Introduction
Cholestasis, which is characterized by accumulation
of bile acids in the liver or systemic circulation, leads to bile
retention and can develop into symptoms such as fibrosis and
cirrhosis, accompanied by inflammation (1,2).
Cholestasis is mainly caused by mechanical obstruction or a genetic
defect at the bile duct (3,4). A
previous study reported that protein deficiency or a dysfunction
caused by genetic defects may have an important role in the
formation, progression and excretion of bile (3).
Oxidative stress and mitochondrial dysfunction are
regarded as the main cellular symptoms of hepatic cholestasis
(5). Excessive production of
reactive oxygen species (ROS) can mediate mitochondrial oxidative
damage, such as destruction of the respiratory chain, along with
destruction of the inner and outer membrane, and various matrix
proteins (6–8). Furthermore, changes in mitochondrial
membrane permeability may result in the increased expression of
various pro-apoptotic factors, such as cytochrome c, thus
initiating the mitochondrial apoptosis pathway to induce cell
apoptosis (9). Catalpol, an
iridoid glycoside abundant in the roots of Rehmannia
glutinosa, has been reported to protect the body from
developing neurodegenerative diseases; it has been shown to improve
the cognitive function of rats in old age (10), reduce oxidative stress in the
cerebral cortex and ameliorate the pathological condition of
Alzheimer's disease in mice (11).
In addition, catalpol has a variety of pharmacological activities,
including anti-inflammatory effects, and has been reported to play
an important role in maintaining a redox balance (12). A recent study revealed that
catalpol may improve neointimal hyperplasia in hyperglycemic rats
through reducing the expression of monocyte chemoattractant
protein-1 in the carotid artery, thus suggesting that catalpol
could have a role in the prevention of hyperglycemia (13). Another study reported that catalpol
exerted a protective effect on lipopolysaccharide/D-
galactosamine-induced acute liver injury through the inhibition of
inflammation and oxidation, and regulation of related signaling
pathways (14). Furthermore, it
has been demonstrated that catalpol can alleviate mitochondrial
damage (15). In addition,
catalpol has been demonstrated to inhibit the production of
inflammatory mediators in human intestinal Caco-2 cells, pointing
to the potential role of catalpol in the prevention of intestinal
inflammation (16).
In view of the antioxidative stress and
anti-inflammatory effects of catalpol, and its ability to improve
mitochondrial membrane potential, it was hypothesized that catalpol
could also improve hepatic injury in cholestasis. Therefore, the
present study aimed to further explore the potential mechanism of
action of catalpol.
Materials and methods
Animals
BALB/C male mice (n=30, weight, 10–25 g; age, 6–8
weeks) were purchased from Shanghai SLAC Laboratory Animal Co.,
Ltd. Mice were housed under a 12-h light/dark cycle at a relative
temperature of 23±2°C and 70% humidity. The mice were provided with
free access to food and water. The animal experiments were
performed strictly in accordance with the Care and Use of
Laboratory Animal Regulations (17). This study was approved by the
Ethics Review Board for Animal Studies of the Affiliated Yantai
Yuhuangding Hospital of Qingdao University (Yantai, China; approval
no. SH20186242).
Model establishment and grouping
A mouse model of cholestasis was established by bile
duct ligation (BDL). Briefly, the mice were anesthetized by
intraperitoneal injection of ketamine (100 mg/kg; Jiangsu Hengrui
Medicine Co., Ltd.,) and xylazine (10 mg/kg; Sigma-Aldrich; Merck
KGaA). Next, the common bile duct was separated from its
surrounding connective tissue, and the hepatic artery from the
portal vein. The common bile duct was then ligated with 5-0 silk
thread at two locations close to the beginning of its
intrapancreatic portion and was transected between the two
ligatures. Mice in the sham group received a laparotomy without
subsequent identification and ligation of the bile duct. Mice in
the sham and BDL groups were administered 0.9% normal saline (20
ml/kg/day) for 7 days. Mice in the catalpol-high (−H) group were
gavaged with 10 mg/kg catalpol (Nanjing Jingzhu Biotechnology Co.,
Ltd.) for 7 days after undergoing sham surgery, whereas those in
the BDL + catalpol groups were gavaged with either 5 mg/kg catalpol
[catalpol-low (−L)] or 10 mg/kg catalpol (catalpol-H) for 7 days
after the BDL model was established. A total of 1 week after the
experiment, all mice were sacrificed with an overdose of
pentobarbital sodium (120 mg/kg) followed by collection of blood
and liver samples. Blood was collected via the tail vein, and then
the blood samples (0.4 ml) were immediately separated by
centrifugation at 2,200 × g at 4°C for 10 min to obtain serum
samples.
