Introduction
Metabolic syndrome (MS) is a cluster of metabolic
derangements that may increase the risk of development of diabetes
and cardiovascular diseases (1).
In general, MS includes abdominal obesity, dyslipidemia, impaired
fasting glucose level and high blood pressure (2,3). The
prevalence of MS is increasing worldwide and, according to data
from the International Diabetes Federation, about one-quarter of
the world's adult population is suffering from it (4).
Nonalcoholic fatty liver disease (NAFLD) is one of
the most common types of chronic liver diseases (5), characterized by a wide range of
histopathological states ranging from simple steatosis to more
severe fibrosis and cirrhosis, which may lead to liver failure or
hepatocellular carcinoma (6).
Hepatic morphology and functions may be adversely affected by MS,
consequently leading to the development of NAFLD (7). NAFLD is also suggested to be a strong
determinant of the development of MS (8). The prevalence of NAFLD has been
steadily increasing along with metabolic conditions (9). The prevalence of MS with NAFLD is
known to increase with a body mass index (BMI) of 18% in
normal-weight NAFLD subjects to 67% in obese subjects (7).
The exact mechanisms underlying NAFLD have yet to be
elucidated. Insulin resistance and chronic oxidative stress are
reported to play major roles and serve as the first
pathophysiological drivers of liver damage and NAFLD (10,11).
The histological hallmark of NAFLD is the accumulation of
triacylglycerol (TG)-rich lipid droplets within hepatocytes
(12). In addition, several
abnormalities in various lipid classes have been identified in
NAFLD (13). In over-nutrition,
adipose tissue insulin resistance leads to inappropriate lipolysis
and release of fatty acids into the circulation, which can be taken
up and induce an overload in the liver (14). The excess fatty acids and glucose
in the blood can be diverted to the oxidative pathways in other
tissues and trigger oxidative stress including mitochondrial
reactive oxygen species production and lipotoxic lipids (14). Nuclear factor κβ (NF-κB) signal
also increases and then induces the production of pro-inflammatory
mediators including tumor necrosis factor α (TNF-α), interleukin
(IL) 6 and IL-1β (15).
At present, the standard of care for patients with
NAFLD and MS focuses on lifestyle modification, including diet and
physical activity targeting visceral adiposity (16,17).
Diverse medications have also been developed in an attempt to treat
NAFLD. Vitamin E has been used for its antioxidant ability to
normalize liver enzymes or improve inflammation and hepatocyte
ballooning (18,19). Pioglitazone, an insulin sensitizer
peroxisome proliferator-activated receptor γ (PPARγ) agonist, is
known to be effective in alleviating NAFLD. The American
Association for Study of Liver Disease usually recommends the
combined use of vitamin E and pioglitazone (20). Although insulin sensitizers such as
pioglitazone and the antioxidant vitamin E have shown promising
results, there are some concerns regarding adverse effects and
long-term safety. Pioglitazone may cause weight gain, edema,
osteoporosis and heart failure (21,22),
while vitamin E is considered to be fairly benign. Vitamin E is
fat-soluble and could exert adverse effects at high doses (23,24).
Therefore, it is of considerable interest to explore safe treatment
regimens for NAFLD and MS.
Molecular hydrogen (H2) is the smallest
gas molecule with a strong ability to penetrate and access cells
and even organelles (25).
H2 has been regarded as biochemically inert for a long
time and little attention has been paid to its biological effect,
although it was shown to inhibit the growth of squamous carcinoma
in mice in 1975 (26). In 2007,
Ohsawa et al (27)
discovered the selective antioxidant mechanism of low concentration
H2 (<4%, v/v) for ischemia/reperfusion injury. In
recent years, several studies have confirmed its protective effects
against various diseases, including central nervous system
pathology, cardiovascular and cerebrovascular diseases, cancer,
organ injuries and dermatologic diseases. Our previous studies have
demonstrated the safety and effectiveness of H2 in
metabolic disorders, especially atherosclerosis, through animal and
clinical experiments (28–31). However, H2 inhalation
has never been tried in alleviating NAFLD.
The present study assessed the potential beneficial
effects of H2 on the general state, lipid metabolism
parameters and liver histology and functionality in a rat model of
MS induced by high fat and fructose diet (HFFD).
Materials and methods
Animals and model establishment
A total of 46 male Sprague-Dawley rats (age, 6 weeks
old; weight, 240–260 g) were purchased from the Beijing Vital River
Laboratory Animal Technology Co., Ltd. The rats were housed under
standard conditions with suitable temperature (22±1°C), humidity
(50–60%) and a 12-h light/dark cycle. Animals were provided with
food and water ad libitum. All the experiments were
conducted following the guidelines of the laboratory animal ethics
committee of Shandong First Medical University and Shandong Academy
of Medical Sciences (http://marxism.sdfmu.edu.cn/info/1126/1104.htm,
version 2011). After one week of acclimatization, the rats were
randomly allocated to four different experimental groups
(n=10-12/group) as follows: i) Control group (Con) exposed to a
regular diet and air; ii) model group (HFFD) exposed to HFFD and
air; iii) low H2 group (HFFD + LH2) exposed
to HFFD and 4% H2; and, iv) high H2 group
(HFFD + HH2) exposed to HFFD and 67% H2.
The model was induced by HFFD feeding. The diet
contained 24.2% protein, 42.1% carbohydrates and 25.4% fat,
providing 19.8, 35.2 and 45% calories, respectively. In addition,
the diet contained 2% cholesterol and 20% fructose. The animals
began HFFD feeding after a one-week acclimation period until the
end of the experiment. The experiment lasted for 10 weeks.
H2 inhalation
Low (4%) and high (67%) concentrations of
H2 were provided by our self-developed device.
