Introduction
Chronic post-surgery pain (CPSP) is a common
complication that occurs in ~10-50% of patients after surgery
(1). CPSP not only affects the
quality of life and work efficiency of patients, but also triggers
depression, anxiety and other adverse emotional problems (2). These negative emotions caused by CPSP
lead to incidences of suicide as high as 10–15% (3). CPSP also brings heavy burden to the
economy of the medical system and society, it is reported >€200
billion in Europe and $150 billion in America every year (4). The mechanism of CPSP is complex and
there is no satisfactory treatment yet. Therefore, it is important
to explore new mechanisms and treatment options for pain that can
effectively prevent the occurrence of CPSP (5,6).
The natural process of childbirth can lead to
serious maternal tissue trauma. Currently approximately one-third
of childbirths are achieved through a caesarean section.
Approximately 20% of puerperae have postpartum back pain following
caesarean delivery and ~5% of puerperae have varying degrees of
abdominal pain (7). The feelings
of pain occur at ~3 months or 10 months postpartum (19 and 12%,
respectively). The aforementioned data reveal that child delivery,
including caesarean section is the main factor of chronic pain in
puerperae. However, it is noted that the incidence of chronic pain
caused by childbirth is far less than expected. The incidence of
chronic pain after caesarean section was much lower compared with
other surgeries, such as transcervical hysterectomy or inguinal
hernia repair (8). Thus, a
protective mechanism may exist for pain relief during childbirth,
which may provide clues for the prevention of CPSP.
A previous study indicated that the protective
mechanisms associated with childbirth may be mediated by the
analgesic effect of hormones that are synthesized and released in
the central nervous system after delivery (9). Tissue injury and inflammation lead to
an increased level of cytokines, growth factors or hormones that
promote the expression and function of central cannabinoid and
opioid receptors, which was considered as a possible mechanism of
the analgesic effect (10,11). However, the specific mechanism of
postpartum pain relief is unclear. Understanding the mechanism of
postpartum pain cannot only provide the molecular basis of the
pathophysiological process of CPSP, but also provide a new
theoretical basis and therapeutic drug target for the prevention
and treatment of CPSP.
In recent years, the discovery of microRNAs
(miRNAs/miRs) provided novel insights for studying disease
mechanisms. These non-coding RNAs are involved in the regulation of
genome expression by negatively regulating downstream target genes
(12). The role of miRNAs in the
pathogenesis of pain is largely unknown. The discovery of
pain-relief mechanism by miRNAs during childbirth will provide a
novel basis for the clinical treatment of pain.
In the present study, microRNAs of spinal cords were
screened in rats with neuropathic pain, with delivery and without
pregnancy, to identify miRNAs that are associated with
delivery-induced pain relief.
Materials and methods
Animals
Specified pathogen-free Sprague Dawley non-pregnant
and pregnant (10–12 days old) female rats were obtained from Vital
River Laboratory Animal Technology Co., Ltd (Beijing). Production
license no. SCXK (Beijing) 2012-0001; certificate no.
11400700185355. All animal experiments in this study were approved
by JiangSu Center for Safety Evaluation of Drug (approval no.
LL-20160915-01) in accordance to the ‘Regulations for the
administration of affairs concerning experimental animals of
Jiangsu Province’. The rats were maintained prior to experiments in
an animal room under standard conditions (23±2°C temperature,
60±10% humidity, 12 h: 12 h light-dark cycle). All efforts were
made to minimize the suffering of animals. The endpoints for
SNI-modelled animals were defined by rapid weight loss, constant
unusual response to pain and 24–36 none or less food and water
uptake.
Animals were allocated into three groups. Control,
non-pregnant rats, n=12; SNI, non-pregnant rats, n=14;
delivery+SNI, pregnant rats, n=20. For delivery+SNI group, 32
pregnant rats were SNI-modelled to ensure that at least 20 mice
could deliver at day 6 after modelling. The SNI modelling and sham
operation (for control rats) were performed at day 0. The paw
withdrawal threshold (PWT) was measured before, immediately after
and every two days after modelling, until day 20. The delivery
occurred at day 6 after modelling and samples for biological assays
were collected at day 9.
Primary spinal cord dorsal horn neuron
isolation and culture
Fetal rats were anesthetized with isoflurane (3% for
induction and 5% for deep anesthesia) and subsequently decapitated.
The back of the rat was sterilized with ethanol, opened with
sterilized surgical instruments and the spinal cord was exposed.
Then the spinal cord was removed and digested with trypsin
containing 0.25% EDTA at 37°C for 10 min, whilst constantly
shaking. The trypsinization was stopped by adding culture media,
and cells were centrifuged at 200 × g at room temperature for 6
min. Spinal neurons were then disseminated into single cells and
inoculated on a 24-well plate and cultured in an incubator at 37°C,
under 5% CO2. The culture medium contained neurobasal +
B27 (1%) (cat. no. A3582801; Gibco; Thermo Fisher Scientific,
Inc.), FBS (20%) (cat. no. 10099141C; Gibco; Thermo Fisher
Scientific, Inc.), glutamine (final concentration 2 mmol/l; cat.
no. G7513; Sigma-Aldrich; Merck KGaA) and penicillin (100
U/ml)-streptomycin solution (0.1 mg/ml) (ScienCell Research
Laboratories, Inc.; cat. no. 0503). Cultured spinal neurons were
starved from B27 for 6 h before the addition of 1 µM OT
(Sigma-Aldrich; Merck KGaA) for 30 min (13).
