Introduction
Diabetes mellitus is a common chronic metabolic
disease with increasing prevalence worldwide (1). Diabetes mellitus may lead to eye
disease and visual impairment as a result of abnormal blood vessels
supplying oxygen and nutrients to the retina (2) Patients with diabetes occasionally
develop fragile blood vessels in the retina that hemorrhage into
the vitreous cavity of the orbit; a process termed vitreous
hemorrhage (VH), which may lead to sudden severe loss of vision
(3). VH is a sign of advanced
diabetic eye disease (1); the
incidence of VH is ~7 cases per 100,000 individuals worldwide and
is one of the most common causes for the development of subacute or
acute visual disorders (3). VH
usually occurs suddenly without any pain and although the symptoms
vary, they usually include sudden appearance of spots, sudden
blurring of vision or even blindness (4). Mild VH can resolve spontaneously,
whereas severe VH is treated through vitrectomy (5).
Since its development in the 1970s and 1980s,
magnetic resonance imaging (MRI) has proven to be a useful
non-invasive technique in diagnostic medicine and biomedical
research, especially for evaluating the structure, function and
neurochemical properties of the brain (6). Functional MRI (fMRI) detects brain
activity by measuring changes related to blood flow (7). Researchers can analyze cerebral blood
flow and metabolism to explore the activation of specific regions
of the brain, such as the visual pathways from the retina to the
cortex and the spatial organization of the brain, and these
analyses can assist in elucidating the pathogenesis of eye diseases
(8,9).
The amplitude of low-frequency fluctuations (ALFF)
is an indicator of regional spontaneous neuronal activity in blood
oxygenation level-dependent signals at low frequencies, based on
the square root integral of the power spectrum (10). ALFF is widely regarded as a highly
accurate and sensitive measurement method that provides an index
for evaluating spontaneous neural activity (10). Previous studies have successfully
used ALFF to assess the neurological status of retinal detachment
(11), acute eye pain (12), primary angle-closure glaucoma
(13) and strabismus with
amblyopia (9). To the best of our
knowledge, the present study is the first to assess the intrinsic
brain activity in type-2 diabetic patients with VH and normal
controls (NCs), as well as the correlation between the intrinsic
brain activity and clinical manifestations, using the ALFF method.
In the present study, it was hypothesized that VH in patients with
type-2 diabetes may induce abnormal activity in the visual
cortex.
Materials and methods
Subjects
A total of 31 type-2 diabetic patients with VH (sex,
16 males and 15 females; mean age, 56.03 years; age range,
56.03±4.61 years) were recruited from June 2017 to September 2018
in the present study. Of these patients, 15 (eight males and seven
females) cases were caused by type-2 diabetic retinopathy and 16
(eight males and eight females) cases were caused retinal vein
occlusion. The inclusion criteria for subjects were: i) Diabetes;
ii) VH caused by type-2 diabetic retinopathy or retinal vein
occlusion (Fig. 1); iii) absence
of bilateral ocular diseases (for example, retinal degeneration,
optic neuritis, strabismus, amblyopia, cataracts and glaucoma); and
(4) normal vision in the affected
eyes prior to sudden loss of vision. The exclusion criteria for the
subjects were: i) History of ophthalmic surgery (for example,
scleral buckle or vitreous and glaucoma surgery); ii) VH with
ocular trauma; and iii) history of systemic diseases (for example,
heart disease, hypertension and psychiatric diseases.
An equal number of NCs (sex, 16 males and 15
females; total of 31; mean age, 56.48 years; age range, 56.48±4.29
years) with similar characteristics (for example, educational
level, sex and age) to patients of the VH group were recruited from
June 2017 to September 2018 to the present study. The inclusion
criteria were: i) Absence of brain parenchymal malformation
reported through MRI; ii) best corrected visual acuity (BCVA) ≥1.0,
without ocular diseases; iii) absence of serious diseases (for
example, heart disease, hypertension and psychiatric diseases),
except type-2 diabetes; iv) ability to undergo MRI examination; and
v) presence of diabetes without VH.
The Ethics Committee of the First Affiliated
Hospital of Nanchang University approved the present study. All
methods complied with the Declaration of Helsinki. Each subject
provided written informed consent and voluntarily participated
after being informed of the purpose, content and potential risks of
the present study.
Anxiety and depression score
assessment
The scores for anxiety and depression were obtained
via questionnaires using the self-rating depression scale (14) and Hamilton anxiety scale (15), respectively. Higher scores on these
scales indicated a higher level of anxiety or depression (16).
MRI parameters
MRI scanning was conducted using a 3-Tesla MRI
scanner (Trio; Siemens Healthineers). The whole-brain T1-weights
were obtained via magnetization-prepared gradient echo images using
parameters described in a previous study (13). The duration of the entire scanning
sequence was 15 min.
fMRI data analysis
The functional diagrams were analyzed as previously
described (9). Briefly, the data
were initially filtered through MRIcro software (www.mricro.com) (17)
and subsequently the rest of the images were pre-processed using
Statistical Parametric Mapping (SPM; http://www.fil.ion.ucl.ac.uk/spm; The MathWorks, Inc.)
and the Data Processing Assistant for rs-fMRI software (DPARSFA;
version 4.0; http://rfmri.org/DPARSF; Institute of
Psychology, Chinese Academy of Sciences). The first ten volumes
from each subject were discarded due to the signal reaching
equilibrium and the participants' adaptation to the scanning noise.
