Introduction
Cervical cancer (CC) frequently occurs in women over
the age of 15 years and is the second leading cause of
cancer-associated mortality (1).
Although the morbidity and mortality rates of CC have been
decreasing over the past 30 years, the 5-year survival rate of
patients with CC at an advanced stage remains <40% (2,3). At
present, the treatments for patients with CC primarily include
surgery, radiotherapy and chemotherapy (4). Despite their wide application in
clinical practice, the outcome remains unsatisfactory, which is
mainly due to the metastasis of CC (5). Therefore, there is an urgent need to
search for effective therapeutic strategies by exploring the
mechanisms underlying CC.
MicroRNAs (miRNAs/miRs) are a class of small RNA
molecules 21–25 nucleotides in length, which serve important roles
in several biological processes, such as cell proliferation,
migration, invasion and tumorigenesis via regulating specific
target genes (6–8). Abnormal dysregulation of miRNAs and
their targets has also been associated with the development of CC.
For example, miR-106a has been reported to enhance cell migration
and invasion, and increased matrix metalloproteinase expression in
CC cells via targeting TIMP metallopeptidase inhibitor 2 (TIMP2)
(9). In addition, miR-149 has been
suggested to inhibit the proliferation and promote the apoptosis of
CC cells via targeting GIT1 (10).
miR-130a has emerged as an important miRNA in the development and
progression of numerous types of malignancy, including
hepatocellular carcinoma (HCC), ovarian cancer, glioblastoma,
prostate carcinoma and CC (11).
Kong et al (12)
demonstrated that miR-130a-3p may be a therapeutic target for
breast cancer. Liu et al (13) further suggested that upregulation
of miR-130a-3p expression suppressed the migration and invasion of
HCC cells through downregulating expression of its target gene
SMAD4. However, the biological function of miR-130a-3p in CC
remains unclear.
Runt-related transcription factor 3 (RUNX3) is
located at chromosome 1p.13-p36.11, and has been demonstrated to
have an anti-tumor role in numerous types of cancer (14). Huang et al (15) revealed that RUNX3 exerted a
tumor-inhibiting effect on breast cancer via modulating estrogen
receptor-α. RUNX3 has also been reported to inhibit the
tumorigenesis of HCC by suppressing cancer stem cells in a jagged
1-mediated manner (16). RUNX3 may
also have a major role in the cellular processes of tumorigenesis
and metastasis, including epithelial-mesenchymal transition (EMT)
(17), adhesion (18), invasion (19) and apoptosis (20). Zhen et al (21) revealed that overexpression of RUNX3
suppressed the proliferation, migration and invasion of CC cells,
thus suggesting that RUNX3 may function as a tumor suppressor in
CC. However, the regulatory relationship between miR-130a-3p and
RUNX3 in CC remains unclear.
In the present study, the expression of miR-130a-3p
was evaluated in both CC tissues and cell lines. Functional
experiments were performed to analyze the regulatory role of
miR-130a-3p in CC in vitro, and in vivo using a mouse
xenograft model. Furthermore, the target genes of miR-130a-3p were
assessed with a focus on the association with RUNX3 in the CC
models. The findings of the present study highlighted miR-130a-3p
as a promising biomarker and therapeutic target for CC.
Materials and methods
Clinical specimens
A total of 50 paired CC tissues and adjacent normal
tissues (within 5 cm of the tumors) were obtained from patients
with CC (age range, 35–66 years; mean age, 46.1±4.9 years) via
surgical resection from April 2017 to July 2018 at Changle County
People's Hospital (Weifang, China). All CC tissues were confirmed
via histopathological examination. The inclusion criterion was
first-time diagnosis. The exclusion criteria included the presence
of other types of malignant tumor and patients who had received CC
treatment before admission. The clinicopathological features of the
patients were recorded, including age, tumor size, tumor grade,
Federation of Gynecology and Obstetrics (FIGO) stage (22), lymphatic metastasis and depth of
cervical invasion. Informed, written consent was obtained from all
patients, and the study received approval from the ethics committee
of Changle County People's Hospital (approval no. 2016013; Weifang,
China).
Cell lines and culture
Normal human cervical epithelial cells (Ect1/E6E7)
and CC cell lines (CaSki and SiHa) were purchased from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences.
The cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare
life Sciences) supplemented with 10% FBS (HyClone; GE Healthcare
life Sciences) in an incubator (MCO-15AC; SANYO Electric Co., Ltd.)
at 37°C and 5% CO2 with saturated humidity.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the tissues and cells
using TRIzol® Plus RNA Isolation reagents (Invitrogen;
Thermo Fisher Scientific, Inc.). RNA (1 µg) was reverse transcribed
into cDNA at 42°C for 45 min using a miScript II RT kit (Qiagen
GmbH). The qPCR reaction was performed using an ABI 7500HT Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with SYBRGreen master mix (CoWin Biosciences). The reaction
conditions were as follows: 95°C for 3 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 30 sec, and a final extension step
at 72°C for 1 min. The mRNA/miRNA expression levels were calculated
using the 2−ΔΔCq method (23). The primer sequences are listed in
Table I. U6 or β-actin was used as
the internal reference for detection of miR-130a-3p or RUNX3,
respectively.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Name of primer | Sequences |
|---|
| miR-130a-3p | Forward:
5′-GATGCTCTCAGTGCAATGTTA-3′ |
|
| Reverse:
5′-CTCTGTCTCTCGTCTTGTTGGTAT-3′ |
| U6 | Forward:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| Reverse:
5′-AACGCTTCACGAATTTGCGT-3′ |
| RUNX3 | Forward:
5′-TCTGTAAGGCCCAAAGTGGGTA-3′ |
|
| Reverse:
5′-ACCTCAGCATGACAATATGTCACAA-3′ |
| β-actin | Forward:
5′-ACACCTTCTACAATGAGCTG-3′ |
|
| Reverse:
5′-CTGCTTGCTGATCCACATCT-3′ |
Cell transfection
When SiHa or CaSki cells reached 80% confluence,
they were seeded into 6-well cell culture plates (6×105
cells/well). miR-130a-3p mimics (cat. no. B01001), miR-130a-3p
inhibitor (cat. no. B03001) and corresponding negative controls
[NCs; mimics-NC (cat. no. B04004) and inhibitor-NC (cat. no.
