Introduction
Osteoarthritis (OA) is a common chronic arthritis
affecting cartilaginous tissues that generally occurs in
middle-aged and elderly patients and affects ~40% of individuals
>70 years of age (1). The
common clinical manifestations of OA are pain, and joint
dysfunction and deformity, and OA is regarded as one of the primary
causes of disability, representing a major healthcare issue for
both governments and researchers (2). Previous studies have demonstrated
that numerous factors contribute to the occurrence of OA, including
obesity, aging, inflammation and physical strain (3–5). Due
to the high prevalence of obesity and age-associated diseases, OA
is associated with significant morbidity worldwide (6). Therefore, an increased understanding
of the pathological mechanisms underlying OA, particularly
degeneration of articular cartilage, may prevent the progression of
the disease.
Nuclear factor erythroid 2 like 1 (NRF1) is a basic
leucine zipper protein that belongs to the cap-N-collar
transcriptional factor family (7).
NRF1 is produced by osteoblasts, and is involved in regulating
osterix expression, osteoblast differentiation and bone formation
(8). NRF1 was shown to serve
important roles in rheumatoid arthritis (9). Additionally, NRF1 serves an important
role in the regulation of antioxidant enzymes during
inflammation-induced oxidative stress in osteoblastic cells
(10). The aforementioned data
suggest that NRF1 is closely associated with the development of
arthritis, partly by regulating cellular differentiation,
inflammation and oxidative stress. However, to the best of our
knowledge, the function of NRF1 in the progression of OA has not
been previously investigated.
MicroRNAs (miRs/miRNAs) are a group of short,
non-coding RNAs that serve an important role in regulating gene
expression. Furthermore, miRNAs have been implicated in the
progression of various diseases, including OA (11). Kong et al (12), revealed that miR-486-5p was
upregulated in patients with knee OA, and was positively correlated
with the severity of the disease. Additionally, miR-486-5p was
reported to target SMAD family member 2, and negatively regulate
the expression of collagen type II and aggrecan in chondrocytes,
aggravating the progression of OA (13). However, to the best of our
knowledge, the role of miR-486-5p in OA has only been investigated
in the aforementioned two studies, and whether miR-486-5p could
regulates NRF1 remains unknown. Therefore, the mechanisms
underlying miR-486-5p and OA require further investigation.
The present study aimed to explore the roles of
miR-486-5p and NRF1 in OA, and to explore the underlying mechanism
of molecular action, which may provide a novel treatment strategy
for OA.
Materials and methods
Cell culture and treatment
The murine chondrogenic ATDC5 cell line was obtained
from the American Type Culture Collection. The cells were cultured
in DMEM: Hams F-12 (1:1; Sigma-Aldrich; Merck KGaA) supplemented
with 2 mM glutamine (Sigma-Aldrich; Merck KGaA) and 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) and maintained at 37°C
in a humidified incubator containing 5% CO2. ATDC5 cells
were then treated with lipopolysaccharide (LPS; Sigma-Aldrich;
Merck KGaA) at different concentrations (0, 1, 2, 4 and 8 µg/ml) at
37°C for 6 h to induce inflammatory injury.
Cell Counting Kit-8 (CCK-8) assay
The proliferation of ATDC5 cells was determined
using a CCK-8 assay kit (Dojindo Molecular Technologies, Inc.),
according to the manufacturers protocol. Cells (5×103
cells/well) were seeded into a 96-well plate and cultured for 24 h.
After treatment with LPS for 6 h, 20 µl CCK-8 solution was added
into each well and incubated for another 4 h at 37°C. The
absorbance was subsequently detected at a wavelength of 450 nm
using a microplate reader.
Western blot analysis
ATDC5 cells were lysed with cold RIPA lysis buffer
(Beyotime Institute of Biotechnology) supplemented with protease
inhibitor. The concentration of protein was measured using the BCA
protein assay (Pierce; Thermo Fisher Scientific, Inc.). The
proteins (20 µg/lane) were separated using 10% SDS-PAGE, and
transferred onto PVDF membranes. Membranes were blocked with 5%
skimmed milk at room temperature for 1 h and incubated with the
primary antibodies with a dilution of 1:1,000 overnight at 4°C. The
antibodies against NRF1 (cat. no. ab137572), Bax (cat. no.
ab32503), Bcl-2 (cat. no. ab59348), cleaved caspase-3 (cat. no.
ab49822), caspase-3 (cat. no. ab32499), superoxide dismutase 1
(SOD1; cat. no. ab13498), heme oxygenase-1 (HO-1; cat. no.
ab13243), NAD(P)H quinone dehydrogenase 1 (NQO-1; cat. no. ab34173)
and GAPDH (cat. no. ab9485) were obtained from Abcam. Following
incubation with the primary antibodies, the membranes were
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.)
for 2 h at room temperature. Bands were visualized using an
enhanced chemiluminescence kit (SuperSignal™ West Pico PLUS
chemiluminescent substrate; Thermo Fisher Scientific, Inc.). The
densitometric analysis was performed using ImageJ software v1.4
(National Institutes of Health).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from ATDC5 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). To
detect the mRNA levels of miR-486-5p, the first strand of cDNA was
synthesized using the PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd.), according to the manufacturers protocol,
and the following temperature conditions: Initial incubation at
37°C for 15 min, followed by an incubation at 85°C for 5 sec.