After that, the head motion artifacts were corrected, and the
interference effect was eliminated using linear regression.
Finally, the data were standardized to meet the space criteria
defined by the Montreal Neurological Institute (18).
ALFF analysis
The RESTing-state fMRI data analysis Toolkit (REST)
toolkit (19) was applied to
divide the brain areas with varying ALFF values of type-2 diabetic
patients with VH and NCs into regions of interest (ROI). The mean
ALFF value of each ROI was obtained by averaging each primitive
ALFF value on all voxels. A linear correlation analysis was
performed to assess the association between the mean ALFF values of
each ROI and the behavioral performance in type-2 diabetic patients
with VH.
Statistical analysis
SPSS software (version 22.0; IBM Corp.) was used to
analyze the cumulative clinical variables between the NC and VH
groups by performing χ2 tests for categorical data and
independent Student's t-tests for continuous data. P<0.05 was
considered to indicate a statistically significant difference. Data
are presented as the mean ± SEM.
The difference in the voxel level between the two
groups was studied using the REST toolkit with a two-sample t-test.
The statistical threshold for the voxel level was set to P<0.05
using Gaussian random field theory for multiple comparisons.
AlphaSim was calibrated with P<0.01 and a cluster size >0.40
voxels.
The mean ALFF values in various brain areas of
type-2 diabetic patients with VH and NCs were classified using
receiver operating characteristic (ROC) curves, which were
performed using SPSS software (version 22.0; IBM Corp.).
Pearson's correlation analysis was performed using
GraphPad Prism (version 7.0; GraphPad Software, Inc.) to assess the
association between the mean ALFF values of multiple brain regions
and related behavioral performances in the VH group.
Results
Demographics and measurements of
vision
The VH and NC groups did not demonstrate significant
differences in terms of age (P=0.967) and body weight (P=0.906).
However, a significant difference was observed between the VH and
NC groups for BCVA-right (P<0.001) and BCVA-left (P<0.001).
In addition, the mean duration of VH was 24.15±20.97 days (Table I).
 | Table I.Demographics and clinical
measurements by group. |
Table I.
Demographics and clinical
measurements by group.
| Condition | VH | NC | t-value | P-value |
|---|
| Male/female | 16/15 | 16/15 | N/A | >0.990 |
| Age (years) | 56.030±4.610 | 56.480±4.290 | 0.041 | 0.967 |
| Weight (kg) | 60.480±3.830 | 61.100±3.490 | 0.118 | 0.906 |
| Handedness | 31R | 31R | N/A | >0.990 |
| Duration of VH
(days) | 24.150±20.970 | N/A | N/A | N/A |
| Best-corrected
VA-right eye | 0.640±0.050 | 1.070±0.030 | −7.771 | <0.001 |
| Best-corrected
VA-left eye | 0.660±0.060 | 1.06±0.020 | −6.231 | <0.001 |
Differences in ALFF
The ALFF values in the VH group were significantly
higher in the bilateral cerebellum posterior lobe (CPL), left
CPL/left lingual gyrus (LG) and bilateral superior frontal gyrus
(SFG)/left postcentral gyrus (PG) compared with those obtained for
the NC group (P<0.05; Fig. 2
and Table II). Additionally,
certain brain areas in the VH group displayed notably lower ALFF
values, including the right middle frontal gyrus (MFG), right
inferior frontal gyrus (IFG), right MFG/left anterior cingulate
(AC), left MFG-1, right SFG, right MFG, right SFG/MFG and left
MFG-2 (Fig. 2 and Table II). Changes in the mean ALFF
values between the VH and NC groups are presented in Fig. 3. Nevertheless, no correlation was
observed between the ALFF values of different brain areas and their
manifestations in type-2 diabetic patients with VH (P>0.05).