SNI model establishment
The SNI rat model was constructed as previously
described (14). Briefly, after
anaesthetizing the rats (3% isoflurane for induction and 2% for
anesthesia maintenance), the furs on the left leg were shaved with
a razor. The skin of the leg was sterilized by alcohol-soaked
cotton, before making an incision on the skin (15–25 mm) from the
proximal femur, followed by blunt dissection of the muscle with a
surgical scissor. The peroneal nerve and tibial nerve were isolated
and ligated. Nerve tissue (2 mm) was intercepted. The wounds were
sealed and disinfected and rats were returned to the cage. The sham
operation was performed on control rats, using the same procedure
as with the SNI modelling, however the nerve tissue was not
intercepted.
Behavioral testing
The mechanical PWT was examined in all rats every
two days after the establishment of SNI model. Briefly, rats were
placed in a cylinder. The calf muscle of the sural nerve region was
vertically penetrated with a VonFreys touch wire (Dynamic). The
pain threshold was the intensity of the touch wire specifications
when the rats withdrew their legs.
RNA extraction
Treated primary neurons or spinal cord tissues were
washed with PBS three times. Subsequently, 1 ml TRIzol (Takara Bio,
Inc.) was added, and incubated for 2 min at RT. The solution was
then transferred to an RNase-free Eppendorf tube and 200 µl
chloroform was added, followed by vigorous shaking for 15 sec.
Samples were then centrifuged at 12,000 × g and 4°C for 15 min.
After centrifugation, the solution was divided into three layers
and the upper colorless liquid was carefully transferred into a new
Eppendorf tube. An equal volume of isopropanol was added and mixed
at room temperature for 5 min, followed by centrifuged at 4°C,
12,000 × g, for 10 min. Following removal of the supernatant, 75%
ethanol (1 ml) was added to the precipitate, and agitated for 15
sec and centrifuged at 12,000 × g and 4°C. Precipitation was
transferred into a clean hood and dried for 3–5 min. Thereafter,
RNAs were dissolved in DEPC water (Thermo Fisher Scientific, Inc.)
and Nanodrop test (Thermo Fisher Scientific, Inc.) was used to
determine the RNA concentration.
miRNA microarray analysis
To prepare for the miRNA microarray analysis, the
reaction was prepared using miRNA complete Labeling and Hyb kit
(24X; Agilent Technologies, Inc.; cat. no. 5190-0456) through
dephosphorylation, denaturation and ligation, according to Agilent
miRNA microarray protocol. Subsequently, the labelled RNA was
purified using Micro Bio-Spin 6 (Bio-Rad Laboratories, Inc.; cat.
no. 732-6221) and dried using a vacuum concentrator at 45–55°C or
on the medium-high heat setting. After preparing the hybridization
samples, hybridization was conducted in the Agilent G2545A
Hybridization Oven (Agilent Technologies, Inc.) at 55°C for 20 h.
In addition, the microarray slides were washed, scanned and finally
the images were added to be extracted using the Agilent Feature
Extraction software v10.5.1.1 (Agilent Technologies, Inc.) to
analyze and generate the miRNAs. In order to perform the
differential expression analysis, a DEGseq package tool (15) was applied and the significantly
differential miRNAs were identified according to the P-value
(<0.05).
Bioinformatic databases
Target genes were predicted with the help of
bioinformatics (Target Scan release 7.2: http://www.targetscan.org/vert_72/, PicTar release
2007: https://pictar.mdc-berlin.de/,
miRanda release 2010: http://www.microrna.org/microrna/home.do and miRase
release 22.1: http://www.mirbase.org/) analysis and
combined with the literature.
Lentiviral vector construction and
animal injection
For the small interfering (si)RNA experiment, the
miR-124a and miR-29c siRNA lentivirus (RNAi-LV) were designed and
synthesized with the following sequences: miR-124a,
5′-CUCUGCGUGUUCACAGCGGACCUUGAUUUAAUGUCUAUACAAUUAAGGCACGCGGUGAAUGCCAAGAG-3′;
and miR-29c,
5′-ATCTCTTACACAGGCTGACCGATTTCTCCTGGTGTTCAGAGTCTGTTTTTGTCTAGCACCATTTGAAATCGGTTATGATGTAGGGGGA-3′.
U6-vshRNA-UBI-GFP was the backbone of the lentiviral vector and the
same vector but carrying scrambled sequence
(5′-GCACGCGTATACTTCGATTATCACGTATTCTTGTACGTTCTGTGTTGTCCAGGTGAAGGTTAACTCGTGGATACGACCGTATTGATTG-3′)
was used as negative control lentivirus (NC-LV). All the
lentiviruses were produced by Shanghai GeneChem Co., Ltd. The
lentiviral vectors were injected into the rats' spinal cord to
create local response 4 days after SNI modelling.
Detecting OT concentration by
enzyme-linked immunosorbent assay (ELISA)
Cerebrospinal fluid (CSF) was isolated from rats in
all groups. Briefly, following anesthesia by 2% pentobarbital
sodium (80 mg/kg), fur on the head and neck of the rat was shaved
and skin was sterilized. Subsequently, the rat's head was fixed on
the orientator. A longitudinal incision (~2 cm) was generated by a
scalpel and the dorsal muscles of the neck were separated with
scissors. In order to avoid bleeding, the deepest muscles attached
to the bone were scraped with the back of the scalpel in order to
expose the foramen magnum of the occipital bone. The CSF was
extracted directly from the foramen magnum. Then the muscles and
skin were sealed and the sulfonamide powder was sprayed at the
wound to prevent infection. After the CSF extraction, the same
amount of sterilized saline was injected to maintain the original
pressure in the spinal cord cavity. The OT concentration in the
fluid was detected by ELISA kit (cat. no. ab133050; Abcam),
according to the manufacturer's instructions.