RT-qPCR was performed using a SYBR-Green Prime Script RT-PCR kit
(Takara Biotechnology Co., Ltd.), according to the manufacturers
instructions. The thermocycler conditions for RT-qPCR were as
follows: 95°C for 5 min, followed by 40 cycles of denaturation at
95°C for 15 sec and annealing/extension at 60°C for 30 sec. The
primer sequences were as follows: miR-486-5p forward,
5-CTCGCTTCGGCAGCACA-3; miR-486-5p reverse 5-ACGCTTCACGAATTTGCGT-3;
NRF1 forward, 5- GGAGAGCTTCCCTGCACAGT-3; NRF1 reverse, 5-
TTACTTCCATAGCCTGCATTTCC-3; β-actin forward, 5-CCGCGAGCACAGCTTCTT-3;
β-actin reverse, 5-CCCACGATGGAGGGGAATAC-3; U6 forward,
5-CTCGCTTCGGCAGCACA-3; and U6 reverse, 5-AACGCTTCACGAATTTGCGT-3.
Data were collected and analyzed using 2−ΔΔCq method
(14) and normalized to the
internal control U6 and β-actin.
Cell transfection
ATDC5 cells were transfected with the miR-486-5p
inhibitor (5-CUCGGGGCAGCUCAGUACAGGA-3; 150 nM; Qiagen GmbH) and
inhibitor control (miR-NC; 5-UUCUCCGAACGUGUCACGUTT-3; 150 nM;
Qiagen GmbH), or transfected with short hairpin RNA (shRNA)1 or
shRNA2 targeting NRF1 (100 nM) and its scramble control shRNA
(shRNA-NC; empty vector), which were cloned into pSilencer 3.1-H1
puro plasmids (Shanghai GenePharma Co. Ltd.), using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturers protocol. The transfection
efficiency was detected by RT-qPCR 48 h post-transfection.
Dual luciferase reporter assay
The binding site of miR-486-5p on NRF1 was predicted
using the miRDB database (www.mirdb.org)
and was validated using the luciferase reporter assay.
PmirGLO-NRF1-WT or pmirGLO-NRF1-Mut reporter plasmids (Promega
Corporation) were co-transfected with the miR-486-5p mimic or
miR-NC into ATDC5 cells using Lipofectamine® 2000
reagent. The luciferase activity was measured using a
Dual-Luciferase® Reporter assay system (Promega
Corporation) 48 h post-transfection. The data were standardized to
Renilla luciferase activity.
Inflammatory cytokines assay
The cell culture medium of ATDC5 cells was
collected. The levels of tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β, IL-6, monocyte chemotactic protein 1 (MCP-1)
and intercellular adhesion molecule 1 (ICAM-1) were determined
using ELISA kits (cat. no. 210-TA-005 for TNF-α; cat. no.
201-LB-005 for IL-1β; cat. no. S6050 for IL-6; cat. no. SCP00 for
MCP-1; cat. no. 796-IC-050 for ICAM-1; R&D Systems, Inc.)
according to the manufacturers instructions.
Oxidative stress factors assay
The cell culture medium of ATDC5 cells was
collected. The levels of reactive oxygen species (ROS; cat. no.
E004-1-1), malondialdehyde (MDA; cat. no. A003-4-1), SOD (cat. no.
A001-3-2) and lactate dehydrogenase (LDH; A020-2-2) were determined
using their corresponding assay test kits (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturers
instructions.
Flow cytometry
The apoptosis of ADTC5 cells was analyzed using an
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis detection kit (Sigma-Aldrich; Merck KGaA). Following
treatment of LPS, the transfected ATDC5 cells were washed with PBS
and incubated with Annexin V-FITC/PI (50 µg/ml) in the presence of
RNase A (50 µg/ml; Sigma-Aldrich; Merck KGaA) at room temperature
for 30 min in the dark. The apoptotic cells were detected by a
FACScan flow cytometer (BD Biosciences) and analyzed using FlowJo
software v7.6 (FlowJo LLC).
Statistical analysis
Statistical analyses were performed using SPSS
software (v.17.0; SPSS, Inc.). Data are presented as the mean ± SD
of 3 independent experiments. Data were analyzed by the one-way
ANOVA followed by the Tukeys post hoc test. P<0.05 was
considered to indicate a statistically significant different
difference.