Analysis of liver function-related
indexes in serum
The serum of mice was collected from blood samples
by centrifugation at 2,200 × g at 4°C for 10 min. Commercial ELISA
kits were used to measure serum levels of alanine aminotransferase
(ALT; cat. no. C009-2), aspartate aminotransferase (AST; cat. no.
C010-2), alkaline phosphatase (ALP; cat. no. A059-2), total
bilirubin (TBIL; cat. no. C019-1) and total bile acid (TBA; cat.
no. E003-2) (all Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's protocol.
Determination of inflammatory factor
index in serum
The blood samples were centrifuged at 2,200 × g for
10 min at 4°C to collect serum, which was then immediately stored
at −80°C until use. Tumor necrosis factor-α (TNF-α; cat. no.
MTA00B), interleukin (IL)-1β (cat. no. MLB00C) and IL-6 (cat. no.
M6000B) were detected in the serum samples using ELISA kits
(R&D Systems, Inc.) according to the manufacturer's
protocol.
Histomorphology
The liver tissues were sliced and fixed in 4%
paraformaldehyde overnight at 4°C. Subsequently, tissue sections
were dehydrated in a diminishing series of ethanol, embedded in
paraffin and sectioned into 4-5-µm slices. The sections were
stained with hematoxylin and eosin (H&E) for 10 min at room
temperature and observed under a light microscope (magnification,
×100; DM4000B; Leica Microsystems, Inc.).
Liver mitochondria isolation
After resection of the liver, the tissue samples
were homogenized in a buffer containing 2 mmol/l HEPES, 0.5 mmol/l
EGTA, 70 mmol/l mannitol, 220 mmol/l sucrose and 0.1% BSA
(Sigma-Aldrich; Merck KGaA) at a ratio of 10:1 buffer:tissue (v/w),
final pH 7.4. The tissue homogenates were centrifuged at 4°C for 10
min at 1,000 × g to pellet the whole cells and nuclei. The
supernatant was further centrifuged at 4°C for 10 min at 10,000 × g
to precipitate the heavy membrane fractions (mitochondria).
Mitochondrial membrane potential
detection
JC-1 staining was performed to detect the
mitochondrial membrane potential. The hepatocytes were isolated
from the liver tissues. Briefly, the liver tissues were digested in
0.1% trypsin for 10 min at 37°C and filtered (40 µm; Hangzhou
Xiaoyou Biotechnology Co., Ltd.) to obtain cell suspension, and the
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. The harvested hepatocytes were subsequently
washed with PBS, and then incubated with 5 µmol/l JC-1 (Cell
Signaling Technology, Inc.) for 30 min prior to being subjected to
flow cytometry (ACEA Bioscience; Agilent Technologies, Inc.).
Mitochondrial adenosine triphosphate
(ATP) content
The mitochondria suspension and 1X Reaction buffer
solution were prepared and subjected to a luciferase luciferin kit
(cat. no. FF2021; Enliten®; Promega Corporation) to
determine mitochondrial ATP content, according to the
manufacturer's protocol. Subsequently, luminous intensity of the
sample was detected at λ=560 nm using the FLUOstar
Omega® multi-function microplate reader (BMG Labtech
GmbH).
Detection of ROS production
Production of intracellular ROS was examined using
the fluorescent probe DCFH-DA (cat. no. HY-D0940; MedChemExpress).
Liver mitochondria were isolated as aforementioned and incubated
with a final concentration of 10 µmol/l DCFH-DA for 30 min at 37°C
and the fluorescence intensity was measured by flow cytometry (ACEA
Bioscience; Agilent Technologies, Inc.) (λ excitation, 488 nm; λ
emission, 525 nm).
Glutathione (GSH) content
detection
The level of GSH in mitochondria and serum was
determined by spectrophotometry. The chemical
5,50-dithio-2-nitrobenzoic acid (DTNB; cat. no. JD499-5G; Beijing
Dingguo Changsheng Biotechnology Co., Ltd.) was used as the
indicator of GSH. To extract mitochondrial GSH, the mitochondrial
suspension was treated with trichloroacetic acid to a final
concentration of 10% (w/v) at room temperature for 5 min. The
denatured proteins were removed by centrifugation at 15,000 × g for
1 min at 4°C and 100 µl DTNB (0.04% phosphate buffer) was then
added at room temperature for 15 min. The yellow intensity was
recorded at 412 nm using an ultraviolet spectrophotometer
(ultrospec 2000® ultraviolet spectrophotometer;
Pharmacia Biotechnology; GE Healthcare).