H2 and oxygen were supplied from cylinders, while the
air was provided by an air generator. The flow rate of each gas was
adjusted by a flow meter. The 3 gases were mixed in a plastic box
and pumped into a sealed animal chamber with a total flow rate of 3
l/min for 2 h once daily. Gas detectors XP-3140 (New Cosmos
Electric Co., Ltd.) and JR2000-O2 (JingRuiBo Technology Co., Ltd.)
were used to monitor the concentrations of H2 and oxygen
(21%) to confirm the stability of each gas component. The gas
intervention started on the day of HFFD feeding and continued until
the end of the experiment.
Sampling
Food intake was recorded every other day in the last
week. After 10 weeks of the experiment, the rats were anesthetized
by an intraperitoneal injection of chloral hydrate (400 mg/kg body
weight) and body weight, abdominal circumference and tibial length
were measured. Blood samples (8–10 ml) were collected from the
inferior vena cava, centrifuged at 3,000 × g for 15 min at 4°C and
stored at −80°C for further biochemical analyses. Rats were
sacrificed by cardiac perfusion using phosphate-buffered saline
(PBS) without fixation. Animal death was defined as mydriasis,
respiratory arrest and cardiac arrest for a period of >5 min.
Liver and abdominal white fats (including perirenal, perigonadal
and mesenteric fats) were dissected and weighed. Parts of the
tissues were fixed in 4% paraformaldehyde for 24 h at room
temperature for histopathological examination, while the remaining
tissues were immediately sliced, frozen under liquid nitrogen and
stored at −80°C for the evaluation of biochemical parameters. Liver
and abdominal fat indices were calculated by dividing the tissue
weight (g) by tibial length (cm).
Oral glucose tolerance test
(OGTT)
OGTT was performed during the last week of the
experiment. Rats were deprived of their respective diets overnight
and then administered with 2 g glucose/kg body weight by oral
gavage. Blood samples were collected from the tail vein at 0, 30,
60, 90 and 120 min after oral glucose loading and blood glucose
levels were measured with a calibrated OneTouch UltraEasy
glucometer (LifeScan). The total area under the curve (AUC) for
OGTT was calculated by the trapezoid method.
Quantification of plasma biochemical
markers
Biochemical parameters indicative of lipid levels
(total cholesterol and total triglycerides) and liver functions
[alanine aminotransferase (ALT), aspartate aminotransferase (AST)
and lactic dehydrogenase (LDH)] were measured with an automatic
biochemistry analyzer Chemray-240 (Rayto Life and Analytical
Sciences Co., Ltd.).
Measurement of intrahepatic lipid
content
Lipid extraction from the liver was carried out
using the methyl-tert-butyl ether (MTBE) method with some
modifications. Briefly, liver tissues (8–10 mg) were homogenized in
280 µl of cold methanol containing an internal standard mixture
[cholesteryl ester (CE) 17:0, TG 17:0/17:1/17:0 and
d7-cholesterol]. In total, 50 µl of homogenates were stored to
analyze protein content using a commercial bicinchoninic acid
protein assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.). MTBE (1 ml) was added to the remaining volume of
homogenates; the samples were vortexed and transferred to a rotary
spinner at 40 rpm for 1 h at room temperature. Phase separation was
induced by adding 325 µl water. After vortexing, the samples were
centrifuged at 10,000 × g for 10 min at 4°C. The upper hydrophobic
fraction (350 µl) was transferred to another tube, dried with
N2 gas and stored at −80°C. The lipid extracts were
reconstituted in 200 µl of acetonitrile:2-propanol (1:1, v/v) prior
to liquid chromatography tandem mass spectrometry (LC-MS/MS)
analysis.
LC-MS/MS was performed using a Shimadzu LC-20 AD
binary pump system coupled to a SIL-20AC autoinjector and
interfaced with an ABI 4000 QTrap mass spectrometer (SCIEX).
Chromatographic separations were carried out on a Waters Symmetry
C18 column (3.5 µm, 2.1 mm i.d.x100 mm) with a Waters C18 guard
column (3.5 µm, 2.1 mm i.d.x10 mm) at 40°C (Waters Corporation).
The injection volume was 10 µl, while the flow rate was 0.3 ml/min.
The mobile phase comprised (A) 10 mM ammonium formate in
acetonitrile: Water: Formic acid (83:17:0.1, v/v/v) and (B) 10 mM
ammonium formate in acetonitrile: 2-propanol: formic acid
(50:50:0.1, v/v/v). Isocratic elution was performed with 95% B for
16 min.
Detection was accomplished at the multiple reaction
monitoring mode with positive-ion detection. For CE and TG,
electrospray ionization source was selected with the following
settings: Ion spray voltage=5,500 V; ion source heater
temperature=400°C; source gas 1=40 psi; source gas 2=40 psi; and
curtain gas=10 psi. For free cholesterol, atmospheric pressure
chemical ionization source was selected with the following
parameters: Nebulizer gas pressure of 55 psi; curtain gas pressure
of 20 psi; nebulizer current of 3 µA, source temperature of 550°C
and medium nitrogen collision gas pressure.
The precursor-to-product ion m/z transitions,
declustering potentials and collision energy are summarized in
Table SI. Relative quantification
of lipids in samples was carried out based on the intensity of each
species divided by the intensity of the internal standards and
protein concentrations.
Liver histology
Fixed tissues were washed with pure water for 12 h,
dehydrated in gradient ethyl alcohol concentrations and embedded in
paraffin. Sections (5 µm) were cut, deparaffinized, hydrated and
stained with hematoxylin at room temperature for 5 min, followed by
1% eosin staining at room temperature for 3 min (H&E).
To assess lipid deposition, liver tissues were
frozen in liquid nitrogen, embedded in optimum cutting temperature
compound, sectioned at 8 µm thickness and stained with 0.5% Oil Red
O for 15 min at 60°C. All the slides were viewed and images
captured using an Olympus BX53 light microscope (Olympus
Corporation) at magnification, ×400.
