Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide. These tumours primarily occur
following a hepatitis B virus (HBV) infection or a medical history
of chronic hepatitis viral infection and cirrhosis (1,2).
Accumulating evidence has suggested that cytokine levels may
predict the progression of HBV infections (3,4).
Interleukin (IL)-10 is an important anti-inflammatory cytokine
secreted by specific cells, including T regulatory lymphocytes
(Treg cells), activated macrophages and T helper 2 cells. The
expression levels of IL-10 regulate the immune function and
determine the balance between cellular and humoral responses
(5). In addition, the levels of
fibrinogen (FIB) and D-dimer have been found to be effective
predictors of adverse tumor profiles and outcomes for HCCs
(6). Therefore, liver function and
coagulation data analyses are useful in understanding the
progression of HBV-associated HCC.
α-fetoprotein (AFP) is one of the most common
diagnostic indicators of liver cancer and previous studies have
shown that patients with AFP-positive liver cancer exhibit lower
survival rates than those with AFP-negative liver cancer (7–9).
Overall, an inflammatory microenvironment plays an important role
in the progression of HCC. The lymphocyte-to-monocyte ratio (LMR)
is a novel inflammatory biomarker that combines estimates of host
immune homoeostasis and of the tumour microenvironment, and has
been identified as a predictor of clinical outcomes for a variety
of malignancies, including breast, renal, lung and colorectal
cancers (10–14). Previously, it has been shown that
the peripheral LMR possesses important prognostic value for
patients with HCC (15–17).
CD4+ T lymphocytes are associated with
the development of liver cancer, while CD8+ T
lymphocytes are associated with the clearance of liver cancer cells
(18). AFP can lead to tumor
immune escape by affecting the ratio of
CD4+/CD8+ T lymphocytes (19). However, it remains to be
investigated whether the numbers of CD4+ and
CD8+ T lymphocytes are associated with AFP levels in
HCC.
CD4+ CD25+ Treg cells are a
subgroup of CD4+ T lymphocytes with negative
immunoregulatory functions. They can exert inhibitory effects by
secreting regulatory cytokines, such as IL-10 and transforming
growth factor (TGF)-β. The secretion of these cytokines occurs via
direct cell contact or via the pathway of programmed cell death
protein 1 (PD-1)/PD-1 ligand (PD-L). The latter pathway contributes
to the malignant transformation of the cells that escape immune
surveillance and immune defense, which in turn results in the
occurrence and development of tumors (20–22).
At present, the IL-10-592 polymorphism within the
IL-10 gene promoter region has been shown to be associated with the
susceptibility to occult HBV infection (OBI), a virological
condition characterized by a low release of HBV from liver cells
and low HBV-DNA levels in serum and/or liver tissue of HBV surface
antigen (HBsAg)-negative subjects, which is the natural course for
patients with a chronic HBV infection and patients who develop an
HBV infection following immunosuppressive therapy (23–26).
According to European guidelines on management of chronic HBV (CHB)
infection, the natural history of CHB can be schematically divided
into five, not necessarily sequential and stable, phases, the 5th
of which is OBI that is defined as the presence of viral DNA in the
liver (with detectable or undetectable HBV DNA in the serum) of
individuals testing negative for the HBsAg (27–29).
The mechanism and clinical implications of OBI are not fully
understood. Previous studies have reported that OBI can potentially
contribute to acute exacerbation, cirrhosis and HCC (30–32).
However, OBI prevalence and its natural course in the general
population have been insufficiently investigated. HBV DNA without
HBs antigenemia has been detected in the following clinical
situations: i) Chronic, presumably viral, hepatitis unrelated to
HCV, atypical alcoholic hepatitis and HCC; ii) viral reactivation
following immunosuppression; and iii) Transmission via
transplantation, transfusion or experimental transmission to
chimpanzees (29,33–34).
Among the immunosuppressive agents, glucocorticoids (GCs) are one
of the earliest medications that can effectively inhibit the
infection of HBV (35). During
this type of infection, IL-10 expression is affected by the GC via
ERK-mediated phosphorylation of the GC receptor (GR) in dendritic
cells (36).
Conversely, the role of the LMR in patients with
AFP-positive and AFP-negative HCC has not been previously explored.
In the present study, the association between the LMR and AFP was
investigated. In addition, peripheral blood CD4+ and
CD8+ T lymphocyte numbers were analyzed in combination
with PD-1, IL-10 and TGF-β levels. Concomitantly, the association
between the IL-10 and GR expression was explored.
Materials and methods
Ethics and patients
The present study was reviewed and approved by the
institutional review board of the Chongqing Medical University at
The First Affiliated Hospital of Chongqing Medical University
(Chongqing, China). Written informed consent was obtained from all
patients. The medical records of 266 in-patients with
HBV-infections, who were negative for non-HBV hepatitis viruses and
did not suffer from autoimmune hepatitis, were retrospectively
reviewed. All patients were 35–55 years old and included 47% women
and 53% men. The patients who were selected were treated between
January 2010 and December 2016 at The First Affiliated Hospital of
Chongqing Medical University. HBsAg, HBV e antigen (HBeAg), HBV-DNA
and alanine aminotransferase (ALT) were the markers used to
classify the patients into the various groups, along with the
findings from histological examinations of their liver tissues. The
patients were divided into 4 groups according to the guidelines for
the prevention and treatment of chronic HBV in China (2015 edition)
(37) and the diagnostic criteria
for HCC, as evaluated by histologic examination of the liver. The
groups consisted of 103 HBV carriers with normal liver function
(ALT normal and HBsAg positive), 68 patients with chronic HBV
infection (ALT normal, HBsAg positive and HBV-DNA positive), 51
patients with AFP-positive HCC (abnormal ALT levels, HBV-DNA
positive, presence of a mass by histological analysis, AFP >20
ng/ml) and 44 patients with AFP-negative HCC (abnormal ALT levels,
HBV-DNA positive, presence of a mass by histological analysis, AFP
≤20 ng/ml). This information is shown in Table I.
 | Table I.Patient information among the four
groups. |
Table I.
Patient information among the four
groups.
| Group | No. patients | ALT | HBsAg | HBV-DNA | Hepatic histologic
examination | AFP, ng/ml |
|---|
| Normal liver
functional HBV carriers | 103 | Normal | + | – | – | NA |
| Chronic HBV
infection | 68 | Normal | + | + | – | NA |
| AFP-positive
HCC | 51 | Abnormal | + | + | + | ≤20 |
| AFP-negative
HCC | 44 | Abnormal | + | + | + | >20 |
Case data analysis
All case data for the markers LMR, AFP, prothrombin
time (PT), activated partial thromboplastin time (APTT), thrombin
time (TT), FIB, D-dimer, fibrin degradation products (FDP), serum
ALT and aspartate aminotransferase (AST) were obtained from the
medical laboratory of The First Affiliated Hospital of Chongqing
Medical University. These data were statistically analyzed using
SPSS software (version 17.0). The parameter LMR for the 4 groups
were compared using one-way ANOVA with Scheffe's multiple
comparison test (P<0.05). Spearman correlation analysis was
performed. The parameters PT, APTT, TT, FIB, D-dimer, FDP, and
ALT/AST for AFP-positive and AFP-negative HCC were compared using a
student's t-test (P<0.05). The optimal cut-off value of the
association of LMR with AFP was assessed using receiver operating
characteristic (ROC) curves. The threshold for significance was set
at 5% for Spearman correlation analysis.