Results
Expression of miR-486-5p and NRF1 in
LPS-induced ATDC5 cells
Following treatment of ATDC cells with LPS (0, 1, 2,
4 and 8 µg/ml), the cell proliferation was detected using a CCK-8
kit, and the mRNA and protein expression levels of miR-486-5p and
NRF1 were detected using RT-qPCR and western blotting,
respectively. As shown in Fig. 1A,
LPS treatment (2, 4 and 8 µg/ml) significantly decreased the
proliferation in a concentration-dependent manner. Furthermore, the
mRNA level of miR-486-5p was increased following LPS treatment (2,
4 and 8 µg/ml; Fig. 1B). Protein
expression of NRF1 was significantly decreased following LPS
treatment, particularly at 4 µg/ml of LPS (Fig. 1C). Therefore, this concentration
was chosen for subsequent experimentation. The results revealed
that miR-486-5p was upregulated while NRF1 was downregulated in
LPS-induced ATDC5 cells. To determine the LPS-induced inflammatory
injury in ATDC5 cells, the levels of inflammatory cytokines
including TNF-α, IL-1β, IL-6, MCP-1 and ICAM-1 were assayed by
ELISA kits. Results in Fig. 1D
indicated that LPS induced a significant increase in these
inflammatory cytokines, revealing a marked inflammatory injury
induced by LPS.
 | Figure 1.Expression of miR-486-5p and NRF1 in
LPS-induced ATDC5 cells. Following induction of LPS (0, 1, 2, 4 and
8 µg/ml) in ATDC cells, (A) cell viability was detected using a
CCK-8 assay, (B) mRNA level of miR-486-5p was detected using
reverse transcription-quantitative PCR, and (C) protein expression
of NRF1 was detected using western blotting. *P<0.05 and
***P<0.001 vs. 0. (D) Levels of inflammatory cytokines,
including TNF-α, IL-1β, IL-6, MCP-1 and ICAM-1 were detected by
ELISA kits. ***P<0.001 vs. control. Data were generated from at
least 3 experiments. miR, microRNA; LPS, lipopolysaccharide; NRF1,
nuclear factor erythroid 2 like 1; TNF-α, tumor necrosis factor α;
IL, interleukin; MCP-1, monocyte chemotactic protein 1; ICAM-1,
intercellular adhesion molecule 1. |
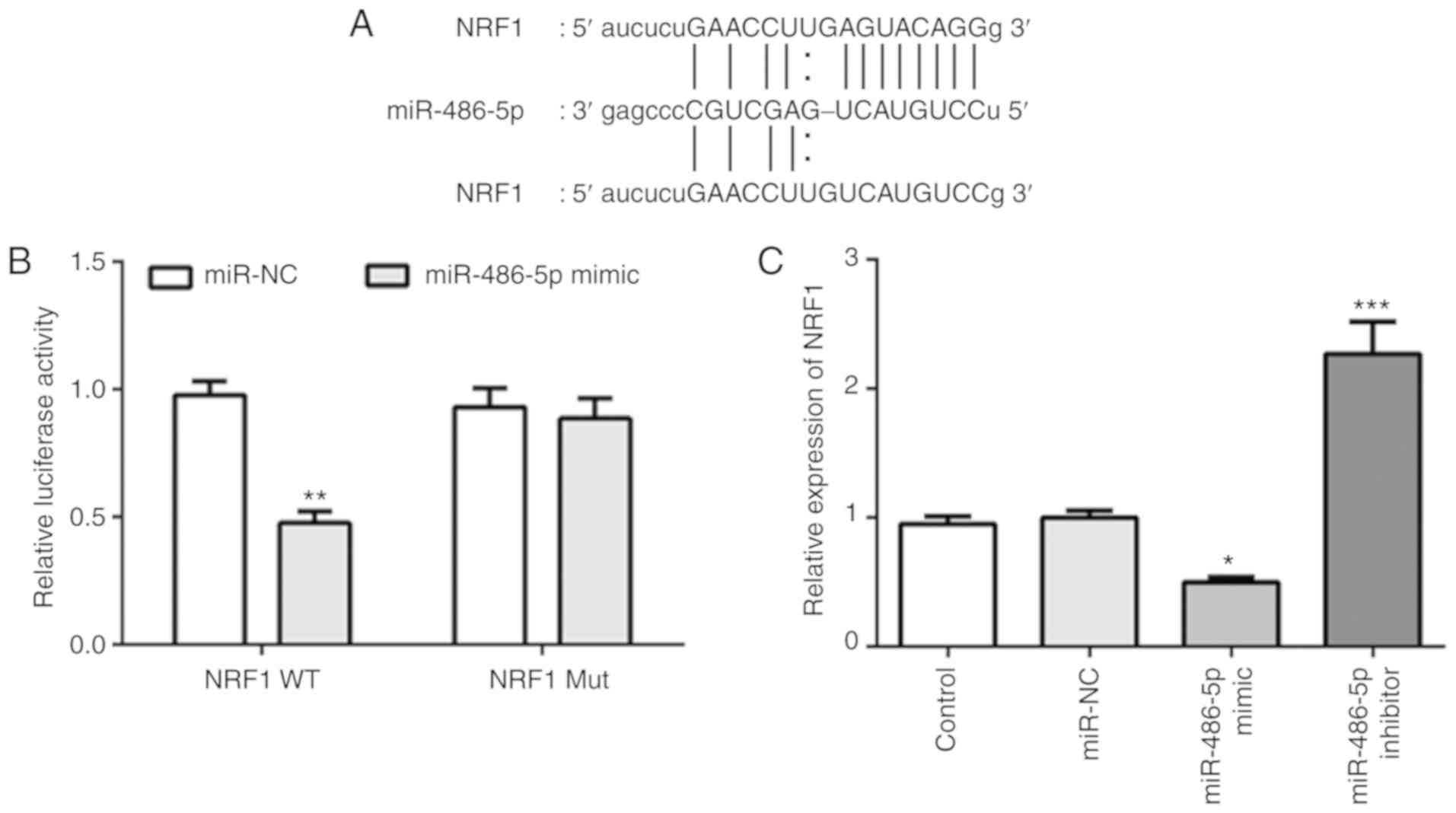
NRF1 is a target of miR-486-5p in ATDC
cells
Analysis of the online databases of miRDB revealed
that the 3-UTR of NRF1 included a binding site for miR-486-5p
(Fig. 2A). A luciferase reporter
assay was subsequently performed to validate the interaction
between miR-486-5p and NRF1 in ATDC cells. As shown in Fig. 2B, overexpression of miR-486-5p
significantly suppressed the activity of the reporter gene, whereas
the plasmid containing the mutated sequence did not affect the
activity of the reporter gene in ADTC cells. Besides,
overexpression of miR-486-5p decreased the expression of NRF1,
while inhibition of miR-486-5p increased the expression of NRF1
(Fig. 2C). These results suggested
that miR-486-5p could directly target NRF1 and regulate the
expression of NRF1 in ATDC5 cells.