Western blotting
Spinal neurons were lysed using RIPA lysis buffer
with 1% PMSF on ice for 30 min and the lysates were centrifuged at
12,000 × g and 4°C for 5 min to remove cell debris. The protein
concentration was determined by BCA assay. Subsequently, the lysate
was heated at 95°C for 10 min with 1X Laemmli sample buffer
(Bio-Rad Laboratories, Inc.) and then loaded 30 µg on a 10% gel
(Bio-Rad Laboratories, Inc.) for electrophoresis by SDS-PAGE. The
proteins were subsequently transferred onto a nitrocellulose
membrane (Bio-Rad Laboratories, Inc.) and blocked with 5% BSA TBST
for 1 h at room temperature. After blocking, the membrane was
probed with 1:1,000 primary antibodies for 2 h at room temperature:
OXTR (cat. no. ab87312), GABA (cat. no. ab17413), GABAA
(cat. no. ab33299), c-Fos (cat. no. ab208942) and β-actin (cat. no.
ab8226; all from Abcam) and corresponding horseradish
peroxidase-conjugated 1:2,000 goat anti-rabbit or goat anti-mouse
secondary antibodies (cat. no. ab97080; cat. no. ab97040 Abcam) at
room temperature for 2 h. Protein bands were detected by the
Western Lightning® ECL Pro Enhanced Chemiluminescence
Substrate (PerkinElmer, Inc.) and imaged using the Tanon 1600/1600R
Gel Imaging System (UVP, LLC). The ImageJ (v1.8.0_112; National
Institutes of Health) was used to quantify the bands.
Paraffin sectioning and
immunohistochemistry
Standard immunohistochemistry was used for c-Fos
detection. Fresh spinal cord tissues were collected from rats in
all groups. Briefly, rats were anesthetized by 3% pentobarbital
sodium, shaved and sterilized. The rats were euthanized by
CO2 (0.5 l/min in a 5-l chamber) inhalation and 2 cm of
T5-L5 spinal cords were collected. The
peripheral soft tissue was removed immediately and cleaned spinal
cord tissue was dehydrated using a graded series of ethanol (50,
70, 85, 90 and 100%), and then paraffin-embedded and cut into
300-µm sections. Heat-mediated antigen retrieval for 20 min at
100°C was achieved using citrate buffer solution (pH 6.0) (Wuhan
Servicebio Technology Co., Ltd.) for c-Fos detection. Sections were
incubated with 3% H2O2 at room temperature
(Wuhan Servicebio Technology Co., Ltd.) for 25 min to block
endogenous peroxidase activity. After blocking for 30 min with 3%
BSA (Beijing Solarbio Science & Technology Co., Ltd.), paraffin
sections were incubated overnight at 4°C with 1:200 c-Fos primary
antibody (cat. no. ab208942; Abcam). The next day, sections were
washed with PBS and subsequently incubated with corresponding
HRP-labelled 1:2,000 secondary antibody (Bio-Rad Laboratories,
Inc.) for 50 min at room temperature. The target antigen was
detected by 3,3′-diaminobenzidine (DAB) (Agilent Technologies,
Inc.) and the nucleus was stained at room temperature by Harris
hematoxylin (Wuhan Servicebio Technology Co., Ltd.) for 3 min.
Luciferase assay
The Oxtr minimal promoter or CpG mutated (C
to A) Oxtr minimal promoter was cloned into pGL3-Basic
Vector (Promega Corporation) between Xba I and
HindIII sites, according to manufacturer's protocol. The
fused vector was transfected into E. coli by the electrical
shock method for amplification. The amplified vector was
subsequently collected by GeneJet Plasmid Miniprep kit (Thermo
Fisher Scientific, Inc.), according to manufacturer's protocol.
Primary neurons were transfected with 1 µg prepared luciferase
vector and 4 µl FuGENE (Promega Corporation) according to
manufacturer's protocol. Thereafter, cells were infected with or
without miR-29c lentivirus. Cells were lysed by lysis buffer
(Promega Corporation) and Renilla luciferase activity was
measured after 24 h using Lucetta Luminometer (Lonza AAL-1001).
Enhanced green fluorescent protein
(EGFP) observation in spinal cord tissue
Rats with SNI rats were sacrificed by CO2
inhalation (0.5 l/min in a 5-l chamber) one week after lentivirus
injection. The spinal cord was collected as aforementioned. The
tissue was subsequently placed under fluorescence microscopy
(magnification, ×100) to capture EGFP images.
Electrophysiology assay
The experiment was recorded under whole cell patch
clamp mode (Molecular Devices, LLC) in which stereomicroscope
(magnification, ×10) was used. The tip of the microelectrode, with
a diameter of 1.5 mm and resistance of 7–10 megohms, was placed
next to the cultured primary spinal cord neurons under the guidance
of the resistance change in the Axon pCLAMP 11.1 software (DL
Naturegene Life Sciences, Inc.). When encountering cells, the test
pulse square wave appears, and the increase in resistance generates
negative pressure to form a giant seal (Giga-Seal). After the
formation of a giant resistance seal, a short negative pressure is
applied to the micro-electrode chamber, the cells are aspirated and
the whole-cell pattern is formed. The membrane current was
amplified by an amplifier (Molecular Devices, LLC) and converted to
a digital signal by a digitizer (Molecular Devices, LLC). Data were
recorded and analyzed using Molecular Devices' Pclamp 10.2
software.