Reverse transcription-quantitative
(RT-q) PCR
Liver tissues were homogenized in cold
TRIzol® reagent (Thermo Fisher Scientific, Inc.) using a
high-speed tissue grinding instrument (KZ-II, Wuhan Servicebio
Technology Co., Ltd.). RNA was extracted following the
manufacturer's instructions (Invitrogen; Thermo Fisher Scientific,
Inc.) and its purity and concentration were measured using an
Ultraviolet-visible spectrophotometer (DS-11, DeNovix Inc.). Total
RNA was reverse transcribed into first-strand cDNA using HiFiScript
gDNA Removal RT MasterMix (CoWin Biosciences) at 37°C for 15 min.
For RT-qPCR analysis, the reaction was carried out using UltraSYBR
Mixture in a total volume of 25 µl prepared in accordance with the
instruction of the reagent kit (CoWin Biosciences). Expression of
SREBP-1c gene was measured and β-actin was used as a
housekeeping gene. The primer sequences were as follows: SREBP-1c,
5′-GCAACACTGGCAGAGATCTACGT-3′ (forward) and
5′-TGGCGGGCACTACTTAGGAA-3′ (reverse); β-actin,
5′-TTCCTTCCTGGGTATGGAAT-3′ (forward) and 5′-GAGGAGCAATGATCTTGATC-3′
(reverse). RT-qPCR reactions were performed under the following
conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 10
sec, 61°C for 32 sec and 72°C for 32 sec. The 2−ΔΔCq
method (32) was used to normalize
mRNA expression level to that of β-actin. This experiment
was repeated three times independently.
Statistical analysis
Results are presented as mean ± standard deviation.
Shapiro-Wilk normality test (α=0.05) was used to determine
whether sample group data were distributed normally. For parametric
data, one-way analysis of variance was performed followed by the
least significant difference (LSD) post hoc test. For
non-parametric data, Kruskal-Wallis tests were performed. All
statistical analyses were performed using SPSS 20.0 (IBM Corp.) for
Windows. P<0.05 was considered to indicate a statistically
significant difference.
Results
H2 inhalation ameliorated
body weight gain and compositional changes
Although the mean daily food intake was reduced
(P<0.05, Fig. 1A) in HFFD-fed
rats, their body weight, BMI, abdominal fat index and liver index
were significantly higher compared with those of normal diet-fed
rats. Low and high concentrations of H2 inhalation could
reduce these parameters in a dose-dependent manner (Fig. 1B-E). Significant differences of BMI
were observed between HFFD and high H2 (HFFD +
HH2) groups (P<0.01). No difference in kidney index
was detected among different groups (Fig. 1F).
 | Figure 1.Effects of H2 inhalation
on body weight gain and compositional changes. (A) Changes in food
intake, (B) body weight, (C) BMI, (D) abdominal fat index, (E)
liver index, and (F) kidney index of rats from Con (control group),
HFFD (model group), HFFD + LH2 and HFFD + HH2
groups. *P<0.05, **P<0.01 and ***P<0.001 vs. the Con
group; ##P<0.01 vs. the HFFD group. n=10-12 per
group. Con, control group; HFFD, high fat and fructose diet group;
LH2, low H2 group; HH2, high
H2 group. |
H2 inhalation increased the
AUC for OGTT
OGTT results revealed that the plasma glucose level
of all rats increased after oral glucose administration. Rats from
the HFFD group demonstrated the highest level of blood glucose.
Following H2 inhalation, the values significantly
decreased in a dose-dependent manner (Fig. 2A). The area under the curve (AUC)
for OGTT was significantly increased for the HFFD-fed rats, which
is indicative of the impairment in glucose tolerance (P<0.05,
Fig. 2B). H2 inhalation
at both low and high concentrations could lower the AUC for OGTT by
9.0 and 14.8%, respectively (Fig. 2A
and B). No significant differences were observed between the
low and high H2 inhalation groups.
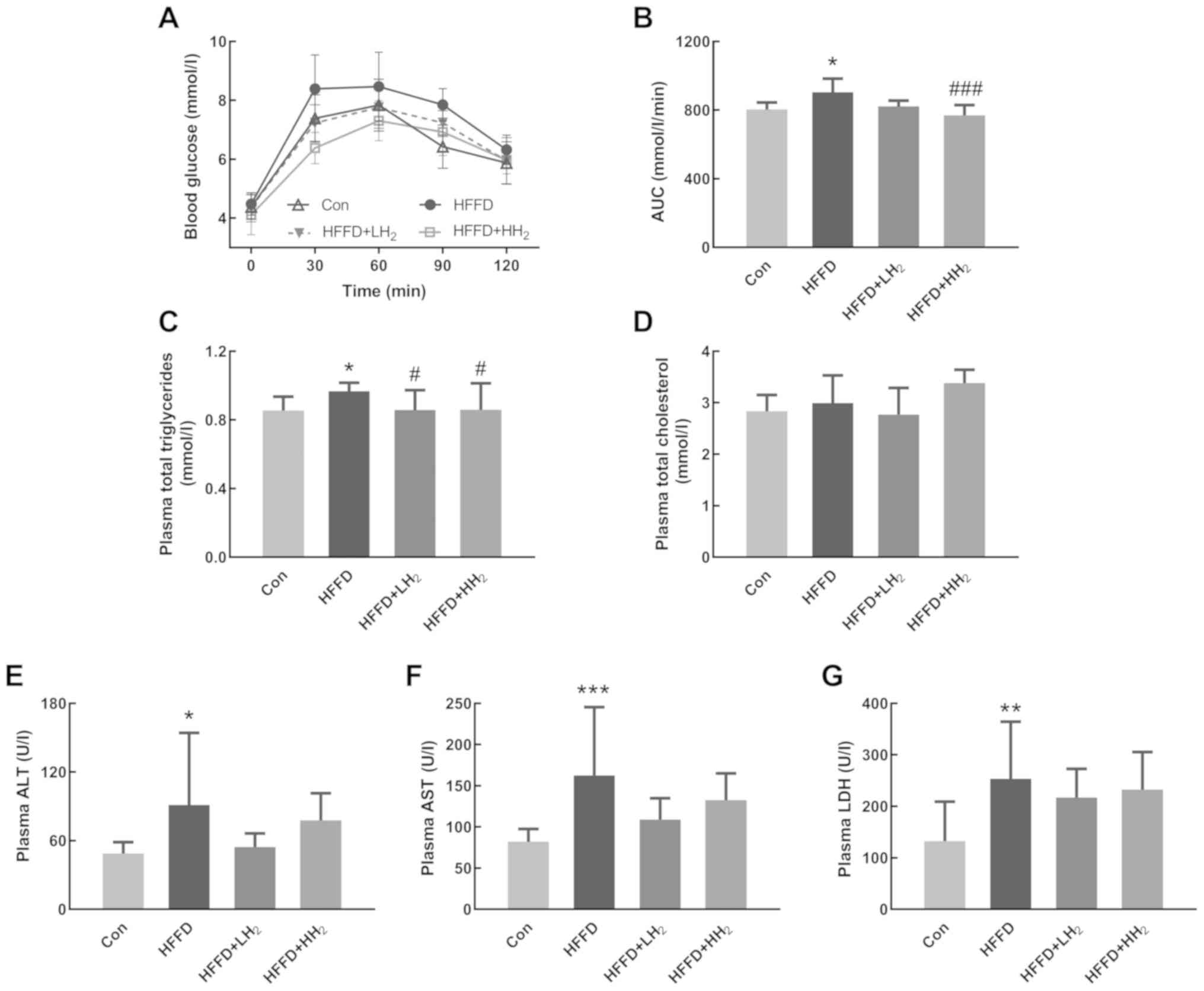 | Figure 2.Effects of H2 inhalation
on OGTT and plasma biochemical values. (A) Curve of OGTT and (B)
AUC of OGTT (n=10 per group). Concentrations of (C) plasma total
triglycerides, (D) total cholesterol, (E) ALT, (F) AST and (G) LDH
(n=8–12 per group). Data are shown as mean ± standard deviation.
