Introduction
Secondary osteoporosis is a common type of
osteoporosis in clinical practice. It is mainly secondary to
diseases, including connective tissue disease or multiple myeloma,
or to the use of glucocorticoids (GCs), and presents as the
destruction of bone microstructure, bone loss and increased risk of
fracture (1–3). GC-induced osteoporosis is the
commonest secondary type of osteoporosis in clinical practice and
has the highest incidence rate after postmenopausal osteoporosis
and gerontological osteoporosis (3,4).
Long-term administration of high doses of GCs causes increased bone
absorption, decreased bone density and osteoporosis (5). A variety of rheumatology-associated
diseases need to be treated with GCs, and the prevalence of
secondary osteoporosis is relatively high, requiring active
prevention and treatment (3). The
incidence of osteoporosis in patients who have received GC
treatment for >0.5 years can be 30–50% (6). In order to improve the quality of
life of patients with rheumatic diseases, effective treatments for
GC-induced osteoporosis are urgently required.
Histone acetylation, catalyzed by histone
deacetylases (HDACs), regulates stem cell and osteoblast
differentiation (7,8). HDACs are involved in the mediation of
molecular signaling pathways that regulate the specification,
maturation and terminal differentiation of osteoblasts (7,9,10).
It has been reported that HDAC inhibitors can promote the
maturation of osteoblasts (11).
Reduction of HDAC1 in osteoblasts induces bone formation via
upregulation of runt-related transcription factor 2 (Runx2)
expression (10). HDAC6 knock-out
mice exhibit a slightly increased bone mineral density, which
indicates that HDAC6 is involved in bone biology (12). Furthermore, it has been
demonstrated that silencing of HDAC induces the differentiation of
stem cells (13–16). Long-term treatment with high doses
of GCs decreases the maturity of osteoblasts, which is mediated by
crosstalk between glucocorticoid receptor (GR) and HDAC6, and the
GR-HDAC6 repressor complex. HDAC6 binding to GR regulates the
process of dexamethasone (Dex)-induced mesenchymal stem cell
differentiation into osteoblasts (17). However, the specific role of HDAC6
in Dex-induced proliferation and differentiation of preosteoblasts
remains to be elucidated. Ricolinostat (ACY-1215; an HDAC6
inhibitor) has been demonstrated to reduce inflammatory damage in
osteoarthritis (18). However,
further studies are required to determine whether it has any effect
in osteoporosis.
The present study constructed Dex-induced MC3T3-E1
cells to simulate the GC-induced osteoporosis referred to in
previous studies (19–21). It aimed to explore the role of
ACY-1215 in Dex-induced proliferation and differentiation of
preosteoblasts to provide a basis for direct clinical
treatment.
Materials and methods
Human serum samples
The present study was approved by the Human Ethics
Committee Review Board of Ningbo Number 6 Hospital (Ningbo, China)
and informed written consent was obtained from each patient
(male/female=5/5; age, 43–65 years) and 10 healthy individuals
(male/female=5/5; age, 41–62 years). Serum specimens were obtained
from 10 patients with osteoporosis induced by GCs and 10 healthy
individuals at Ningbo No. 6 Hospital between March 2018 and March
2019. The serum specimens were stored at −80°C prior to further
experiments.
Cell culture and treatment
MC3T3-E1 cells were provided by the American Type
Culture Collection. MC3T3-E1 cells were routinely cultured in 90%
DMEM-H containing 10% FBS (HyClone; Cytiva) in an incubator with 5%
CO2 at 37°C. The cell culture liquid was exchanged every
3 days and cells were subcultured until the cell confluence reached
80–90%. MC3T3-E1 cells were treated with various concentrations of
Dex (0.01, 0.1, 1 and 10 µM) at 37°C for 24 h to select the optimal
concentration of Dex. Additionally, MC3T3-E1 cells were pre-treated
with various concentrations of ACY-1215 (1, 5 and 10 mM) at 37°C
(14) and then treated with the
optimal concentration of Dex.
Cell counting kit-8 (CCK-8) assay
MC3T3-E1 cells were collected and inoculated into
96-well plates at a density of 3×103 cells/well.
Following treatment with only Dex for 24 h, or pretreatment with
ACY-1215 for 2 h followed by treatment with Dex at 37°C for 24 h,
MC3T3-E1 cells in each well were incubated with 10 µl CCK-8
solution at 37°C for 2 h in the dark. Finally, the absorbance value
of each well was measured at a wavelength of 450 nm using a
Synergy™ 2 Multi-function microplate reader (BioTek Instruments,
Inc.).
Reverse transcription-quantitative
(RT-q) PCR analysis
MC3T3-E1 cells were seeded into 12-well plates
(3×104 cells/well). Total RNA was extracted using
TRIzol® (Thermo Fisher Scientific, Inc.) and reverse
transcription was performed using the Reserve Transcription System
kit (Thermo Fisher Scientific, Inc.). Quantitative detection was
performed using the Real-Time Fluorescence Quantitative Universal
kit (DRR041A; Takara Biotechnology Co., Ltd.). The relative
expression levels of HDAC6, osteopontin (OPN), Runx2, osterix
(Osx), collagen I (COL1A1) and GR were detected. GAPDH was used as
an internal control, and semi-quantitative analysis was performed
using the 2−∆∆Cq method (22). The amplification conditions were as
follows: 95°C for 10 min, followed by 40 cycles at 95°C for 10 sec
and 58°C for 60 sec. The following primers were used for RT-qPCR:
GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′; HDAC6 forward,
5′-GGAAAAGGTCGCCAGAAACTT-3′ and reverse,
5′-GGCCGGTTGAGGTCATAGTT-3′; OPN forward,
5′-AGACCTGACATCCAGTACCCTG-3′ and reverse,
5′-GTGGGTTTCAGCACTCTGGT-3′; Runx2 forward,
5′-TCCACACCATTAGGGACCATC-3′ and reverse,
5′-TGCTAATGCTTCGTGTTTCCA-3′; Osx forward,
5′-AGCGACCACTTGAGCAAACAT-3′ and reverse,
5′-GCGGCTGATTGGCTTCTTCT-3′; COL1A1 forward,
5′-CGGCTCCTGCTCCTCTTA-3′ and reverse, 5′-GGTGGGATGTCTTCGTCTT-3′; GR
forward, 5′-CATTACCACAGCTCACCCCTAC-3′ and reverse,
5′-GCAATCACTTGACGCCCAC-3′. The experiment was repeated three
times.