Quantitative (q)PCR
Total RNA was extracted from treated primary neurons
or spinal cord tissues as above. qPCR was performed using
SYBR® Green Real-time PCR Master mix (Takara) in the
StepOnePlus Real-Time PCR system (Applied Biosystems, Inc.). The
quantitative PCR conditions were as follows: 95°C for 10 min,
followed by 40 cycles of 15 sec at 95°C and 60°C for 30 sec. Actin
housekeeping gene was measured as an internal control for all
samples. The relative expression of each microRNA was calculated
using the 2−ΔΔCq method (16). Each experiment was replicated three
times. The forward and reverse sequences of primers are shown in
Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward sequence
(5′ to 3′) | Reverse sequence
(5′ to 3′) |
|---|
| GABA |
TGTAAACTCAGATGTCCCGCAG′ |
CGGGCTTGTCCAGCAGAAATA |
| OXTR |
CGTACTGGCCTTCATCGTGT |
GAATTCTCCTCTCCGCCCAC |
|
GABAA |
AGACTCAGGATGGGCCTGAT |
GCCCTTCTCCTCCAGTTCAC |
| c-Fos |
TTATTTTGGCAGCCCACCGA |
TCAAGTCCAGGGAGGTCACA |
| Actin |
CCTGGCACCCAGCACAAT |
GCCGATCCACACGGAGTACT |
Statistical analysis
SPSS v20 (IBM Corp.) and GraphPad Prism version 5.0
(GraphPad Software, Inc.) were used for statistical analysis. All
values are expressed as mean ± SD. The unpaired 2-tailed Student's
t-test was used. When comparing among two or more groups, one-way
analysis of variance or repeated measures ANOVA test, followed by
Tukey's test, was performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Delivery-induced pain relief in rats
with SNI
To confirm if delivery can induce pain relief in
animal models, an SNI rat model was established and the mechanical
PWT was investigated. C-Fos is a well-documented marker for neural
pain in previous literature, as such, c-Fos was used as an
indicator of neural pain level in the present study (17). The results showed that rats
presented with decreased mechanical PWT after SNI modelling,
compared with those in the control group (Fig. 1A). Delivery+SNI rats presented with
significantly increased PWT after the delivery of pups (Fig. 1A), while the PWT in SNI group
remained very low. However, the PWT of delivery+SNI rats could not
recover back to the level of the control group. In addition, the
downregulation of c-Fos after delivery was observed in delivery+SNI
group compared with the SNI only counterparts by qPCR, which was in
corroboration with the trend observed with the PWT (Fig. 1C). These results were further
confirmed by western blotting and immunohistochemistry staining
(Fig. 1B and D).
miR-29c is associated with pain
relief
The spinal cords of SNI rats with or without
delivery were collected at the third day after delivery and
analyzed using miRNA microarray. The samples were collected at the
third day after delivery to minimize any other unknown factors
caused by delivery on the delivery day. As shown in Fig. 1A, the pain threshold still remains
at an improved level at the third day after delivery, and was
significantly higher, compared with the SNI group; thus, this time
point was considered optimal for collecting samples. The result
showed that 71 miRNAs had significant differential expressions
between delivery+SNI and SNI groups of rats. There were 37
upregulated miRNAs and 34 downregulated miRNAs in delivery+SNI
group (Fig. 2A). qPCR was used to
verify 10 miRNAs with more than 5-fold difference, and the results
were consistent with the microarray results (Fig. 2B). The upregulated miR-124a and
downregulated miR-29c, which have the largest differences between
the two groups, were selected for further investigation.
miR-124a-overexpressed and miR-29c-silenced lentiviruses were
constructed and injected into SNI rats. The transfection was
confirmed by qPCR (Fig. S1). It
was found that miR-124a-overexpression had no effects on the pain
threshold, but miR-29c-silencing increased the threshold of
mechanical PWT 24 h following transfection (Fig. 2C and D).
miR-29c inhibited OXTR expression
A search online on miRase was performed and it was
found that miR-29c is highly conserved during evolution. The base
sequence of UAGCACCAUUGAAAUCAGU is highly conserved among humans,
rats, mice, dogs and other species (Fig. 3A). The expression levels of miR-29c
in different organs were examined and it was found that it is
highly expressed in spinal cord, suggesting miR-29c plays an
important role in neural tissues (Fig.
3B). Target Scan (http://www.targetscan.org/) analysis revealed a fully
matched base sequence ‘UGGUGCU’ of numerous target genes conserved
with the sequence ‘ACCACGA’ of miR-29c, including spinal oxytocin
receptor (OXTR) that is involved in delivery (Fig. 3C). The luciferase assay showed that
miR-29c can inhibit the activity of OXTR 3′UTR, suggesting that
OXTR might be a target of miR-29c (Fig. 3D).