Malondialdehyde (MDA) content
detection
After isolation of liver mitochondria, MDA in
mitochondria and serum was detected using a thiobarbituric acid
reactive substance (TBARS) assay kit (cat. no. KA4409; AmyJet
Scientific, Inc.) according to the manufacturer's protocol.
Isolated mitochondria were washed in an ice-cold
3-(N-norpholino)propanesulfonic acid (MOPS)-KCl buffer (50 mM MOPS,
100 mM KCl) to remove sucrose, and re-suspended in fresh MOPS-KCl
buffer. Then, the mitochondrial suspension was mixed with 15%
trichloroacetic acid, 0.375% thiobarbituric acid, 0.24 M HCl, and
0.5 mM Trolox. The mixture was heated for 15 min at 100°C.
Following centrifugation (15,000 × g; 5 min; 4°C), the absorbance
of the supernatant was assessed, MDA in reaction with
thiobarbituric acid produces the MDA-TBA adduct, which could be
read at 532 nm using an Epoch plate reader (BioTek Instruments,
Inc.).
Western blot analysis
Liver tissues (0.1 g) were homogenized by incubation
with 1 ml protein lysis buffer (Beyotime Institute of
Biotechnology) and centrifuged at 12,000 × g for 10 min at 4°C to
collect the supernatant. The concentration of the protein was
evaluated using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Proteins (30 µg) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes (Sigma-Aldrich; Merck KGaA). The
membranes were then blocked with 5% skimmed milk powder for 3 h at
room temperature and incubated with the relevant primary antibodies
[cytochrome c (1:1,000; cat. no. ab90529; 15 kD), Bcl-2
(1:1,000; cat. no. ab59348; 26 kD), Bax (1:1,000; cat. no. ab32503;
21 kD), cleaved caspase-3 (1:1,000; cat. no. ab2302; 17 kD) and
caspase-3 (1:5,000; cat. no. ab32351; 32 kD) (all from Abcam)]
overnight at 4°C. The membranes were subsequently incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG H&L
(1:2,000; cat. no. ab205718; Abcam) secondary antibodies for 1 h.
GAPDH (1:1,000; cat. no. ab181602; 36 kD; Abcam) served as the
internal control. Chemiluminescence detection was conducted using
an ECL Western Blotting Substrate kit (cat. no. ab65623; Abcam).
Protein expression levels were semi-quantified using the Image-Pro
Plus 6.0 software (Media Cybernetics, Inc.).
Statistical analysis
The experiments were performed in triplicate. Data
are presented as the mean ± standard deviation. The data were
analyzed using one-way ANOVA followed by Bonferroni's post-hoc test
using SPSS 20.0 software (IBM Corporation). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of catalpol on serum
biochemical indexes of cholestatic mice
Mice in the catalpol-H treatment group showed no
significant alterations in the serum levels of ALT, AST, ALP, TBIL
or TBA (Fig. 1A-E) compared with
mice in the sham control group. However, addition of catalpol-H and
catalpol-L could significantly reduce serum ALT, AST, ALP, TBIL and
TBA levels in the BDL model group (Fig. 1A-E), with catalpol-H producing a
significantly greater protective effect compared with
catalpol-L.
 | Figure 1.Effects of catalpol on serum
biochemical indexes and inflammatory factor indexes in mice of
different treatment groups. Liver function parameters of (A) ALT,
(B) AST, (C) ALP, (D) TBIL and (E) TBA in mice in different
treatment groups. Inflammatory factors, including (F) TNF-α, (G)
IL-1β and (H) IL-6, were assessed in the serum. ***P<0.001 vs.
Sham; #P<0.05, ##P<0.01 and
###P<0.001 vs. Catalpol-H; ^P<0.05,
^^P<0.01 and ^^^P<0.001 vs. BDL. ALT,
alanine aminotransferase; AST, aspartate aminotransferase; ALP,
alkaline phosphatase; TBIL, total bilirubin; TBA, total bile acid;
TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6,
interleukin-6; BDL, bile duct ligation; catalpol-H, catalpol-high
treatment; catalpol-L, catalpol-low treatment. |
Effects of catalpol on serum
inflammatory factor indexes of cholestatic mice
As shown in Fig.
1F-H, it was revealed that mice treated with catalpol-H had no
significant change in the serum levels of TNF-α, IL-1β and IL-6
compared with the sham control group. Serum levels of TNF-α, IL-1β
and IL-6 all significantly increased following BDL. Furthermore,
compared with the BDL model group, catalpol-L and catalpol-H
significantly reduced the levels of TNF-α, IL-1β and IL-6, with
catalpol-H producing a greater effect compared with catalpol-L.