Cell preparation
A total of 30 patients (age, 35–45 years; 13 women
and 17 men) with HCC were diagnosed on the basis of HBV infection
and selected from The First Affiliated Hospital of Chongqing
Medical University between October 2017 and June 2018. Among them,
15 patients who were AFP-positive and had an LMR <2.01 were
selected as the AFP-positive liver cancer group, and 15 patients
who were AFP-negative and had an LMR >2.01 were selected as the
AFP-negative liver cancer group. A total of 15 patients with an HBV
infection without liver cancer were selected as the control group.
All patients in the liver cancer group met the diagnostic criteria
for primary liver cancer with HBV infection (38), and were excluded from other types
of tumors and immune diseases, such as diabetes, arthritis and HIV
as well as blood diseases, such as leukemia, lymphoma and other
fungal and bacterial infections. All patients were treated with
agents that did not affect the results of the study and any
interventions affecting peripheral blood T lymphocyte subsets, such
as surgery, minimally invasive operations, blood transfusion,
radiotherapy or chemotherapy were conducted two months prior to
enrollment. The clinical characteristics of the patients are
summarized in Table II.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Factor | HBV+
NHCC (n=15) | AFP+ HCC
(n=15) | AFP− HCC
(n=15) |
|---|
| Age, years | 49.4±7.68 | 55.3±15.59 | 55.1±12.46 |
| ALT/AST | 1.16±0.24 | 10.99±0.97 | 0.83±0.37 |
| LMR | 5.28±1.5 | 1.55±0.37 | 4.55±2.42 |
| PLT | 146±63 | 205±107 | 128±77 |
| FDP | 3.64±3.31 | 11.62±13.14 | 3.54±4.67 |
| D-Dimer | 1.58±0.86 | 6.10±5.63 | 0.72±0.81 |
Flow cytometry for CD4, CD8 and PD-1
expression analysis
A total of 100 µl whole blood was collected from the
patients and transferred to test tubes. Subsequently, 20 µl
CD3-FITC, 5 µl CD4-BV510, 20 µl PD-1-PE (CD279-PE) and 5 µl
CD8-BV421 antibodies were added. An equal volume of these
antibodies were added to the non-HCC (NHCC) tubes. Mouse anti-human
monoclonal antibodies CD3-FITC (cat. no. 561806), CD4-BV510 (cat.
no. 562970), CD8-BV421 (cat. no. 562428) and PD-1-PE (CD279-PE;
cat. no. 557946) were purchased from BD Biosciences, used according
to the manufacturer's instruction (1×106 cells were
used). The samples were mixed with the antibodies under oscillating
conditions for 3–5 sec and incubated at room temperature in the
dark for 30 min.
A total of 1,000 µl red blood cell lysis buffer
(Beyotime Institute of Biotechnology) was added to the tubes and
the samples were mixed gently with the antibodies. Following
incubation at room temperature for 10 min, the samples were
centrifuged for 4 min at 500 × g at room temperature and the
supernatant was discarded. A total of 1,000 µl PBS were added and
the samples were centrifuged for 4 min at 500 × g at room
temperature, after which the milky white precipitate was collected.
Following a final resuspension in 400 µl PBS, the samples were
examined using a FACSCalibur™ flow cytometer (BD Biosciences). The
percentage of the samples expressing a particular cell surface
marker was analyzed on a FACSCalibur using the Cell Quest Pro™
software (v 5.2; BD Sciences).
ELISA analysis of the expression of
IL-10 and TGF-β1
Human IL-10 ELISA kit (cat. no. BH-DB047) and Human
TGF-β1 ELISA kit (cat. no. BH-ELISA 1805) were purchased from
Shanghai Bohu Biotechnology Co. (https://bhbta.biomart.cn/) Serum samples were stored
at −20°C, thawed and then centrifuged (400 × g; 10 min; room
temperature). Serum samples for TGF-β1 detection were acidified and
neutralized according to the manufacturer's instructions to
activate TGF-β1. A total of 100 µl sample or a standard of a known
concentration was added to each well and the plate was incubated
for 2 h at 37°C. Subsequently, 100 µl Biotin-antibody (1X) was
added to each well and incubated for 1 h at 37°C. The samples were
then aspirated and washed three times. A total of 100 µl
horseradish peroxidase-avidin (1X) was added to each well and the
samples were incubated for 1 h at 37°C. The samples were aspirated
and washed 5 times, then incubated with an additional 90 µl
3,3′,5,5′-tetramethylbenzidine substrate in the dark for 15–30 min
at 37°C. Finally, 50 µl stop solution was added to each well and
the absorbance was monitored at 450 nm within 5 min. A standard
curve was produced according to the absorbance readings of the
known concentration standards, which was then used to determine the
concentration of the samples.
Preparation of human peripheral blood
mononuclear cells (PBMCs)
Human PBMCs were isolated from blood samples
collected from AFP-positive and AFP-negative patients using the
lymphocyte separation medium (TBD Science) according to the
manufacturer's instructions. Subsequently, the cells were cultured
in serum-free haematopoietic cell medium (Takara Bio, Inc.) at 37°C
in an incubator with 5% CO2.
ELISA for determining the effect of
dexamethasone or RU486 on IL-10 expression
Dexamethasone (Sigma-Aldrich; Merck KGaA) or RU486
(Sigma-Aldrich; Merck KGaA) at three different concentrations (3, 7
and 10 µmol/l), were added to 4–6×105 PBMCs (200 µl) in
a 96-well plate and incubated for 48 h at 37°C with 5%
CO2. The dexamethasone and RU486 concentrations were
selected according to Chasserot-Golaz et al (39). Subsequently, the supernatant was
collected and the expression levels of IL-10 were assessed using an
ELISA kit (Human IL-10 ELISA kit; cat. no. CSB-E04593h; Cusabio
Biotech Co., Ltd.) and MB-530 microplate reader to determine the
cut-off value. Each group analysis was replicated 3 times.
IL-10-592 promoter binding site gene
assay
The binding site of the IL-10-592 promoter to the GR
required IL-10-592 wild-type and mutant promoters that were
inserted into the pMCS-green-Renilla-luciferase plasmid. The GR
gene was inserted into the pGL3-basic plasmid which was transferred
into 293T cells using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) over the course of 48 h, according
to the manufacturer's instructions.
Total RNA was extracted using Total RNA Isolation
reagent (SuPerfecTRI™; cat. no. 3101-100; www.pufei.com) at 43.5°C for 1 h and then 73.5°C for 3
min. Each RNA sample was reverse transcribed according to the
Promega M-MLV kit (cat. no. M1705) content. Then, 2 ul dNTPs were
the mixture of 10 mM dATP, dCTP, dGTP and dTTP and 6 ul
Nuclease-Free Water. The 5 µl buffer consisted of 250 mM Tris HCl,
375 mM HCl, 15 mM MgCl2 and 50 mM DTT. The primers of GR
used were as follows: 5′-GCTGTCGCGCTCACTGGCTGTC-3′ and reverse,
5′-GCATCGTTCCGATCACTTCGCA-3′. Reverse transcription-quantitative
PCR was performed on an ABI 7000 RT PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.), using SYBR green PCR master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used: Initial denaturation at 95°C
for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30
sec, then dissociating at 95°C for 15 sec, 60°C for 30 sec and 95°C
for 15 sec. The reference gene was GAPDH. The following specific
primers were used: GAPDH forward, 5′-CAATGACCCCTTCATTGACC-3′ and
reverse, 5′-GATCTCGCTCCTGGAAGATG-3′; pGL3 forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′; and pGL3-GR forward,
5′-GTCTTCACCCTCACTGGCTGTC-3′ and reverse,
5′-GGTCATTTCCCATCACTTTTGT-3′. mRNA expression levels were
quantified using the 2−ΔΔCq method of quantification
(40).