miR-486-5p regulates the LPS-induced
inflammatory response in ATDC5 cells by targeting NRF1
The effect of miR-486-5p and NRF1 on LPS-induced
ATDC5 cells was investigated. A miR-486-5p inhibitor or shRNA
targeting NRF1 was transfected into cells. As shown in Fig. 3A and B, the increased mRNA level of
miR-486-5p induced by LPS was significantly decreased following
transfection with the miR-486-5p inhibitor. Furthermore, the
expression of NRF1 was significantly decreased when cells were
transfected with shRNA1 or shRNA2 targeting NRF1. Due to a higher
transfection efficacy, shRNA1-NRF1 was used for the further
experimentation.
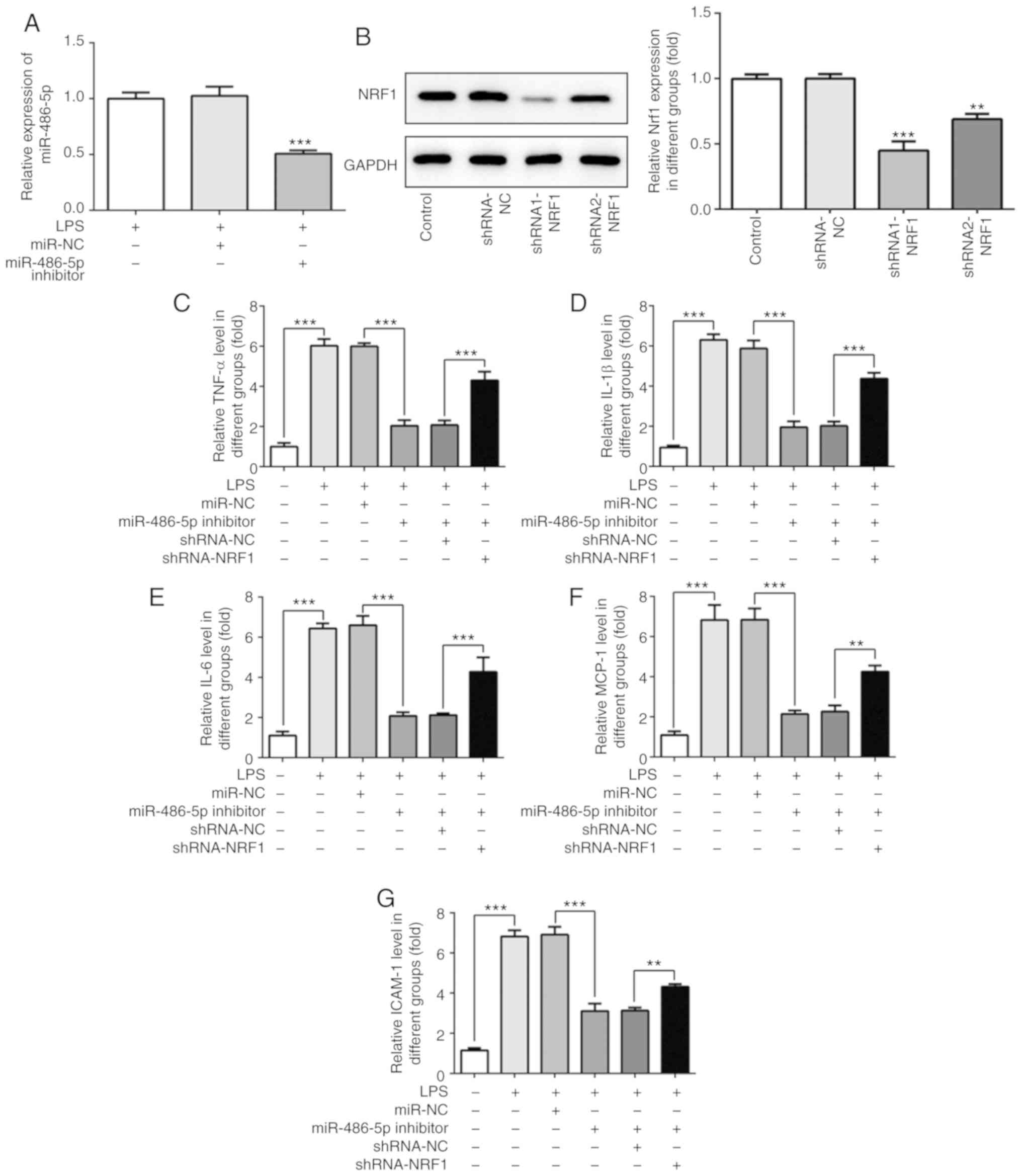 | Figure 3.Effect of miR-486-5p on inflammatory
response in LPS-induced ATDC5 cells. (A) Following transfection
with the miR-486-5p inhibitor, the mRNA level of miR-486-5p was
detected using reverse transcription-quantitative PCR.