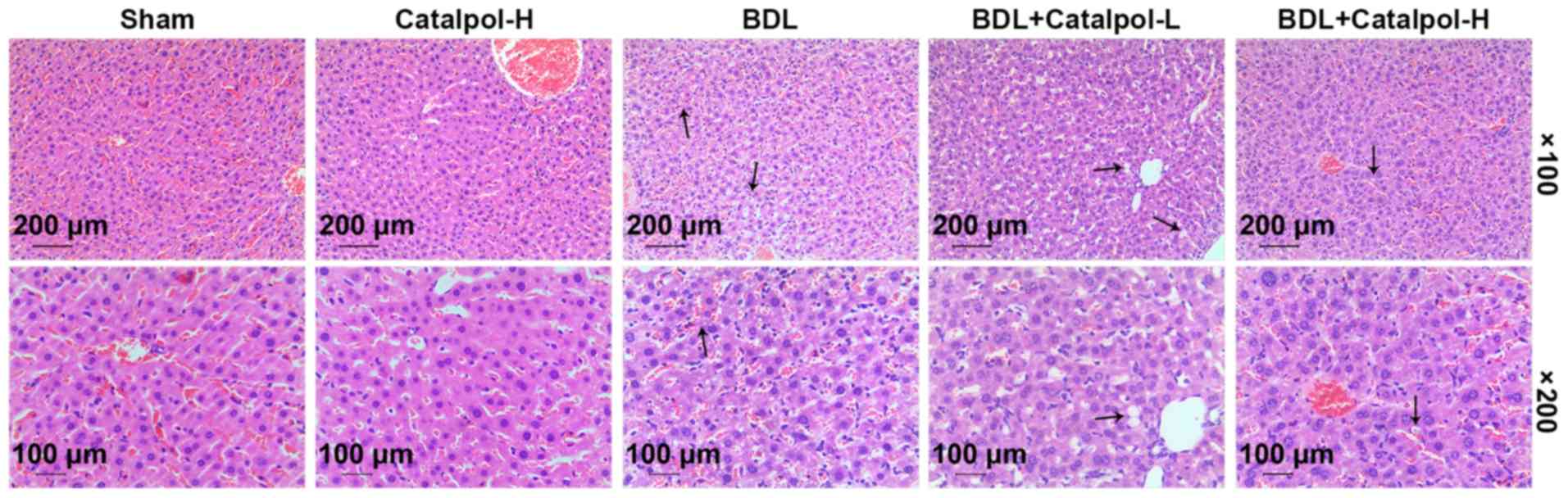
Effects of catalpol on histological
alterations of cholestatic mice
The histological alterations in liver tissues
derived from cholestatic mice were evaluated by H&E staining.
As shown in Fig. 2, treatment with
catalpol-H alone had no marked effect on the histological
morphology of the mouse liver tissues. In the BDL model group,
inflammatory infiltration and hepatocyte damage in the liver
tissues were observed, and subsequent treatment with catalpol
markedly reduced hepatocyte necrosis and neutrophil infiltration,
with catalpol-H producing a greater protective effect compared with
catalpol-L.
Effects of catalpol on hepatocyte
mitochondria and oxidative stress of cholestatic mice
Flow cytometry was performed to detect mitochondrial
membrane potential of the extracted hepatocytes. The subsequent
results of JC-1 distribution demonstrated that the mitochondrial
membrane potential in the BDL model group was reduced compared with
that in the sham group. In addition, catalpol-H had no notable
effect on mitochondrial membrane potential in normal mouse liver
cells; however, treatment with catalpol-L and catalpol-H
significantly improved the mitochondrial membrane potential of
liver cells in the cholestatic model mice (Fig. 3A). The ATP content of mitochondria
was determined using a luciferase luciferin kit; the results
revealed that ATP content was significantly lower in mitochondria
in the BDL group compared with the sham control group, whereas
treatment with catalpol significantly increased the content of ATP
in mitochondria within the cholestatic mice compared with the BDL
model group (Fig. 3B). The
production of ROS in the mitochondria was measured by flow
cytometry, and the results indicated that ROS generation was
increased in the BDL model group compared with the sham group,
whereas catalpol significantly reduced the production of ROS
compared with in the BDL model group, with catalpol-H producing a
more significant effect compared with catalpol-L (Fig. 3C).