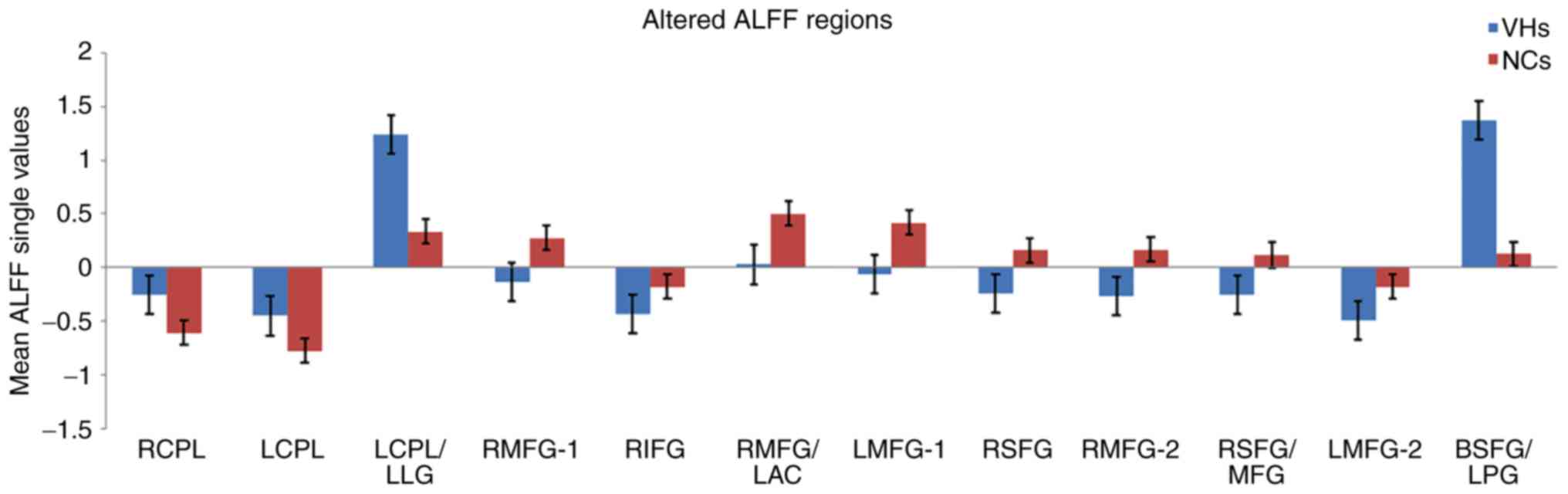 | Figure 3.Mean ALFF values between the VH and
NC groups in the different regions of the brain. ALFF, amplitude of
low-frequency fluctuation; VH, vitreous hemorrhage; NC, normal
control; RCPL, right cerebellum posterior lobe; LCPL, left
cerebellum posterior lobe; LLG, left lingual gyrus; RMFG, right
middle frontal gyrus; RIFG, right inferior frontal gyrus; LAC, left
anterior cingulate; LMFG, left middle frontal gyrus; RSFG, right
superior frontal gyrus; MFG, middle frontal gyrus; BSFG/LPG,
bilateral superior frontal gyrus/left postcentral gyrus. |
 | Table II.Brain areas with significantly
different amplitude of low-frequency fluctuations values between VH
and NC groups. |
Table II.
Brain areas with significantly
different amplitude of low-frequency fluctuations values between VH
and NC groups.
| A, VHs >
NCs |
|---|
|
|---|
|
| MNI
coordinates |
|
|
|
|---|
|
|
|
|
|
|
| Brain regions | X | Y | Z | BA | Peak voxels | t-value |
|---|
| RCPL | 27 | −66 | −57 | / | 446 | 5.1342 |
| LCPL | −42 | −54 | −57 | / | 185 | 5.7220 |
| RCPL/LCPL | −54 | −51 | −30 | 18 | 244 | 5.0742 |
| BSFG/LPG | −3 | −9 | 78 | 6 | 340 | 7.0388 |
|
| B, VHs <
NCs |
|
|
| MNI
coordinates |
|
|
|
|
|
|
|
|
|
| Brain
regions | X | Y | Z | BA | Peak
voxels | t-value |
|
| RMFG | 39 | 42 | 18 | 10 | 213 | −4.4753 |
| RIFG | 33 | 27 | 0 | 45 | 92 | −3.5367 |
| RMFG/LAC | 3 | 51 | 15 | 9/32 | 610 | −5.2619 |
| LMGF | −51 | 9 | 21 | 10 | 283 | −6.5002 |
| RSFG | 24 | 30 | 51 | 6 | 156 | −4.5000 |
| RMFG | 48 | 6 | 54 | 10 | 117 | −3.8469 |
| RSFG/MFG | 6 | −3 | 60 | 10 | 97 | −3.8568 |
| LMFG | −27 | −6 | 51 | 10 | 94 | −4.6431 |
Correlation analysis
In the VH group, the mean ALFF signal values of the
right MFG/left AC were negatively correlated with the anxiety score
(r=−0.906; P<0.0001) and depression score (r=−0.854;
P<0.0001; Fig. 4A and B).
Notably, in the VH group, BCVA of the contralateral eye was
positively correlated with the mean ALFF signal values of the left
MFG (r=0.634; P<0.0001) and right MFG (r=0.494; P=0.004;
Fig. 4C and D).
ROC curves
It was hypothesized that the difference in ALFF
values may serve as a potential diagnostic marker for
differentiating type-2 diabetic patients with VH from NCs. To
verify this hypothesis, a ROC curve was constructed by collecting
the mean ALFF values of various brain areas in type-2 diabetic
patients with VH. An area under the curve (AUC) value of 0.5–0.7
and 0.7–0.9 represented lower and higher accuracy, respectively, of
the ALFF value in the different areas of the brain as diagnostic
markers. The AUC values of the ALFF values in each area were
calculated. The areas of the brain with ALFF values that displayed
high accuracy as diagnostic markers (VHs > NCs) were as follows:
Right CPL (0.893; P<0.001), left CPL (0.823; P<0.001), left
CPL/left LG (0.856; P<0.001) and bilateral SFG/left PG
(0.839; P<0.001; Fig. 5A).
Contrastingly, the areas of the brain with ALFF values that
displayed lower accuracy as diagnostic markers (VHs < NCs) were
as follows: Right MFG-1 (0.847; P<0.001), right IFG (0.837;
P<0.001), right MFG/left AC (0.890; P<0.001), left MFG-1
(0.868; P<0.001), right SFG (0.864; P<0.001), right MFG-2
(0.832; P<0.001), right SFG/MFG (0.781; P<0.001) and left
MFG-2 (0.850; P<0.001; Fig.