Western blot analysis
After passaging, MC3T3-E1 cells were seeded into
12-well plates (3×104 cells/well). Following
pretreatment with ACY-1215 for 2 days, and treatment with Dex for 7
days, the medium was discarded and cells were lysed using RIPA
buffer (Roche Applied Science) to extract protein, which was
quantified using bicinchoninic acid kits. Protein (30 µg) was added
to 10% SDS-PAGE gel for electrophoresis, and then
electrotransferred onto nitrocellulose membranes. Following
blocking of the nitrocellulose membranes with 5% skimmed milk for 2
h at room temperature, the membranes were incubated with primary
antibodies against HDAC6 (cat. no. ab1440; dilution, 1:1,000;
Abcam), OPN (cat. no. ab8448; dilution, 1:1,000; Abcam), Runx2
(cat. no. ab76956; dilution, 1:1,000; Abcam), Osx (cat. no.
sc-393325; dilution, 1:1,000; Santa Cruz Biotechnology, Inc.),
COL1A1 (cat. no. ab34710; dilution, 1:1,000; Abcam), GR (cat. no.
ab3578; dilution, 1:1,000; Abcam) and GAPDH (cat. no. ab9485;
dilution, 1:2,500; Abcam) overnight at 4°C. The following day, the
nitrocellulose membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (cat. no. 7074; dilution,
1:2,000; Cell Signaling Technology, Inc.) at room temperature for 1
h. Finally, protein bands were visualized with ECL Detection
reagents (Amersham; Cytiva) and the gray value of the bands was
analyzed using Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc.).
Alkaline phosphatase (ALP)
activity
MC3T3-E1 cells were gently washed twice with PBS,
and MC3T3-E1 cells in each well were incubated with 1 ml 0.2%
Triton X-100 at 4°C overnight. Using ALP assay kits (Beyotime
Institute of Biotechnology), cells were mixed with 5 µl cell lysis,
matrix solution and buffer per well of a 96-well plate at 37°C for
15 min. Following the addition of chromogenic agent
(para-nitrophenyl phosphate) to cells at 37°C for 15 min, the
absorbance value of each well was detected at a wavelength of 490
nm using a microplate reader. Additionally, standard wells and
blank control wells were used. ALP activity was calculated
according to the definition of enzyme activity.
Alizarin red staining
After the cells occupied 80–90% of the bottom of the
culture bottle, the cells were digested and inoculated into 24-well
plates at a density of 2×105 cells/well. Subsequently,
600 µl culture solution was added to each well. Following
pretreatment with ACY-1215 for 2 days and treatment with Dex for 7
days, the culture medium was discarded. MC3T3-E1 cells were washed
with PBS three times, fixed with 10% formaldehyde for 10 min at 4°C
and washed with distilled water three times. Subsequently, 500 µl
0.1% alizarin red dye was added to each well and incubated at 37°C
for 30 min. Finally, MC3T3-E1 cells were washed with distilled
water three times and incubated with PBS at 37°C for 10 min. The
stained samples were observed using an inverted fluorescence
microscope (magnification, ×200).
Statistical analysis
SPSS v22.0 statistical software (IBM Corp.) was used
to analyze the data, and the measurement data are presented as the
mean ± standard deviation. A t-test was used for comparisons
between two groups, and one-way analysis of variance with Tukey's
post hoc test was used for comparisons among multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
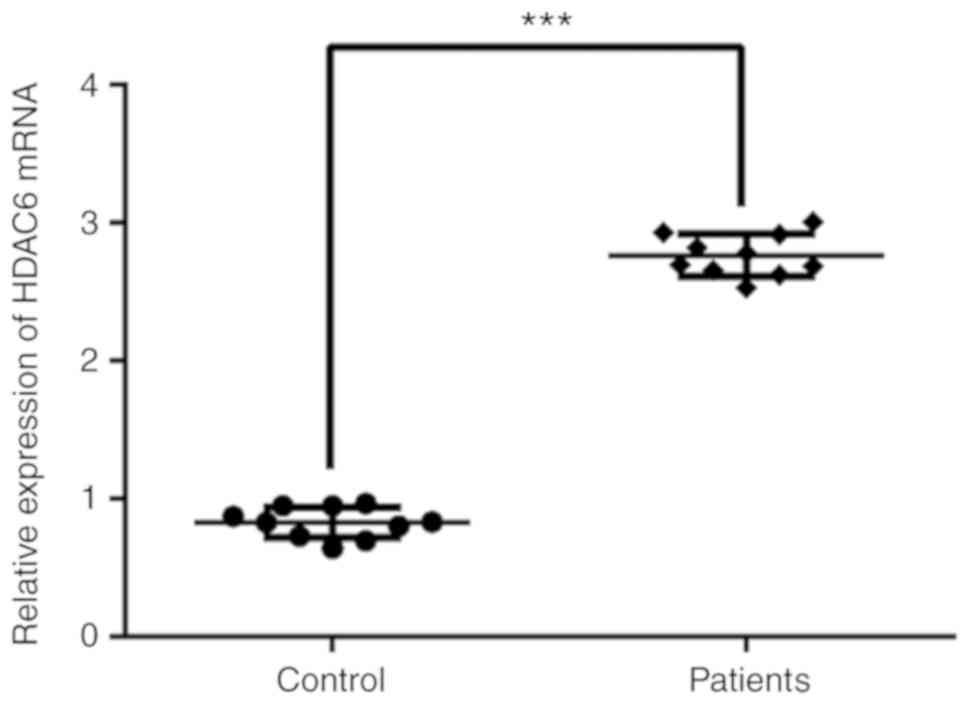
HDAC6 expression in the serum of
patients with GC-induced osteoporosis
The expression levels of HDAC6 in the serum of
patients with GC-induced osteoporosis were determined by RT-qPCR
analysis (Fig. 1). HDAC6 serum
expression was significantly increased in patients with GC-induced
osteoporosis compared with healthy individuals (control).