The detection of luciferase activity was performed
with the Dual-Glo Luciferase Assay System (Promega Corporation)
after 48 h transfection according to the manufacturer's
instructions. The compound-induced increases in luciferase activity
were estimated by dividing the value of the firefly luminescence to
the value of the Renilla luminescence (transfection
efficiency control). The data were normalized to the luciferase
activity of the non-treated controls, which were set to 1 (25%).
Each concentration was tested in triplicate and the assay was
conducted three times.
Statistical tests for flow cytometry,
ELISA and the value of the luminescence assay
These data were statistically analyzed using the
SPSS software (version 17.0; SPSS, Inc.). All experimental results
are presented as the mean ± SD. They were compared using one-way
ANOVAs with Scheffe's multiple comparison tests. P<0.05 was
considered statistically significant.
Results
Comparison of the LMR for the four
groups
The LMR was decreased in the treated groups compared
with the levels noted in the normal liver function HBV carrier
group (Table III). However, the
results of the chronic HBV group did not differ significantly from
those of the normal liver function HBV carrier group. A marked
decline was noted in the LMR of the AFP-positive and AFP-negative
HCC groups. These results indicated that the LMR was higher in the
non-HCC groups than that of the HCC groups. Therefore, the normal
liver function of the HBV carrier group (non-HCC group) was
compared with the chronic HBV group. Both the AFP-positive and
AFP-negative HCC groups exhibited significant differences with
regards to the LMR compared with the non-HCC group (Table IV).
 | Table III.Statistical analysis of the LMR among
the four groups. |
Table III.
Statistical analysis of the LMR among
the four groups.
| Comparison
group | Comparison LMR
(mean ± SD) |
|---|
| Normal liver
functional HBV carriers vs. chronic HBV infection | 4.52±2.6 vs.
4.17±1.99 |
| Normal liver
functional HBV carriers vs. AFP negative HCC | 4.52±2.6 vs.
2.93±1.87a |
| Normal liver
functional HBV carriers vs. AFP positive HCC | 4.52±2.6 vs.
1.76±1.17a |
| Chronic HBV
infection vs. AFP negative HCC | 4.17±1.99 vs.
3.72±2.65a |
| Chronic HBV
infection vs. AFP positive HCC | 4.17±1.99 vs.
3.72±2.65a |
| AFP negative HCC
vs. AFP positive HCC | 2.93±1.87 vs.
1.76±1.17a |
 | Table IV.Statistical analysis of the LMR among
three groups. |
Table IV.
Statistical analysis of the LMR among
three groups.
| Comparison
group | Comparison LMR
(mean ± SD) |
|---|
| Non-HCC vs. AFP
positive | 4.37±2.36 vs.
1.76±1.17a |
| Non-HCC vs. AFP
negative | 4.37±2.36 vs.
2.93±1.87a |
| AFP positive vs.
negative | 1.76±1.17 vs.
2.93±1.87a |
Statistical analysis of liver function
and coagulation data for AFP-negative and AFP-positive HCC
groups
The liver function and coagulation were assessed in
the AFP-negative and -positive HCC groups. Notably, the following
parameters, AST/ALT, PT, APTT, FIB, D-dimer and FDP were assessed.
The levels of PT, APTT or FIB between the two groups were not
significantly altered, whereas significant between-group
differences were noted with regards to the AST/ALT, D-dimer and FDP
(Table V). Spearman correlation
analysis (Table VI) indicated
that D-dimer and FDP were associated with the LMR in the
AFP-negative group. This association was not noted for the AST and
ALT markers.
 | Table V.Statistical analysis of data among
two groups. |
Table V.
Statistical analysis of data among
two groups.
| Factor | AFP positive HCC
(n=44) | AFP negative HCC
(n=51) |
|---|
| APTT | 37.25±6.59 | 37.23±8.76 |
| PT | 14.83±1.55 | 14.38±1.80 |
| FIB | 3.40±1.35 | 3.70±1.43 |
| D-Dimer | 3.89±6.83 |
1.51±1.38a |
| FDP | 9.32±11.67 |
4.79±4.15a |
| AST/ALT | 1.91±1.83 |
1.30±0.79a |
 | Table VI.Spearman analysis of the LMR between
D-Dimer, FDP and AST/ALT. |
Table VI.
Spearman analysis of the LMR between
D-Dimer, FDP and AST/ALT.
| Value | D-Dimer | FDP | AST/ALT |
|---|
| P-value | <0.001 | <0.001 | 0.059 |
| R-value | −0.42 | −0.432 | −0.107 |
Optimal cut-off values of the LMR for
AFP-negative and -positive groups
Based on the aforementioned results, the
associations of the LMR with the AFP-negative and AFP-positive
patient groups were examined. The optimal cut-off value of LMR for
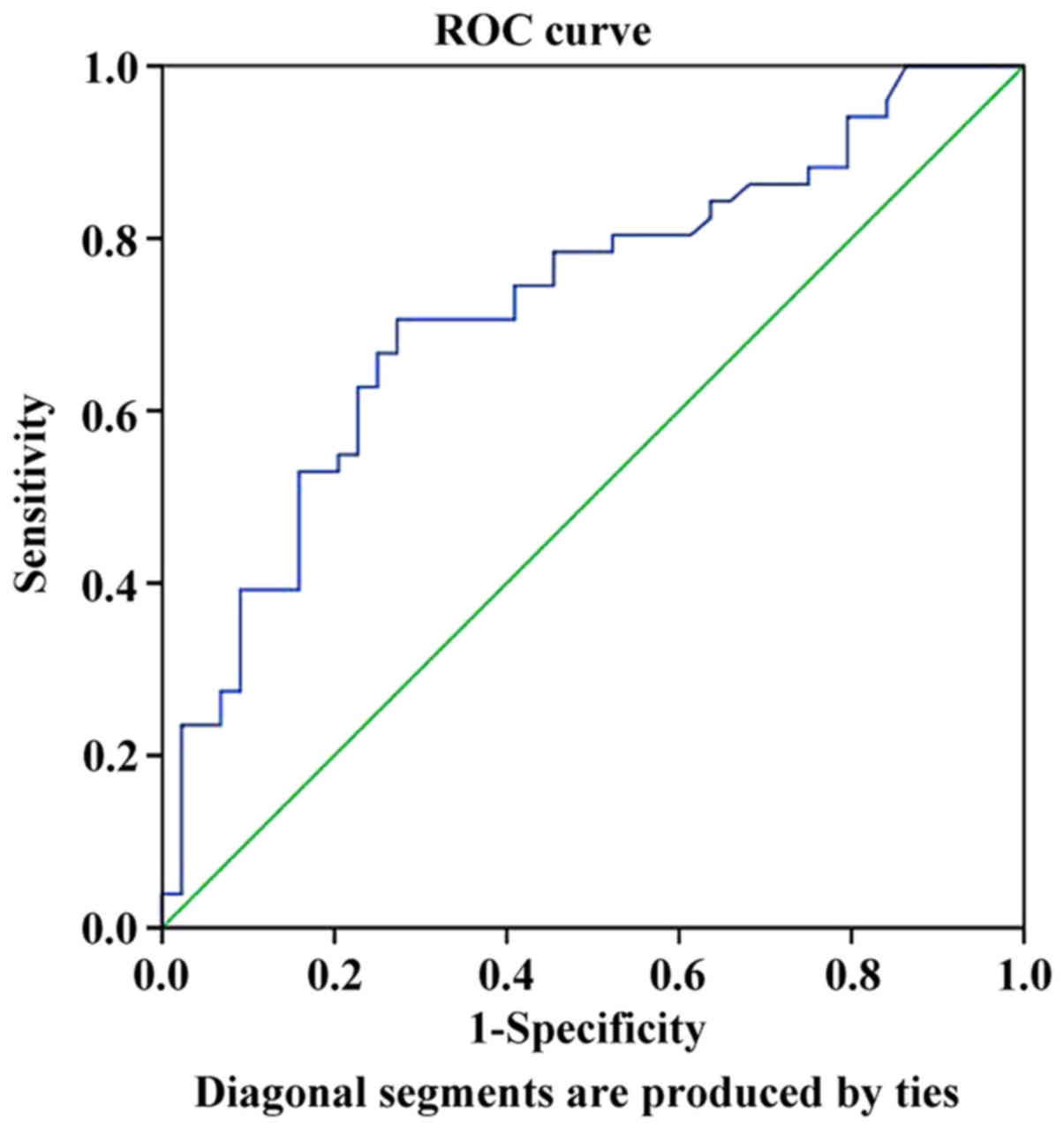
AFP was 2.01 with an area under the curve of 0.724, a sensitivity
of 68.6% and a specificity of 75.0% (Fig. 1). The mean LMR was associated with
the AFP status in the AFP-negative and -positive groups. Therefore,
Spearman correlation analysis was used to assess the LMR values in
these two groups and the results indicated significant correlations
(R=0.387, P<0.001).