B04006)] were synthesized by Shanghai GenePharma Co., Ltd.
miR-130a-3p mimics or mimics-NC (20 nM) were transfected into SiHa
cells, and miR-130a-3p inhibitor or inhibitor-NC (20 nM) were
transfected into CaSki cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells without
transfection were considered as the mock group. In addition,
pcDNA3.1-RUNX3 (pcDNA3.1, carrying the RUNX3 coding sequence) and
pcDNA3.1-NC (empty vector), synthesized by Sangon Biotech Co.,
Ltd., were co-transfected in SiHa cells (20 nM) with miR-130a-3p
mimics/mimics-NC (20 nM) using Lipofectamine® 2000.
After transfection for 48 h at 37°C, the cells were collected for
follow-up experiments.
Dual-luciferase reporter gene
assay
TargetScan release 5.2 (http://www.targetscan.org) was used to predict the
presence of a binding site for miR-130-3p on the RUNX3 sequence.
Based on this prediction, the 3′-untranslated region (UTR) of RUNX3
containing wild-type (Wt) or mutated (Mut) binding sites were
synthesized by Shanghai GenePharma Co., Ltd., and were then
inserted into the PsiCHECK-2 vector (Promega Corporation) to obtain
RUNX3-Wt and RUNX3-Mut, respectively. SiHa cells (2×105
cells/well) were co-transfected with RUNX3-Mut/RUNX3-Wt and
miR-130a-3p mimics/mimics-NC (Shanghai GenePharma Co., Ltd.) using
Lipofectamine® 2000. Following 48 h of incubation at
37°C, Renilla and firefly luciferase activities were
detected using a Dual-Luciferase Reporter assay system (Promega
Corporation), according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Western blotting
Total proteins were extracted from SiHa or CaSki
cells using RIPA lysis buffer (Beyotime Institute of Biotechnology)
and quantified using a Bicinchoninic Acid Protein Assay kit (Thermo
Fisher Scientific, Inc.). The protein samples (40 µg) were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 10% gels and electroblotted onto a
polyvinylidene fluoride membrane. The membrane was blocked with 5%
skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST)
for 1 h at room temperature and incubated with anti-GAPDH (1:1,000;
cat. no. ab9485; Abcam) and anti-RUNX3 (1:1,000; cat. no. ab224641;
Abcam) antibodies at 4°C overnight. After three washes with TBST,
the membrane was incubated with a horseradish peroxidase-conjugated
goat anti-rabbit IgG secondary antibody (1:10,000; cat. no. 7074;
Cell Signaling Technology, Inc.) for 1 h at 25°C. Protein bands
were visualized using a chemiluminescent substrate kit (Invitrogen;
Thermo Fisher Scientific, Inc.) and analyzed using Gel-Pro Analyzer
software (version 4.0; Media Cybernetics, Inc.). GAPDH was used as
the internal reference.
MTT assay
Cells were seeded into 96-well plates
(6×103 cells/well, 200 µl/well) and were incubated at
37°C. At 0, 24, 48 and 72 h of culture, 20 µl MTT reagent (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added to each well. After 4 h of
incubation at 37°C, 150 µl dimethyl sulfoxide was added to
terminate the reaction. The optical density at 450 nm
(OD450) was detected using a microplate reader (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The growth curves were
drawn as a plot of the OD value over time. This experiment was
performed in triplicate and was repeated three times.
Annexin V-propidium iodide (PI) double
staining assay
To detect the early apoptosis of CC cells resulting
from the various treatments, cells (1×105) were
suspended in 500 µl binding buffer and then stained with 5 µl
Annexin V-enhanced green-fluorescent protein and 5 ml PI using the
eBioscience™ Annexin V-FITC apoptosis detection kit (Invitrogen;
Thermo Fisher Scientific, Inc.) at 25°C for 10 min in the dark.
Subsequently, apoptosis was detected using a FACScan flow cytometer
(version 2.0; BD Biosciences) and the data were analyzed using
CellQuest version 5.1 software (BD Biosciences).
Wound-healing assay
Cells were seeded into 6-well plates
(1×106/well). When cells reached 90% confluence, a
scratch was created using a 10-µl pipette tip. After 48 h of
culturing in serum-free medium, the wound gaps were observed under
an inverted light microscope (magnification, ×200) and measured
using ImageJ software (version 1.46; National Institutes of
Health). The relative migration rate was calculated as (original
gap distance-gap distance at 48 h)/original gap distance ×100 and
normalized to that of the mock group. This experiment was performed
in triplicate and was repeated three times.
Transwell invasion assay
The upper chamber of Transwell inserts was precoated
at 37°C for 30 min with Matrigel (BD Biosciences) according to the
manufacturer's instructions 1 day before the experiment. The cells
(1×106) were resuspended in serum-free medium and then
transferred onto the Matrigel-coated upper chamber, and RPMI 1640
containing 10% FBS was added to the lower chamber. After incubation
for 24 h at 37°C, the non-invaded cells in the upper chamber were
scraped off with cotton swabs, and the cells that invaded the lower
inserts were fixed with 90% ethanol and stained with Coomassie
brilliant blue at 37°C for 30 min. The stained cells were imaged
and counted in five random fields under an inverted light
microscope (magnification, ×200). The number of invading cells was
calculated by normalizing to that of the mock group. This
experiment was performed in triplicate and was repeated three
times.
Xenograft tumor mouse model
All experimental procedures with mice were performed
according to the Chinese legislation regarding research with
experimental animals. The animal study received approval from the
Ethics Committee of Changle County People's Hospital (approval no.