*P<0.05, **P<0.01 and ***P<0.001 vs. the Con group;
#P<0.05 and ###P<0.001 vs. the HFFD
group. OGTT, Oral glucose tolerance test; AUC, area under the
curve; ALT, aminotransferase; AST, aspartate aminotransferase; LDH,
lactic dehydrogenase; Con, control group; HFFD, high fat and
fructose diet group; LH2, low H2 group;
HH2, high H2 group. |
H2 inhalation improved
plasma biomarkers
Plasma total triglyceride levels were significantly
higher in the rats from HFFD groups compared with those from the
Con group. H2 inhalation at both low and high
concentrations could significantly prevent the increase in plasma
total triglycerides (P<0.05, Fig.
2C). Rats fed with HFFD and subjected to H2
inhalation demonstrated no significant changes in plasma total
cholesterol (Fig. 2D). In
comparison with the rats from the Con group, those from the HFFD
feeding group had significantly increased plasma activities for
ALT, AST and LDH, the biomarkers of liver functions (P<0.05,
P<0.001 and P<0.01, respectively). Rats from the HFFD +
LH2 group had lower plasma ALT, AST and LDH activities
compared with the HFFD group (Fig.
2E-G). A low concentration of H2 seemed to exert
better effects than a high concentration.
H2 inhalation regulated
hepatic fat accumulation
HFFD consumption led to the development of fatty
liver. Hepatic lipid profiles for cholesterol and TG species were
analyzed and are presented in Fig.
3. In comparison with the control rats, those fed with HFFD
demonstrated a significant increase in the sum of hepatic
cholesterol and TG species (16.4- and 6.0-fold, respectively).
After inhalation of low concentration of H2, the total
hepatic cholesterol and TG levels were suppressed by 11.1 and
26.2%, respectively. Inhalation of a high concentration of
H2 resulted in a significant decrease in the levels of
total hepatic cholesterol and TG by 32.2 and 40.5%, respectively
(P<0.05, Fig. 3). The relative
fold increase in different cholesterol and TG species demonstrated
great variations. The contents of most species decreased after
H2 inhalation.
H2 inhalation regulated
hepatic fat accumulation
Evaluation of the general morphology of the liver
tissue from Con rats revealed a uniform color and texture. The
volume of the liver tissue seemed to slightly increase for the rats
from HFFD group, consistent with the observations such as round
blunt edges, loose texture, light beige color and greasy feeling
with dense small holes. H2 intervention could partly
alleviate these phenomena (Fig.
S1). After H&E staining, the hepatocytes from the Con group
were normal and distinct, regularly arranged and formed clear and
complete hepatic cords (Fig. 4A).
Only few tiny lipid droplets were observed after Oil Red O staining
(Fig. 4E). By contrast, the tissue
from the HFFD group demonstrated round fat vacuoles (lipid
droplets) of different sizes in the cytoplasm and fuzzy or even
broken intercellular boundaries. In addition, the hepatic cord
demonstrated disordered arrangements (Fig. 4B and F). This condition was
alleviated following H2 treatment, especially at high
concentration (Fig. 4C and D).
Smaller and less red-dyed fatty droplets were observed after
intervention with low and high concentrations of H2
(Fig. 4G and H). These findings
are in agreement with the changes in the hepatic cholesterol and TG
contents (Fig. 3). The expression
of the lipid synthesis gene SREBP-1c was significantly
upregulated in HFFD rats (P<0.001, Fig. 4I). Inhalation of low and high
concentrations of H2 significantly decreased
SREBP-1c mRNA expression in a dose-dependent manner
(P<0.05 and P<0.01, respectively; Fig. 4I).
Discussion
As a small molecular gas, H2 may diffuse
into the target tissues without any hindrance. H2
administration can be achieved through inhalation, drinking
H2-rich water, or injection of H2-rich saline
(25). The beneficial effects of
the inhalation of low concentrations (2 and 4%) of H2
gas against ischemia/reperfusion injuries were first described by
Ohsawa et al (27).