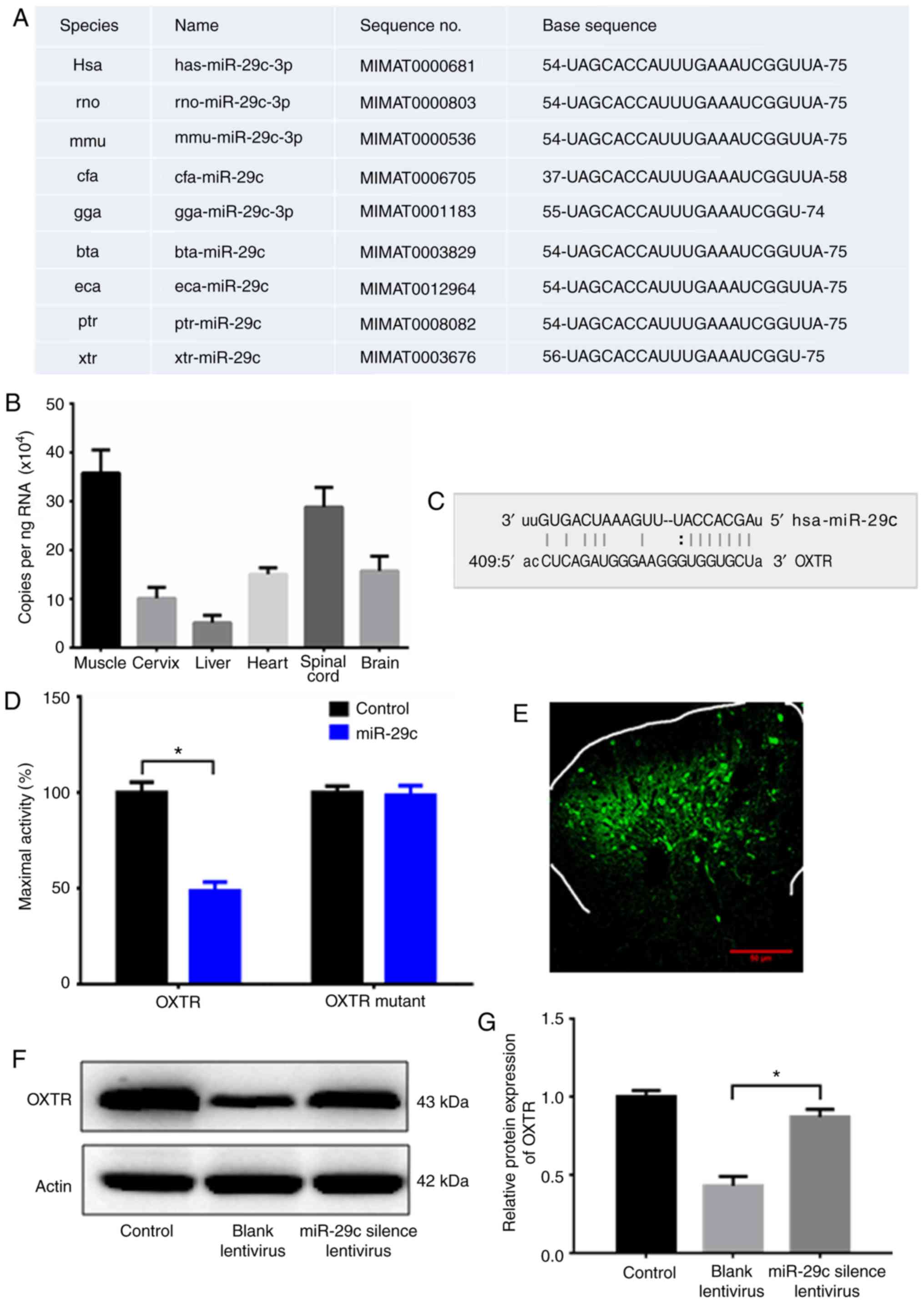 | Figure 3.miR-29c regulates OXTR expression.
(A) Online analysis (miRase) revealed that miR-29c is highly
conserved among human, rat, mouse, dog and other species. (B) The
expression of miR-29c in various organs examined by qPCR. (C) A
fully matched base sequence ‘UGGUGCU’ of OXTR and conserved
sequence ‘ACCACGA’ of miR-29c. (D) Luciferase assay measuring the
activity of OXTR. (E) SNI rats injected with miR-29c-silencing
lentivirus. One week later, the spinal cord of the rat was isolated
and the virus was identified by green fluorescence. (F and G) OXTR
expression was examined by western blotting. Control, rats with
sham operation (not SNI). NC lentivirus, SNI rats injected with NC
lentivirus. Silenced miR-29c, SNI rats injected miR-29c silencing
lentivirus. Data shown are means ± SD. Statistical significance was
determined by one-way analysis of variance, followed by Tukey's
post hoc test. *P<0.05. n=5. miR, microRNA; OXTR, oxytocin
receptor; SNI, spared nerve injury; NC, negative control. |
For further confirmation, the negative control
lentivirus, containing a scrambled sequence, and miR-29c-silencing
lentivirus was injected into two groups of SNI model rats.
miR-29c-silencing lentivirus was detected in spinal cords by green
immunofluorescence (Fig. 3E). OXTR
expression level in the spinal cord of these rats was analyzed by
western blotting. The result showed that rats injected with
miR-29c-silencing lentivirus presented with significantly higher
OXTR expression compared with SNI rats injected with negative
control vector lentivirus (Fig. 3F and
G), confirming that silencing miR-29c can restore OXTR
expression in SNI model rats.
Delivery induced OT-GABA mediated pain
relief response in neuronal cells
Furthermore, the mechanism of pain relief induced by
delivery was investigated. OT has received much attention in recent
years, due to its role in spinal antinociception to reduce the
symptoms of pain in inflammatory and neuropathic conditions.
Therefore, the concentration of OT in CSF was tested, and as
expected it was found that on the day of delivery, delivery+SNI
rats presented with higher levels of OT in their CSF compared with
SNI-only rats (Fig. 4A). This
indicated that the OT-GABA pathway may participate in the pain
relief response. To mimic the hormone environment of delivery in
vitro, primary neuronal cells were isolated from spinal cord of
the fetus at day 22 of gestation and treated with OT. The proposed
OT-mediated pain relief response was analyzed by measuring the
relative protein expression levels and electrophysiology. The
results of western blotting and qPCR showed that OT treatment
induced the upregulation of GABA, GABAA and OXTR, but
the downregulation of c-Fos, indicating the activation of
OXTR-mediated pain relief response (Fig. 4B, C and D). Electrophysiology assay
was performed to test the miniature inhibitory postsynaptic
currents (mIPSCs) in neurons. OT increased the frequency and
amplitude of the mIPSCs, however no influences on the decay time
were observed (Fig. 4E), which
suggested that the spinal inhibitory neurons became more active,
subsequently reducing the feeling of pain.