5B).
 | Figure 5.ROC curve analysis of the mean ALFF
values of the altered brain regions in VH. (A) The areas under the
ROC curve were: RCPL 0.893, (P<0.001; 95% CI, 0.814–0.972), LCPL
0.823 (P<0.001; 95% CI, 0.723–0.923), LCPL/LLG 0.856
(P<0.001; 95% CI, 0.761–0.951) and BSFG/LPG 0.839 (P<0.001;
95% CI, 0.732–0.945). (B) The areas under the ROC curve were:
RMFG-1 0.847 (P<0.001; 95% CI, 0.748–0.946), RIFG 0.837
(P<0.001; 95% CI, 0.739–0.934), RMFG/LAC 0.890 (P<0.001; 95%
CI, 0.807–0.972), LMFG-1 0.868 (P<0.001; 95% CI, 0.782–0.954),
RSFG 0.864 (P<0.001; 95% CI, 0.773–0.954), RMFG-2 0.832
(P<0.001; 95% CI, 0.724–0.941), RSFG/MFG 0.781 (P<0.001; 95%
CI, 0.666–0.897) and LMFG-2 0.850 (P<0.001; 95% CI,
0.754–0.947). ROC, receiver operating characteristic; ALFF,
amplitude of low-frequency fluctuation; RCPL, right cerebellum
posterior lobe; CI, confidence interval; LCPL, left cerebellum
posterior lobe; LLG, left lingual gyrus; BSFG/LPG, bilateral
superior frontal gyrus/left postcentral gyrus; RMFG, right middle
frontal gyrus; RIFG, right inferior frontal gyrus; LAC, left
anterior cingulate; LMFG, left middle frontal gyrus; RSFG, right
superior frontal gyrus; MFG, middle frontal gyrus; AUC, area under
the curve. |
Relationship between MRI and VH
Based on the results of the present study, the
hypothesized mechanism by which intracerebral hemorrhage may cause
VH was that the brain regions associated with the processing of
emotions may promote abnormal neural activities. Subsequently, the
abnormal neural activities may lead to cerebral hemorrhage, thus
promoting VH, impaired visual function and mood swings, initiating
a vicious circle (Fig. 6).
Schematic diagram of the mean ALFF
values of altered brain regions in the VH group
ALFF values of the following regions in diabetic
patients with VH were increased to various extents compared with
those obtained for NCs: (1)
Cerebellum posterior lobe. L/lingual gyrus. L (BA 6; t=7.0388),
(3) cerebellum posterior lobe. L
(t=5.7220), (5) cerebellum
posterior lobe. R (t=5.1342), and (6) cerebellum posterior lobe. L/lingual
gyrus. L (BA 18; t=5.0742). By contrast, the ALFF values of the
following regions were decreased: (2) Middle frontal gyrus. L (BA 10;
t=−6.5002), (4) medial frontal
gyrus. R (BA 9; t=−5.2619), (7)
middle frontal gyrus. L (BA 10; t=−4.6431), (8) superior frontal gyrus. R (BA 6;
t=−4.5000), (9) middle frontal
gyrus. R (BA 10; t=−4.4753), (10)
superior frontal gyrus. R/middle frontal gyrus (BA 10; t=−3.8568),
(11) middle frontal gyrus. R (BA
10; t=−3.8469) and (12) inferior
frontal gyrus. R (BA 45; t=−3.5367) (Fig. 7).
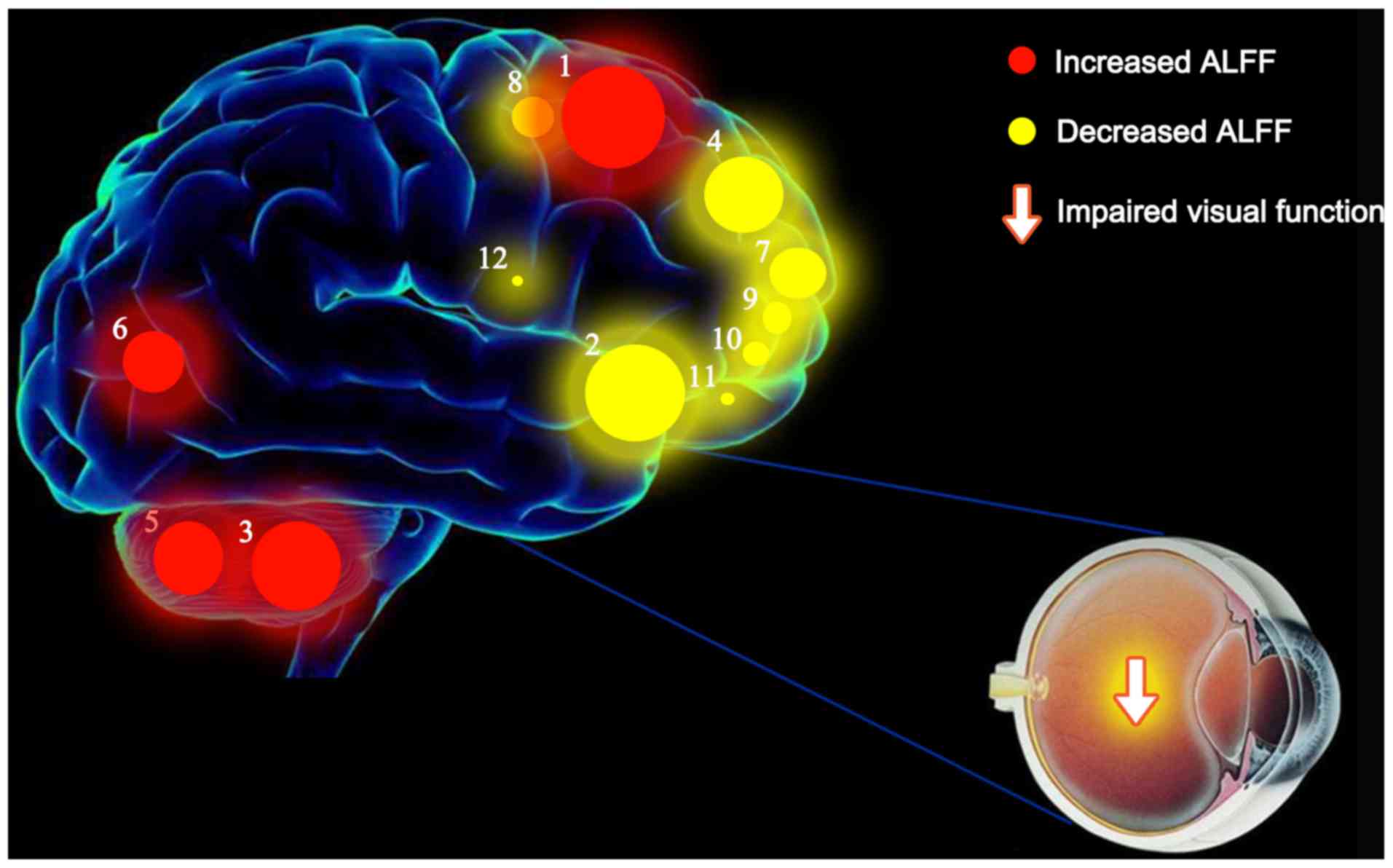 | Figure 7.Mean ALFF values of altered brain
regions in the VH group. ALFF values of the following regions in
diabetic patients with VH were increased to various extents
compared with those obtained for NCs: (1) Cerebellum posterior lobe. L/lingual
gyrus. L (BA 6; t=7.0388), (3)
cerebellum posterior lobe. L (t=5.7220), (5) cerebellum posterior lobe. R
(t=5.1342), and (6) cerebellum
posterior lobe. L/lingual gyrus. L (BA 18; t=5.0742). By contrast,
