to investigate the
effects of miR-340-5p on a commonly used cell model of
ischemia-reperfusion injury, oxygen-glucose deprivation/reperfusion
(OGD/R)-induced PC12 cells (23–25).
The target gene of miR-340-5p was also investigated.
Materials and methods
Cell culture and OGD/R induction
PC12 cells (CRL-1721.1) were obtained from the
American Type Culture Collection and were seeded into 96-well
plates at a density of 1×104 cells/well. Cells were
cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA),
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin, and maintained in a humidified
atmosphere with 5% CO2 at 37°C. To study the effects of
microRNA-340-5p, the cells were divided into the control group,
OGD/R group, miR-NC group and miR-340-5p mimic group. For further
study on the underlying mechanism, the cells were divided into an
OGD/R group, miR-NC group, miR-340-5p mimic group, miR-340-5p mimic
+ empty plasmid group and a miR-340-5p mimic + Neurod4
overexpression group. OGD/R was performed in all the groups except
for control groups.
OGD/R was performed by culturing the cells in
glucose-free medium (RPMI-1640; Gibco; Thermo Fisher Scientific,
Inc.) in an incubator with CO2 (5%; v/v) and
N2 (95%; v/v) at 37°C. After 2 h of incubation, the
medium was replaced with normal medium and cells were incubated in
an atmosphere of 5% CO2 and 95% air at 37°C for 12
h.
Cell transfection
Cells were cultured in 6-well plates
(1×104 cells/well) containing RPMI-1640 medium without
antibiotics. Upon reaching 70% confluence, cells were transfected
with a miR-340-5p mimic, miR-negative control (NC), neuronal
differentiation 4 (Neurod4) pcDNA3.1 plasmid (10 µl/ml) or an empty
pcDNA3.1 plasmid (10 µl/ml) using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) under
serum-free conditions for 6 h. After 48 h of transfection, the
cells were collected for subsequent experiments. The miR-340-5p
mimic (5′-UUAUAAAGCAAUGAGACUGAUU-3′), miR-NC
(5′-UUCUCCGAACGUGUCACGUTT-3′), pcDNA3.1 empty plasmid and Neurod4
overexpression plasmid were all purchased from Shanghai GenePharma
Co., Ltd.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
qScript miRNA cDNA Synthesis kit (Quantabio), according to the
manufacturer's protocol. qPCR was subsequently performed using a
TaqMan™ Real Time PCR Mix (Thermo Fisher Scientific, Inc.). The
following primer pairs were used for qPCR: GAPDH forward,
5′-AATGGATTTGGACGCATTGGT-3′ and reverse,
5′-TTTGCACTGGTACGTGTTGAT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; Neurod4 forward,
5′-AGCTGGTCAACACACAATCCT-3′ and reverse,
5′-TTCCATAAGAGCCCGGTCTTC-3′; and miR-340-5p forward,
5′-GCGGTTATAAAGCAATGAGA-3′ and reverse, 5′-GTGCGTGTCGTGGAGTCG-3′.
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 8 min; followed by 40 cycles at 95°C for
15 sec and 60°C for 30 sec; and a final extension at 70°C for 35
sec. Expression levels were quantified using the 2−ΔΔCq
method (26) and normalized to
GAPDH or U6.
Detection of tumor necrosis factor
(TNF)-a, interleukin (IL)-1β, monocyte chemoattractant protein-1
(MCP-1) and IL-6
TNF-α (cat. no. BMS662), IL-1β (cat. no. ERIL1B),
MCP-1 (cat. no. BMS631INST) and IL-6 (cat. no. BMS625) ELISA kits
(Thermo Fisher Scientific, Inc.) were used to detect the levels of
TNF-α, IL-1β, MCP-1 and IL-6. Briefly, cells in the different
groups were lysed in lysis buffer (Beyotime Institute of
Biotechnology) and centrifuged (16,000 × g for 10 min at 4°C) to
collect the supernatants. The levels of TNF-α, IL-1β, MCP-1 and
IL-6 in the supernatants of each group were analyzed using the
corresponding kits, according to the manufacturer's protocols.
Flow cytometric analysis of
apoptosis
Flow cytometry was used to analyze the effects of
miR-340-5p overexpression on cell apoptosis with an Annexin V-FITC
Apoptosis Detection kit (eBioscience; Thermo Fisher Scientific,
Inc.). Cells were grouped into a control group, OGD/R group, miR-NC
group and miR-340-5p mimic group. To determine the effects of the
target binding between Neurod4 and miR-340-5p on the rate of cell
apoptosis, the cells were divided into an OGD/R group, miR-NC
group, miR-340-5p mimic group, miR-340-5p mimic + empty plasmid
group and a miR-340-5p mimic + Neurod4 overexpression group. OGD/R
was performed in all the groups except for control group. Following
their respective treatments, cells (1×105 cells/well) in
the different groups were washed with PBS and resuspended in the
binding buffer. Cells were subsequently incubated with 5 µl Annexin
V-FITC and 10 µl propidium iodide staining solution for 15 min at
room temperature in the dark. Apoptotic cells were analyzed using a
BD FACSCalibur™ flow cytometer (BD Biosciences) and BD CellQuest
software (version 5.1; BD Biosciences).