***P<0.001 vs. LPS and miR-NC groups. (B) Following transfection
with the shRNA-NRF1, the protein expression of NRF1 was detected
using western blotting. **P<0.01 and ***P<0.001 vs. control.
(C-G) Levels of inflammatory cytokines (TNF-α, IL-1β, IL-6, MCP-1
and ICAM-1) were detected using ELISA. Data were generated from at
least 3 experiments. **P<0.01 and ***P<0.001. miR, microRNA;
LPS, lipopolysaccharide; NRF1, nuclear factor erythroid 2 like 1;
TNF-α, tumor necrosis factor α; IL, interleukin; MCP-1, monocyte
chemotactic protein 1; ICAM-1, intercellular adhesion molecule 1;
shRNA, short hairpin RNA; NC, negative control. |
The change of inflammatory cytokines, directly
reflecting the degree of inflammatory injury in LPS-induced ATDC5
cells, is presented in Fig. 3C-G.
As observed from the results, LPS induction significantly increased
the production of TNF-α, IL-1β, IL-6, MCP-1 and ICAM-1. As
expected, the production of these inflammatory cytokines was
decreased following transfection with the miR-486-5p inhibitor, and
co-transfection with shRNA-NRF1 abrogated this effect. These
results suggested that the inhibition of miR-486-5p may
significantly alleviate LPS-induced inflammatory injury in ATDC5
cells by regulating NRF1.
miR-486-5p regulates LPS-induced cell
apoptosis by targeting NRF1
The effect of miR-486-5p on cell apoptosis in
LPS-induced ATDC5 cells was investigated. Flow cytometry analysis
revealed an increased apoptosis rate following LPS induction,
whereas in the miR-486-5p inhibitor group the apoptotic rate of the
cells was decreased compared with the LPS group (Fig. 4A). Additionally, the expression
levels of apoptosis-associated proteins were investigated by
western blotting. LPS induction significantly decreased the protein
expression of Bcl-2, and increased the protein expression of Bax
and cleaved caspase-3 (Fig. 4B),
indicating that LPS treatment resulted in ATDC cell apoptosis by
regulating the expression of apoptosis-associated proteins.
Furthermore, the expressions of Bax and cleaved caspase-3 was
decreased, and Bcl-2 was increased in the miR-486-5p inhibitor
group, suggesting an inhibitory effect of miR-486-5p in LPS-induced
ATDC cells. The inhibitory effect of miR-486-5p was decreased by
co-transfection with shRNA-NRF1. These results suggested that
miR-486-5p could attenuate cell apoptosis in LPS-induced ATDC5
cells by targeting NRF1.
miR-486-5p regulates LPS-induced
oxidative stress in ADTC5 cells by targeting NRF1
The results presented in Fig. 5A-E demonstrate the changes in
oxidative stress markers in the LPS-induced ATDC5 cells. As shown
in Fig. 5A-D, LPS induction
significantly increased the activity of ROS and the levels of MDA
and LDH, and decreased the activity of SOD. Additionally,
antioxidant enzymes such as SOD1, HO-1 and NQO-1 were significantly
decreased following treatment with LPS, which is indicative of
oxidase and anti-oxidase imbalance and may result in oxidative
stress injury. Inhibition of miR-486-5p significantly decreased the
activity of ROS, decreased the levels of MDA and LDH, and increased
the activity of SOD, as well as increased the expression of
antioxidant enzymes. These data indicated a decrease in oxidative
stress following transfection with the miR-486-5p inhibitor.
However, co-transfection with shRNA-NRF1 abrogated this effect.