miR-29c reduces pain through the
spinal OT-GABA pathway
Based on the aforementioned results, it was
hypothesized that the silencing of miR-29c in the spinal cord can
alleviate pain, through the synaptic release of GABA, regulating
the expression of OXTR. To verify this hypothesis, the expression
levels of OXTR, GABA and GABAA in the spinal dorsal horn
were investigated by western blotting. The results showed a
significant increase in the aforementioned activated factors (OXTR
and GABA) that are associated with the OT-GABA pathway in SNI rats
with spinal injection of miR-29c-silenced lentivirus (Fig. 5A and B). Furthermore, the
miR-29c-silencing lentiviral vector and OXTR antagonist were used
to intervene cultured neurons, that were treated with OT (1 µM) for
30 min. Electrophysiology assay was performed to test mIPSCs in
neurons. It was found that silencing miR-29c significantly
increased the release of GABA in the culture medium (Fig. 5C) and increased the frequency and
amplitude of the mIPSCs compared with the control group (Fig. 5D). Interestingly, the addition of
OXTR antagonist L-368,899 hydrochloride (2 nM) (APExBIO) reversed
the amplified neuronal inhibitory current flows induced by
silencing miR-29c. This result indicated that miR-29c might reduce
pain through the OT-GABA pathway (Fig.
5E).
 | Figure 5.miR-29c regulates pain through spinal
oxytocin-GABA pathway. (A and B) SNI rats were injected with
miR-29-silencing lentivirus or NC lentivirus. One week later, rats
were sacrificed and the expressions of c-Fos, OXTR, GABA and GABAA
in spinal dorsal horn were analyzed by western blotting. Data shown
are mean ± SD of triplicates. Statistical significance was
determined by Student's t-test. (C) Cultured neurons were
pre-treated with OT (1 µM) for 30 min and subsequently intervened
by miR-29c-silencing lentiviral or NC lentiviral vectors. The
concentration of GABA in the culture medium was measured by
enzyme-linked immunosorbent assays. (D) mIPSCs were measured in
neurons with same treatments described in (C). (E) Cultured neurons
were pre-treated with OT (1 µM) for 30 min and subsequently
intervened with miR-29c silencing lentiviral vector with or without
OXTR antagonist, L-368,899. The mIPSCs were then measured.
NSP>0.05; *P<0.05; **P≤0.01. miR, microRNA; OXTR,
oxytocin receptor; SNI, spared nerve injury; GABA, γ-aminobutyric
acid; OT, oxytocin; mIPSCs, miniature inhibitory postsynaptic
currents. |
Discussion
It has been reported that the incidence of chronic
pain caused by childbirth is far below expected compared with other
tissue lesions, however the mechanism remains unclear. This project
explored the association between pain relief and childbirth. In
recent years, the discovery of miRNAs has provided a new avenue for
studying disease mechanism. miRNAs are mainly involved in the
regulation of gene expression at the post-transcriptional level
through the negative regulation of downstream target genes and play
important roles in the field of life sciences. Thus far, studies on
miRNAs are mainly involved in tumorigenesis, cell differentiation
and cardiovascular complications (12,18,19).
Recently, the role of miRNAs in pain regulation has begun to draw
attention. For example, there were reports that the upregulation of
miR-146 and miR-183 in the central nervous system can reduce the
expression of inflammatory mediators, which can effectively
alleviate pain caused by osteoarthritis (20); blocking the expression of miRNA-21
could effectively alleviate neuropathic hyperalgesia (21). In addition, the differentially
expressed miRNAs could also be presented and easily detected in the
body fluid of patients, in the form of exosomes or cell-free
miRNAs, which provides a potentially non-invasive detection method
for patients (22,23). In the study, an SNI rat model was
constructed during pregnancy to mimic the change in peripheral
nerve injury-induced hypersensitivity during the postpartum period.
For the control, sham operation was performed on control rats, in
an attempt to minimize the differences between control, SNI and
delivery+SNI groups. The sham operation may have some impacts on
the pain sensitivity of control rats initially but with recovery in
the following days, this impact was diminished and the pain
threshold of control rats returned to normal. The rats in the
delivery+SNI group were not allowed to perform maternal behaviors,
as this may have an impact on the OT level, leading to unknown
effects to the pain threshold. The rats did not show obvious
negative moods or behaviors as their control or SNI-only
counterparts. The results showed a significant improvement in the
neuropathic hyperalgesia on day 3 after delivery, which was
consistent with a previous report (9). A microarray analysis was performed on
the spinal cord of rats with neuropathic pain with or without
pregnancy at the third day after delivery. As expected, several
miRNAs were found to be differentially expressed indicating the
potential effects of miRNAs that are associated with pain
regulation.
To understand the specific candidate miRNAs that are
responsible for the protective effects of vaginal delivery,
upregulated miR-124a and downregulated miR-29c were selected as the
microRNAs of interest, since they demonstrated the largest
differences (~8-fold) between delivery+SNI and SNI groups
respectively (Fig. 2B). The
lentivirus was applied in order to increase the expression of
spinal miR-124a and decrease the expression of spinal miR-29c. The
downregulation of spinal miR-29c significantly improved neuropathic
hyperalgesia. Furthermore, bioinformatics analysis and previous
studies showed that: i) miR-29c was highly conserved during
evolution, with 100% homology in many species (human, rat, dog,
horse.), therefore, functional and mechanistic study in animal
models can provide a valuable direction for clinical application;
ii) the expression levels of miR-29c in different organs were
investigated and it was found that miR-29c is highly expressed in
spinal cord, suggesting that miR-29c may play an important role in
the central nervous system; iii) many studies on miR-29c
demonstrated its involvement in tumorigenesis and immunoregulation
(24,25). A recent study demonstrated that the
downregulation of miR-29c was involved in the initial pain
response, through recapitulating the profile of activated microglia
and TNFα (26). These findings
indicate that spinal miR-29c is involved in pain relief induced by
childbirth.