Thereafter, many studies have demonstrated the therapeutic effects
of low concentrations of H2 inhalation on different
diseases (33–35). In comparison with other methods,
inhalation of H2 gas could provide more H2,
especially at high concentrations. The recent development of the
H2 generator has led to a gradual increase in the number
of studies using high concentrations of H2. Inhalation
of high concentrations of H2 has been proved to
ameliorate ischemia/reperfusion injury (36,37),
endometriosis (38) and
glyoxylate-induced calcium oxalate deposition (39) in animal models. Commercially
available machines for inhalation of high concentrations of
H2 have also been used in patients (40). Although both low and high
concentrations of H2 have been applied in experiments, a
comparison of their therapeutic effects has not been made. Thus, 4
and 67% H2 were chosen in this study to investigate the
dosage effect on NAFLD in MS rats. As the oxygen concentration
directly generated from the commercial machine was 33%, our
self-made device was used to maintain the oxygen level in the mixed
gas to approximately 21% to avoid effects of high concentrations of
oxygen.
The occurrence and development of MS are influenced
by several factors, particularly diet. Fat and fructose have been
used in combination to induce MS and a HFFD-induced rodent model is
the best model to study human MS (41). Thus, HFFD was used in this study to
mimic human diets associated with the development of MS.
MS is a cluster of pathological conditions related
to obesity, insulin resistance and dyslipidemia (3). The present study demonstrated that
10-week HFFD feeding could lead to MS in rats by causing abdominal
obesity and glucose intolerance as well as by increasing liver
damage marker levels and dyslipidemia. Previous studies have shown
the beneficial role of drinking H2-rich water in
potential patients with MS (28,42)
or MS rat model SHR. Cg-Leprcp/NDmcr (SHR-cp)
(43). However, the effects of
H2 inhalation on HFFD-induced MS in rats remain to be
elucidated.
The present study provided direct evidence that
inhalation of H2 during the 10-week experimental period
decreased the body weight, BMI and abdominal fat index in a
dose-dependent manner in rats fed with HFFD, demonstrating the
anti-obesity effect of H2, particularly at a high
concentration. A previous clinical study demonstrated that the oral
administration of H2-generating minerals can
significantly reduce body fat percentage and arm fat index in
middle-aged overweight women, which is indicative of the beneficial
effects of H2 in the management of body composition in
obesity (44). The present study
confirmed the effects of H2 on visceral adipose depots
and highlighted the beneficial effects of H2 against
MS.
After challenging rats with a glucose load, both low
and high concentrations of H2 could decrease the AUC
values for OGTT. This observation is suggestive of the increase in
glucose disposal, consistent with the results of previous findings
that H2-rich saline improved glucose tolerance in a
high-fat and high-sugar diet rat model after a single injection of
streptozotocin (45). The results
of the present study are suggestive of the increase in insulin
sensitivity after treatment with H2 although no
significant difference was observed between 4 and 67% H2
inhalation. Therefore, different administration strategies and
dosages of H2 play a positive role in the improvement of
glucose tolerance. Plasma insulin level was also measured but no
significant difference was observed (data not shown), which may due
to limitations of the current animal model.
The liver is the most important tissue involved in
the regulation of glucose and lipid metabolism (5). NAFLD is the most frequent liver
disease commonly associated with MS (7). Intraperitoneal administration of
H2-rich saline can improve NAFLD in rats (45). The present study investigated the
effects of H2 inhalation on NAFLD in MS rats. The
consumption of HFFD can lead to hepatic fat accumulation and liver
dysfunctions (46), as is evident
from the leakage of cellular enzymes such as ALT, AST and LDH
(47). In the present study, the
treatment with H2 in HFFD-fed rats could reduce the
plasma levels of ALT, AST and LDH, although the differences were
not significant. The statistical insignificance may be related to
the insufficient number of animals in each group and the relatively
short experimental period. It demonstrated better effects for
plasma ALT and AST levels after low H2 treatment. The
results suggested that inhalation of H2, especially at
low concentrations, could attenuate hepatic necrosis by maintaining
hepatocyte integrity. The mechanism underlying the enhanced
effectiveness of low concentration of H2 requires
further investigation.
Alterations in hepatic lipids are important
pathophysiological hallmarks of fatty liver disease (12). In the present study, histological
evaluation revealed the increase in fat vacuoles in the liver of
rats fed with HFFD and this condition improved following
H2 treatment in a dose-dependent manner. Rats fed with
HFFD demonstrated a marked increase in all cholesterol species in
the liver compared with those fed with the control diet. This
observation is in line with a previously reported study, wherein
the liver tissues from high fat, high cholesterol, cholate diet-fed
mice demonstrated a significant increase in free cholesterol and
different CE levels (48).
Multiple defects in production, secretion and clearance of lipids
in patients with NAFLD result in the accumulation of triglycerides
in hepatocytes (12). A previous
study reported that TG species are notably upregulated in the fatty
liver of a genetically obese insulin-resistant ob/ob mouse model
(49). In the present study, the
liver from HFFD-fed rats demonstrated a marked increase in
different triglyceride levels. Some triglycerides may serve as more
specific biomarkers than total triglycerides. In obesity, the
increase in triglycerides containing more saturated fatty acid
moieties (e.g., TG 16:0/16:0/16:0) compared with those containing
more unsaturated fatty acids (e.g., TG 18:2/18:2/18:2) in the
plasma is associated with an increase in BMI (50). Individual species could have
distinct roles in the progression of NAFLD. Tu et al
(48) reported a significant
decrease in the levels of triglycerides with higher degrees of
saturation (0–3 double bonds) compared with those with higher
degrees of unsaturation (>3 double bonds) in the liver of mice
fed with a high fat, high cholesterol, cholate diet. In the present
study, different triglycerides demonstrated varied levels of
increment in HFFD group compared with those observed in the Con
group. The increase in triglycerides with less unsaturated fatty
acids was higher compared with those with polyunsaturated fatty
acids. It is known that high-fat diet will increase saturated fatty
acids such as palmitic acid and n-6 unsaturated fatty acids such as
arachidonic and then promote the inflammatory and oxidative
responses (51,52). In the present study, H2
treatment may alleviate the progress of NAFLD via changing the
composition of fatty acids in liver lipids.