Statistical analysis of
CD4+ and CD8+ T lymphocytes among the three
groups
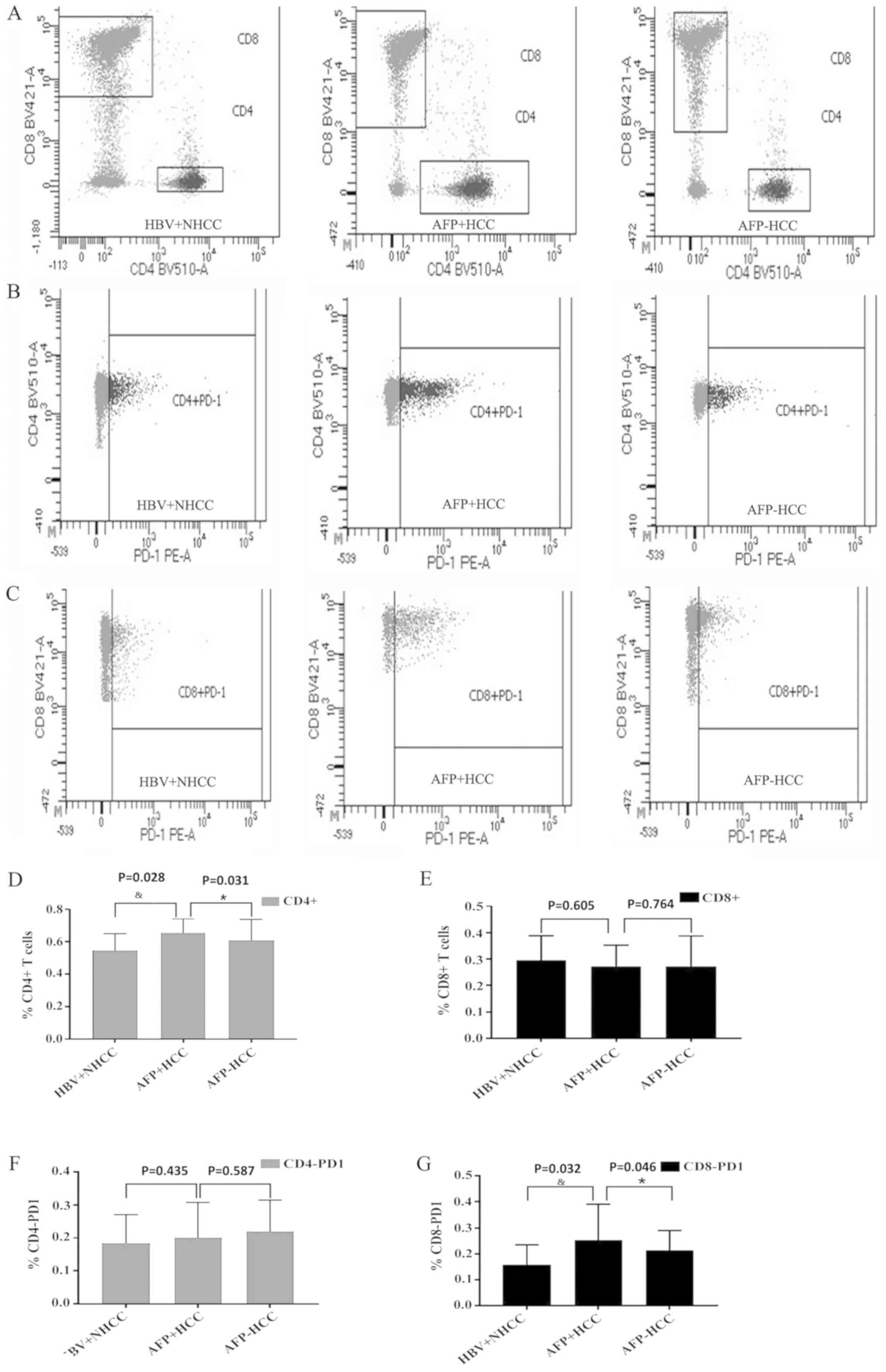
Based on the cutoff value, the proportion of
CD4+ T and CD8+ T lymphocytes in the
peripheral blood of patients in the HBV+ non-liver cancer,
AFP-positive and AFP-negative groups was assessed by flow
cytometry. The percentages of CD4+ T lymphocytes for the
aforementioned groups were 55±10, 65±8 and 61±13, respectively
(Fig. 2A and D). A significant
difference was noted among the three groups (P<0.05) and the
highest increase was evident in the AFP-positive group indicating
that CD4+ T lymphocyte number correlated with AFP
levels. The percentages of CD8+ T lymphocytes in the
three groups were 30±9, 27±8 and 27±11, respectively (Fig. 2A and E). The number of
CD8+ T lymphocytes was markedly reduced in the liver
cancer group, but no significant differences were noted in the
AFP-positive and negative groups (P>0.05), indicating that the
number of CD8+ T lymphocytes did not exhibit a
correlation with the AFP concentration.
Statistical analysis of CD4-PD-1 and
CD8-PD-1 in each group
The expression levels of PD-1 in CD4+ T
and CD8+ T lymphocytes from the peripheral blood of
patients in the non-liver cancer, AFP-positive and AFP-negative
groups were assessed by flow cytometry. The expression levels of
CD4-PD-1 were 18±8, 20±10 and 22±9, respectively (Fig. 2B and F). The expression levels of
CD4-PD-1 appeared to be increased in the liver cancer group, but no
significant differences were noted in the three groups (P>0.05),
suggesting that CD4-PD-1 was independent of AFP expression. The
expression levels of CD8-PD-1 in the three groups were 16±7, 25±13
and 21±7, respectively (Fig. 2C and
G). The expression levels of CD8-PD-1 in the liver cancer group
were significantly increased compared with the HBV+NHCC group
(P<0.05), whereas the expression levels of the AFP-positive
group were higher than those of the AFP-negative group, indicating
that CD8-PD-1 was associated with AFP levels.