These results suggested that inhibition of miR-486-5p could
alleviate LPS-induced oxidative stress in ATDC5 cells by targeting
NRF1.
 | Figure 5.Effect of miR-486-5p on oxidative
stress in LPS-induced ATDC5 cells. Levels of (A) ROS, (B) MDA, (C)
LDH and (D) SOD were determined using corresponding test kits. (E)
Protein expression levels of antioxidant enzymes (SOD1, HO-1 and
NQO-1) were detected using western blotting. Data were generated
from at least 3 experiments. *P<0.0.5, **P<0.01 and
***P<0.001. miR, microRNA; LPS, lipopolysaccharide; NRF1,
nuclear factor erythroid 2 like 1; ROS, reactive oxygen species;
MDA, malondialdehyde; LDH, lactate dehydrogenase; SOD, superoxide
dismutase; HO-1, heme oxygenase 1; NQO-1, NAD(P)H quinone
dehydrogenase 1; NC, negative control; shRNA, short hairpin RNA |
Discussion
The present study revealed that miR-486-5p was
upregulated while NRF1 was downregulated in LPS-treated ATDC5
cells. Further investigation demonstrated that NRF1 was a direct
target of miR-486-5p, and that miR-486-5p serves an important role
in regulating cell damage in LPS-induced ATDC5 cells by targeting
NRF1. OA is generally accompanied by oxidative stress, apoptosis
and inflammation, which are involved in cartilage damage (15). In the present study, an increased
production of inflammatory cytokines, severe oxidative stress
injury and increased apoptosis were observed in LPS-induced ATDC5
cells. Inhibition of miR-486-5p effectively suppressed
inflammation, oxidative stress and apoptosis induced by LPS,
whereas the inhibitor effect of downregulated miR-486-5p was
decreased by inhibiting NRF1. Additionally, the decreased
expressions of SOD1, HO-1 and NQO-1 as a result of LPS treatment
was improved by the inhibition of miR-486-5p. Decreasing the
expression of NRF1 abrogated this effect. These results indicated
that the inhibition of miR-486-5p may have significant therapeutic
potential in OA.
The degeneration of articular cartilage is one of
the primary causes underlying the development of OA (16). The inflammatory response of
articular cartilage accompanied by the release of inflammatory
cytokines from chondrocytes is responsible for the degeneration of
articular cartilage, and therefore for the occurrence and
development of OA (17,18). LPS induced inflammatory injury in
various cells by promoting the production and release of
inflammatory cytokines, including TNF-α, IL-6 and IL-1β, which were
involved in the inflammatory response of OA (19,20).
At present, LPS-induced chondrocytes are widely used as a cell
model to simulate the inflammation of articular cartilage in order
to investigate the mechanisms underlying OA and to identify more
effective therapeutic targets (18,21).
An increase in the release of TNF-α, IL-6, IL-1β and MCP-1 was
detected in LPS-induced chondrogenic ATDC5 cells in the present
study. Inhibition of miR-486-5p decreased the release of
inflammatory cytokines, suggesting that the inhibition of
miR-486-5p was beneficial and suppressed LPS-induced inflammatory
injury in ATDC5 cells. ICAM-1, a type of cell adhesion molecule, is
an immunoglobulin-like transmembrane protein that is induced by
inflammatory cytokines in endothelial cells (22). Therefore, the expression of ICAM-1
is generally upregulated by inflammatory cytokines such as TNF-α
and IL-6 (23,24). The present study revealed that
ICAM-1 was upregulated in LPS-induced ATDC5 cells in response to
the inflammatory cytokines. The expression of ICAM-1 was
downregulated following the inhibition of miR-486-5p, reflecting
the decrease in inflammatory injury.
The pathological process of OA is complex and
multifactorial, and chondrocyte apoptosis is crucial in the
progression of OA (25).
Chondrocyte apoptosis has been observed in cell and animal models
of OA (25,26). Inhibition of cell apoptosis has
been an effective approach to screen for beneficial drugs in the
treatment of OA (25,26). The present study revealed that LPS
significantly increased the rate of apoptosis, which was then
decreased after inhibition of miR-486-5p. In addition, the
increased expression of Bcl-2, and decreased expression levels of
Bax and cleaved caspase-3 following the inhibition of miR-486-5p
indicated that miR-486-5p may exert its function by regulating the
expression of apoptosis-related proteins.
Previous studies have revealed that HO-1 is an
inducible enzyme that exerts anti-inflammatory, antioxidant and
anti-apoptotic functions (27,28).
It has been reported that the activation of HO-1 may protect
against oxidative stress induced by ethanol in LO2 cells (29). Inhibition of HO-1 promoted the
activation of the NF-κB signaling pathway and the production of
pro-inflammatory cytokines (30).
It was also reported that HO-1 regulated oxidative stress and
apoptosis in OA chondrocytes (31). In the present study, HO-1 was
significantly downregulated in chondrocytes induced by LPS-induced
ATDC5 cells, and was upregulated following miR-486-5p inhibition.
This suggested that the anti-inflammatory, antioxidant and
anti-apoptotic effects of miR-486-5p inhibition in LPS-induced
ATDC5 cells may be attributed to the downregulation of HO-1.
Oxidative stress and generation of free radicals, as primary and
secondary events, have contributed to a large number of diseases,
including OA (32). The gene of
SOD1, NQO-1, as well as ROS, MDA, LDH and total SOD serve an
important role in cytoprotection against oxidative stress (33–35).
The present study revealed that the anti-oxidative activity of
miR-486-5p inhibition was reflected in the increased expression of
antioxidant-associated genes such as HO-1, NQO-1 and SOD, and the
decreased expression of ROS, MDA and LDH.