2016013). The healthy male BALB/c nude mice (weight, 20±2 g; age, 4
weeks; n=15) were obtained from Shanghai Experimental Animal Center
of Chinese Academy of Sciences. The animals were housed in a
sterile environment at a controlled temperature of 20°C and 40%
relative humidity, under a 12-h light/dark cycle with free access
to food and water. Subsequently, the mice were randomly divided
into three groups: Mock, mimics-NC and miR-130a-3p mimics
(n=5/group). The mock and transfected SiHa cells at the logarithmic
growth phase (1×107 cells/nude mice, 200 µl) were
resuspended in PBS and injected into the intradermal left axilla of
the mice. The longest diameter (L) and the shortest diameter (W) of
the xenograft tumors were measured with a Vernier caliper every 7
days after injection, and the tumor volume was calculated using the
following formula: V=L × W2/2. At the end of week 4, the
mice were anesthetized by an intraperitoneal injection of 50 mg/kg
pentobarbital sodium and were then sacrificed by cervical
dislocation. The xenograft tumors were dissected completely and
weighed.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
statistical software (IBM Corp.) and Prism v7.01 (GraphPad
Software, Inc.). Data are presented as the mean ± standard
deviation. A paired Student's t-test was used to compare
differences between two groups. One-way analysis of variance
followed by Tukey's post-hoc test was applied for analyzing more
than two groups. Differences in clinicopathological features
between patients with CC with high or low expression of miR-130a-3p
were determined by χ2 test (group size >5) or
Fisher's exact test (group size ≤5). Pearson's correlation analysis
was used to determine the correlation between the expression levels
of miR-130a-3p and RUNX3 in CC tissues. The diagnostic analysis was
performed through receiver operating characteristic (ROC) curve
analysis with healthy controls as true negative cases and patients
with CC as true positive cases. All experiments were conducted in
triplicate, with ≥3 independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-130a-3p expression is upregulated
in CC
The expression levels of miR-130a-3p were
significantly higher in the tumor tissues compared with in the
normal tissues of patients with CC (P<0.0001; Fig. 1A). Patients with CC were then
divided into two groups (high and low expression) according to the
median miR-130a-3p expression levels (3.901). As indicated in
Table II, high expression of
miR-130a-3p in patients with CC exhibited strong associations with
FIGO stage (P=0.003), lymph node metastasis (P=0.0009) and depth of
cervical invasion (P=0.0244), but not with age, tumor size or tumor