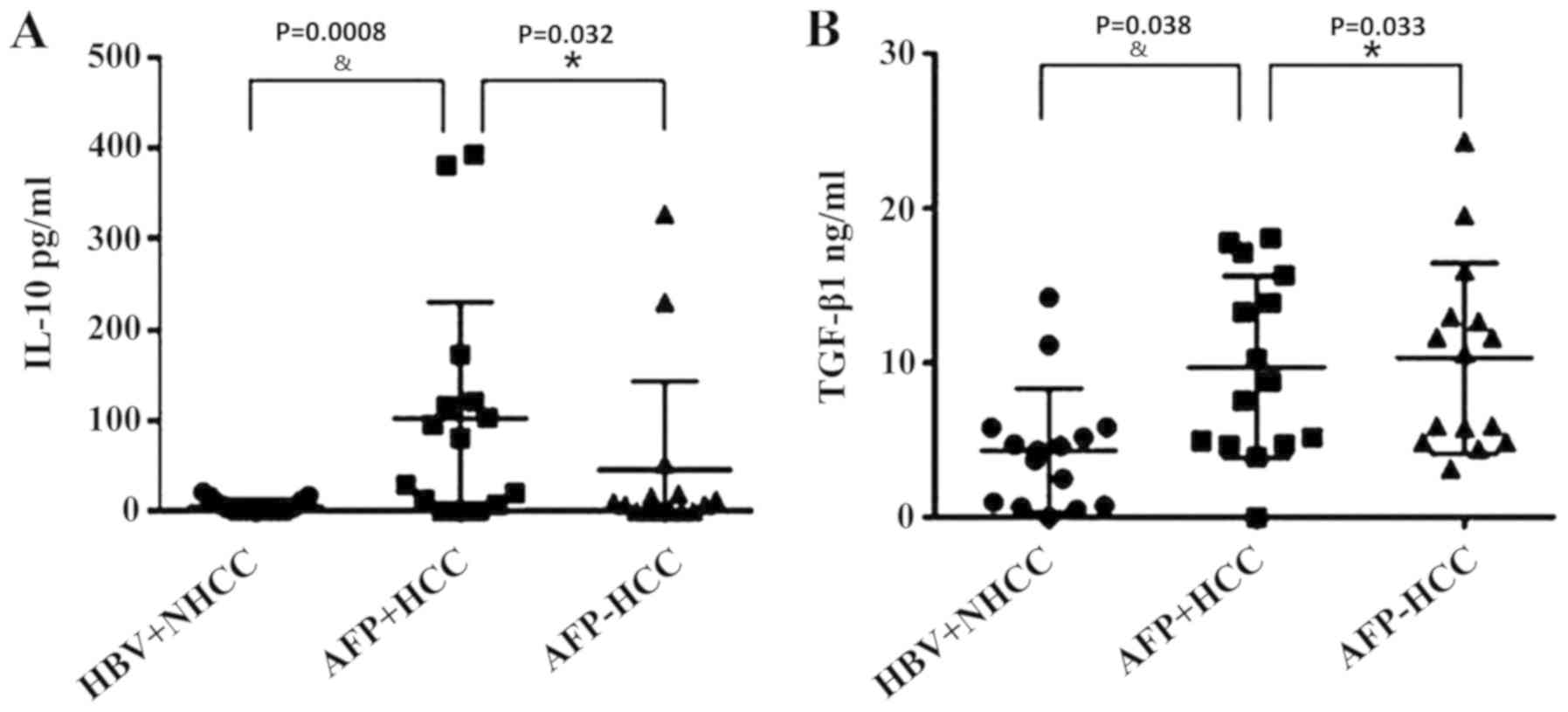
Statistical analysis of serum IL-10
and TGF-β in each group
The serum levels of IL-10 and groups were measured
using ELISAs. The IL-10 concentration levels were 5.58±7.30,
102.11±123.56 and 45.52±94.23, respectively (Fig. 3A). The TGF-β1 levels in the
aforementioned groups were 4.38±3.89, 9.74±5.69 and 10.33±5.98,
respectively (Fig. 3B). The
one-way ANOVA analysis indicated that IL-10 and TGF-β1 levels
exhibited significant differences between the three groups
(P<0.05). IL-10 and TGF-β1 exhibited the lowest levels in the
HBV+ non-liver cancer group, and the highest of IL-10 in the
AFP-positive group, indicating that IL-10 expression levels were
associated with AFP levels. However, TGF-β1 levels in AFP-positive
group were lower compared with the AFP-negative group
(P<0.05).
Dexamethasone promotes the expression
of IL-10 in AFP-positive patients
ELISAs indicated that following incubation of the
samples with 3, 7 and 10 µmol/l dexamethasone for 48 h, the optical
density values of IL-10 were higher in the AFP-positive group than
in the AFP-negative group (Fig.
S1). These results indicated that the expression levels of
IL-10 were higher in the AFP-positive group than in the
AFP-negative group.
RU486 inhibits the expression of IL-10
in HBV-infected patients
The samples of the AFP-negative and AFP-positive
group were incubated with RU486 for 24 h, an inhibitor of
dexamethasone (41). The
expression levels of IL-10 were gradually decreased in
aforementioned two groups with increasing concentrations of RU486,
but the expression of IL-10 was not markedly decreased at 7 µmol
(Fig. S2).
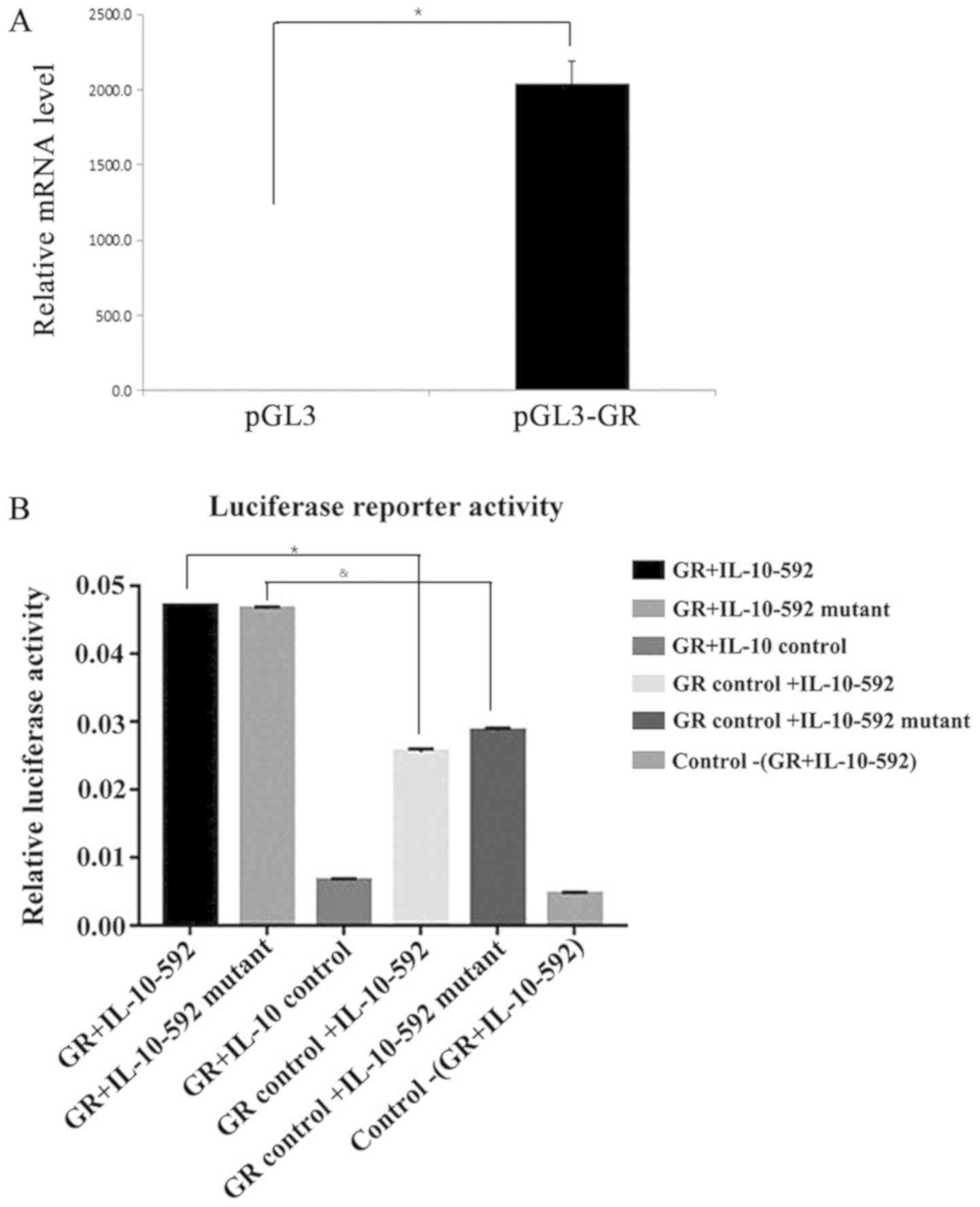
IL-10 promoter is the binding site for
GR regardless of the presence of the polymorphic sites
To determine whether the IL-10-592 promoter is the
binding site for GR, luciferase reporter activity was assessed
following the overexpression of GR (Fig. 4A). The IL-10 promoter produced a
strong induction of relative luciferase activity that peaked at 48
h in the 293T-cotransfected luciferase cells that overexpressed GR
(Fig. 4B). The relative luciferase
activity caused by the IL-10 promoter was higher than that of the
control cells (P<0.05). However, following cotransfection of the
cells with the IL-10-592 polymorphic promoter and the GR gene, a
similar induction in the relative luciferase activity was noted
compared with that noted for the wild type variant (P=0.15). These
results indicated that the IL-10 promoter was the binding site for
GR, regardless of the presence of the IL-10-592 polymorphism.