Intracellular GSH contents in the serum were also
detected; as shown in Fig. 4A, GSH
content was significantly reduced in the BDL model group compared
with that in the sham control group, whereas treatment with
catalpol significantly increased GSH content compared with the BDL
model group. Once again, catalpol-H produced a greater protective
effect compared with catalpol-L. Moreover, similar results were
found with regards to GSH concentration in the mitochondria
(Fig. 4B). The contents of MDA in
the serum and mitochondria were also assessed, and the data
revealed that catalpol significantly reduced the total MDA content
in the BDL cholestatic model mice (Fig. 4C and D), with catalpol-H producing
a significantly more protective effect than catalpol-L.
Effects of catalpol on protein
expression levels in the liver of cholestatic mice
As shown in Fig. 5,
expression levels of proteins related to apoptosis and oxidative
stress were measured. The results revealed that cytochrome c
protein expression was increased in the BDL model group, whereas
cytochrome c levels were significantly reduced by treatment
with catalpol in the cholestatic model mice, with catalpol-H
producing a greater protective effect compared with catalpol-L.
With regards to the expression levels of proteins related to cell
apoptosis, it was revealed that Bcl-2 expression was significantly
reduced, whereas Bax and cleaved caspase-3 were significantly
increased in the BDL model group. Conversely, following treatment
with catalpol, the protein expression levels were significantly
altered and closer to the sham control sample levels. Once again,
catalpol-H produced a more protective effect than catalpol-L. There
were no notable changes to total caspase-3 expression levels among
the different control and treatment groups.
Discussion
Catalpol, which is an iridoid glycoside extracted
from Rehmannia glutinosa, has an anti-ischemic effect
(18–21). It has been suggested that catalpol
could effectively protect promyelinating oligodendrocytes from
apoptosis and myelination defects. Myelination defects may be
induced by oxygen glucose deprivation, which catalpol can protect
against through suppressing Ca2+ overload. Catalpol has
also been shown to inhibit mitochondrial damage and production of
ROS (18). A recent study reported
that catalpol had a protective role in diabetic nephropathy by
regulating the synthesis of nitric oxide (22). The present study revealed that in a
mouse model of cholestatic mice, catalpol exerted a protective
function potentially via its antioxidant activities and regulation
of mitochondrial membrane potential.
In this study, treatment with catalpol-H (10 mg/kg)
had no significant effect on serum levels of ALT, AST, ALP, TBIL or
TBA in mice in the sham control group, but it effectively reduced
signs of liver damage caused by BDL in the cholestatic mouse model.
ALT, AST, ALP, TBIL and TBA are all indicative of liver function
(23). Recently, a study on the
mechanism of andrographolide in the protection of cholestatic liver
injury found that andrographolide exerted a certain protective
effect on cholestatic liver injury, and the serum levels of the
aforementioned indicators were decreased (24). The present results were consistent
with this previous study, and preliminarily indicated that catalpol
had a protective effect on liver injury caused by cholestasis.
Cholestasis, which is characterized by disordered
bile formation and/or bile flow, is either an inherited or acquired
disease, and can lead to the interruption of liver excretion of
bile acids, enterohepatic circulation and may eventually develop
into severe liver damage (25).
Mitochondrial dysfunction is known to eventually induce cellular
apoptosis and oxidative stress. Oxidative stress and mitochondrial
damage are associated with the pathogenesis of liver cholestasis;
oxidative stress can lead to cell death and organ damage, whereas
the mitochondria are the main target of cytotoxic molecules, and
mitochondrial damage can cause a cell energy crisis and release of
cell death mediators (26).
Excessive ROS is known to directly impair mitochondrial function,
resulting in subsequent translocation of apoptosis-inducing factors
from the mitochondria to the nuclei and eventually resulting in
cell death (27). In addition,
reduced mitochondrial membrane potential affects the opening of the
mitochondrial permeability transition pore, which may cause
mitochondria-dependent cell death (28). Studies have revealed that catalpol
could scavenge ROS and enhance antioxidant abilities through
improving mitochondrial functions that protect against oxidative
attack (29–31). In the present study, the results
demonstrated that catalpol could significantly increase
mitochondrial membrane potential and reduce the levels of ROS in
the hepatocytes of cholestatic mice. In addition, a previous study
demonstrated that catalpol reduced oxidative stress in a colitis
model, which in turn reversed the increase in MDA, and reductions
in GSH and superoxide dismutase (SOD) in the colitis group
(32). Cai et al (18) previously reported that catalpol
ameliorated overproduction of ROS and protected pre-myelinating
oligodendrocytes against ischemia-induced oxidative injury via the
ERK1/2 signaling pathway.