grade. The area under the ROC curve value for discriminating
between CC and normal tissues was 0.938 (95% confidence interval,
0.888-0.988), and the sensitivity and specificity were 82.5 and
92.5%, respectively (Fig. 1B).
 | Table II.Association between miR-130a-3p
expression and clinicopathological features of patients with
cervical cancer. |
Table II.
Association between miR-130a-3p
expression and clinicopathological features of patients with
cervical cancer.
| Clinicopathological
features | Total no. of
cases | High miR-130a-3p
(n=17) | Low miR-130a-3p
(n=23) | P-value |
|---|
| Age (years) |
|
|
| 0.45 |
|
<55 | 23 | 9 | 14 |
|
|
≥55 | 17 | 8 | 9 |
|
| Tumor size
(cm) |
|
|
| 0.08 |
|
<4 | 18 | 5 | 13 |
|
| ≥4 | 22 | 12 | 10 |
|
| Tumor grade |
|
|
| 0.21 |
|
G1/G2 | 20 | 7 | 13 |
|
| G3 | 20 | 10 | 10 |
|
| FIGO stage |
|
|
| 0.003a |
|
I/II | 23 | 4 | 19 |
|
|
III/IV | 17 | 13 | 4 |
|
| Lymph node
metastasis |
|
|
| 0.0009a |
| No | 24 | 5 | 19 |
|
|
Yes | 16 | 12 | 4 |
|
| Depth of cervical
invasion (mm) |
|
|
| 0.0244a |
|
<2/3 | 25 | 7 | 17 |
|
|
≥2/3 | 15 | 10 | 6 |
|
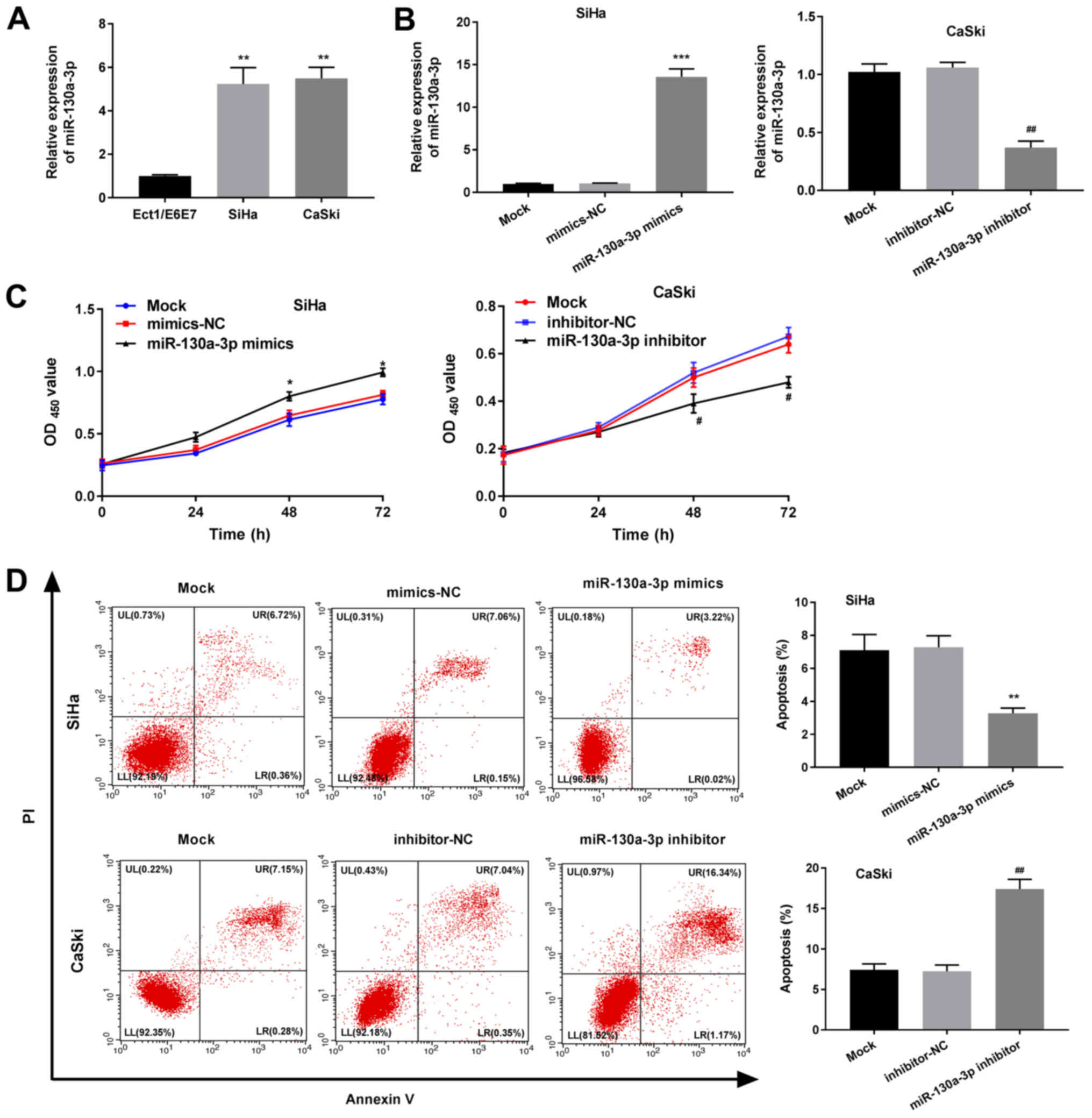
miR-130a-3p enhances the proliferation
and suppresses the apoptosis of CC cells
As revealed in Fig.
2A, the expression levels of miR-130-3p were significantly
higher in CaSki and SiHa cells than in normal cervical epithelial
cells (Ect1/E6E7) (P<0.01). RT-qPCR further revealed that the
expression levels of miR-130-3p were significantly elevated in the
miR-130a-3p mimics group compared with in the mimics-NC group
(P<0.001; Fig. 2B). The
proliferation detected via MTT analysis was also significantly
increased in the miR-130a-3p mimics group compared with that in the
mimics-NC group (P<0.05; Fig.
2C). As demonstrated in Fig.
2D, apoptosis of the miR-130a-3p mimics group was significantly
reduced compared with that of the mimics-NC group (P<0.01). All
of the effects observed in the miR-130-3p mimics group were the
opposite of those observed in the miR-130a-3p inhibitor group.
miR-130a-3p promotes the migration and
invasion abilities of CC cells
As demonstrated in Fig.
3A, the relative migration rate of cells in the miR-130a-3p
mimics group was significantly enhanced compared with that of the
cells in the mimics-NC group (P<0.01). The relative number of
invasive cells was also significantly enhanced in the miR-130a-3p
mimics group compared with that in the mimics-NC group (P<0.01;
Fig. 3B). The opposite effects
were observed in the miR-130-3p inhibitor group. Transfection with
mimics-NC or inhibitor-NC did not affect the relative migration
rate or relative number of invasive cells.
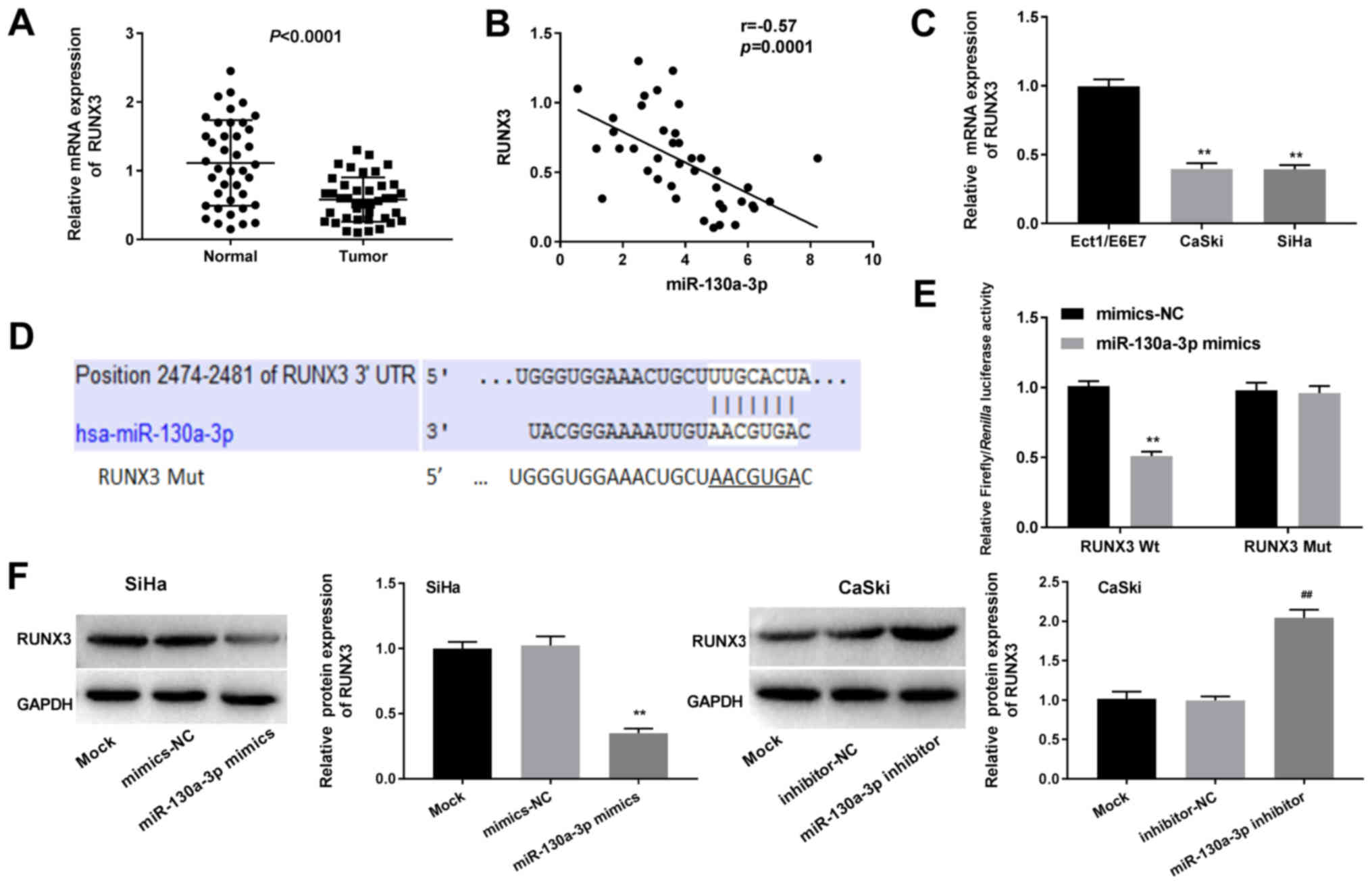
RUNX3 is a target gene of
miR-130a-3p
As shown in Fig.