Discussion
In the present study, a cohort of 266 in-patients
with HBV infections were examined, who were negative for non-HBV
hepatitis viruses and did not suffer from autoimmune hepatitis. The
data indicated that a high LMR correlated with AFP negativity and
lower levels of D-dimer and FDP in the HCC group.
Previous studies have demonstrated that AFP
contributes to the development, growth, invasion and metastasis of
HCC (42–45). For example, Bihari et al
(42) reported that increased
levels of AFP following HCC therapy were indicative of incomplete
response or recurrence, which indicated that the release of AFP
from HCC cells to the circulation was a major source of HCC
metastasis. In addition, Yang et al (44) reported that HCC patients with no
contraindications for surgery and serum AFP levels ≤20 ng/ml could
mainly benefit from hepatectomy as a primary treatment as opposed
to HCC patients with serum AFP levels >20 ng/ml who required
comprehensive therapy other than surgical resection and close
follow-up. Moreover, several studies have suggested that the
D-dimer can predict survival of several types of malignancies
(42,46,47).
To the best of the authors' knowledge, the present study is the
largest scale study to date that has explored the association
between the LMR and the clinicopathological parameters for AFP. The
results suggested that patients with AFP-negative HCC exhibited
higher LMR and lower levels of D-dimer and FDP. Additional studies
have reported that AFP-negative HCC patients with high LMR exhibit
optimal disease-free survival and lower metastasis rate than
AFP-positive patients with low LMR (48,49).
Multiple mechanisms may be associated with HCC
invasion and metastasis, including recombinant human AFP
(rhAFP)-induced expression of matrix metallopeptidase 9; C-C
chemokine receptor type 5 and AFP mRNA levels; and absent in
melanoma 2 protein levels (43,50,51).
The measurement of the levels of these markers in various
neoplasms, limits their wider application for tracking the disease
status during the clinical course of treatment (41,49).
In contrast to these markers, LMR is a readily measured biomarker
that can be used to predict the HCC status (49,50,52).
The present study indicated that the LMR correlated significantly
with the D-dimer and FDP. Patients with HCC who were AFP-negative
and had low levels of D-dimer and FDP, exhibited a high LMR
compared with patients with HCC who were AFP-positive. Liu et
al (53) further reported that
FIB and D-dimer levels were elevated following carcinogenesis.
However, in the present study, the FIB levels of patients with HCC
who were AFP-positive or -negative, were not significantly
different. In addition, the cut-off value used in the present study
for the LMR, which was selected by ROC curve analysis, was not the
same as that reported by Hong et al (54). Nonetheless, the present study
contains specific limitations. Firstly, selection bias may exist
due to the retrospective nature of the study. Secondly, diabetes
mellitus, ischemia, renal disease and systemic inflammation, which
could potentially affect LMR, were not evaluated. Thirdly, the
correlations of FIB with HCC and the cut-off value of LMR were
different than those reported in other studies due to different
endpoints assessed and different populations enrolled (47,48).
Therefore, the conclusions of the present study may need to be
validated by a prospective investigation.
Various in vitro studies have shown that AFP
can play an immunosuppressive role in the tumor microenvironment.
Previously, AFP has been shown to induce apoptosis in immune cells,
such as lymphocytes and dendritic cells, by upregulating the
expression of the pro-apoptotic genes, including Bax, BH3
interacting-domain death agonist, Bad and apoptotic protease
activating factor 1. Concomitantly, AFP has been shown to enhance
the activity of pro-apoptotic factors [Fas, Fas ligand (FasL) and
tumor necrosis factor-related apoptosis-inducing ligand] in immune
cells. These pro-apoptotic markers are usually induced to increase
forkhead box 3 expression in CD4+ T lymphocytes and
promote the transformation of CD4+ CD25− T
lymphocytes into CD4+ CD25+ Treg cells
(55).
Previous clinical studies have shown that serum AFP
levels are closely associated with the development, recurrence rate
and survival rate of primary liver cancer (44,55).
The malignant degree and long-term recurrence rate of AFP negative
liver cancer are low, which results in high survival rate and
optimal prognosis (44). In the
present study, it was concluded that CD4+ T lymphocytes
were significantly different from each other among the three
groups. The high level of CD4+ T lymphocytes was
associated with poor prognosis of liver cancer in the AFP positive
group. The majority of previous studies have reported that
CD4+ CD25+ T lymphocytes are involved in
immunosuppression (44,55,56).
CD4+ CD25+ T lymphocytes are a subgroup of T
lymphocytes with negative immunoregulatory functions. They exert
negative effects on tumor immunity by inhibiting T lymphocytes and
may also act as a prognostic factor for patients with liver cancer
(44). It has been shown that
higher Treg cell numbers are associated with various clinical
pathological parameters, such as increased AFP levels, presence of
multiple tumors, poor differentiation, advanced tumor stage and
poor prognosis of vascular infiltration (56). In the present study, the cell
surface marker molecule CD25 was not detected, whereas the
CD25+ Treg usually accounted for 5–10% of
CD4+ T lymphocytes (57). Therefore, the number of
CD4+ T lymphocytes detected in the present study was of
particular importance.
It also has been reported that PD-1/PD-L can
upregulate the expression of Fas and FasL in Treg cells, inhibit
the proliferation of CD8+ T lymphocytes and promote the
apoptosis of CD8+ T cells (56–58).
In the present study, flow cytometry and ELISA assays indicated
that the expression levels of CD8-PD-1 and IL-10 were different in
the three groups (P<0.05). The expression levels of the
aforementioned markers were significantly increased in the liver
cancer group, whereas the expression levels in the AFP-positive
group were higher than those of the AFP-negative group. PD-1 was
expressed in a variety of activated immune cells and played a key
role in immune regulation. The combination of PD-1 and PD-L
inhibited the activation and proliferation of T lymphocytes,
induced apoptosis of T cells and caused tumor cells to evade immune
attack, which showed that the PD-1/PD-L ratio played a negative
role in the anti-tumor immune response. In addition, PD-1 is
significantly unregulated in tumor-infiltrated lymphocytes, notably
in effector CD8+ T cells (58,59).