The present study revealed the effect of miR-486-5p
on the LPS-induced inflammatory response, oxidative stress and
apoptosis in ATDC5 cells. Therefore, further investigation was
performed to elucidate the target gene and regulatory mechanism of
miR-486-5p. The results revealed that NRF1 is a direct target of
miR-486-5p. NRF1 is an important regulator involved in cellular
oxidative stress and inflammatory injury. In NRF1-knockout mice,
the expression levels of HO-1 and NQO-1 genes were significantly
increased, indicating that NRF1 knockout alleviates the oxidative
stress response (36,37). In addition to mediating the
expression of antioxidant genes, NRF1 is also implicated in
cellular immune responses and mitochondrial homeostasis (38). In the present study, the effect of
miR-496-5p inhibition was diminished following co-transfection with
shRNA-NRF1, indicating that miR-496-5p may exert its functions by
regulating the expression of NRF1. This result demonstrated that
NRF1 participated in the inflammatory response and oxidative stress
during the progression of OA.
In conclusion, the present study demonstrated that
inhibition of miR-486-5p alleviated LPS-induced cell damage in
ATDC5 cells. Furthermore, NFR1 was shown to be a target of
miR-486-5p, and inhibition of miR-486-5p mitigated inflammation,
oxidative stress and apoptosis by regulating NFR1. The results of
the present study may provide a novel viewpoint regarding
miR-486-5p as potential therapeutic target for OA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors contributions
QC designed and conceived the experiments. QC, MJ
and CL performed the experiments. QC and RG analyzed the data. QC,
MJ and CL wrote the manuscript. MJ and RG performed the software
analyses. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dieppe PA and Lohmander LS: Pathogenesis
and management of pain in osteoarthritis. Lancet. 365:965–973.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Y, Ming J, Li Y, Du X, Deng M, He B,
Zhou J, Wang G and Liu S: Surfactant protein D attenuates nitric
oxide-stimulated apoptosis in rat chondrocyte by suppressing p38
MAPK signaling. Biochem Biophys Res Commun. 495:526–532. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sacitharan PK: Ageing and Osteoarthritis.
Subcell Biochem. 91:123–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones G: Whats new in osteoarthritis
pathogenesis? Intern Med J. 46:229–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yucesoy B, Charles LE, Baker B and
Burchfiel CM: Occupational and genetic risk factors for
osteoarthritis: A review. Work. 50:261–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin H, Zhang H, Ma T, Lan H, Feng S, Zhu H
and Ji Y: Resveratrol protects murine chondrogenic ATDC5 cells
against LPS-induced inflammatory injury through up-regulating
miR-146b. Cell Physiol Biochem. 47:972–980. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bugno M, Daniel M, Chepelev NL and
Willmore WG: Changing gears in Nrf1 research, from mechanisms of
regulation to its role in disease and prevention. Biochim Biophys
Acta. 1849:1260–1276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim J, Xing W, Wergedal J, Chan JY and
Mohan S: Targeted disruption of nuclear factor erythroid-derived
2-like 1 in osteoblasts reduces bone size and bone formation in
mice. Physiol Genomics. 40:100–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li G, Han N, Li Z and Lu Q: Identification
of transcription regulatory relationships in rheumatoid arthritis
and osteoarthritis. Clin Rheumatol. 32:609–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SY, Kim SH, Yoon HK, Yim CH and Lim
SK: The role of nuclear factor-E2-related factor 1 in the oxidative
stress response in MC3T3-E1 osteoblastic cells. Endocrinol Metab
(Seoul). 31:336–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nugent M: MicroRNAs: Exploring new
horizons in osteoarthritis. Osteoarthritis Cartilage. 24:573–580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong R, Gao J, Si Y and Zhao D:
Combination of circulating miR-19b-3p, miR-122-5p and miR-486-5p
expressions correlates with risk and disease severity of knee
osteoarthritis. Am J Transl Res. 9:2852–2864. 2017.PubMed/NCBI
|
|
13
|
Shi J, Guo K, Su S, Li J and Li C:
miR-486-5p is upregulated in osteoarthritis and inhibits
chondrocyte proliferation and migration by suppressing SMAD2. Mol
Med Rep. 18:502–508. 2018.PubMed/NCBI
|
|
14
|
Livak KJST and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mongkhon JM, Thach M, Shi Q, Fernandes JC,
Fahmi H and Benderdour M: Sorbitol-modified hyaluronic acid reduces
oxidative stress, apoptosis and mediators of inflammation and
catabolism in human osteoarthritic chondrocytes. Inflamm Res.
63:691–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata K, Kanemura N, Kokubun T, Fujino T,
Morishita Y, Onitsuka K, Fujiwara S, Nakajima A, Shimizu D and
Takayanagi K: Controlling joint instability delays the degeneration
of articular cartilage in a rat model. Osteoarthritis Cartilage.