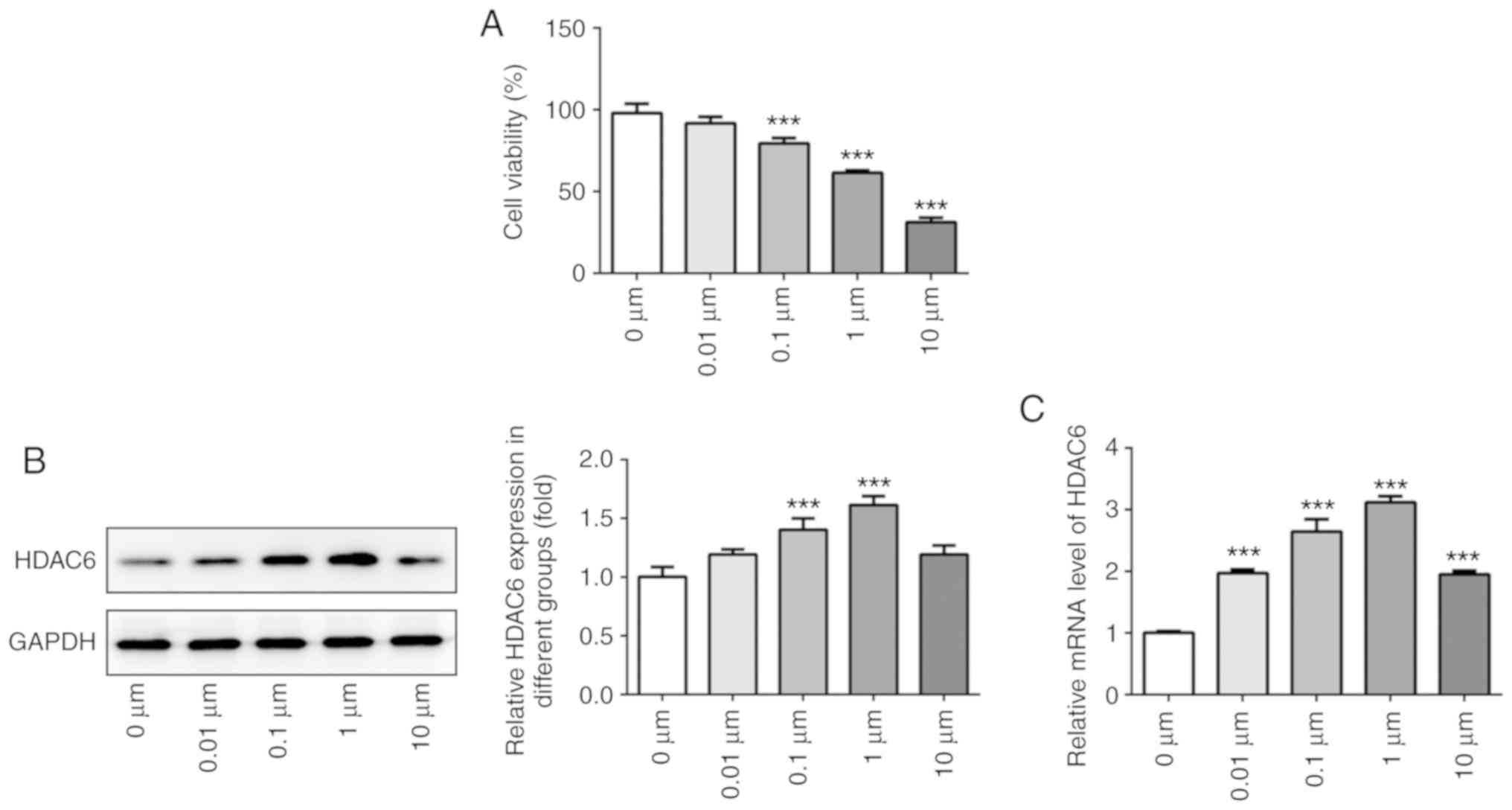
Dex promotes HDAC6 expression in
osteoblast MC3T3-E1 cells
MC3T3-E1 cells were treated with Dex at various
concentrations for 24 h. Cell viability was gradually decreased
with increasing Dex concentration (Fig. 2A). HDAC6 expression was gradually
upregulated in MC3T3-E1 cells treated with 0–1 µM Dex, whereas
HDAC6 expression was decreased in MC3T3-E1 cells treated with 10 µM
Dex (Fig. 2B and C). Therefore, 1
µM Dex was selected for the following experiments.