4A, the relative mRNA expression levels of RUNX3 were reduced
in CC tissues compared with in normal tissues (P<0.0001).
Pearson's correlation analysis revealed a negative correlation
between the expression levels of miR-130a-3p and RUNX3 in CC
tissues (r=−0.57; P=0.0001; Fig.
4B). Consistently, compared with in normal Ect1/E6E7 cells, the
mRNA expression levels of RUNX3 were significantly reduced in CaSki
and SiHa CC cells (P<0.01; Fig.
4C).
 | Figure 4.miR-130a-3p directly targets RUNX3.
(A) Relative mRNA expression levels of RUNX3 in CC tissues, as
determined by RT-qPCR. (B) Pearson's correlation analysis was used
to detect the correlation between the expression levels of
miR-130a-3p and RUNX3. (C) Relative mRNA expression levels of RUNX3
in CC cell lines, as determined by RT-qPCR. **P<0.01 vs.
Ect1/E6E7. (D) TargetScan was used to predict the binding site
between RUNX3 and miR-130a-3p. (E) Dual-luciferase reporter gene
assays were used to detect the luciferase activity of SiHa cells
co-transfected with miR-130a-3p mimics or mimics-NC, and RUNX3-Wt
or RUNX3-Mut. **P<0.01 vs. mimics-NC. (F) Relative protein
expression of RUNX3, as determined via western blotting.
##P<0.01 vs. inhibitor-NC; **P<0.01 vs. mimics-NC.
RUNX3, Runt-related transcription factor 3; CC, cervical cancer;
miR, microRNA; RT-qPCR, reverse transcription-quantitative PCR; NC,
negative control; Wt, wild-type; Mut, mutated. |
TargetScan predicted a binding site for miR-130a-3p
at the 3′-UTR of RUNX3 (Fig. 4D).
The miR-130a-3p mimics significantly decreased the luciferase
activity of RUNX3-Wt compared with the mimics-NC, but did not
influence the luciferase activity of RUNX3-Mut (P<0.01; Fig. 4E). As indicated in Fig. 4F, the relative protein expression
of RUNX3 was significantly downregulated by miR-130a-3p
upregulation and upregulated by miR-130a-3p downregulation compared
with the mimics-NC groups (P<0.01). Transfection with mimics-NC
or inhibitor-NC did not influence the luciferase activity or
relative protein expression of RUNX3. These findings suggested that
RUNX3 may represent a target gene of miR-130a-3p.
RUNX3 eliminates the tumor-promoting
effects of miR-130a-3p on CC cells
Western blot analysis revealed that the relative
protein expression of RUNX3 was significantly enhanced in the
pcDNA3.1-RUNX3 group compared with that in the pcDNA3.1-NC group
(P<0.01). However, transfection with pcDNA3.1-NC did not
influence the relative protein expression of RUNX3 (Fig. 5A). In addition, the
OD450 value, relative migration rate and relative number
of invasive cells were significantly increased in the pcDNA3.1-NC +
miR-130a-3p mimics group, and were significantly decreased in the
RUNX3 + mimics-NC group compared with those in the pcDNA3.1-NC +
mimics-NC group (P<0.05; Fig. 5B, D
and E). However, the overexpression of RUNX3 reversed the
promoting effects of miR-130a-3p mimics on the proliferation,
migration and invasion of SiHa cells. The results of the double
staining assay for apoptosis revealed the opposite pattern to those
of the MTT assay (P<0.05; Fig.
5C).