Furthermore, the prediction of downstream target
genes regulated by miR-29c was conducted using bioinformatics and
molecular biology techniques. As a result, the conserved sequence
‘ACCACGA’ of miR-29c matched with the base sequences ‘UGGUGCU’ of
numerous target genes, including spinal OXTR, which is associated
with pain relief after delivery (27). The luciferase assay demonstrated
that the of luciferase reporter activity with the OXTR 3′UTR was
inhibited by miR-29c, indicating that miR-29c can inhibit OXTR
expression in rats. Animal models also indicated a negative
association between spinal miR-29c and OXTR mRNA expression in SNI
rats injected with miR-29c inhibitory lentivirus, confirming that
silencing miR-29c can restore OXTR expression level in neuropathic
pain. A previous study suggested that the protective mechanism for
the reversal of pain after childbirth may be due to the function of
spinal OT and OXTR (27). It was
reported that OXTR, a G protein-coupled transmembrane receptor, can
induce a series of biological effects when combined with OT
(28). Previous studies proposed
an antinociceptive role for intrathecal OT (27,28),
in which OT was synthesized in the paraventricular nucleus and
supraoptic nucleus of the hypothalamus, then entered into the CSF
and interacted with increased OXTR in the dorsal horn of the spinal
cord. This enhanced the release of inhibitory neurotransmitters
GABA, resulting in an increased activity of GABAergic interneurons
(29,30). The expression of OXTR, GABA and
GABAA in the spinal dorsal horn was confirmed with
western blotting and qPCR. The data of the present study showed a
significant increase in OXTR and GABA, which are associated with
the OT-GABA pathway in SNI rats with spinal injection of
miR-29c-silencing lentivirus, indicating a potential mechanism for
pain modulation by miR-29c.
Increased OT concentration in the CSF was observed
in the delivery + SNI rats compared with non-pregnant SNI rats.
This was similar to a previous study, which showed increased OT
release in the central nervous system after delivery (31). In addition, OT was used in the
present study to trigger an enhanced GABAergic inhibitory activity
in spinal neurons (13,32). Electrophysiology assay was
performed to test mIPSCs in neurons. The in vitro data from
the present study revealed that postsynaptic mIPSP frequency and
amplitude can be increased by OT treatment in primary spinal cord
neuron cells by OXTR, which is consistent with the improved
hyperalgesia induced by childbirth in SNI rats (9). Furthermore, the downregulation of
miR-29c by miR-29c-silencing lentivirus for 5 h does not only
increase the GABA concentration in culture medium but also
increases OT-triggered mIPSP frequency and amplitude, suggesting a
GABAergic activation in the central nervous system. Interestingly,
this OT-induced GABAergic pathway can be blocked by OXTR
antagonist. Furthermore, it was confirmed that the downregulation
of miR-29c can reverse hyperalgesia though the OT-GABA pathway.
However, it should be noted that the present study only
demonstrated that miR-29c can regulate the OT-OXTR pathway, leading
to pain relief in an animal model; however, whether and how the
OT-OXTR pathway regulates miR-29c was not investigated, which
should be further investigated.
Taken together, the present study demonstrated a new
mechanism of pain modulation, in which delivery reduced
hyperalgesia by downregulating the expression of spinal miR-29c.
The spinal OT-GABA pathway plays an important role in reversing
childbirth-induced pain. Furthermore, the downregulation of miR-29c
in the spinal cord can result in the activation of the OT-GABA
pathway. Thus, the present study offers new insights into pain
research and therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81500944, 81600960 and
81271242), the Talent Fund (grant no. 2015-WSW-059) and Nanjing
Municipal Health Bureau general project (grant no. YKK14127).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and XS conceived and designed the experiments,
and drafted the manuscript. XW carried out the molecular genetic
studies and participated in the sequence alignment. GZ conducted
the immunoassays. YZ and XF participated in the sequence analysis
and conducted the statistical analysis. SX conceived the study,
participated in its design and coordination, and helped draft the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by JiangSu Center for Safety
Evaluation of Drug (Approval ID: LL-20160915-01) in accordance to
the ‘Regulations for the administration of affairs concerning
experimental animals of Jiangsu Province’. All efforts were made to
minimize suffering of animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rashiq S and Dick BD: Post-surgical pain
syndromes: A review for the non-pain specialist. Can J Anaesth.
61:123–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salama-Hanna J and Chen G: Patients with
chronic pain. Med Clin North Am. 97:1201–1215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rihmer Z: Antidepressants, depression and
suicide. Neuropsychopharmacol Hung. 15:157–164. 2013.(In
Hungarian). PubMed/NCBI
|
|
4
|
Tracey I and Bushnell MC: How neuroimaging
studies have challenged us to rethink: Is chronic pain a disease? J
Pain. 10:1113–1120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marchand F, Jones NG and McMahon SB:
Future treatment strategies for neuropathic pain. Handb Exp
Pharmacol. 589–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaasa T, Romundstad L, Roald H, Skolleborg
K and Stubhaug A: Hyperesthesia one year after breast augmentation
surgery increases the odds for persisting pain at four years A
prospective four-year follow-up study. Scand J Pain. 1:75–81. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hannah ME, Whyte H, Hannah WJ, Hewson S,
Amankwah K, Cheng M, Gafni A, Guselle P, Helewa M, Hodnett ED, et
al: Maternal outcomes at 2 years after planned cesarean section
versus planned vaginal birth for breech presentation at term: The
international randomized term breech trial. Am J Obstet Gynecol.