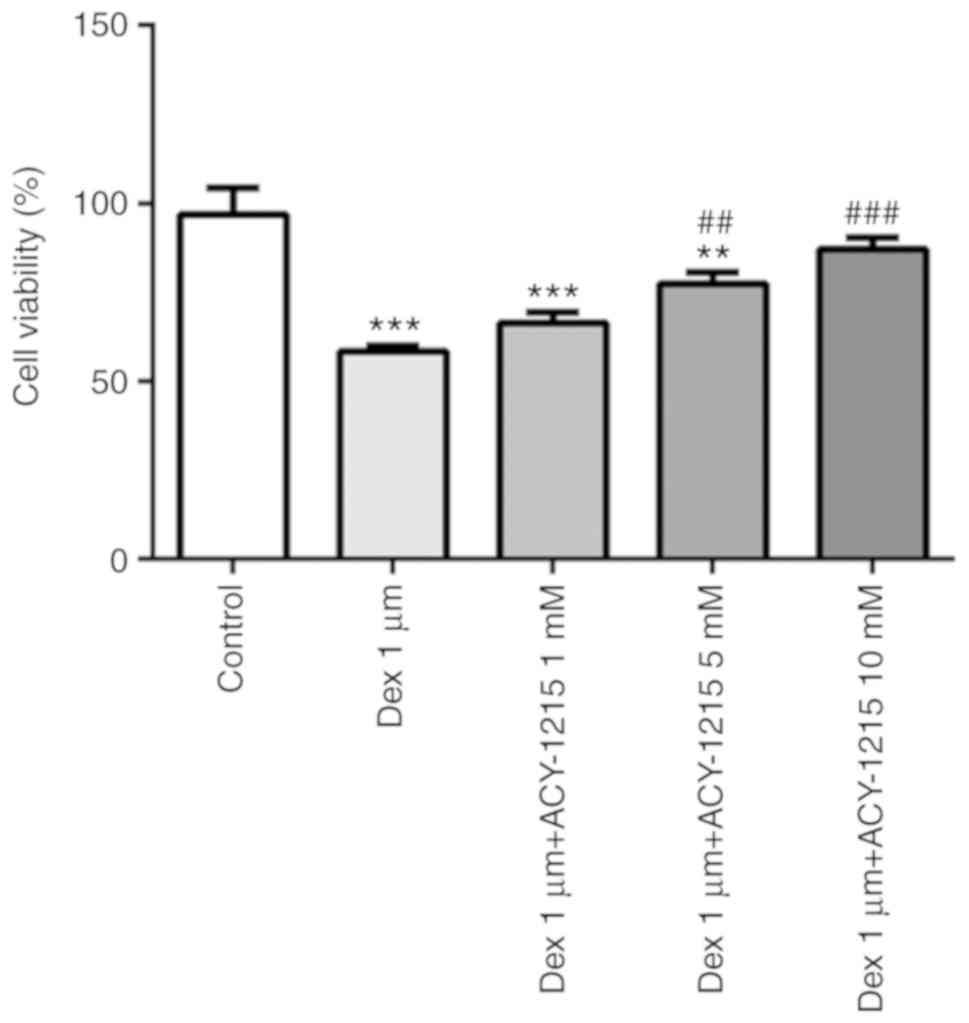
ACY-1215 decreases the effect of Dex
on cell viability
MC3T3-E1 cells were pre-treated with ACY-1215 (1, 5
and 10 mM) for 2 h, and then treated with 1 µM Dex for 24 h. The
treatment of MC3T3-E1 cells with 1 µM Dex suppressed the cell
viability, and this was reversed by treatment with ACY-1215 (1, 5
and 10 mM; Fig. 3).
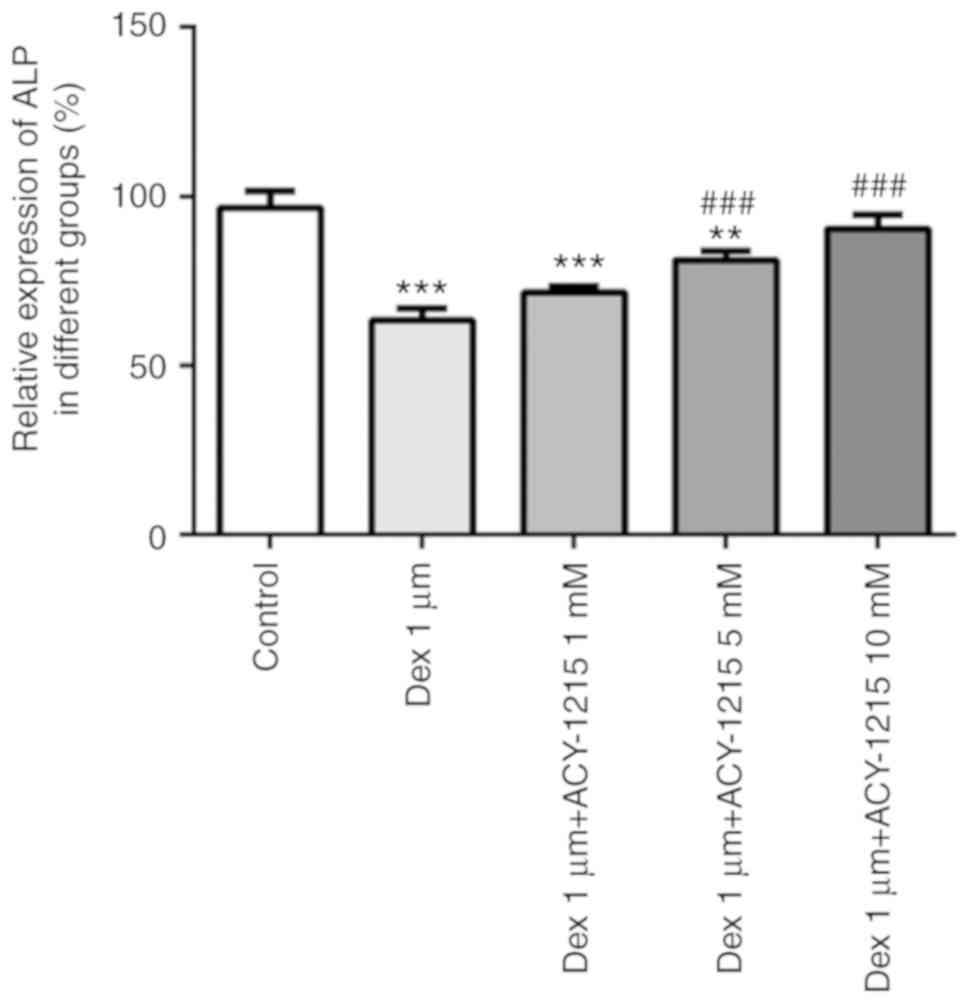
ACY-1215 decreases the Dex-induced
suppression of ALP activity in osteoblast MC3T3-E1 cells
MC3T3-E1 cells were treated with ACY-1215 and Dex as
aforementioned. After 7 days, ALP activity was significantly
decreased in the Dex group. When ACY-1215 (1, 5 and 10 mM) was used
to treat MC3T3-E1 cells treated with Dex, ALP activity was
increased in a concentration-dependent manner (Fig. 4).