Due to the inhibition of antiviral and anticancer immune function,
the increase in the expression levels of PD-1/PD-L may lead to the
poorer prognosis of HCC patients and may promote tumor invasion and
postoperative recurrence in liver cancer patients (59). Previously, the expression levels of
PD-1 in Treg cells were investigated in combination with the
expression levels of Fas and FasL. These biomarkers were shown to
promote the secretion of IL-10 via the PD-1/PD-L pathway in order
to exert negative immune regulation. IL-10 inhibits the stimulation
of immune cells. In addition, IL-10 can assist the differentiation
of infantile T cells into Treg cells, while Treg cells can secrete
IL-10. Positive feedback regulation between Treg cells and IL-10
may promote immune tolerance (60–62).
Therefore, AFP can cause upregulation of Fas and FasL expression in
Treg cells and promote the secretion of IL-10 by PD-1/PD-L
signaling, leading to the inhibition of the proliferation of
CD8+ T lymphocytes. TGF-β1 is an important cytokine,
which induces the production of Treg cells. It has been shown that
Treg cell infiltration is higher in cells with high expression of
TGF-β1 than that in low TGF-β1 expression cells, suggesting that
the expression of TGF-β1 in tumor tissues may increase the local
Treg cell infiltration in the tumor, while the infiltration of Treg
cells in the liver tissue is often associated with poor prognosis.
In addition to the direct effect on tumor progression and
infiltration, TGF-β exhibits a potent immunosuppressive effect.
TGF-β1 can inhibit the proliferation of effector T cells, control
the differentiation of various CD4+ subtypes and reduce
the generation of effector T cells. In a previous study, following
the stimulation of TGF-β1, CD4+ immature T cells were
differentiated into Treg cells and the number of Treg cells in
liver cancer tissues was positively associated with the expression
of TGF-β1 (20,63). TGF-β1 promotes tumor immune escape
by maintaining a normal Treg cell number and enhancing the
induction of Treg cell differentiation (20). In the present study, ELISA
indicated that TGF-β1 levels in the liver cancer group were
significantly higher than those in the non-liver cancer group,
while the TGF-β1 levels in the AFP-negative group were slightly
higher than those in the AFP-positive group. Therefore, the
immunosuppressive effect of TGF-β1 and its association with AFP
expression requires further investigation. Taken collectively, the
data suggested that AFP may upregulate the expression of Fas and
FasL on Treg cells and promote the secretion of IL-10 via the
PD-1/PD-L signaling pathway, thereby inhibiting the proliferation
of CD8+ T lymphocytes. Therefore, the decrease in the
number of CD8+ T lymphocytes should be consistent with
the increase in the AFP levels. However, the results of the present
study indicated that the number of CD8+ T lymphocytes
was significantly different in the liver cancer and non-liver
cancer groups, whereas no significant difference was noted in the
AFP-negative and AFP positive groups. Unfortunately, it has been
reported that only 20% of patients with liver cancer are sensitive
to PD-1/PD-L treatment (20).
Moreover, certain patients that respond to the treatment still
relapse (64). This suggests that
other factors play a role in the suppression of CD8+ T
lymphocytes. Inhibition of PD-1/PD-L signaling may not solely
account for the number of CD8+ T lymphocytes. Therefore,
it was concluded that the number of CD8+ T lymphocytes
exhibited no significant differences between the AFP-positive and
AFP-negative groups.
It has been proposed that upregulation of IL-10
expression may be a potential mechanism by which GR exerts its
beneficial effects (62).
Therefore, the association of the −592 polymorphism, within the
promoter of the IL-10 gene, with the GR gene was examined.
Increased luciferase activity was noted due to the presence of the
IL-10-592 promoter. The results indicated that there were no
significant differences in luciferase activity between the cells
with the mutant IL-10-592 promoter and the cells with the wild-type
IL-10-592 promoter. Moreover, it has been previously shown that GR
recognizes a specific nucleotide sequence in the HBV genome and
that ERK-mediated phosphorylation of the GR modulates the
expression of IL-10 following infection of dendritic cells with HBV
(38,65). In addition, the binding site of the
GR with IL-10 is a factor that has to be considered. The results
suggested that the expression levels of IL-10 were increased by the
association of the GR with the IL-10 promoter, regardless of the
presence of the polymorphism. LMR could be affected by other
diseases, including diabetes mellitus (63). Therefore, the association of the GR
with the IL-10 promoter in HCC requires further investigation to
exclude the influence of diabetes mellitus and other factors.
In conclusion, AFP-negative HCC patients exhibited
higher LMR and lower levels of D-dimer and FPD compared with those
noted in AFP-positive HCC patients. The number of CD4+
and CD8-PD-1 T lymphocytes and the expression levels of IL-10 in
the serum of HCC patients were consistent with the expression
levels of AFP. The latter may be involved with the secretion of
IL-10 via PD-1/PD-L signaling, thereby inhibiting the proliferation
of CD8+ T lymphocytes. Furthermore, IL-10 expression was
associated with GR regardless of the presence of the polymorphism.
In conclusion, the number of peripheral blood T lymphocytes, the
expression of PD-1 and the content of AFP could be used as
prognostic indicators for patients with liver cancer, which is
essential for understanding the immunopathological mechanisms of
liver cancer and for the development of effective therapeutic
strategies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Foundation for the
National Science Foundation of China (grant no. 81401728).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW designed the study. HW, SL and XL wrote the
manuscript. HW and YX performed the reverse
transcription-quantitative PCR experiments and luciferase activity
assay. LL and SL performed the case data analysis. XL and HW
performed the ELISA and flow cytometry experiments. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the ethics
committee of the Institutional Review Board of Chongqing Medical
University First Affiliated Hospital. (Clinical trial registration
no.: 2014-71). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakamoto Y: Promising new strategies for
hepatocellular carcinoma. Hepatol Res. 47:2673–265. 2017.
View Article : Google Scholar
|
|
2
|
Pu C, Jiang C, Lang L, Hui H, Wang Z, Ma D
and Zhang Y: Combination of microRNAs and cytokines: A method for
better evaluation of acute-on-chronic liver failure. Clin Lab.
64:247–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Yang J, Xing Z, Zhang H, Wen Y, Qi
F, Zuo Z, Xu J and Yao Z: IL-4 mediates the delayed neurobehavioral
impairments induced by neonatal hepatitis B vaccination that
involves the down-regulation of the IL-4 receptor in the
hippocampus. Cytokine. 110:137–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tao NN, Gong R, Chen X, He L, Ren F, Yu
HB, Chen J and Ren JH: Interleukin-35 stimulates hepatitis B virus
transcription and replication by targeting transcription factor
HNF4α. J Gen Virol. 99:645–654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao QJ, Liu DW, Zhang SY, Jia M, Wang LM,
Wu LH, Wang SY and Tong LX: Polymorphisms of some cytokines and
chronic hepatitis B and C virus infection. World J Gastroenterol.
15:5610–5619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Guo H, Gao F, Shan Q, Li J, Xie H,
Zhou L, Xu X and Zheng S: Fibrinogen and D-dimer levels elevate in
advanced hepatocellular carcinoma: High pretreatment fibrinogen
levels predict poor outcomes. Hepatol Res. 47:1108–1117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bowyer C, Lewis AL, Lloyd AW, Phillips GJ
and Macfarlane WM: Hypoxia as a target for drug combination therapy
of liver cancer. Anticancer Drugs. 28:771–780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pesapane F, Nezami N, Patella F and
Geschwind JF: New concepts in embolotherapy of HCC. Med Oncol.