GSH has a key role in the antioxidant defense system
of cells and is also a key factor in maintaining the redox
environment of cells. GSH protects cells from excessive ROS by
reacting directly with free radicals in non-enzymatic reactions and
acting as an electronic donor for GSH peroxidase to catalyze
peroxide reduction (33). MDA is a
marker of oxidative stress; a significant increase in MDA indicates
a significant increase in lipid peroxidation (34). The results of the present study
revealed that treatment with catalpol greatly increased
mitochondrial ATP and GSH contents, and reduced the level of MDA,
which suggested that catalpol may protect hepatocytes against
oxidative stress through the induction of antioxidant generation
and a reduction in the generation of oxidizing products. These
results indicated that the antioxidant pharmacological activity of
catalpol may be produced through the enhancement of endogenous
antioxidant enzymatic activities and through the inhibition of free
radical generation (indicated by decreased levels of ROS). Catalpol
was previously shown to increase the activity of GSH and reduce MDA
levels, which thereby ameliorated cognitive deficits and attenuated
oxidative damage in the brain of elderly mice induced by
D-galactose (29). In addition, a
previous study indicated that the protected effect of glycine on
cholestasis may be related to its ability to protect the redox
environment, prevent oxidative stress and maintain mitochondrial
function (35). Consistently, the
present study reported that following treatment with catalpol, the
pathological changes of liver tissues in the BDL model mice could
be alleviated. In addition, the functional indexes of liver
mitochondria in cholestatic mice were evaluated, and the damage to
the liver caused by cholestasis was found to result in the
dissipation of the liver mitochondrial membrane potential, which
was markedly reversed after the drug administration.
The current findings also revealed that catalpol
greatly reduced the contents of pro-inflammatory factors in the
serum derived from model mice in a concentration-dependent manner.
A recent study indicated that IL-6 and IL-1β may enhance the bile
canaliculi dilatation caused by cholestatic antibiotics (36). It has also been found that
ginsenoside can attenuate oxidative stress and the inflammatory
response in rats with intrahepatic cholestasis (37). The present data demonstrated that
the serum levels of AST, ALT, ALP and TBA, liver MDA, SOD activity
and pathological damage of the cholestatic model mice were markedly
reduced by catalpol. In addition, ginsenoside treatment was found
to noticeably reduce the protein expression levels of TNF-α, IL-6
and IL-1β (37). These results are
consistent with the present research results. In addition, the
apoptotic and oxidative stress proteins, such as cytochrome
c, Bcl-2, Bax and caspase-3 were detected in the liver
tissues, and a high concentration of catalpol significantly
downregulated the expression of Bax, cytochrome c and
cleaved caspase 3, and upregulated Bcl-2 expression in the
hepatocytes of cholestatic mice.
In conclusion, the current study revealed that
catalpol protected the liver from cholestasis-mediated injury
through its antioxidant effects, and its ability to improve
mitochondrial membrane potential and regulate inflammatory
cytokines. However, there are still some limitations in this study;
for example, catalpol plays a protective role in the oxidative
damage of cholestasis by targeting genes or regulating pathways,
and the specific genes and pathways that catalpol interacts with
need to be further studied. In addition, the findings need to be
confirmed in clinical studies. Taken together, catalpol may act to
protect hepatocytes from oxidative stress and could improve
mitochondrial membrane potential, which is closely related to the
recovery of cholestasis-mediated liver injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG and JX made substantial contributions to study
conception and design. Data acquisition, analysis and
interpretation was conducted by XG, JX and HL, along with drafting
and critically revising the manuscript. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Review Board
for Animal Studies of the Affiliated Yantai Yuhuangding Hospital of
Qingdao University (approval no. SH20186242).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
H&E
|
hematoxylin and eosin
|
|
ATP
|
adenosine triphosphate
|
|
ROS
|
reactive oxygen species
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
BDL
|
bile duct ligation
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
ALP
|
alkaline phosphatase
|
|
TBIL
|
total bilirubin
|
|
TBA
|
total bile acid
|
References
|
1
|
Afonso MB, Rodrigues PM, Simao AL,
Ofengeim D, Carvalho T, Amaral JD, Gaspar MM, Cortez-Pinto H,
Castro RE, Yuan J, et al: Activation of necroptosis in human and
experimental cholestasis. Cell Death Dis. 7:e23902016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vinken M: In vitro prediction of
drug-induced cholestatic liver injury: A challenge for the
toxicologist. Arch Toxicol. 92:1909–1912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Squires JE and McKiernan P: Molecular
mechanisms in pediatric cholestasis. Gastroenterol Clin North Am.
47:921–937. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gomez-Ospina N, Potter CJ, Xiao R,
Manickam K, Kim MS, Kim KH, Shneider BL, Picarsic JL, Jacobson TA,
Zhang J, et al: Mutations in the nuclear bile acid receptor FXR
cause progressive familial intrahepatic cholestasis. Nat Commun.