191:917–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenach JC, Pan P, Smiley RM,
Lavand'homme P, Landau R and Houle TT: Resolution of pain after
childbirth. Anesthesiology. 118:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gutierrez S, Liu B, Hayashida K, Houle TT
and Eisenach JC: Reversal of peripheral nerve injury-induced
hypersensitivity in the postpartum period: Role of spinal oxytocin.
Anesthesiology. 118:152–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lavand'homme P: Chronic pain after
childbirth. Curr Opin Anaesthesiol. 26:273–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gintzler AR and Liu NJ: Importance of sex
to pain and its amelioration; relevance of spinal estrogens and its
membrane receptors. Front Neuroendocrinol. 33:412–424. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiu H, Alqadah A and Chang C: The role of
microRNAs in regulating neuronal connectivity. Front Cell Neurosci.
7:2832014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YT, Huang CC and Hsu KS: Oxytocin
promotes long-term potentiation by enhancing epidermal growth
factor receptor-mediated local translation of protein kinase Mζ. J
Neurosci. 32:15476–15488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Decosterd I and Woolf CJ: Spared nerve
injury: An animal model of persistent peripheral neuropathic pain.
Pain. 87:149–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Feng Z, Wang X, Wang X and Zhang
X: DEGseq: An R package for identifying differentially expressed
genes from RNA-seq data. Bioinformatics. 26:136–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris JA: Using c-fos as a neural marker
of pain. Brain Res Bull. 45:1–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Braicu C, Cojocneanu-Petric R, Chira S,
Truta A, Floares A, Petrut B, Achimas-Cadariu P and Berindan-Neagoe
I: Clinical and pathological implications of miRNA in bladder
cancer. Int J Nanomedicine. 10:791–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Lyu H, Wang J and Liu B: MicroRNA
regulation and therapeutic targeting of survivin in cancer. Am J
Cancer Res. 5:20–31. 2014.PubMed/NCBI
|
|
20
|
Li X, Kroin JS, Kc R, Gibson G, Chen D,
Corbett GT, Pahan K, Fayyaz S, Kim JS, van Wijnen AJ, et al:
Altered spinal microRNA-146a and the microRNA-183 cluster
contribute to osteoarthritic pain in knee joints. J Bone Miner Res.
28:2512–2522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakai A and Suzuki H: Nerve injury-induced
upregulation of miR-21 in the primary sensory neurons contributes
to neuropathic pain in rats. Biochem Biophys Res Commun.
435:176–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petersen B and Kingsley K: Differential
expression of miR-21, miR-133 and miR-155 from exosome fractions
isolated from oral squamous cell carcinomas in vitro. J Med Discov.
1:jmd160102016.
|
|
23
|
Ma C, Nguyen HPT, Luwor RB, Stylli SS,
Gogos A, Paradiso L, Kaye AH and Morokoff AP: A comprehensive
meta-analysis of circulation miRNAs in glioma as potential
diagnostic biomarker. PLoS One. 13:e01894522018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Yi J, Zheng X, Liu S, Fu W, Ren L,
Li L, Hoon DSB, Wang J and Du G: miR-29c plays a suppressive role
in breast cancer by targeting the TIMP3/STAT1/FOXO1 pathway. Clin
Epigenetics. 10:642018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liston A, Papadopoulou AS, Danso-Abeam D
and Dooley J: MicroRNA-29 in the adaptive immune system: Setting
the threshold. Cell Mol Life Sci. 69:3533–3541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong H, Na YJ, Lee K, Kim YH, Lee Y, Kang
M, Jiang BC, Yeom YI, Wu LJ, Gao YJ, et al: High-resolution
transcriptome analysis reveals neuropathic pain gene-expression
signatures in spinal microglia after nerve injury. Pain.
157:964–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Juif PE, Breton JD, Rajalu M, Charlet A,
Goumon Y and Poisbeau P: Long-lasting spinal oxytocin analgesia is
ensured by the stimulation of allopregnanolone synthesis which
potentiates GABA(A) receptor-mediated synaptic inhibition. J
Neurosci. 33:16617–16626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gimpl G and Fahrenholz F: The oxytocin
receptor system: Structure, function, and regulation. Physiol Rev.
81:629–683. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun W, Zhou Q, Ba X, Feng X, Hu X, Cheng
X, Liu T, Guo J, Xiao L, Jiang J, et al: Oxytocin relieves
neuropathic pain through GABA release and presynaptic TRPV1
inhibition in spinal cord. Front Mol Neurosci. 11:2482018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Severino AL, Chen R, Hayashida K,
Aschenbrenner CA, Sun H, Peters CM, Gutierrez S, Pan B and Eisenach
JC: Plasticity and function of spinal oxytocin and vasopressin
signaling during recovery from surgery with nerve injury.
Anesthesiology. 129:544–556. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takeda S, Kuwabara Y and Mizuno M: Effects
of pregnancy and delivery on oxytocin levels in human plasma and
cerebrospinal fluid. Endocrinol Jpn. 32:875–880. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han RT, Kim YB, Park EH, Kim JY, Ryu C,
Kim HY, Lee J, Pahk K, Shanyu C, Kim H, et al: Long-term isolation
elicits depression and anxiety-related behaviors by reducing
oxytocin-induced GABAergic transmission in central amygdala. Front
Mol Neurosci. 11:2462018. View Article : Google Scholar : PubMed/NCBI
|



