Measurement of nitric oxide (NO)/NADPH
levels
The levels of NO and NADPH were analyzed using a
Nitric Oxide Synthase Activity assay kit (colorimetric; cat. no.
ab211083; Abcam) and NADPH assay kit (colorimetric; cat. no.
ab186031; Abcam), respectively. Briefly, cells in the different
groups were lysed in lysis buffer (Beyotime Institute of
Biotechnology) and collected prior to being analyzed for the levels
of NO and NADPH using their corresponding kits, according to the
manufacturer's protocols.
Western blotting
Protein expression levels were analyzed using
western blotting. Total protein was extracted from cells using
lysis buffer (Beyotime Institute of Biotechnology) and lysates were
centrifuged (16,000 × g for 10 min at 4°C). Total protein was
quantified using a bicinchoninic acid assay kit [Yeasen
Biotechnology (Shanghai) Co., Ltd.] and proteins (30 µg/lane) were
separated via SDS-PAGE on a 10% gel. The separated proteins were
transferred to PVDF membranes (EMD Millipore) and blocked in 5%
skimmed milk for 2 h at room temperature. The membranes were
incubated at 4°C overnight with the following primary antibodies:
Anti-Bcl-2 (1:1,000; cat. no. ab196495; Abcam), anti-Bax (1:1,000;
cat. no. ab3250; Abcam), anti-Bad (1:1,000; cat. no. ab32445;
Abcam), anti-cleaved caspase 3 (1:1,000; cat. no. ab49822; Abcam),
anti-caspase 3 (1:1,000; cat. no. ab13847; Abcam),
anti-phosphorylated (p)-endothelial NOS (eNOS; 1:500; cat. no.
ab215717; Abcam), anti-eNOS (1:500; cat. no. ab199956; Abcam) and
anti-GADPH (1:500; cat. no. ab9485; Abcam). Following the primary
antibody incubation, the membranes were incubated with a
horseradish peroxidase-conjugated secondary antibody (1:5,000; cat.
no. ab7090; Abcam) for 1 h at room temperature. Protein bands were
visualized using an ImageQuant™ LAS 500 (GE Healthcare) and an ECL
western blotting substrate kit (cat. no. ab65623; Abcam).
ImageQuant TL software (version 7.0; Cytiva) was used to perform
densitometry.
Dual-luciferase reporter assay
Neurod4 was predicted as a target gene of
miR-340-5-5p using TargetScan software (version 7.2; http://www.targetscan.org/vert_72/). Thus, a
dual-luciferase reporter assay was performed to verify the target
binding of miRNA-340-5p and Neurod4. The wild-type (WT) 3′
untranslated region (3′UTR) binding site of Neurod4 was amplified
using PCR and cloned into a pmirGLO reporter plasmid (Promega
Corporation). The 3′UTR fragment of Neurod4 was also mutated,
resulting in a mutant (MUT) 3′UTR, using the Fast MultiSite
Mutagenesis System (Beijing Transgen Biotech Co., Ltd.) and cloned
into the pmirGLO reporter plasmid. Cells (5×104)
cultured in 24-well plates were co-transfected with an equal
concentration (450 ng/µl) of Neurod4 (WT or MUT) and miR-340-5p
mimic or miR-NC using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. After transfection for 48 h, the relative luciferase
activity was detected using a Dual-Luciferase Reporter assay system
(Promega Corporation), according to the manufacturer's protocols.
Luciferase activity was normalized to Renilla luciferase
activity.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc.) and data from three
independent experiments are presented as the mean ± SD. Statistical
differences were determined using a one-way ANOVA, followed by
Tukey's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-340-5p expression levels are
decreased in the OGD/R group and miR-340-5p overexpression reduces
the OGD/R-induced inflammatory status
The expression levels of miR-340-5p were
significantly reduced in the OGD/R group compared with the control
group (Fig. 1A), indicating that
miR-340-5p may have a certain role in OGD/R-induced cells. The
miR-340-5p mimic was successfully transfected into OGD/R-induced
cells; significantly increased expression levels of miR-340-5p were
observed in the miR-340-5p mimic group compared with the OGD/R and
miR-NC groups (Fig. 1B).