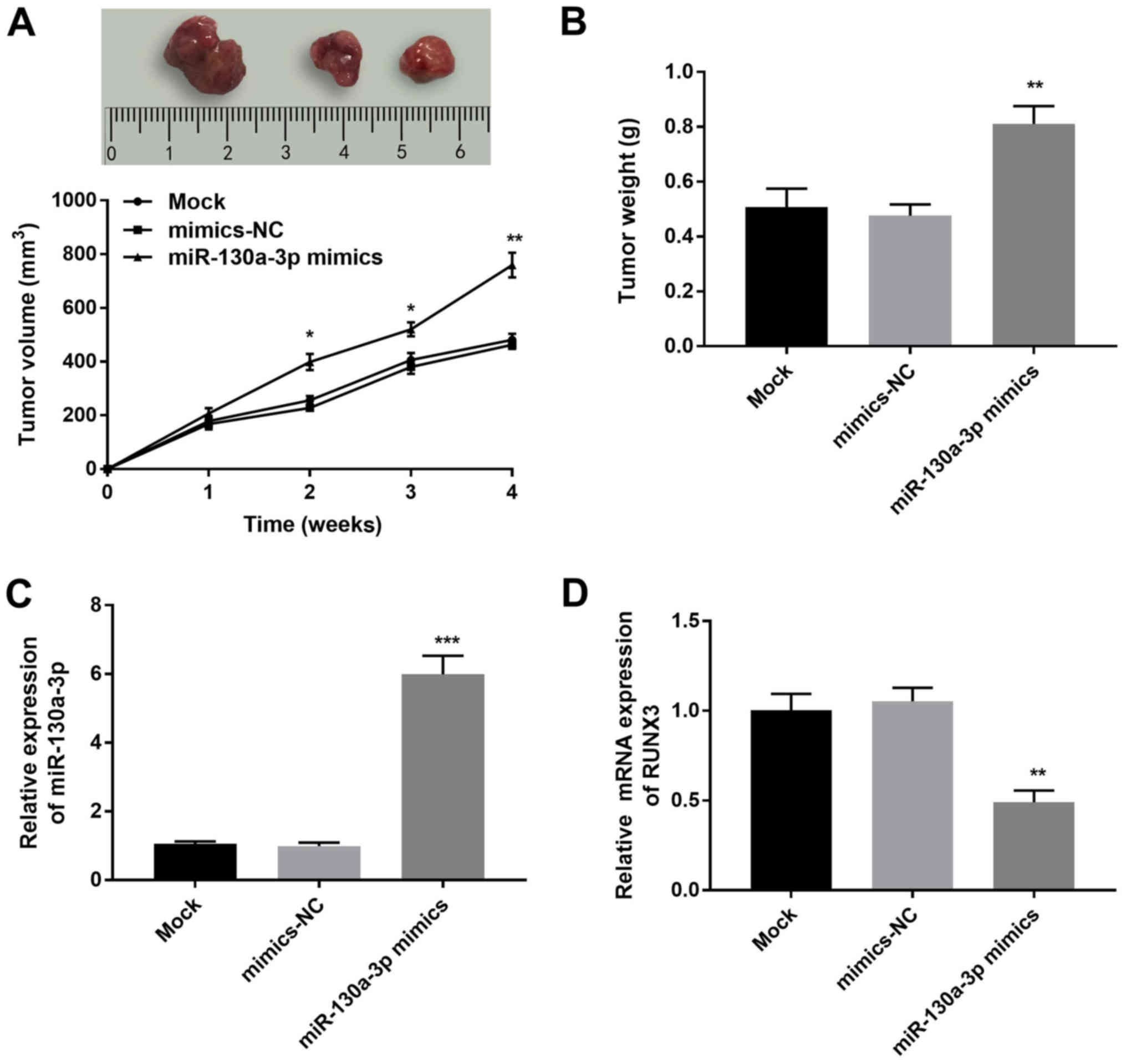
miR-130a-3p promotes tumor growth in
nude mice
To confirm the effect of miR-130a-3p on CC
tumorigenesis, SiHa cells transfected with mimics-NC or miR-130a-3p
mimics were injected into the intradermal left axilla of nude mice.
As shown in Fig. 6A and B, the
tumor volume and weight were significantly increased in the
miR-130a-3p mimics group compared with in the mimics-NC group
(P<0.05). Moreover, the relative expression levels of
miR-130a-3p in tumor xenograft tissues were elevated in the
miR-130a-3p mimics group in comparison with those in the mimics-NC
group (P<0.001; Fig. 6C),
whereas the relative mRNA expression levels of RUNX3 were
significantly reduced in the miR-130a-3p mimics group in comparison
with those in the mimics-NC group (P<0.01; Fig. 6D).
Discussion
miRNAs function as oncogenes or anti-tumor genes and
serve critical roles in tumor progression (10). Numerous oncomiRs have been
discovered in CC to date, including miR-21 (24), miR-10a (25) and miR-133b (26). In the present study, it was
revealed that the expression levels of miR-130a-3p were
significantly increased in CC tissues compared with in adjacent
normal tissues, indicating that miR-130a-3p may be an oncogene in
CC. Abnormal expression of miR-130a has been reported to be closely
associated with tumor progression. Chen et al (27) reported that upregulation of
miR-130a expression was associated with the TNM stage and lymph
node metastasis of colorectal cancer. Yin et al (28) demonstrated that upregulation of
miR-130a was significantly associated with lymph node metastasis
and an advanced clinical stage of CC. Consistent with these
previous studies, the present study demonstrated that high
expression of miR-130a-3p was significantly associated with the
FIGO stage, lymph node metastasis and depth of cervical invasion in
patients with CC. These findings further support that miR-130a-3p
may act as a tumor promoter in CC. It was therefore hypothesized
that the upregulation of miR-130a-3p expression may have clinical
value in the prediction of CC. This hypothesis was supported by the
ROC curve revealing the diagnostic value of miR-130a-3p for CC. The
current findings demonstrated that miR-130a-3p may be a potential
biomarker for the diagnosis and prognosis of CC.