In a previous study, drinking H2-rich
water was shown to slightly decrease the levels of hepatic
cholesterol and triglycerides without any statistical significance
in a methionine-choline-deficient diet-induced nonalcoholic
steatohepatitis mouse model (53).
The present study revealed the dose-dependent effect of
H2 inhalation on the alleviation of liver lipid
accumulation and 66% H2 inhalation was found to
significantly decrease hepatic cholesterol and triglycerides. Thus,
inhalation of high concentrations of H2 may be a better
choice for reducing liver lipid accumulation.
A previous study on human hepatoma HepG2 cells
revealed that the mechanism underlying the effects of H2
gas on lipid metabolism disorders may involve the modulation of
signal transduction pathways such as the c-Jun N-terminal kinase
pathway (54). Sterol regulatory
element-binding proteins are a family of regulated transcription
factors that stimulate lipid synthesis in the liver (55). SREBP-1c plays a unique role
in the expression of the genes involved in hepatic triglyceride
synthesis and may contribute to the pathogenesis of NAFLD (48). SREBP-1c in normal livers is
low, but it shows an increase in liver steatosis and NAFLD
(56). Recent findings have
demonstrated that some agents can relieve metabolic disorders in
the liver via SREBP-1c downregulation, PPAR-α
upregulation and NF-κB inactivation (57). In the present study,
SREBP-1c mRNA increased in HFFD-fed rats, but decreased in
H2 inhalation groups, which indicated the lipogenesis
was inhibited. PPAR-α is a key transcriptional regulator of
fatty acid oxidation systems in the liver and is usually used
together with SREBP-1c to estimate hepatic lipid homeostasis
(57). Changes in the expression
of SREBP-1c in the present study may be associated with
PPAR-α expression (58).
Meanwhile, the mRNA expression of fatty acid synthase (FAS)
was also examined, but no significant difference was achieved (data
not shown). Further lipid synthesis- and lipolysis-related genes
warrant further study.
In conclusion, the findings of the present study
emphasized that H2 inhalation at both low and high
concentrations could ameliorate the physical, metabolic and hepatic
disorders in MS rats induced by HFFD. It reported a dosage effect
of H2 on most indicators, except ALT, AST and LDH, and
the lower concentration of H2 exerted better effects.
The potential mechanism was inferred as the reduced synthesis of
fatty acids and lipid accumulation in the liver. The results of the
present study provided the basic data for clinical trials and
H2 inhalation may be considered as a novel adjuvant for
clinical treatment of NAFLD. However, the present study also has
some limitations such as the lack of statistical significance for
some factors and lack of detection of more genes associated with
lipid metabolism. Further investigation is required to clarify the
underlying mechanism and provide the basis for the selection of the
best dose and concentration.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81770855), Taishan
Scholars Program of Shandong Province (grant no. ts201511057) and
the Academic promotion programme of Shandong First Medical
University (grant nos. 2019QL010 and 2019PT009).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
SQ was responsible for funding acquisition.
Experiments were performed by BL, JX, MZ, MW, TM, MZ and QG. BL, JX
and SQ conceived the methodology. BL and JX wrote the original
draft of the manuscript and further writing, review and editing was
by SQ. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The ethics committee of the Shandong First Medical
University and Shandong Academy of Medical Sciences approved and
supervised the research proposal (approval no. 2017049).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kapravelou G, Martinez R, Andrade AM,
Nebot E, Camiletti-Moiron D, Aparicio VA, Lopez-Jurado M, Aranda P,
Arrebola F, Fernandez-Segura E, et al: Aerobic interval exercise
improves parameters of nonalcoholic fatty liver disease (NAFLD) and
other alterations of metabolic syndrome in obese Zucker rats. Appl
Physiol Nutr Metab. 40:2860–1252. 2015. View Article : Google Scholar
|
|
2
|
Eckel RH, Grundy SM and Zimmet PZ: The
metabolic syndrome. Lancet. 365:1415–1428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eckel RH, Alberti KG, Grundy SM and Zimmet
PZ: The metabolic syndrome. Lancet. 375:181–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaur J: A comprehensive review on
metabolic syndrome. Cardiol Res Pract. 2014:9431622014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bugianesi E, Leone N, Vanni E, Marchesini
G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L,
Salizzoni M and Rizzetto M: Expanding the natural history of
nonalcoholic steatohepatitis: From cryptogenic cirrhosis to
hepatocellular carcinoma. Gastroenterology. 123:134–140. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marchesini G, Bugianesi E, Forlani G,
Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N,
Melchionda N and Rizzetto M: Nonalcoholic fatty liver,
steatohepatitis, and the metabolic syndrome. Hepatology.
37:917–923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lonardo A, Ballestri S, Marchesini G,
Angulo P and Loria P: Nonalcoholic fatty liver disease: A precursor
of the metabolic syndrome. Dig Liver Dis. 47:181–190. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Younossi ZM, Stepanova M, Afendy M, Fang
Y, Younossi Y, Mir H and Srishord M: Changes in the prevalence of
the most common causes of chronic liver diseases in the United
States from 1988 to 2008. Clin Gastroenterol Hepatol. 9:524–530.e1.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanyal AJ, Campbell-Sargent C, Mirshahi F,
Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML and Clore
JN: Nonalcoholic steatohepatitis: association of insulin resistance
and mitochondrial abnormalities. Gastroenterology. 120:1183–1192.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Browning JD and Horton JD: Molecular
mediators of hepatic steatosis and liver injury. J Clin Invest.