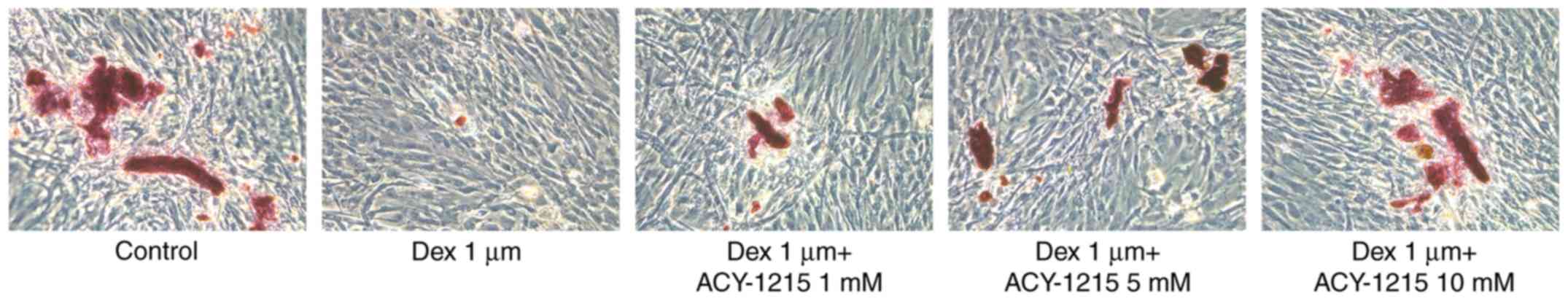
ACY-1215 decreases the Dex-induced
suppression of the capacity for mineralization of osteoblast
MC3T3-E1 cells
Following treatment of MC3T3-E1 cells with ACY-1215
and Dex for 7 days, mineralization was impaired in the Dex group
compared with the control group. However, following pretreatment
with ACY-1215 (1, 5 and 10 mM), mineralization was enhanced in
MC3T3-E1 cells treated with Dex (Fig.
5).
ACY-1215 decreases the Dex-induced
suppression of osteogenesis of osteoblast MC3T3-E1 cells
Following treatment of MC3T3-E1 cells with ACY-1215
and Dex for 7 days, the protein and mRNA expression levels of
mineralization-associated proteins were detected by western
blotting and RT-qPCR. As revealed in Fig. 6A and B, the protein and mRNA
expression levels of OPN, Runx2, Osx and COL1A1 were decreased in
MC3T3-E1 cells treated with Dex. Increasing concentrations of
ACY-1215 reversed the inhibitory effect of Dex and increased the
protein and mRNA expression levels of OPN, Runx2, Osx and COL1A1 in
MC3T3-E1 cells treated with Dex.
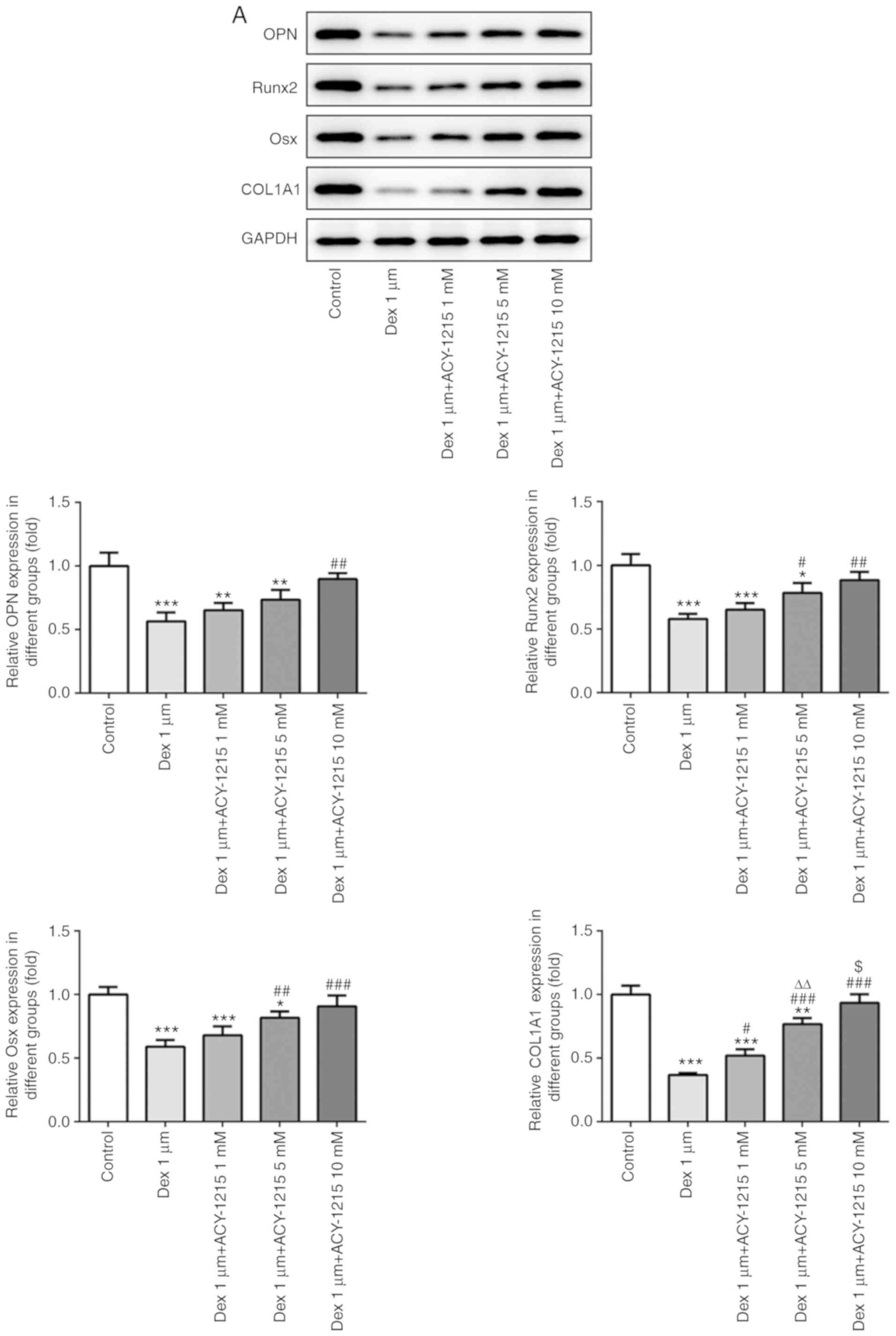 | Figure 6.ACY-1215 decreases the Dex-induced
suppression of osteogenesis of osteoblast MC3T3-E1 cells. (A) The
protein expression of OPN, Runx2, Osx and COL1A1 in
ACY-1215-pretreated MC3T3-E1 cells treated by Dex was determined by
western blot analysis. *P<0.05, **P<0.01 and ***P<0.001
vs. the control group. #P<0.05,
##P<0.01 and ###P<0.001 vs. the Dex
1-µm group. ∆∆P<0.01 vs. the Dex 1-µm+ACY-1215 1-mM
group. $P<0.05 vs. the Dex 1-µm+ACY-1215 5-mM group.