Subsequently, the effects of miR-340-5p overexpression on the
inflammatory status of cells were investigated. Compared with the
control group, the levels of TNF-α, IL-1β, MCP-1 and IL-6 were all
significantly increased in the OGD/R group (Fig. 1C), which demonstrated that the
OGD/R cell model was successfully induced. The OGD/R-induced
inflammatory status, which is indicated by the levels of TNF-α,
IL-1β, MCP-1 and IL-6, was decreased in the miR-340-5p mimic group
when compared with the OGD/R group (Fig. 1C).
 | Figure 1.miR-340-5p expression levels are
increased in the OGD/R group and the effect of miR-340-5p
overexpression on the inflammatory status. Expression levels of
miR-340-5p in the (A) control and OGD/R groups, and (B) OGD/R,
miR-NC and miR-340-5p mimic groups (C) Levels of TNF-α, IL-1β,
MCP-1 and IL-6 were analyzed in the different groups. ***P<0.001
vs. control or miR-NC group; ###P<0.001 vs. OGD/R
group. IL, interleukin; MCP-1, monocyte chemoattractant protein-1;
miR, microRNA; NC, negative control; OGD/R, oxygen-glucose
deprivation/reperfusion; TNF-α, tumor necrosis factor α. |
miR-340-5p overexpression reduces the
cell apoptotic rate induced by OGD/R in PC12 cells
The rate of cell apoptosis in the OGD/R group was
significantly increased compared with the control group (Fig. 2A and B), which further confirmed
that the OGD/R cell model was successfully induced. However,
OGD/R-induced apoptosis was significantly reduced by the miR-340-5p
mimic. To further validate these findings, the expression levels of
apoptotic proteins were analyzed. The expression levels of the
anti-apoptotic protein Bcl-2 were significantly decreased following
OGD/R induction compared with the control group, whereas
OGD/R-induced decreases in the Bcl-2 expression levels were
significantly increased in the miR-340-5p mimic group (Fig. 3). Furthermore, the expression
levels of pro-apoptotic proteins, Bax and cleaved caspase 3/caspase
3, were significantly increased in the OGD/R group compared with
the control group, and this effect was significantly reduced
following miR-340-5p overexpression (Fig. 3). All these findings suggested that
miR-340-5p overexpression may inhibit cell apoptosis through
increasing the expression levels of Bcl-2, and decreasing those of
Bax, cleaved caspase 3 and caspase 3.
miR-340-5p overexpression inhibits NO
production, reduces NADPH levels and increases the relative
expression levels of p-eNOS in OGD/R-induced PC12 cells
The relative expression levels of p-eNOS were
determined as the ratio of p-eNOS/eNOS/GADPH. NO, which is induced
by OGD/R in PC12 cells, was evaluated herein. The NO levels were
significantly increased in the OGD/R group compared with the
control group, whereas these OGD/R-induced increased levels were
significantly reduced in the miR-340-5p mimic group (Fig. 4A). Furthermore, p-eNOS/eNOS was
significantly decreased in the OGD/R group compared with the
control group, and this effect was partly reversed in the
miR-340-5p mimic group (Fig. 4C and
D). NADPH is an important indicator of the presence of
oxidative stress, which generates ROS (27). OGD/R-induced increases in NADPH
levels were significantly reduced by miR-340-5p overexpression
(Fig. 4B). All these results
indicated that miR-340-5p overexpression may exert a protective
effect over OGD/R-induced PC12 cells through increasing eNOS
activity, and reducing NADPH levels and NO production.
Neurod4 is a target of miR-340-5p in
OGD/R-induced PC12 cells
It was predicted by TargetScan that Neurod4 was a
target gene of miR-340-5p (Fig.
5A), thus a dual-luciferase reporter assay was used for further
validation. The relative luciferase activity was significantly
reduced in the 3′UTR-WT- Neurod4 + miR-340-5p mimic group compared
with the 3′UTR-MUT-Neurod4 + miR-340-5p mimic group (Fig. 5B), indicating that miR-340-5p may
target Neurod4 in OGD/R-induced PC12 cells. Furthermore, Neurod4
expression levels were significantly increased in the OGD/R group
compared with the control group, and this effect was significantly
reversed following the addition of the miR-340-5p mimic (Fig. 5C). This observation further
validated that Neurod4 may be a target of miR-340-5p in
OGD/R-induced PC12 cells.
miR-340-5p overexpression reduces the
inflammatory status, whereas Neurod4 overexpression counteracts the
effects of miR-340-5p overexpression on OGD/R-induced PC12
cells
Neurod4 expression levels were significantly
increased in the Neurod4 overexpression group compared with the
empty plasmid group (Fig. 6A),
indicating that Neurod4 overexpression was successful in the PC12
cells. The OGD/R-induced levels of TNF-α, IL-1β, MCP-1 and IL-6
were significantly reduced following miR-340-5p overexpression in
the OGD/R + miR-340-5p mimic group (Fig. 6B-E). However, following Neurod4
overexpression, the anti-inflammatory effects of miR-340-5p
overexpression on OGD/R-induced PC12 cells were reversed and the
inflammatory levels were significantly increased. These findings
indicated that the anti-inflammatory effects of miR-340-5p
overexpression on OGD/R-induced PC12 cells may be achieved by
decreasing the levels of TNF-α, IL-1β, MCP-1 and IL-6 through
downregulating Neurod4 expression.