To further confirm the oncogenic effect of
miR-130a-3p on CC, a series of functional experiments were
performed to identify the regulatory role of miR-130a-3p on CC
cells. As expected, it was demonstrated that overexpression of
miR-130a-3p enhanced the proliferation, migration and invasiveness,
and inhibited the apoptosis of CC cells. A previous study
demonstrated that the miR-130 family (miR-130b, miR-301a and
miR-301b) promoted the migration and invasion of bladder cancer
cells via negative regulation of phosphatase and tensin homolog
(PTEN) (18). Jiang et al
(29) revealed that miR-130a
promoted the proliferation, migration and invasion of gastric
cancer cells via targeting RUNX3, and Yin et al (28) verified that miR-130a enhanced the
proliferation and invasion of CC cells via modulating TIMP2. In
accordance with the results of these previous studies, in the
present study it was concluded that the upregulation of miR-130a-3p
contributed to the progression of CC. Therefore, silencing
miR-130a-3p may represent a potential therapeutic strategy for
CC.
miRNAs exert their functions via regulating the
expression of corresponding target genes. For example, miR-130a has
been reported to enhance the migration, invasion and EMT of
osteosarcoma cells through suppressing PTEN (30). miR-130a may also promote cell
proliferation, migration and invasion via targeting RUNX3 in
gastric cancer (29). In the
present study, RUNX3 was identified as a target gene of
miR-130a-3p. Supporting this result, a negative correlation was
observed between the expression levels of miR-130a-3p and RUNX3 in
CC tissues. RUNX3 is mapped on human chromosome 1p36, and has been
reported to serve as an anti-tumor gene in lung (31), bladder (32) and gastric cancer (33). Li et al (21) revealed that knockdown of RUNX3
promoted the proliferation, migration and invasiveness of CC cells.
Similarly, Gao et al (34)
reported that upregulation of RUNX3 inhibited the proliferation of
CC cells. Consistent with these previous studies, it was
demonstrated in the present study that the expression levels of
RUNX3 were significantly decreased in CC cell lines. Moreover,
RUNX3 overexpression eliminated the tumor-promoting effects of
miR-130a-3p on the proliferation, migration, invasiveness and
apoptosis of CC cells. These results indicated that miR-130a-3p may
promote the tumorigenesis of CC through targeting RUNX3. The
present in vivo assay further confirmed this hypothesis.
RUNX3 can also be regulated by promoter methylation
(35). Aberrant methylation of CpG
islands of RUNX3 has been demonstrated to reduce the expression
levels of RUNX3 in a variety of human malignancies (36–38).
However, in the present study, the methylation status of RUNX3 in
CC tissues, cells or xenografts was not analyzed; this will be
considered in our future work.
In conclusion, miR-130a-3p was highly expressed in
CC tissues and cells, and exhibited high diagnostic value.
miR-130a-3p promoted the proliferation, migration and invasiveness,
and inhibited the apoptosis of CC cells through targeting RUNX3.
The results of the present study proposed miR-130a-3p as a
promising therapeutic target for the treatment of CC and provided
new research directions to further understand the pathogenesis of
CC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW made substantial contributions to the conception
and design of the study; and MW, XW and WL acquired, analyzed and
interpreted the data, and drafted and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed, written consent was obtained from all
patients, and the study received approval from the Ethics Committee
of Changle County People's Hospital (approval no. 2016013). The
animal study also received approval from the Ethics Committee of
Changle County People's Hospital (approval no. 2016013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krieger N, Bassett MT and Gomez SL: Breast
and cervical cancer in 187 countries between 1980 and 2010. Lancet.
379:2990–1392. 2012. View Article : Google Scholar
|
|
2
|
Bosch FX and de Sanjosé S: The
epidemiology of human papillomavirus infection and cervical cancer.
Dis Markers. 23:213–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosch FX, Lorincz A, Muñoz N, Meijer CJ
and Shah KV: The causal relation between human papillomavirus and
cervical cancer. J Clin Pathol. 55:244–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franco EL, Duarte-Franco E and Ferenczy A:
Cervical cancer: Epidemiology, prevention and the role of human
papillomavirus infection. CMAJ. 164:1017–1025. 2001.PubMed/NCBI
|
|
5
|
Dizon DS, Mackay HJ, Thomas GM, Werner TL,
Kohn EC, Hess D, Rose PG and Covens AL: State of the science in
cervical cancer: Where we are today and where we need to go.
Cancer. 120:2282–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu K, He Y, Xia C, Yan J, Hou J, Kong D,
Yang Y and Zheng G: MicroRNA-15a inhibits proliferation and induces
apoptosis in CNE1 nasopharyngeal carcinoma cells. Oncol Res.
24:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Yang C, Wang K, Liu X and Liu Q:
MicroRNA-33b inhibits the proliferation and migration of
osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol
Res. 25:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Hui Y, Li X and Jia Q: Silencing
of lncRNA CCDC26 restrains the growth and migration of glioma cells
in vitro and in vivo via targeting miR-203. Oncol
Res. 26:1143–1154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Zhou Q, Tao L and Yu C:
MicroRNA-106a promotes cell migration and invasion by targeting
tissue inhibitor of matrix metalloproteinase 2 in cervical cancer.
Oncol Rep. 38:1774–1782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian B, Zhao L, Wang X, Xu J, Teng F, Gao
L and Shen R: RETRACTED: miR-149 regulates the proliferation and
apoptosis of cervical cancer cells by targeting GIT1. Biomed
Pharmacother. 105:1106–1116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang HD, Jiang LH, Sun DW, Jian L and Ji
ZL: The role of miR-130a in cancer. Breast Cancer. 24:521–527.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong X, Zhang J, Li J, Shao J and Fang L:
MiR-130a-3p inhibits migration and invasion by regulating RAB5B in
human breast cancer stem cell-like cells. Biochem Biophys Res
Commun. 501:486–493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Li Y, Wang R, Qin S, Liu J, Su F,
Yang Y, Zhao F, Wang Z and Wu Q: MiR-130a-3p regulates cell
migration and invasion via inhibition of Smad4 in gemcitabine
resistant hepatoma cells. J Exp Clin Cancer Res. 35:192016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Subramaniam MM, Chan JY, Yeoh KG, Quek T,
Ito K and Salto-Tellez M: Molecular pathology of RUNX3 in human
carcinogenesis. Biochim Biophys Acta. 1796:315–331. 2009.PubMed/NCBI
|
|
15
|
Huang B, Qu Z, Ong CW, Tsang YH, Xiao G,
Shapiro D, Salto-Tellez M, Ito K, Ito Y and Chen LF: RUNX3 acts as
a tumor suppressor in breast cancer by targeting estrogen receptor
α. Oncogene. 31:527–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishina S, Shiraha H, Nakanishi Y, Tanaka
S, Matsubara M, Takaoka N, Uemura M, Horiguchi S, Kataoka J,
Iwamuro M, et al: Restored expression of the tumor suppressor gene
RUNX3 reduces cancer stem cells in hepatocellular carcinoma by
suppressing Jagged1-Notch signaling. Oncol Rep. 26:523–531.