7:107132016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ommati MM, Farshad O, Niknahad H,
Arabnezhad MR, Azarpira N, Mohammadi HR, Haghnegahdar M, Mousavi K,
Akrami S, Jamshidzadeh A, et al: Cholestasis-associated
reproductive toxicity in male and female rats: The fundamental role
of mitochondrial impairment and oxidative stress. Toxicol Lett.
316:60–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin F, Yan J, Zhao Y, Guo KJ, Zhang ZL, Li
AP, Meng CY and Guo L: Bone marrow mesenchymal stem cells repair Cr
(VI)-injured kidney by regulating mitochondria-mediated apoptosis
and mitophagy mediated via the MAPK signaling pathway. Ecotoxicol
Environ Saf. 176:234–241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rajavel T, Packiyaraj P, Suryanarayanan V,
Singh SK, Ruckmani K and Pandima Devi K: β-sitosterol targets
Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS
mediated mitochondrial dysregulation and p53 activation. Sci Rep.
8:20712018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao X, Ren X, Zhu R, Luo Z and Ren B:
Zinc oxide nanoparticles induce oxidative DNA damage and
ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos.
Aquat Toxicol. 180:56–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang LL, Han L, Ma XL, Yu QL and Zhao SN:
Effect of mitochondrial apoptotic activation through the
mitochondrial membrane permeability transition pore on yak meat
tenderness during postmortem aging. Food Chem. 234:323–331. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia Z, Wang F, Zhou S, Zhang R, Wang F,
Huang JH, Wu E, Zhang Y and Hu Y: Catalpol protects synaptic
proteins from beta-amyloid induced neuron injury and improves
cognitive functions in aged rats. Oncotarget. 8:69303–69315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang JZ, Wu J, Xiang S, Sheng S, Jiang Y,
Yang Z and Hua F: Catalpol preserves neural function and attenuates
the pathology of Alzheimer's disease in mice. Mol Med Rep.
13:491–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao J, An L, Xu Y and Huang Y: Catalpol
exerts an anti-epilepticus effect, possibly by regulating the
Nrf2-Keap1-ARE signaling pathway. Med Sci Monit. 24:9436–9441.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CM, Wang BW, Fang WJ, Pan CM, Shyu KG
and Hou SW: Catalpol ameliorates neointimal hyperplasia in diabetic
rats. Planta Med. 85:406–411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Jia R, Wang F, Qiu G, Qiao P, Xu
X and Wu D: Catalpol protects mice against
Lipopolysaccharide/D-galactosamine-induced acute liver injury
through inhibiting inflammatory and oxidative response. Oncotarget.
9:3887–3894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Wang C, Jin Y, Yang Q, Meng Q,
Liu Q, Dai Y, Cai L, Liu Z and Liu K; Sun H Activating the
PGC-1α/TERT pathway by catalpol ameliorates atherosclerosis via
modulating ROS production, DNA damage, telomere function, :
Implications on mitochondria and telomere link. Oxid Med Cell
Longev. 2018:28763502018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park KS: Catalpol reduces the production
of inflammatory mediators via PPAR-gamma activation in human
intestinal Caco-2 cells. J Natural Med. 70:620–626. 2016.
View Article : Google Scholar
|
|
17
|
National Research Council Committee for
the Update of the Guide for the C and use of laboratory A, . The
National Academies Collection: Reports funded by National
Institutes of Health. Guide for the care and use of laboratory
Animals. National Academies Press (US) Copyright © 2011, National
Academy of Sciences. (Washington (DC)). 2011.PubMed/NCBI
|
|
18
|
Cai Q, Ma T, Li C, Tian Y and Li H:
Catalpol protects Pre-myelinating oligodendrocytes against
Ischemia-induced oxidative injury through ERK1/2 signaling pathway.
Int J Biol Sci. 12:1415–1426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu D, Wang L, Jiang Z, Zhao G, Hassan HM,
Sun L, Fan S, Zhou Z, Zhang L and Wang T: A new hypoglycemic
mechanism of catalpol revealed by enhancing MyoD/MyoG-mediated
myogenesis. Life Sci. 209:313–323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng YF, Wang R, Bian Y, Chen WS and Peng
L: Catalpol attenuates IL-1β induced matrix catabolism, apoptosis
and inflammation in rat chondrocytes and inhibits cartilage
degeneration. Med Sci Monit. 25:6649–6659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin C, Lu Y, Yan X, Wu X, Kuai M, Sun X,
Chen Q, Kong X, Liu Z, Tang Y, et al: Catalpol protects
glucose-deprived rat embryonic cardiac cells by inducing mitophagy
and modulating estrogen receptor. Biomed Pharmacother. 89:973–982.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun W, Gao Y, Ding Y, Cao Y, Chen J, Lv G,
Lu J, Yu B, Peng M, Xu H, et al: Catalpol ameliorates advanced
glycation end product-induced dysfunction of glomerular endothelial
cells via regulating nitric oxide synthesis by inducible nitric
oxide synthase and endothelial nitric oxide synthase. IUBMB Life.