the ALFF values of the following regions were decreased: (2) Middle frontal gyrus. L (BA 10;
t=−6.5002), (4) medial frontal
gyrus. R (BA 9; t=−5.2619), (7)
middle frontal gyrus. L (BA 10; t=−4.6431), (8) superior frontal gyrus. R (BA 6;
t=−4.5000), (9) middle frontal
gyrus. R (BA 10; t=−4.4753), (10)
superior frontal gyrus. R /middle frontal gyrus (BA 10; t=−3.8568),
(11) middle frontal gyrus. R (BA
10; t=−3.8469) and (12) inferior
frontal gyrus. R (BA 45; t=−3.5367). The sizes of the spots denote
the degree of quantitative changes in the ALFF values between the
VH and NC groups. ALFF, amplitude of low-frequency fluctuations;
VH, vitreous hemorrhage; NCs, normal controls; R, right; L, left;
BA, Brodmann's area. |
Discussion
VH is defined as the presence of extravasated blood
in the vitreous humor (3).
Cerebral hemorrhages, such as aneurysmal subarachnoid hemorrhages,
may cause VH (20). In addition,
these hemorrhagic brain regions are associated with emotional
processing, which may lead to abnormal neurological activity.
fMRI is a technique for measuring brain activity by
detecting blood flow-related changes, that has been increasingly
used in research to improve understanding of the function of the
healthy brain and the disruption of normal cerebral function by
disease (21). The ALFF method is
used in resting-state fMRI to detect and quantify the activation
sites in the brain, as well as to determine the local
synchronization of spontaneous fMRI signals (22). Alterations in the mean ALLF values
have been found in a variety of ophthalmic diseases (13,23),
suggesting that this method may be helpful in the diagnosis and
treatment of such diseases (Table
III).
 | Table III.Amplitude of low-frequency
fluctuations method applied in ophthalmological diseases. |
Table III.
Amplitude of low-frequency
fluctuations method applied in ophthalmological diseases.
|
|
| Brain areas |
|
|---|
|
|
|
|
|
|---|
| Author, year | Disease | UDs > NCs | UDs < NCs | (Refs.) |
|---|
| Huang et al,
2015 | Optic neuritis | LCPL, RCPL, RITG,
LPG, RIT/FG, LFG, LCF, LIPL, LC | RCPL, RCAL, RP,
RIFG, RI, LMFG, LSTG, RSG, BAC/MFG, BP, RSG, RIPL | (17) |
| Huang et al,
2015 | Primary
angle-closure glaucoma | RPG | RMFG, LMFG, RSFG,
RP, LPG, RAG, LSFG | (13) |
| Li et al,
2014 | Primary open-angle
glaucoma | RMFG, RSMA | RLG, RITG,
LPCG | (34) |
| Tan et al,
2016 | Congenital comitant
strabismus | BCPL, LAG | BMFG | (30) |
| Tan et al,
2016 | Unilateral acute
open globe injury | LC, LMCC, BP | No brain
region | (45) |
| Li et al,
2016 | Late monocular
blindness | RMFG, LMFG,
LSG | LCAL, RPG, RC,
LPG/PL | (23) |
| Pan et al,
2018 | Acute eye pain | RPG, LPG, LC | LP/PG, RP/PG,
LP | (12) |
| Min et al,
2018 | Strabismus with
amblyopia | RSFG, RP, LC,
BPG | LCPL, LT, RT,
LMFG | (9) |
| Kang et al,
2019 | Retinal
detachment | FSO, ITG | OL, MFG | (11) |
To the best of our knowledge, the present study is
the first to assess alterations in the inherent activity patterns
in different regions of the brain of type-2 diabetic patients with
VH vs. NCs, via the ALFF method (Fig.