 | Figure 6.Neurod4 overexpression counteracts
the effects of miR-340-5p overexpression on inflammatory status in
OGD/R-induced PC12 cells. (A) Neurod4 expression levels were
analyzed in the different groups. Levels of the inflammatory
markers (B) TNF-α, (C) IL-6, (D) IL-1β and (E) MCP-1 were
determined in the different groups. *P<0.05, **P<0.01,
***P<0.001 vs. (A) empty plasmid or (B-E) OGD/R group; (B-E)
###P<0.001 vs. OGD/R + miR-NC group;
ΔΔΔP<0.001 vs. miR-340-5p mimic + empty plasmid
group. IL, interleukin; MCP-1, monocyte chemoattractant protein-1;
miR, microRNA; NC, negative control; Neurod4, neuronal
differentiation 4; OGD/R, oxygen-glucose deprivation/reperfusion;
TNF-α, tumor necrosis factor α. |
miR-340-5p overexpression reduces
apoptosis and Neurod4 overexpression counteracts the effects of
miR-340-5p overexpression on OGD/R-induced PC12 cells
The OGD/R-induced increases in cell apoptosis were
significantly reduced following miR-340-5p overexpression (Fig. 7A and B); however, the apoptotic
rate in OGD/R-induced cells transfected with the miR-340-5p mimic
was significantly increased following Neurod4 overexpression. These
findings suggested that Neurod4 overexpression may reverse the
effects of miR-340-5p overexpression on cell apoptosis in
OGD/R-induced PC12 cells. As an anti-apoptotic protein, Bcl-2
expression levels were observed to be significantly increased in
the OGD/R + miR-340-5p mimic group; however, this effect was
reversed following Neurod4 overexpression (Fig. 7C). In addition, the expression
levels of the pro-apoptotic proteins Bax, Bad, cleaved caspase 3
and caspase 3 demonstrated the opposite trend compared with the
Bcl-2 expression levels in each group. Taken together, these
findings suggested that miR-340-5p overexpression may protect
OGD/R-induced cells from apoptosis through increasing the
expression levels of Bcl-2, and decreasing the expression levels of
Bax, Bad, cleaved caspase 3 and caspase 3 through decreasing
Neurod4 expression.
 | Figure 7.Neurod4 overexpression counteracts
the effect of miR-340-5p overexpression on the apoptotic rate in
OGD/R-induced PC12 cells. (A) Apoptotic rate was determined in the
different groups. (B) Semi-quantification of (A). (C) Expression
levels of Bax, Bcl-2, cleaved caspase and cleaved caspase 3 in
different groups. *P<0.05, **P<0.01, ***P<0.001 vs. OGD/R
group; ###P<0.001 vs. OGD/R + miR-NC group;
ΔP<0.05, ΔΔP<0.01,
ΔΔΔP<0.001 vs. miR-340-5p mimic + empty plasmid
group. miR, microRNA; NC, negative control; Neurod4, neuronal
differentiation 4; OGD/R, oxygen-glucose
deprivation/reperfusion. |
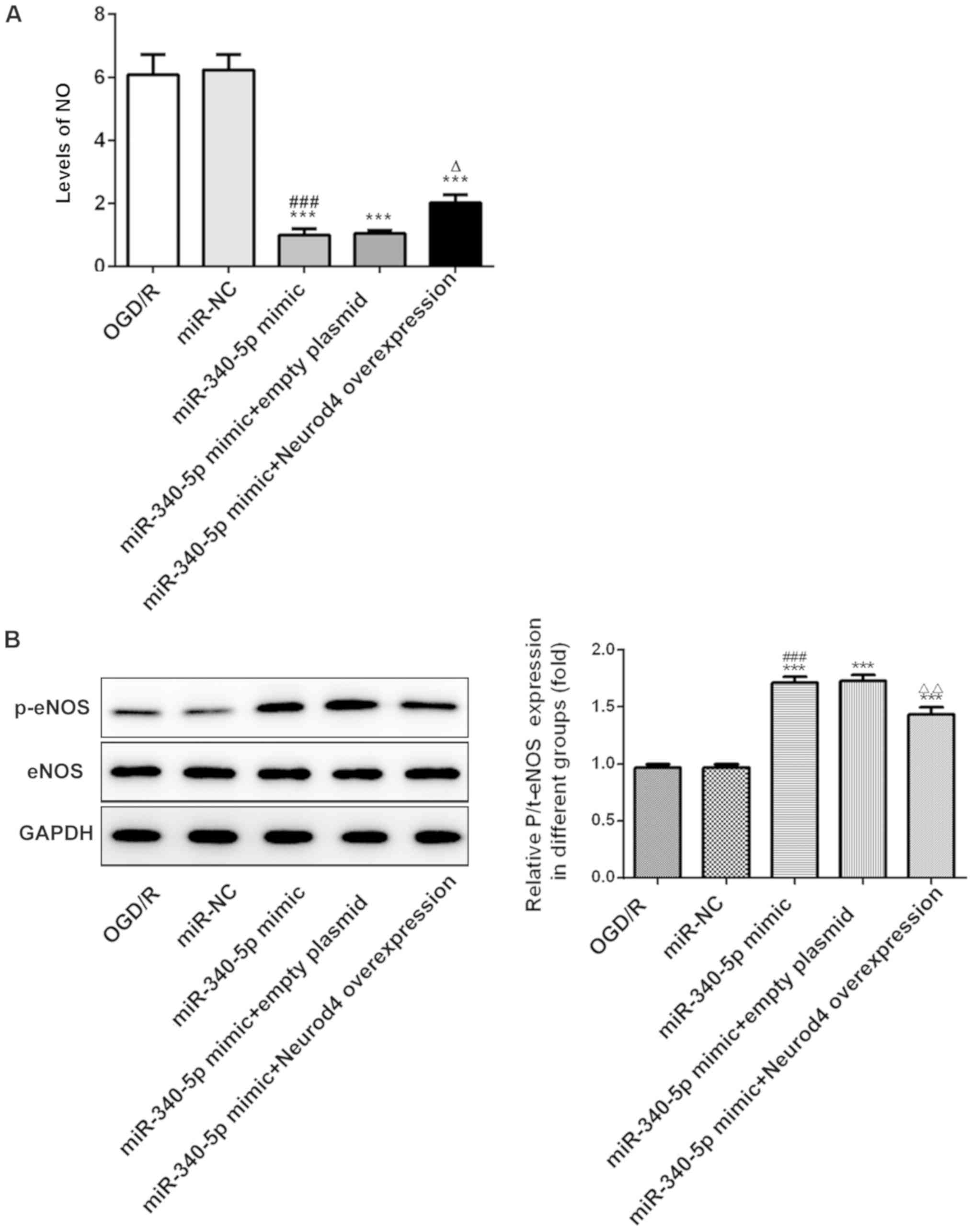
miR-340-5p overexpression protects
OGD/R-induced PC12 cells by reducing NO levels and increasing