2011.PubMed/NCBI
|
|
17
|
Voon DC, Wang H, Koo JK, Nguyen TA, Hor
YT, Chu YS, Ito K, Fukamachi H, Chan SL, Thiery JP and Ito Y: Runx3
protects gastric epithelial cells against epithelial-mesenchymal
transition-induced cellular plasticity and tumorigenicity. Stem
Cells. 30:2088–2099. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Egawa H, Jingushi K, Hirono T, Ueda Y,
Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
The miR-130 family promotes cell migration and invasion in bladder
cancer through FAK and Akt phosphorylation by regulating PTEN. Sci
Rep. 6:205742016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakakura C, Hasegawa K, Miyagawa K,
Nakashima S, Yoshikawa T, Kin S, Nakase Y, Yazumi S, Yamagishi H,
Okanoue T, et al: Possible involvement of RUNX3 silencing in the
peritoneal metastases of gastric cancers. Clin Cancer Res.
11:6479–6488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yano T, Ito K, Fukamachi H, Chi XZ, Wee
HJ, Inoue K, Ida H, Bouillet P, Strasser A, Bae SC and Ito Y: The
RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells
undergoing transforming growth factor beta-induced apoptosis. Mol
Cell Biol. 26:4474–4488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Fan P, Deng M and Zeng C: The roles
of RUNX3 in cervical cancer cells in vitro. Oncol Lett.
15:8729–8734. 2018.PubMed/NCBI
|
|
22
|
Takeshima N, Yanoh K, Tabata T, Nagai K,
Hirai Y and Hasumi K: Assessment of the revised international
federation of gynecology and obstetrics staging for early invasive
squamous cervical cancer. Gynecol Oncol. 74:165–169. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao T and Lin Z: MiR-21 is involved in
cervical squamous cell tumorigenesis and regulates CCL20. Biochim
Biophys Acta. 1822:248–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long MJ, Wu FX, Li P, Liu M, Li X and Tang
H: MicroRNA-10a targets CHL1 and promotes cell growth, migration
and invasion in human cervical cancer cells. Cancer Lett.
324:186–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin W, Dong P, Ma C, Mitchelson K, Deng T,
Zhang L, Sun Y, Feng X, Ding Y, Lu X, et al: MicroRNA-133b is a key
promoter of cervical carcinoma development through the activation
of the ERK and AKT1 pathways. Oncogene. 31:4067–4075. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen W, Tong K and Yu J: MicroRNA-130a is
upregulated in colorectal cancer and promotes cell growth and
motility by directly targeting forkhead box F2. Mol Med Rep.
16:5241–5248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin S, Zhang Q, Wang Y, Li S and Hu R:
MicroRNA-130a regulated by HPV18 E6 promotes proliferation and
invasion of cervical cancer cells by targeting TIMP2. Exp Ther Med.
17:2837–2846. 2019.PubMed/NCBI
|
|
29
|
Jiang H, Yu WW, Wang LL and Peng Y:
miR-130a acts as a potential diagnostic biomarker and promotes
gastric cancer migration, invasion and proliferation by targeting
RUNX3. Oncol Rep. 34:1153–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Yan D, Wu W, Zhu J, Ye W and Shu
Q: MicroRNA-130a promotes the metastasis and epithelial-mesenchymal
transition of osteosarcoma by targeting PTEN. Oncol Rep.
35:3285–3292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato K, Tomizawa Y, Iijima H, Saito R,
Ishizuka T, Nakajima T and Mori M: Epigenetic inactivation of the
RUNX3 gene in lung cancer. Oncol Rep. 15:129–135. 2006.PubMed/NCBI
|
|
32
|
Zhang Z, Wang S, Wang M, Tong N, Fu G and
Zhang Z: Genetic variants in RUNX3 and risk of bladder cancer: A
haplotype-based analysis. Carcinogenesis. 29:1973–1978. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai KW, Koh KX, Loh M, Tada K, Subramaniam
MM, Lim XY, Vaithilingam A, Salto-Tellez M, Iacopetta B, Ito Y, et
al: MicroRNA-130b regulates the tumour suppressor RUNX3 in gastric
cancer. Eur J Cancer. 46:1456–1463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao QQ, Zhou B, Yu XZ, Zhang Z, Wang YY,
Song YP, Zhang L, Luo H and Xi MR: Transcriptome changes induced by
RUNX3 in cervical cancer cells in vitro. Oncol Lett.
19:651–662. 2010.
|
|
35
|
Cortez CC, Liang G, Van Rietschoten A, Jia
L, Tsai YC, Egger G and Jones PA: RUNX3: Promoter 1 methylation
status. Cancer Res. 66 (8 Supp):S3722006.
|
|
36
|
Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW,
Bang YJ and Kang GH: Methylation of RUNX3 in various types of human
cancers and premalignant stages of gastric carcinoma. Lab Invest.
84:479–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park WS, Cho YG, Kim CJ, Song JH, Lee YS,
Kim SY, Nam SW, Lee SH, Yoo NJ and Lee JY: Hypermethylation of the
RUNX3 gene in hepatocellular carcinoma. Exp Mol Med. 37:276–281.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li QL, Kim HR, Kim WJ, Choi JK, Lee YH,
Kim HM, Li LS, Kim H, Chang J, Ito Y, et al: Transcriptional
silencing of the RUNX3 gene by CpG hypermethylation is associated
with lung cancer. Biochem Biophys Res Commun. 314:223–228. 2004.
View Article : Google Scholar : PubMed/NCBI
|