(B) The mRNA expression of OPN, Runx2, Osx and COL1A1 in
ACY-1215-pretreated MC3T3-E1 cells treated by Dex was determined by
reverse transcription-quantitative PCR analysis. *P<0.05,
**P<0.01 and ***P<0.001 vs. the control group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. the Dex 1-µm group.
∆∆P<0.01 and ∆∆∆P<0.001 vs. the Dex
1-µm+ACY-1215 1-mM group. $P<0.05 and
$$P<0.01 vs. the Dex 1-µm+ACY-1215 5-mM group. Dex,
dexamethasone; OPN, osteopontin; Runx2, runt-related transcription
factor 2; Osx, osterix; COL1A1, collagen I. |
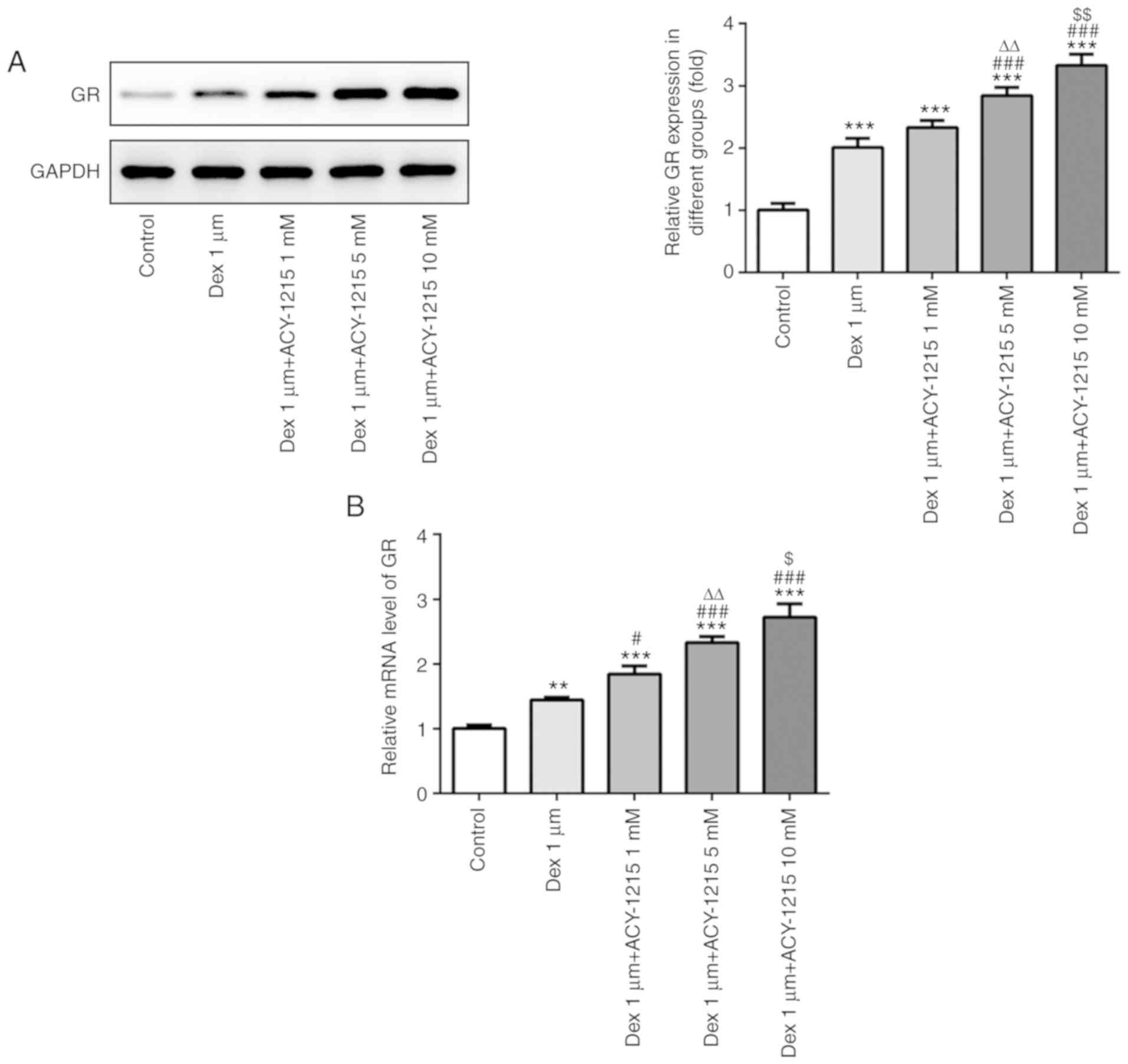
ACY-1215 regulates the expression
levels of GR in osteoblast MC3T3-E1 cells
Following treatment of MC3T3-E1 cells with ACY-1215
and Dex for 7 days, the protein and mRNA expression levels of GR
were analyzed by western blotting and RT-qPCR. As revealed in
Fig. 7A and B, Dex (1 µM)
treatment increased GR receptor expression and ACY-1215 with
increasing concentrations of 1 to 10 mM gradually further increased
the expression of GR receptor.
Discussion
The present study investigated the effect of
ACY-1215 on the proliferation and differentiation of MC3T3-E1 cells
treated with Dex. The results indicated that ACY-1215 reversed the
inhibitory effect of Dex on the proliferation and differentiation
of MC3T3-E1 cells.