p-eNOS/eNOS expression levels, whereas this effect is counteracted
by Neurod4 overexpression
The OGD/R-induced increases in NO levels were
significantly decreased in the OGD/R + miR-340-5p mimic group
(Fig. 8A). However, the effect of
miR-340-5p overexpression was significantly reversed following
Neurod4 overexpression, suggesting that miR-340-5p overexpression
may exert a protective effect by decreasing NO levels (Fig. 8A). The expression levels of p-eNOS
were significantly increased in the OGD/R + miR-340-5p mimic group
compared with the OGD/R group, but this effect was reduced
following Neurod4 overexpression (Fig.
8B), indicating that miR-340-5p overexpression may protect
OGD/R-induced PC12 cells from injuries by decreasing NO levels and
the expression levels of p-eNOS/eNOS through downregulating Neurod4
expression.
Discussion
Ischemic stroke remains one of the major causes of
morbidity; however, how to treat the condition remains largely
unknown. The current therapeutic options for ischemic stroke are
unsatisfactory; therefore, there is an urgent requirement to
further study the underlying mechanisms to identify novel treatment
options for ischemic stroke (28,29).
In the present study, miR-340-5p was observed to exert protective
effects over OGD/R-induced PC12 cells through targeting Neurod4
expression.
The insufficient presence of oxygen and glucose for
normal metabolism is the main cause of injury in stroke (30) and as the therapeutic window is
limited, early recanalization has proved helpful in preventing
ischemic neuronal loss (31).
Thrombolytic therapies are commonly used for the treatment of
ischemic stroke (32); however,
the recovery of blood flow has been found to promote secondary
injuries (33). Thus, reducing
reperfusion injuries is of great significance for improving the
therapy available for ischemic stroke.
In the present study, PC12 cells that received OGD/R
served as the cellular model, which has been used in numerous
previous studies (24,25,34,35).
Consistent with the previous studies, the cell apoptotic rate, the
inflammatory status and NO levels were increased following OGD/R
induction, indicating that the cellular model was successfully
established (36,37).
In a previous study, the expression levels of
miR-340-5p were rapidly decreased in the peripheral blood of
patients who had suffered from an acute ischemic stroke, which
indicated a potentially protective role for miR-340-5p in ischemic
stroke (22). Consistent with this
previous study, miR-340-5p expression levels were decreased in the
OGD/R group in the present study.
Cell apoptosis and inflammation are two important
factors involved in ischemia-reperfusion injury (38–40).