114:147–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang A, Desai A, Hamilton G, Wolfson T,
Gamst A, Lam J, Clark L, Hooker J, Chavez T, Ang BD, et al:
Accuracy of MR imaging-estimated proton density fat fraction for
classification of dichotomized histologic steatosis grades in
nonalcoholic fatty liver disease. Radiology. 274:416–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanyal AJ and Pacana T: A lipidomic
readout of disease progression in a diet-induced mouse model of
nonalcoholic fatty liver disease. Trans Am Clin Climatol Assoc.
126:271–288. 2015.PubMed/NCBI
|
|
14
|
Tsatsoulis A, Mantzaris MD, Bellou S and
Andrikoula M: Insulin resistance: An adaptive mechanism becomes
maladaptive in the current environment-an evolutionary perspective.
Metabolism. 62:622–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valenzuela R and Videla LA: The importance
of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in
development of non-alcoholic fatty liver associated with obesity.
Food Funct. 2:644–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim D, Touros A and Kim WR: Nonalcoholic
fatty liver disease and metabolic syndrome. Clin Liver Dis.
22:133–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ: The diagnosis and
management of non-alcoholic fatty liver disease: Practice guideline
by the American association for the study of liver diseases,
American college of gastroenterology, and the american
gastroenterological association. Hepatology. 55:2005–2023. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hickman I and Macdonald G: Is vitamin E
beneficial in chronic liver disease? Hepatology. 46:288–290. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanyal AJ, Mofrad PS, Contos MJ, Sargeant
C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J and
Mills AS: A pilot study of vitamin E versus vitamin E and
pioglitazone for the treatment of nonalcoholic steatohepatitis.
Clin Gastroenterol Hepatol. 2:1107–1115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American association for the study of
liver diseases. Hepatology. 67:328–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Juurlink DN, Gomes T, Lipscombe LL, Austin
PC, Hux JE and Mamdani MM: Adverse cardiovascular events during
treatment with pioglitazone and rosiglitazone: Population based
cohort study. BMJ. 339:b29422009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hossain N, Kanwar P and Mohanty SR: A
comprehensive updated review of pharmaceutical and
nonpharmaceutical treatment for NAFLD. Gastroenterol Res Pract.
2016:71092702016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gee PT: Unleashing the untold and
misunderstood observations on vitamin E. Genes Nutr. 6:5–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bjelakovic G, Nikolova D, Gluud LL,
Simonetti RG and Gluud C: Mortality in randomized trials of
antioxidant supplements for primary and secondary prevention:
Systematic review and meta-analysis. JAMA. 297:842–857. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicolson GL, de Mattos GF, Settineri R,
Costa C, Ellithorpe R, Rosenblatt S, La Valle J, Jimenez A and Ohta
S: Clinical Effects of Hydrogen Administration: From Animal and
Human Diseases to Exercise Medicine. Int J Clin Med. 7:322016.
View Article : Google Scholar
|
|
26
|
Dole M, Wilson FR and Fife WP: Hyperbaric
hydrogen therapy: A possible treatment for cancer. Science.
190:152–154. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song G, Li M, Sang H, Zhang L, Li X, Yao
S, Yu Y, Zong C, Xue Y and Qin S: Hydrogen-rich water decreases
serum LDL-cholesterol levels and improves HDL function in patients
with potential metabolic syndrome. J Lipid Res. 54:1884–1893. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song G, Tian H, Qin S, Sun X, Yao S, Zong
C, Luo Y, Liu J, Yu Y, Sang H and Wang X: Hydrogen decreases
athero-susceptibility in apolipoprotein B-containing lipoproteins
and aorta of apolipoprotein E knockout mice. Atherosclerosis.
221:55–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zong C, Song G, Yao S, Li L, Yu Y, Feng L,
Guo S, Luo T and Qin S: Administration of hydrogen-saturated saline
decreases plasma low-density lipoprotein cholesterol levels and
improves high-density lipoprotein function in high-fat diet-fed
hamsters. Metabolism. 61:794–800. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song G, Zong C, Zhang Z, Yu Y, Yao S, Jiao
P, Tian H, Zhai L, Zhao H, Tian S, et al: Molecular hydrogen
stabilizes atherosclerotic plaque in low-density lipoprotein
receptor-knockout mice. Free Radic Biol Med. 87:58–68. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi K, Kim H, Hee S, Jin S and Yi H:
Neuroprotective effects of hydrogen inhalation in an experimental
rat intracerebral hemorrhage model. Brain Res Bull. 142:122–128.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Zhao C, Wu S, Xiao G, Zhuge X, Lei
P and Xie K: Hydrogen gas treatment improves the neurological
outcome after traumatic brain injury via increasing miR-21
expression. Shock. 50:308–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou H, Fu Z, Wei Y, Liu J, Cui X, Yang W,
Ding W, Pan P and Li W: Hydrogen inhalation decreases lung graft
injury in brain-dead donor rats. J Heart Lung Transpl. 32:251–258.
2013. View Article : Google Scholar
|
|
36
|
Chen O, Cao Z, Li H, Ye Z, Zhang R, Zhang
N, Huang J, Zhang T, Wang L, Han L, et al: High-concentration
hydrogen protects mouse heart against ischemia/reperfusion injury
through activation of the PI3K/Akt1 pathway. Sci Rep. 7:148712017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Chen O, Ye Z, Zhang R, Hu H, Zhang
N, Huang J, Liu W and Sun X: Inhalation of high concentrations of
hydrogen ameliorates liver ischemia/reperfusion injury through A2A
receptor mediated PI3K-Akt pathway. Biochem Pharmacol. 130:83–92.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He Y, Shi JZ, Zhang RJ, Zhai DX, Zhang D,
Yu CQ and Liu YH: Effects of hydrogen gas inhalation on
endometriosis in rats. Reprod Sci. 24:324–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peng Z, Chen W, Wang L, Ye Z, Gao S, Sun X
and Guo Z: Inhalation of hydrogen gas ameliorates
glyoxylate-induced calcium oxalate deposition and renal oxidative
stress in mice. Int J Clin Exp Pathol. 8:2680–2689. 2015.PubMed/NCBI
|
|
40
|
Chen JB, Kong XF, Lv YY, Qin SC, Sun XJ,
Mu F, Lu TY and Xu KC: ‘Real world survey’ of hydrogen-controlled
cancer: A follow-up report of 82 advanced cancer patients. Med Gas
Res. 9:1152019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Panchal SK and Brown L: Rodent models for
metabolic syndrome research. J Biomed Biotechnol.