71:1268–1283. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Wen JX, Gao SJ, He X, Li PY, Yang
YX, Wei SZ, Zhao YL and Xiao XH: Paeonia lactiflora Pall. regulates
the NF-κB-NLRP3 inflammasome pathway to alleviate cholestasis in
rats. J Pharm Pharmacol. 70:1675–1687. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Cao F, Zhu LL, Liu P, Shang YR,
Liu WH, Dong X, Bao HD, Gong P and Wang ZY: Andrographolide impairs
alpha-naphthylisothiocyanate-induced cholestatic liver injury in
vivo. J Natural Med. 73:388–396. 2019. View Article : Google Scholar
|
|
25
|
Wagner M and Trauner M: Recent advances in
understanding and managing cholestasis. F1000Research. 5:F1000
Faculty Rev–705. 2016. View Article : Google Scholar
|
|
26
|
Heidari R and Niknahad H: The role and
study of mitochondrial impairment and oxidative stress in
cholestasis. Methods Mol Biol. 1981:117–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baud O, Li J, Zhang Y, Neve RL, Volpe JJ
and Rosenberg PA: Nitric oxide-induced cell death in developing
oligodendrocytes is associated with mitochondrial dysfunction and
apoptosis-inducing factor translocation. Eur J Neurosci.
20:1713–1726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Javadov S, Karmazyn M and Escobales N:
Mitochondrial permeability transition pore opening as a promising
therapeutic target in cardiac diseases. J Pharmacol Exp Ther.
330:670–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XL, Jiang B, Li ZB, Hao S and An LJ:
Catalpol ameliorates cognition deficits and attenuates oxidative
damage in the brain of senescent mice induced by D-galactose.
Pharmacol Biochem Behav. 88:64–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bi J, Wang XB, Chen L, Hao S, An LJ, Jiang
B and Guo L: Catalpol protects mesencephalic neurons against MPTP
induced neurotoxicity via attenuation of mitochondrial dysfunction
and MAO-B activity. Toxicol In Vitro. 22:1883–1889. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riddle A, Luo NL, Manese M, Beardsley DJ,
Green L, Rorvik DA, Kelly KA, Barlow CH, Kelly JJ, Hohimer AR and
Back SA: Spatial heterogeneity in oligodendrocyte lineage
maturation and not cerebral blood flow predicts fetal ovine
periventricular white matter injury. J Neurosc. 26:3045–3055. 2006.
View Article : Google Scholar
|
|
32
|
Xiong Y, Shi L, Wang L, Zhou Z, Wang C,
Lin Y, Luo D, Qiu J and Chen D: Activation of sirtuin 1 by
catalpol-induced down-regulation of microRNA-132 attenuates
endoplasmic reticulum stress in colitis. Pharmacol Res. 123:73–82.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Antus B: Oxidative stress markers in
sputum. Oxid Med Cell Longev. 2016:29304342016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shaker RA, Abboud SH, Assad HC and Hadi N:
Enoxaparin attenuates doxorubicin induced cardiotoxicity in rats
via interfering with oxidative stress, inflammation and apoptosis.
BMC Oharmacol Toxicol. 19:32018. View Article : Google Scholar
|
|
35
|
Heidari R, Ghanbarinejad V, Mohammadi H,
Ahmadi A, Ommati MM, Abdoli N, Aghaei F, Esfandiari A, Azarpira N
and Niknahad H: Mitochondria protection as a mechanism underlying
the hepatoprotective effects of glycine in cholestatic mice. Biomed
Pharmacother. 97:1086–1095. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharanek A, Burban A, Ciriaci N and
Guillouzo A: Pro-inflammatory cytokines enhance dilatation of bile
canaliculi caused by cholestatic antibiotics. Toxicol In Vitro.
58:51–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu YJ, Yu ZQ, Zhang CL, Li XP, Feng CY,
Lei K, He WX and Liu D: Protective effects of ginsenosides on
17[Formula: See text]-Ethynyelstradiol-Induced intrahepatic
cholestasis via anti-oxidative and anti-inflammatory mechanisms in
rats. Am J Chin Med. 45:1613–1629. 2017. View Article : Google Scholar : PubMed/NCBI
|