In the current study, following the overexpression of miR-340-5p in
PC12 cells, the cell apoptotic rate, inflammatory status and NO
levels induced by OGD/R were reduced, providing validation for the
protective role of miR-340-5p in cells induced by OGD/R.
Neurod4 is an important factor involved in neuronal
differentiation; its expression levels have been reported to be
increased under various stimuli, which was negatively correlated
with the degree of neuron maturation (41). In the present study, Neurod4 was
identified as a target gene of miR-340-5p. Neurod4 expression
levels have also previously been reported to be increased following
maternal hypoxia (42). In the
present study, Neurod4 expression levels were increased in the
OGD/R group compared with control group, and the OGD/R-induced
increases in Neurod4 expression levels were reduced following
miR-340-5p overexpression. To the best of our knowledge, the latter
finding was reported for the first time in the present study, which
suggested that miR-340-5p may protect PC12 cells from OGD/R injury
through targeting Neurod4 expression. In addition, in a previous
study, the inhibition of Neurod4 expression reduced the
inflammatory levels and suppressed oxidative stress in spinal cord
injury (41,43). These injuries caused by
inflammation and oxidative stress are suggested to be the two major
factors involved in ischemia-reperfusion injury (40,44–46).
In the present study, following Neurod4 overexpression, the
apoptotic rate and inflammatory status of OGD/R-induced cells
transfected with the miR-340-5p mimic were elevated. These findings
suggested that the Neurod4 gene may be an important therapeutic
target and targeting the Neurod4 gene with miR-340-5p may provide a
novel strategy for treating ischemia-reperfusion injury. However,
future studies are required to determine other targets that could
be targeted for the treatment of ischemic stroke.
In a cerebral ischemia-reperfusion injury model, the
levels of NO, a physiological messenger, were reported to be
upregulated and the expression levels of p-eNOS/eNOS were
decreased, which subsequently resulted in ischemia-reperfusion
injuries (47). In the present
study, increased levels of NO induced by OGD/R were subsequently
reduced following miR-340-5p overexpression. The opposite trend was
observed to occur to the expression levels of p-eNOS/eNOS following
miR-340-5p overexpression. Notably, the effects of miR-340-5p
overexpression on NO levels and p-eNOS/eNOS expression levels were
reversed by Neurod4 overexpression. These findings further
indicated that miR-340-5p may protect against injuries from OGD/R
through inhibiting the production of NO and increasing the
expression levels of p-eNOS/eNOS through targeting Neurod4.
In conclusion, the findings of the present study
suggested that miR-340-5p may exert protective effects over OGD/R
in PC12 cells through targeting Neurod4 expression. These results
may provide novel strategies for alleviating the injuries obtained
from ischemia-reperfusion and pave the way for future research on
ischemia-reperfusion injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW designed the present study, prepared the
manuscript and was involved in performing the experiments. GL
performed some parts of the experiments and helped to design the
study. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Satue E, Vila-Corcoles A, Ochoa-Gondar O,
de Diego C, Forcadell MJ, Rodriguez-Blanco T, Barnes L and Jariod
M: Incidence and risk conditions of ischemic stroke in older
adults. Acta Neurol Scand. 134:250–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Q, Wang Y, Song H, Hou C, Cao Q,
Dong K, Huang X, Feng W, Ovbiagele B, Wang M and Ji X: Clopidogrel
and ischemic stroke outcomes by smoking status: Smoker's paradox? J
Neurol Sci. 373:41–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alloubani A, Saleh A and Abdelhafiz I:
Hypertension and diabetes mellitus as a predictive risk factors for
stroke. Diabetes Metab Syndr. 12:577–584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Y, Li R, Sun H, Li J, He B, Xiao S, Li
L and Wang J: Protective effects of oroxylin a on oxygen-glucose
deprivation/reperfusion-induced PC12 cells by activating the sonic
hedgehog signal pathway. Nat Product Commun.
14:1934578X198815442019.
|
|
6
|
Huang J, Upadhyay UM and Tamargo RJ:
Inflammation in stroke and focal cerebral ischemia. Surg Neurol.
66:232–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peyravian N, Dikici E, Deo S, Toborek M
and Daunert S: Opioid antagonists as potential therapeutics for
ischemic stroke. Prog Neurobiol. 182:1016792019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yue XY, Feng ZQ, Yu XY, Hu JM, He XJ and
Shu S: Fire-needle acupuncture for upper limb spastic paralysis
after stroke: Study protocol for a randomized controlled trial. J
Integr Med. 17:167–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barthels D and Das H: Current advances in
ischemic stroke research and therapies. Biochim Biophys Acta Mol
Basis Dis. 1866:1652602020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farzaneh M, Alishahi M, Derakhshan Z,
Sarani NH, Attari F and Khoshnam SE: The expression and functional
roles of miRNAs in embryonic and lineage-specific stem cells. Curr
Stem Cell Res Ther. 14:278–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang
H, Zhang J, Jiang X, Wang Y and Chen YE: Peroxisome
proliferator-activated receptor delta regulation of miR-15a in
ischemia-induced cerebral vascular endothelial injury. J Neurosci.