HDAC6 is closely associated with ubiquitination
imbalance, microtubulin deficiency and oxidative stress, and it
also serves an important regulatory role in the occurrence and
development of ovarian, breast, esophageal and gastric cancers
(23). Additionally, HDAC6 has a
function in bone diseases. Li et al (24) revealed that HDAC6 activity was
increased following high-dose silicate treatment, and HDAC6
inhibition could protect bone mesenchymal stem cells by improving
the microtubule structure and autophagic activity. Wang et
al (25) indicated that HDAC6
knockdown promoted ALP activity and mineralized nodule formation in
dental mesenchymal stem cells. In an achondroplasia model, HDAC6
inhibition and the HDAC6 inhibitor tubacin promoted endochondral
bone growth by decreasing fibroblast growth factor receptor 3
accumulation (26). HDAC6 has been
revealed to be overexpressed in osteoarthritis, and ACY-1215
treatment to promote apoptosis of osteoarthritis osteoblasts and
inhibit aberrant subchondral bone formation (27). In the present study, HDAC6
expression was increased in patients with GC-induced osteoporosis
and MC3T3-E1 cells treated with Dex. Additionally, HDAC6 inhibition
could promote the viability, mineralization and osteogenesis of
MC3T3-E1 cells. Overall, HDAC6 inhibition could effectively improve
ALP activity and mineralized nodule formation, which was also
demonstrated in the present study.
GR has multiple effects on osteocytes in
osteoporosis; however, these have been only rarely investigated
(28). Plotkin et al
(29) demonstrated that GCs
mediate the apoptosis of bone cells via GR. In vitro, high
doses of GCs could inhibit the formation of osteoclasts through GR
in osteoblasts and osteoclasts (30). Furthermore, Kim et al
(31) observed that Dex inhibited
osteoclast formation and proliferation depending on GR in
osteoclasts. HPOB, an HDAC6 inhibitor, alleviated
corticosterone-induced injury in PC12 cells by suppressing GR
translocation (32). In the
present study, GR expression was upregulated in MC3T3-E1 cells
treated with Dex. Furthermore, ACY-1215 promoted GR expression, and
improved the viability, mineralization and osteogenesis of MC3T3-E1
cells. The prolonged use of glucocorticoids could lead to decreased
expression level of the GR gene, which leads to GC resistance
(33). In the present study, the
expression of GR gradually increased with pretreatment by ACY-1215.
It was hypothesized that ACY-1215 may recruit GR receptors to
decrease the number of GR receptors combined with DEX, inhibiting
the role of DEX and thereby improving the growth of MC3T3-E1 cells.
The underlying mechanism of the proliferation promoting effect of
ACY-1215 on Dex-induced MC3T3-E1 cells related to GR receptors
requires further investigation.
Previous studies have demonstrated the role of HDAC6
in regulating GR. For example, HDAC6 can cause deacetylation of
heat shock protein 90, which is crucial for GR chaperone activity,
ligand binding, translocation and gene activation (34–36).
When mesenchymal stromal cells (MSCs) are treated with Dex, HDAC6
translocates to the nucleus in a similar way to GR and the complex
of HDAC6 and GR is formed on the third day of osteogenic induction.
When MSCs are treated with high concentrations of Dex, the GR-HDAC6
complex is weakened, although it is not completely absent. This
could either be explained by partial degradation of GR following
prolonged treatment with high concentrations of Dex or a decrease
in HDAC6 protein expression. In the presence of RU-486 (GR
inhibitor), HDAC6 expression in MSCs is decreased which indicates
that GR could regulate HDAC6 expression. In addition, tubacin
(HDAC6 inhibitor) can weaken the complex formation between GR and
HDAC6 (18). The association
between HDAC6 and GR in the pathogenesis of GC-induced secondary
osteoporosis will be investigated in a future study.
In conclusion, HDAC6 expression was upregulated in
serum samples of patients with osteoporosis and MC3T3-E1 cells
treated with Dex at a certain concentration range (0.01–1 µm).
ACY-1215 treatment improved the cell viability, ALP activity,
mineralization capacity and osteogenesis of MC3T3-E1 cells treated
with Dex. Additionally, ACY-1215 increased the expression levels of
GR in MC3T3-E1 cells treated with Dex. However, there were
limitations in the present study. Studying the effect of ACY-1215
on osteoclastogenesis would render the research more complete. It
is hoped to perform experiments to explore the effect of ACY-1215
on osteoclastogenesis in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Medical and Health Research Project (grant no. 2018KY722).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW and JC contributed to the conception and design
of the study. NW performed the experiments and wrote the
manuscript. FW, SW, QZ and QY helped perform the experiments. SH,
PW and FY analyzed and interpreted the data. HW and JC critically
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee Review Board of Ningbo Number 6 Hospital (Ningbo, China),
and informed written consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirza F and Canalis E: Management of
endocrine disease: Secondary osteoporosis: Pathophysiology and
management. Eur J Endocrinol. 173:R131–R151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheu A and Diamond T: Secondary
osteoporosis. Aust Prescr. 39:85–87. 2016.PubMed/NCBI
|
|
3
|
Colangelo L, Biamonte F, Pepe J, Cipriani
C and Minisola S: Understanding and managing secondary
osteoporosis. Expert Rev Endocrinol Metab. 14:111–122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rooney M, Bishop N, Davidson J, Beresford
MW, Pilkington C, Donagh JM, Wyatt S, Gardner-Medwin J, Satyapal R,
Clinch J, et al: The prevention and treatment of
glucocorticoid-induced osteopaenia in juvenile rheumatic disease: A
randomised double-blind controlled trial. EClinicalMedicine.
12:79–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caplan L and Saag KG: Glucocorticoids and
the risk of osteoporosis. Expert Opin Drug Saf. 8:33–47. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buckley L, Guyatt G, Fink HA, Cannon M,
Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, et
al: 2017 American college of rheumatology guideline for the
prevention and treatment of glucocorticoid-induced osteoporosis.
Arthritis Rheumatol. 69:1521–1537. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGee-Lawrence ME and Westendorf JJ:
Histone deacetylases in skeletal development and bone mass
maintenance. Gene. 474:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Westendorf JJ: Histone deacetylases in
control of skeletogenesis. J Cell Biochem. 102:332–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jensen ED, Schroeder TM, Bailey J,
Gopalakrishnan R and Westendorf JJ: Histone deacetylase 7
associates with Runx2 and represses its activity during osteoblast
maturation in a deacetylation-independent manner. J Bone Miner Res.