7). The ALFF values of the bilateral CPL, left CPL/left LG and
bilateral SFG/left PG were significantly higher in type-2 diabetic
patients with VH vs. NCs (P<0.05). By contrast, the ALFF values
of the right MFG-1, right IFG, right MFG/left AC, left MFG-1, right
SFG, right MFG-2, right SFG/MFG and left MFG-2 were notably lower
in the VH group compared to the NC group (P<0.05).
The CPL, also termed the new cerebellum, is the part
below the primary fissure of the cerebellum (24,25).
This region receives input from the brain stem and cortex and plays
a vital role in fine motor coordination (26), happiness-related activation
(27,28) and saccadic eye movements (29). In addition, dysfunction of the CPL
is linked to amblyopia with strabismus (9), congenital comitant strabismus
(30) and optic neuritis (31). The present study suggested that
type-2 diabetic patients with VH have elevated ALFF values in the
bilateral CPL, indicating abnormal brain activity in this region of
the brain. Therefore, it can be speculated that type-2 diabetic VH
may result in motor control dysfunction and depression in
patients.
The LG, also termed the occipitotemporal gyrus, is
related to visual processing, analysis of logical conditions and
encoding of visual memory (32,33).
In a previous study, patients with primary open-angle glaucoma
exhibited decreased ALFF values in the right LG compared with NCs
(34). However, in the present
study it was revealed that the ALFF values in the left CPL/left LG
of type-2 diabetic patients with VH increased, indicating that VH
may disrupt vision, logical analysis and visual memory function in
the brain.
The PG is located in the lateral parietal lobe of
the brain and plays a key role in sensory function (35). In previous studies, the ALFF values
in patients with primary open-angle glaucoma decreased, whereas
those of patients with acute eye pain increased (12,34).
The results of the present study suggested that ALFF values
increased in the bilateral SFG/left PG of type-2 diabetic patients
with VH, suggesting that VH may affect the sensory functions of
such patients.
The medial frontal gyrus (MFG) lies in the middle of
the frontal gyrus, along with the frontal eye fields, playing a
vital role in the regulation of eye movement (36,37).
Previous studies have shown that ocular muscle dysfunction may
occur in ocular diseases; for example, Kang et al (11) reported that patients with retinal
detachment had decreased ALFF values in the right MFG. Tan et
al (30) reported that
patients with congenital concomitant strabismus had significantly
lower ALFF values in the bilateral MFG. Huang et al
(17) demonstrated that
individuals with optic neuritis had decreased ALFF values in the
left MFG. Similar to these findings, the data from the present
study suggested that the ALFF value of the bilateral MFG was
decreased in type-2 diabetic patients with VH, suggesting that VH
may lead to impairment of the local synchronization of brain
activity, which may subsequently lead to visually-related motor
dysfunction.
The IFG located in Broca's area of the brain
participates in language processing and speech production (38). Huang et al (17) observed decreased ALFF values in the
IFG of patients with optic neuritis. In the present study, a
notable decline in ALFF values in the right IFG of type-2 diabetic
patients with VH was observed, suggesting that VH may cause
impairment of language function in this setting.
The SFG is the marginal gyrus, accounting for
approximately one-third of the human frontal lobe (39). Using fMRI experiments, Goldberg
et al (40) demonstrated
that the SFG was related to self-consciousness and coordinated with
the movement of the sensory system. It has been previously reported
that the ALFF value of the right SFG in strabismus patients with
amblyopia is higher than that obtained in NCs (9). By contrast, the present study
revealed that the ALFF values in the right SFG were lower in type-2
diabetic patients with VH than in NCs. This finding suggested that
VH may have negative effects on self-consciousness and the sensory
system.
The AC cortex is located on the anterior part of the
cingulate cortex and plays a vital role in various functions, such
as the autonomic function of regulation of blood pressure and heart
rate, attention and emotional control (41–43).
Huang et al (17) revealed
that lower ALFF values occurred in the left MFG, left superior
temporal gyrus, right inferior parietal lobule and bilateral AC/MFG
of patients with optic neuritis. In the present study, type-2
diabetic patients with VH displayed lower ALFF values in the right
MFG/left AC, indicating that VH may cause fluctuations in blood
pressure, heart rate, attention and mood.
The main treatment options for severe cases of VH in
patients with diabetes are laser photocoagulation, cryo-coagulation
and vitrectomy (44). In the
future, fMRI may become a widely used method to observe the
treatment of diabetic patients with VH via the detection of brain
activity by measuring changes related to the flow of blood.
However, the present study did not compare brain activity in type-2
diabetic patients with VH prior to and after treatment. The present
study reported changes in different brain areas of type-2 diabetic
patients with VH and therefore provides insight for further
exploration into the pathogenesis of VH.