30:6398–6408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao
L, Yan F, Liu X, Yu S, Ji X and Luo Y: MicroRNA-424 protects
against focal cerebral ischemia and reperfusion injury in mice by
suppressing oxidative stress. Stroke. 46:513–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu LJ, Ouyang YB, Xiong X, Stary CM and
Giffard RG: Post-stroke treatment with miR-181 antagomir reduces
injury and improves long-term behavioral recovery in mice after
focal cerebral ischemia. Exp Neurol. 264:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harraz MM, Eacker SM, Wang X, Dawson TM
and Dawson VL: MicroRNA-223 is neuroprotective by targeting
glutamate receptors. Proc Natl Acad Sci USA. 109:18962–18967. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao L, Pu X, Huang Y and Huang J:
MicroRNA-340-5p relieved chronic constriction injury-induced
neuropathic pain by targeting Rap1A in rat model. Genes Genomics.
41:713–721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Zhou J, Yang B and Yu Y:
microRNA-340-5p inhibits hypoxia/reoxygenation-induced apoptosis
and oxidative stress in cardiomyocytes by regulating the Act1/NF-kB
pathway. J Cell Biochem. 120:14618–14627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long M, Wang Z, Zheng D, Chen J, Tao W,
Wang L, Yin N and Chen Z: Electroacupuncture pretreatment elicits
neuroprotection against cerebral ischemia-reperfusion injury in
rats associated with transient receptor potential vanilloid
1-mediated anti-oxidant stress and anti-inflammation. Inflammation.
42:1777–1787. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai Y, Zhang H, Zhang J and Yan M:
Isoquercetin attenuates oxidative stress and neuronal apoptosis
after ischemia/reperfusion injury via Nrf2-mediated inhibition of
the NOX4/ROS/NF-kB pathway. Chem Biol Interact. 284:32–40. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Li L, Tan X, Liu B, Zhang Y and
Li C: miR-210 mediates vagus nerve stimulation-induced antioxidant
stress and anti-apoptosis reactions following cerebral
ischemia/reperfusion injury in rats. J Neurochem. 134:173–181.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palencia G, Medrano JAN, Ortiz-Plata A,
Farfán DJ, Sotelo J, Sánchez A and Trejo-Solís C: Anti-apoptotic,
anti-oxidant, and anti-inflammatory effects of thalidomide on
cerebral ischemia/reperfusion injury in rats. J Neurol Sci.
351:78–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saad MA, Abdelsalam RM, Kenawy SA and
Attia AS: Ischemic preconditioning and postconditioning alleviates
hippocampal tissue damage through abrogation of apoptosis modulated
by oxidative stress and inflammation during transient global
cerebral ischemia-reperfusion in rats. Chem Biol Interact.
232:21–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoo H, Kim J, Lee AR, Lee JM, Kim OJ, Kim
JK and Oh SH: Alteration of microRNA 340-5p and arginase-1
expression in peripheral blood cells during acute ischemic stroke.
Mol Neurobiol. 56:3211–3221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu H, Wang X and Chen S: Downregulation
of MiR-218-5p protects against oxygen-glucose
deprivation/reperfusion-induced injuries of PC12 cells via
upregulating n-myc downstream regulated gene 4 (NDRG4). Med Sci
Monit. 26:e9201012020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu K and Zhang Y: Protodioscin protects
PC12 cells against oxygen and glucose deprivation-induced injury
through miR-124/AKT/Nrf2 pathway. Cell Stress Chaperones.
24:1091–1099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JK, Wang LC, Jiang Y, Tu PF and Zeng
KW: Neuroprotective effects and mechanism of ethanol extract of
cistanche tubulosa against oxygen-glucose deprivation/reperfusion.
Zhongguo Zhong Yao Za Zhi. 44:2686–2690. 2019.(In Chinese).
PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kovac S, Angelova PR, Holmstrom KM, Zhang
Y, Dinkova-Kostova AT and Abramov AY: Nrf2 regulates ROS production
by mitochondria and NADPH oxidase. Biochim Biophys Acta.
1850:794–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Ma J, Fang Q, Li H, Shen H, Li X,
Xue Q, Zhu J and Chen G: Botch protects neurons from ischemic
insult by antagonizing Notch-mediated neuroinflammation. Exp
Neurol. 321:1130282019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urdaneta AE and Bhalla P: Cutting edge
acute ischemic stroke management. Emerg Med Clin North Am.