23:361–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee HW, Suh JH, Kim AY, Lee YS, Park SY
and Kim JB: Histone deacetylase 1-mediated histone modification
regulates osteoblast differentiation. Mol Endocrinol. 20:2432–2443.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schroeder TM and Westendorf JJ: Histone
deacetylase inhibitors promote osteoblast maturation. J Bone Miner
Res. 20:2254–2263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Kwon S, Yamaguchi T, Cubizolles
F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, et al:
Mice lacking histone deacetylase 6 have hyperacetylated tubulin but
are viable and develop normally. Mol Cell Biol. 28:1688–1701. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dudakovic A, Evans JM, Li Y, Middha S,
McGee-Lawrence ME, van Wijnen AJ and Westendorf JJ: Histone
deacetylase inhibition promotes osteoblast maturation by altering
the histone H4 epigenome and reduces Akt phosphorylation. J Biol
Chem. 288:28783–28791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eslaminejad MB, Fani N and Shahhoseini M:
Epigenetic regulation of osteogenic and chondrogenic
differentiation of mesenchymal stem cells in culture. Cell J.
15:1–10. 2013.PubMed/NCBI
|
|
15
|
Kretsovali A, Hadjimichael C and
Charmpilas N: Histone deacetylase inhibitors in cell pluripotency,
differentiation, and reprogramming. Stem Cells Int.
2012:1841542012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menegola E, Di Renzo F, Broccia ML and
Giavini E: Inhibition of histone deacetylase as a new mechanism of
teratogenesis. Birth Defects Res C Embryo Today. 78:345–353. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rimando MG, Wu HH, Liu YA, Lee CW, Kuo SW,
Lo YP, Tseng KF, Liu YS and Lee OK: Glucocorticoid receptor and
Histone deacetylase 6 mediate the differential effect of
dexamethasone during osteogenesis of mesenchymal stromal cells
(MSCs). Sci Rep. 6:373712016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng C, Shan W, Huang W, Ding Z, Cui G,
Liu F, Lu W, Xu J, He W and Yin Z: ACY-1215 exhibits
anti-inflammatory and chondroprotective effects in human
osteoarthritis chondrocytes via inhibition of STAT3 and NF-κB
signaling pathways. Biomed Pharmacother. 109:2464–2471. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing L, Zhang X, Feng H, Liu S, Li D,
Hasegawa T, Guo J and Li M: Silencing FOXO1 attenuates
dexamethasone-induced apoptosis in osteoblastic MC3T3-E1 cells.
Biochem Biophys Res Commun. 513:1019–1026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Jiang H and Gu X: Echinacoside
suppresses dexamethasone-induced growth inhibition and apoptosis in
osteoblastic MC3T3-E1 cells. Exp Ther Med. 16:643–648.
2018.PubMed/NCBI
|
|
21
|
Han D, Gu X, Gao J, Wang Z, Liu G, Barkema
HW and Han B: Chlorogenic acid promotes the Nrf2/HO-1
anti-oxidative pathway by activating p21 Waf1/Cip1 to resist
dexamethasone-induced apoptosis in osteoblastic cells. Free Radic
Biol Med. 137:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen HT, Liu H, Mao MJ, Tan Y, Mo XQ, Meng
XJ, Cao MT, Zhong CY, Liu Y, Shan H and Jiang GM: Crosstalk between
autophagy and epithelial-mesenchymal transition and its application
in cancer therapy. Mol Cancer. 18:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Liu S, Fu T, Peng Y and Zhang J:
Microtubule destabilization caused by silicate via HDAC6 activation
contributes to autophagic dysfunction in bone mesenchymal stem
cells. Stem Cell Res Ther. 10:3512019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Shi ZY, Feng J and Cao JK: HDAC6
regulates dental mesenchymal stem cells and osteoclast
differentiation. BMC Oral Health. 18:1902018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ota S, Zhou ZQ, Romero MP, Yang G and
Hurlin PJ: HDAC6 deficiency or inhibition blocks FGFR3 accumulation
and improves bone growth in a model of achondroplasia. Hum Mol
Genet. 25:4227–4243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Liu F, Huang W, Wang J, Wan YP, Li
M, Pang YQ and Yin ZS: Ricolinostat (ACY-1215) inhibits VEGF
expression via PI3K/AKT pathway and promotes apoptosis in
osteoarthritic osteoblasts. Biomed Pharmacother. 118:1093572019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komori T: Regulation of skeletal
development by the Runx family transcription factors. J Cell
Biochem. 95:445–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plotkin L, Manolagas S and Bellido T:
Glucocorticoids induce osteocyte apoptosis by blocking focal
adhesion kinase-mediated survival. Evidence for inside-out
signaling leading to anoikis. J Biol Chem. 282:24120–24130. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rauch A, Seitz S, Baschant U, Schilling
AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A,
schmidt-ullrich R, et al: Glucocorticoids suppress bone formation
by attenuating osteoblast differentiation via the monomeric
glucocorticoid receptor. Cell Metab. 11:517–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ, Zhao H, Kitaura H, Bhattacharyya
S, Brewer JA, Muglia LJ, Ross FP and Teitelbaum SL: Glucocorticoids
suppress bone formation via the osteoclast. J Clin Invest.
116:2152–2160. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li ZY, Li QZ, Chen L, Chen BD, Zhang C,
Wang X and Li WP: HPOB, an HDAC6 inhibitor, attenuates
corticosterone-induced injury in rat adrenal pheochromocytoma PC12
cells by inhibiting mitochondrial GR translocation and the
intrinsic apoptosis pathway. Neurochem Int. 99:239–251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li RJ and Liu J: Pharmacogenetics advances
of glucocorticoid resistance and polymorphism of glucocorticoid
receptor. Chin J New Drugs. 24:1246–1254. 2015.
|
|
34
|
Vandevyver S, Dejager L and Libert C: On
the trail of the glucocorticoid receptor: Into the nucleus and
back. Traffic. 13:364–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kovacs JJ, Murphy PJ, Gaillard S, Zhao X,
Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB and Yao TP: HDAC6
regulates Hsp90 acetylation and chaperone-dependent activation of
glucocorticoid receptor. Mol Cell. 18:601–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Espallergues J, Teegarden SL, Veerakumar
A, Boulden J, Challis C, Jochems J, Chan M, Petersen T, Deneris E,
Matthias P, et al: HDAC6 regulates glucocorticoid receptor
signaling in serotonin pathways with critical impact on stress
resilience. J Neurosci. 32:4400–4416. 2012. View Article : Google Scholar : PubMed/NCBI
|