The present study revealed changes in spontaneous
activity in multiple areas of the brain of type-2 diabetic patients
with VH. These findings may point to altered neural mechanisms
present in type-2 diabetic patients with VH and provide a
foundation for conducting further research studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81660158, 81460092
and 81400372), the Natural Science Key Project of Jiangxi Province
(grant no. 20161ACB21017) and the Health Development Planning
Commission Science Foundation of Jiangxi Province (grant no.
20175116).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WQS and LYT were major contributors; both conceived
and designed the experiments, analyzed the data and wrote and
revised the manuscript. QL, BL and NJ recruited the healthy
controls to the study and performed the MRI experiments. PWZ, QY
and LYT collected the data, and YS designed the study and obtained
the financial support. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed under the approval
of the Medical Research Ethics Committee of the First Affiliated
Hospital of Nanchang University. The methods in the present study
were conducted under relevant guidelines and regulations. All
participants involved were offered the whole study design and
signed the informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ong SR, Crowston JG, Loprinzi PD and
Ramulu PY: Physical activity, visual impairment, and eye disease.
Eye (Lond). 32:1296–1303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spraul CW and Grossniklaus HE: Vitreous
hemorrhage. Surv Ophthalmol. 42:3–39. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoonessi R, Hussain A and Jang TB: Bedside
ocular ultrasound for the detection of retinal detachment in the
emergency department. Acad Emerg Med. 17:913–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cuevas P, Outeirino LA, Azanza C, Angulo J
and Gimenez- Gallego G: Dramatic resolution of vitreous hemorrhage
after an intravitreal injection of dobesilate. Mil Med Res.
2:232015.PubMed/NCBI
|
|
6
|
Brown HD, Woodall RL, Kitching RE, Baseler
HA and Morland AB: Using magnetic resonance imaging to assess
visual deficits: A review. Ophthalmic Physiol Opt. 36:240–265.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katwal SB, Gore JC, Gatenby JC and Rogers
BP: Measuring relative timings of brain activities using fMRI.
NeuroImage. 66:436–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conner IP, Odom JV, Schwartz TL and
Mendola JD: Monocular activation of V1 and V2 in amblyopic adults
measured with functional magnetic resonance imaging. J AAPOS.
11:341–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Min YL, Su T, Shu YQ, Liu WF, Chen LL, Shi
WQ, Jiang N, Zhu PW, Yuan Q, Xu XW, et al: Altered spontaneous
brain activity patterns in strabismus with amblyopia patients using
amplitude of low-frequency fluctuation: A resting-state fMRI study.
Neuropsychiatr Dis Treat. 14:2351–2359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuo XN, Di Martino A, Kelly C, Shehzad ZE,
Gee DG, Klein DF, Castellanos FX, Biswal BB and Milham MP: The
oscillating brain: Complex and reliable. Neuroimage. 49:1432–1445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang HH, Shu YQ, Yang L, Zhu PW, Li D, Li
QH, Min YL, Ye L, Zhou Q and Shao Y: Measuring abnormal intrinsic
brain activities in patients with retinal detachment using
amplitude of low-frequency fluctuation: A resting-state fMRI study.
Int J Neurosci. 129:681–686. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q,
Freeberg S, Zhu PW, Ye L, Ma MY, Huang X and Shao Y: Altered
intrinsic brain activities in patients with acute eye pain using
amplitude of low-frequency fluctuation: A resting-state fMRI study.
Neuropsychiatr Dis Treat. 14:251–257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Zhong YL, Zeng XJ, Zhou F, Liu
XH, Hu PH, Pei CG, Shao Y and Dai XJ: Disturbed spontaneous brain
activity pattern in patients with primary angle-closure glaucoma
using amplitude of low-frequency fluctuation: A fMRI study.
Neuropsychiatr Dis Treat. 11:1877–1883. 2015.PubMed/NCBI
|
|
14
|
Zung WW: A self-rating depression scale.
Arch Gen Psychiatry. 12:63–70. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamilton M: The assessment of anxiety
states by rating. Br J Med Psychol. 32:50–55. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zigmond AS and Snaith RP: The hospital
anxiety and depression scale. Acta Psychiatr Scand. 67:361–370.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang X, Cai FQ, Hu PH, Zhong YL, Zhang Y,
Wei R, Pei CG, Zhou FQ and Shao Y: Disturbed spontaneous
brain-activity pattern in patients with optic neuritis using
amplitude of low-frequency fluctuation: A functional magnetic
resonance imaging study. Neuropsychiatr Dis Treat. 11:3075–3083.
2015.PubMed/NCBI
|
|
18
|
Evans AC, Janke AL, Collins DL and Baillet
S: Brain templates and atlases. Neuroimage. 62:911–922. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang J and Yu R: Acute social stress
modulates coherence regional homogeneity. Brain Imaging Behav.