37:365–379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Geng J, Zhang Y, Li S, Wang J, Wang H, Aa
J and Wang G: Metabolomic profiling reveals that reprogramming of
cerebral glucose metabolism is involved in ischemic
preconditioning-induced neuroprotection in a rodent model of
ischemic stroke. J Proteome Res. 18:57–68. 2019.PubMed/NCBI
|
|
31
|
Cisse FA, Damien C, Bah AK, Touré ML,
Barry M, Djibo Hamani AB, Haba M, Soumah FM and Naeije G: Minimal
setting stroke unit in a sub-saharan African public hospital. Front
Neurol. 10:8562019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moussaddy A, Demchuk AM and Hill MD:
Thrombolytic therapies for ischemic stroke: Triumphs and future
challenges. Neuropharmacology. 134:272–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piccardi B, Arba F, Nesi M, Palumbo V,
Nencini P, Giusti B, Sereni A, Gadda D, Moretti M, Fainardi E, et
al: Reperfusion injury after ischemic stroke study (RISKS):
Single-centre (Florence, Italy), prospective observational protocol
study. BMJ Open. 8:e0211832018.PubMed/NCBI
|
|
34
|
Li Y, Shi J, Sun X, Li Y, Duan Y and Yao
H: Theaflavic acid from black tea protects PC12 cells against
ROS-mediated mitochondrial apoptosis induced by OGD/R via
activating Nrf2/ARE signaling pathway. J Nat Med. 74:238–246. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Wu X, An J, Lv W, Geng Y, Lou T and
Zhang Y: Glaucocalyxin B protects against
oxygen-glucose-deprivation/reperfusion-induced neuronal injury in
PC-12 cells. J Cell Biochem. 120:6137–6144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu Y, Zhang X, Han Z, Zhao W and Zhang L:
Expression and regulation of miR-449a and AREG in cerebral ischemic
injury. Metab Brain Dis. 34:821–832. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang H, Wei W, Lan X, Liu N, Li Y, Ma H,
Sun T, Peng X, Zhuang C and Yu J: Neuroprotective effect of
swertiamain on cerebral ischemia/reperfusion injury by inducing the
Nrf2 protective pathway. ACS Chem Neurosci. 10:2276–2286. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang XP, Ding H, Lu JD, Tang YH, Deng BX
and Deng CQ: Autophagy in cerebral ischemia and the effects of
traditional Chinese medicine. J Integr Med. 13:289–296. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nan L, Xie Q, Chen Z, Zhang Y, Chen Y, Li
H, Lai W, Chen Y and Huang M: Involvement of PARP-1/AIF signaling
pathway in protective effects of gualou guizhi decoction against
ischemia-reperfusion injury-induced apoptosis. Neurochem Res.
45:278–294. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wicha P, Tocharus J, Janyou A, Jittiwat J,
Changtam C, Suksamrarn A and Tocharus C: Hexahydrocurcumin protects
against cerebral ischemia/reperfusion injury, attenuates
inflammation, and improves antioxidant defenses in a rat stroke
model. PLoS One. 12:e01892112017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang L, Ge D, Chen X, Jiang C and Zheng S:
miRNA-544a regulates the inflammation of spinal cord injury by
inhibiting the expression of NEUROD4. Cell Physiol Biochem.
51:1921–1931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Golan MH, Mane R, Molczadzki G, Zuckerman
M, Kaplan-Louson V, Huleihel M and Perez-Polo JR: Impaired
migration signaling in the hippocampus following prenatal hypoxia.
Neuropharmacology. 57:511–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai J, Xu LJ, Han GD, Sun HL, Zhu GT,
Jiang HT, Yu GY and Tang XM: MiR-137 attenuates spinal cord injury
by modulating NEUROD4 through reducing inflammation and oxidative
stress. Eur Rev Med Pharmacol Sci. 22:1884–1890. 2018.PubMed/NCBI
|
|
44
|
Gao XJ, Xie GN, Liu L, Fu ZJ, Zhang ZW and
Teng LZ: Sesamol attenuates oxidative stress, apoptosis and
inflammation in focal cerebral ischemia/reperfusion injury. Exp
Ther Med. 14:841–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang DD, Zou MJ, Zhang YT, Fu WL, Xu T,
Wang JX, Xia WR, Huang ZG, Gan XD, Zhu XM and Xu DG: A novel
IL-1RA-PEP fusion protein with enhanced brain penetration
ameliorates cerebral ischemia-reperfusion injury by inhibition of
oxidative stress and neuroinflammation. Exp Neurol. 297:1–13. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo
C, Peng J, Li J, Yung KK and Mo Z: Neuroprotective effects of
bilobalide on cerebral ischemia and reperfusion injury are
associated with inhibition of pro-inflammatory mediator production
and down-regulation of JNK1/2 and p38 MAPK activation. J
Neuroinflammation. 11:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He D, Song X and Li L:
Geranylgeranylacetone protects against cerebral ischemia and
reperfusion injury: HSP90 and eNOS phosphorylation involved. Brain
Res. 1599:150–157. 2015. View Article : Google Scholar : PubMed/NCBI
|