2011:3519822010.PubMed/NCBI
|
|
42
|
Nakao A, Toyoda Y, Sharma P, Evans M and
Guthrie N: Effectiveness of hydrogen rich water on antioxidant
status of subjects with potential metabolic syndrome-an open label
pilot study. J Clin Biochem Nutr. 46:140–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hashimoto M, Katakura M, Nabika T, Tanabe
Y, Hossain S, Tsuchikura S and Shido O: Effects of hydrogen-rich
water on abnormalities in a SHR Cg-Lepr cp/NDmcr rat-a metabolic
syndrome rat model. Med Gas Res. 1:262011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Korovljev D, Trivic T, Drid P and Ostojic
S: Molecular hydrogen affects body composition, metabolic profiles,
and mitochondrial function in middle-aged overweight women. Ir J
Med Sci. 187:85–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhai X, Chen X, Lu J, Zhang Y, Sun X,
Huang Q and Wang Q: Hydrogen-rich saline improves non-alcoholic
fatty liver disease by alleviating oxidative stress and activating
hepatic PPARα and PPARγ. Mol Med Rep. 15:1305–1312. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Panchal SK, Poudyal H, Iyer A, Nazer R,
Alam MA, Diwan V, Kauter K, Sernia C, Campbell F, Ward L, et al:
High-carbohydrate high-fat diet-induced metabolic syndrome and
cardiovascular remodeling in rats. J Cardiovasc Pharmacol.
57:611–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang O, Liu J, Cheng Q, Guo X, Wang Y,
Zhao L, Zhou F and Ji B: Effects of ferulic acid and γ-oryzanol on
high-fat and high-fructose diet-induced metabolic syndrome in rats.
PLoS One. 10:e01181352015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tu LN, Showalter MR, Cajka T, Fan S,
Pillai VV, Fiehn O and Selvaraj V: Metabolomic characteristics of
cholesterol-induced non-obese nonalcoholic fatty liver disease in
mice. Sci Rep. 7:61202017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yetukuri L, Katajamaa M, Medina-Gomez G,
Seppänen-Laakso T, Vidal-Puig A and Orešič M: Bioinformatics
strategies for lipidomics analysis: characterization of obesity
related hepatic steatosis. BMC Syst Biol. 1:122007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sanders F, McNally B and Griffin JL: Blood
triacylglycerols: A lipidomic window on diet and disease. Biochem
Soc Tran. 44:638–644. 2016. View Article : Google Scholar
|
|
51
|
Hernández-Rodas MC, Valenzuela R,
Echeverría F, Rincón-Cervera MA, Espinosa A, Illesca P, Muñoz P,
Corbari A, Romero N, Gonzalez-Mañan D and Videla LA:
Supplementation with docosahexaenoic acid and extra virgin olive
oil prevents liver steatosis induced by a high-fFat diet in mice
through PPAR-α and Nrf2 upregulation with concomitant SREBP-1c and
NF-kB downregulation. Mol Nutr Food Res. 61:2017. View Article : Google Scholar
|
|
52
|
Echeverría F, Valenzuela R, Espinosa A,
Bustamante A, Álvarez D, Gonzalez-Mañan D, Ortiz M, Soto-Alarcon SA
and Videla LA: Reduction of high-fat diet-induced liver
proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol
supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J Nutr
Biochem. 63:35–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kawai D, Takaki A, Nakatsuka A, Wada J,
Tamaki N, Yasunaka T, Koike K, Tsuzaki R, Matsumoto K, Miyake Y, et
al: Hydrogen-rich water prevents progression of nonalcoholic
steatohepatitis and accompanying hepatocarcinogenesis in mice.
Hepatology. 56:912–921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Iio A and Ito M, Itoh T, Terazawa R,
Fujita Y, Nozawa Y, Ohsawa I, Ohno K and Ito M: Molecular hydrogen
attenuates fatty acid uptake and lipid accumulation through
downregulating CD36 expression in HepG2 cells. Med Gas Res.
3:62013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ahmed MH and Byrne CD: Modulation of
sterol regulatory element binding proteins (SREBPs) as potential
treatments for non-alcoholic fatty liver disease (NAFLD). Drug
Discov Today. 12:740–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Aragno M, Tomasinelli CE, Vercellinatto I,
Catalano MG, Collino M, Fantozzi R, Danni O and Boccuzzi G:
SREBP-1c in nonalcoholic fatty liver disease induced by
Western-type high-fat diet plus fructose in rats. Free Radical Bio
Med. 47:1067–1074. 2009. View Article : Google Scholar
|
|
57
|
Valenzuela R and Videla LA: Impact of the
co-administration of N-3 fatty acids and olive oil components in
preclinical nonalcoholic fatty liver disease models: A mechanistic
view. Nutrients. 12:4992020. View Article : Google Scholar
|
|
58
|
Paquette A, Wang D, Jankowski M, Gutkowska
J and Lavoie JM: Effects of ovariectomy on PPAR alpha, SREBP-1c,
and SCD-1 gene expression in the rat liver. Menopause.
15:1169–1175. 2008. View Article : Google Scholar : PubMed/NCBI
|


