13:762–770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farnarier G, Sobrepere G and Croisy A:
Syndrome of hematoma of the vitreous body with Terson's spontaneous
intracranial hemorrhage. Bull Soc Ophtalmol Fr. 8:556–560. 1955.(In
French).
|
|
21
|
Bandettini PA: Twenty years of functional
MRI: The science and the stories. NeuroImage. 62:575–588. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ,
Liang M, Tian LX, Jiang TZ and Wang YF: Altered baseline brain
activity in children with ADHD revealed by resting-state functional
MRI. Brain Dev. 29:83–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Huang X, Ye L, Wei R, Zhang Y, Zhong
YL, Jiang N and Shao Y: Altered spontaneous brain activity pattern
in patients with late monocular blindness in middle-age using
amplitude of low-frequency fluctuation: A resting-state functional
MRI study. Clin Interv Aging. 11:1773–1780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stoodley CJ and Schmahmann JD: Functional
topography of the human cerebellum. Handb Clin Neurol. 154:59–70.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paulin MG: The role of the cerebellum in
motor control and perception. Brain Behav Evol. 41:39–50. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Casula EP, Pellicciari MC, Ponzo V,
Stampanoni Bassi M, Veniero D, Caltagirone C and Koch G: Cerebellar
theta burst stimulation modulates the neural activity of
interconnected parietal and motor areas. Sci Rep. 6:361912016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stoodley CJ and Schmahmann JD: Functional
topography in the human cerebellum: A meta-analysis of neuroimaging
studies. Neuroimage. 44:489–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schienle A and Scharmuller W: Cerebellar
activity and connectivity during the experience of disgust and
happiness. Neuroscience. 246:375–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayakawa Y, Nakajima T, Takagi M, Fukuhara
N and Abe H: Human cerebellar activation in relation to saccadic
eye movements: A functional magnetic resonance imaging study.
Ophthalmologica. 216:399–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan G, Huang X, Zhang Y, Wu AH, Zhong YL,
Wu K, Zhou FQ and Shao Y: A functional MRI study of altered
spontaneous brain activity pattern in patients with congenital
comitant strabismus using amplitude of low-frequency fluctuation.
Neuropsychiatr Dis Treat. 12:1243–1250. 2016.PubMed/NCBI
|
|
31
|
Ru Y, Huang Y, Liu H, Du J, Meng Z, Dou Z,
Liu X, Wei RH, Zhang Y and Zhao S: α-Melanocyte-stimulating hormone
ameliorates ocular surface dysfunctions and lesions in a
scopolamine-induced dry eye model via PKA-CREB and MEK-Erk
pathways. Sci Rep. 5:186192015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mechelli A, Humphreys GW, Mayall K, Olson
A and Price CJ: Differential effects of word length and visual
contrast in the fusiform and lingual gyri during reading. Proc Biol
Sci. 267:1909–1913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bogousslavsky J, Miklossy J, Deruaz JP,
Assal G and Regli F: Lingual and fusiform gyri in visual
processing: A clinico-pathologic study of superior altitudinal
hemianopia. J Neurol Neurosurg Psychiatry. 50:607–614. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li T, Liu Z, Li J, Liu Z, Tang Z, Xie X,
Yang D, Wang N, Tian J and Xian J: Altered amplitude of
low-frequency fluctuation in primary open-angle glaucoma: A
resting-state FMRI study. Invest Ophthalmol Vis Sci. 56:322–329.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaukoranta E, Hamalainen M, Sarvas J and
Hari R: Mixed and sensory nerve stimulations activate different
cytoarchitectonic areas in the human primary somatosensory cortex
SI. Neuromagnetic recordings and statistical considerations. Exp
Brain Res. 63:60–66. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seitz RJ, Nickel J and Azari NP:
Functional modularity of the medial prefrontal cortex: Involvement
in human empathy. Neuropsychology. 20:743–751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cameron IG, Riddle JM and D'Esposito M:
Dissociable roles of dorsolateral prefrontal cortex and frontal eye
fields during saccadic eye movements. Front Hum Neurosci.
9:6132015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Greenlee JD, Oya H, Kawasaki H, Volkov IO,
Severson MA III, Howard MA III and Brugge JF: Functional
connections within the human inferior frontal gyrus. J Comp Neurol.
503:550–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi WQ, Wu W, Ye L, Jiang N, Liu WF, Shu
YQ, Su T, Lin Q, Min YL, Li B, et al: Altered spontaneous brain
activity patterns in patients with corneal ulcer using amplitude of
low-frequency fluctuation: An fMRI study. Exp Ther Med. 18:125–132.
2019.PubMed/NCBI
|
|
40
|
Goldberg II, Harel M and Malach R: When
the brain loses its self: Prefrontal inactivation during
sensorimotor processing. Neuron. 50:329–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pardo JV, Pardo PJ, Janer KW and Raichle
ME: The anterior cingulate cortex mediates processing selection in
the stroop attentional conflict paradigm. Proc Natl Acad Sci USA.
87:256–259. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Decety J and Jackson PL: The functional
architecture of human empathy. Behav Cogn Neurosci Rev. 3:71–100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jackson PL, Brunet E, Meltzoff AN and
Decety J: Empathy examined through the neural mechanisms involved
in imagining how I feel versus how you feel pain. Neuropsychologia.
44:752–761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
El Annan J and Carvounis PE: Current
management of vitreous hemorrhage due to proliferative diabetic
retinopathy. Int Ophthalmol Clin. 54:141–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tan G, Huang X, Ye L, et al: Altered
spontaneous brain activity patterns in patients with unilateral
acute open globe injury using amplitude of low-frequency
fluctuation: a functional magnetic resonance imaging study.
Neuropsychiatric Disease and Treatment. 12:2015–2020. 2016.
View Article : Google Scholar : PubMed/NCBI
|





