25:297–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saklatvala J: Inflammatory signaling in
cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use
of inhibitors for research into pathogenesis and therapy of
osteoarthritis. Curr Drug Targets. 8:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan L, Liu D, Zhao L, Wang L, Xin M and Li
X: Long noncoding RNA MALAT1 alleviates lipopolysaccharide-induced
inflammatory injury by upregulating microRNA-19b in murine
chondrogenic ATDC5 cells. J Cell Biochem. 119:10165–10175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu DP, Zhang JL, Wang JY, Cui MX, Jia JL,
Liu XH and Liang QD: MiR-1246 promotes LPS-induced inflammatory
injury in chondrogenic cells ATDC5 by targeting HNF4γ. Cell Physiol
Biochem. 43:2010–2021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scotece M, Conde J, Abella V, López V,
Francisco V, Ruiz C, Campos V, Lago F, Gomez R, Pino J, et al:
Oleocanthal inhibits catabolic and inflammatory mediators in
LPS-activated human primary osteoarthritis (OA) chondrocytes
through MAPKs/NF-κB pathways. Cell Physiol Biochem. 49:2414–2426.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y and Kong D: MicroRNA-136 promotes
lipopolysaccharide-induced ATDC5 cell injury and inflammatory
cytokine expression by targeting myeloid cell leukemia 1. J Cell
Biochem. 119:9316–9326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hubbard AK and Rothlein R: Intercellular
adhesion molecule-1 (ICAM-1) expression and cell signaling
cascades. Free Radic Biol Med. 28:1379–1386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dadgar Pakdel F, Keramatipour M,
Noorbakhsh F, Talebi S and Vodjgani M: Investigating the effect of
rs3783605 single-nucleotide polymorphism on the activity of VCAM-1
promoter in human umbilical vein endohelial cells. Iran J Allergy
Asthma Immunol. 14:179–187. 2015.PubMed/NCBI
|
|
24
|
Yu GI, Jun SE and Shin DH: Associations of
VCAM-1 gene polymorphisms with obesity and inflammation markers.
Inflamm Res. 66:217–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Z and Ren C: Emodin attenuates
apoptosis and inflammation induced by LPS through up-regulating
lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed
Pharmacother. 103:897–902. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z,
Zhang J and Pan J: Geniposide suppresses interleukin-1β-induced
inflammation and apoptosis in rat chondrocytes via the
PI3K/Akt/NF-κB signaling pathway. Inflammation. 41:390–399. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu DY, Chen JH, Tan TW, Huang CY, Yeh WL
and Hsu HC: Resistin protects against 6-hydroxydopamine-induced
cell death in dopaminergic-like MES23.5 cells. J Cell Physiol.
228:563–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiong J, Wang K, Yuan C, Xing R, Ni J, Hu
G, Chen F and Wang X: Luteolin protects mice from severe acute
pancreatitis by exerting HO-1-mediated anti-inflammatory and
antioxidant effects. Int J Mol Med. 39:113–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng T, Zhang CL, Song FY, Zhao XL, Yu LH,
Zhu ZP and Xie KQ: The activation of HO-1/Nrf-2 contributes to the
protective effects of diallyl disulfide (DADS) against
ethanol-induced oxidative stress. Biochim Biophys Acta.
1830:4848–4859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo D, Guo Y, Cheng Y, Zhao J, Wang Y and
Rong J: Natural product celastrol suppressed macrophage M1
polarization against inflammation in diet-induced obese mice via
regulating Nrf2/HO-1, MAP kinase and NF-κB pathways. Aging (Albany
NY). 9:2069–2082. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun J, Wei X, Lu Y, Cui M, Li F, Lu J, Liu
Y and Zhang X: Glutaredoxin 1 (GRX1) inhibits oxidative stress and
apoptosis of chondrocytes by regulating CREB/HO-1 in
osteoarthritis. Mol Immunol. 90:211–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Unnikrishnan A, Deepa SS, Liu Y,
Li Y, Ikeno Y, Sosnowska D, Van Remmen H and Richardson A: A new
role for oxidative stress in aging: The accelerated aging phenotype
in Sod1-/- mice is correlated to increased cellular senescence.
Redox Biol. 11:30–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lv D, Zhou Q, Xia Y, You X, Zhao Z, Li Y
and Zou H: The association between oxidative stress alleviation via
sulforaphane-induced Nrf2-HO-1/NQO-1 signaling pathway activation
and chronic renal allograft dysfunction improvement. Kidney Blood
Press Res. 43:191–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Samarghandian S, Azimi-Nezhad M,
Farkhondeh T and Samini F: Anti-oxidative effects of curcumin on
immobilization-induced oxidative stress in rat brain, liver and
kidney. Biomed Pharmacother. 87:223–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohtsuji M, Katsuoka F, Kobayashi A,
Aburatani H, Hayes JD and Yamamoto M: Nrf1 and Nrf2 play distinct
roles in activation of antioxidant response element-dependent
genes. J Biol Chem. 283:33554–33562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Biswas M and Chan JY: Role of Nrf1 in
antioxidant response element-mediated gene expression and beyond.
Toxicol Appl Pharmacol. 244:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim HM, Han JW and Chan JY: Nuclear factor
erythroid-2 Like 1 (NFE2L1): Structure, function and regulation.
Gene. 584:17–25. 2016. View Article : Google Scholar : PubMed/NCBI
|