34:582017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou X, Chen J, Chen L, Feng X, Liu Z, Hu
L, Zeng Z, Jia X, Liang M, Shi B, et al: Negative regulation of
Sirtuin 1 by AMP-activated protein kinase promotes
metformin-induced senescence in hepatocellular carcinoma
xenografts. Cancer Lett. 411:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia W, Wu J, Jia H, Yang Y, Zhang X, Chen
K and Su F: The peripheral blood Neutrophil-to-Lymphocyte ratio is
superior to the Lymphocyte-to-Monocyte ratio for predicting the
long-term survival of triple-negative breast cancer patients. PLoS
One. 10:e01430612015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang Y, Fu Q, Xu L, Zhou L, Liu Z, Yang
Y, Lin Z and Xu J: Prognostic value of preoperative lymphocyte to
monocyte ratio in patients with nonmetastatic clear cell renal cell
carcinoma. Tumour Biol. 37:4613–4620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z
and Xu J: Systemic inflammation score predicts postoperative
prognosis of patients with clear-cell renal cell carcinoma. Br J
Cancer. 113:626–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YM, Lai CH, Chang HC, Chao TY, Tseng
CC, Fang WF, Wang CC, Chung YH, Wang YH, Su MC, et al: Baseline and
trend of Lymphocyte-to-Monocyte ratio as prognostic factors in
epidermal growth factor receptor mutant non-small cell lung cancer
patients treated with first-line epidermal growth factor tyrosine
kinase inhibitors. PLoS One. 10:e01362522015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H,
et al: Prognostic significance of the lymphocyte-to-monocyte ratio
in patients with metastatic colorectal cancer. World J
Gastroenterol. 21:9966–9973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Memon K, Kulik L, Lewandowski RJ, Wang E,
Ryu RK, Riaz A, Nikolaidis P, Miller FH, Yaghmai V, Baker T, et al:
Alpha-fetoprotein response correlates with EASL response and
survival in solitary hepatocellular carcinoma treated with
transarterial therapies: A subgroup analysis. J Hepatol.
56:1112–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao B, Yang FM, Yu ZT, Li R, Xie F, Chen
J, Luo HJ and Zhang JC: Relationship between the expression of MDR1
in hepatocellular cancer and its biological behaviors. Int J Clin
Exp Pathol. 8:6995–7001. 2015.PubMed/NCBI
|
|
17
|
Ji X, Shen Y, Sun H and Gao X: A novel
anti-alpha-fetoprotein single-chain variable fragment displays
anti-tumor effects in HepG2 cells as a single agent or in
combination with paclitaxel. Tumour Biol. 37:10085–10096. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang XH, Yamagiwa S, Ichida T, Matsuda Y,
Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA and Aoyag Y:
Increase of CD4+ CD25+ Regulatory T-Cells in
the liver of patients with hepatocellular carcinoma. J Hepatol.
45:254–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X and Wang Q: Alpha-fetoprotein and
hepatocellular carcinoma immunity. Can J Gastroenterol Hepatol.
2018:90492522018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen Y, Wei Y, Wang Z, Jing Y, He H, Yuan
J, Li R, Zhao Q, Wei L, Yang T and Lu J: TGF-β regulates
hepatocellular carcinoma progression by inducing Treg cell
polarization. Cell Physiol Biochem. 35:1623–1632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dinney CM, Zhao LD, Conrad CD, Duker JM,
Karas RO, Hu Z, Hamilton MA, Gillis TR, Parker TM, Fan B, et al:
Regulation of HBV-specific CD8(+) T Cell-mediated inflammation is
diversified in different clinical presentations of HBV infection. J
Microbiol. 53:718–724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue H, Lin F, Tan H, Zhu ZQ, Zhang ZY and
Zhao L: Overrepresentation of IL-10-Expressing B Cells Suppresses
Cytotoxic CD4+ T cell activity in HBV–Induced
hepatocellular carcinoma. PLoS One. 11:e01548152016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmadabadi BN, Hassanshahi G, Arababadi
MK, Leanza C and Kennedy D: The IL-10 promoter polymorphism at
position −592 is correlated with susceptibility to occult HBV
infection. Inflammation. 35:818–821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liaw YF and Chu CM: Hepatitis B virus
infection. Lancet. 373:582–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan JL, Chen YM, Hsieh TY, Chen YH, Hsieh
CW, Chen DY and Yang SS: Kinetics of viral loads and risk of
hepatitis B virus reactivation in hepatitis B core
antibody-positive rheumatoid arthritis patients undergoing
anti-tumour necrosis factor alpha therapy. Ann Rheum Dis.
70:1719–1725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chemin I and Trépo C: Clinical impact of
Occult HBV Infections. J Clin Virol. 34 (Suppl 1):S15–S21. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
European Association For The Study Of The
Liver, . EASL clinical practice guidelines: Management of chronic
hepatitis B. J Hepatol. 50:227–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raimondo G, Allain JP, Brunetto MR,
Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta
GB, et al: Statements from the Taormina expert meeting on occult
hepatitis B virus infection. J Hepatol. 49:652–657. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raimondo G, Caccamo G, Filomia R and
Pollicino T: Occult HBV Infection. Semin Immunopathol. 35:39–52.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmeltzer P and Sherman KE: Occult
hepatitis B: Clinical implications and treatment decisions. Dig Dis
Sci. 55:3328–3335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen XP, Long X, Jia WL, Wu HJ, Zhao J,
Liang HF, Laurence A, Zhu J, Dong D, Chen Y, et al: Viral
integration drives multifocal HCC during the Occult HBV Infection.
J Exp Clin Cancer Res. 38:2612019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muto J, Sugiyama M, Shirabe K, Mukaide M,
Kirikae-Muto I, Ikegami T, Yoshizumi T, Yamashita YI, Maehara Y and
Mizokami M: Frequency and characteristics of occult hepatitis B
infection among hepatocellular carcinoma patients in Japan. Ann
Hepatol. 17:596–603. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sagnelli C, Macera M, Pisaturo M, Zampino
R, Coppola M and Sagnelli E: Occult HBV Infection in the
oncohematological setting. Infection. 44:575–582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Makvandi M: Update on occult hepatitis B
virus infection. World J Gastroenterol. 22:8720–8734. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schalm SW, Summerskill WH, Gitnick GL and
Elveback LR: Contrasting features and responses to treatment of
severe chronic active liver disease with and without hepatitis BS
antigen. Gut. 17:781–786. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Giqiang W, Fusheng W, Jun C, et al: The
guideline of prevention and treatment for chronic hepatitis B (2015
version). J Practical Hepatol. 19:389–400. 2016.
|
|
37
|
Cong WM, Bu H and Chen J, Dong H, Zhu YY,
Feng LH and Chen J; Guideline Committee, : Practice Guidelines for
the pathological diagnosis of primary liver cancer: 2015 Update.
World J Gastroenterol. 22:9279–9287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ng SS, Li A, Pavlakis GN, Ozato K and Kino
T: Viral infection increases glucocorticoid-induced interleukin-10
production through ERK-mediated phosphorylation of the
glucocorticoid receptor in dendritic cells: Potential clinical
implications. PLoS One. 8:e635872013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chasserot-Golaz S, Beck G and Venetianer
A: Inhibition of growth by the antihormone RU486 in different
hepatoma cell lines. Mol Cell Endocrinol. 82:151–158. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mitre-Aguilar IB, Barrios-Garcia T,
Ruiz-Lopez VM, Cabrera-Quintero AJ, Mejia-Dominguez NR,
Ventura-Gallegos JL, Moreno-Mitre D, Aranda-Gutierrez A,
Mejia-Rangel J, Escalona-Guzman AR, et al: Glucocorticoid-dependent
expression of IAP participates in the protection against
TNF-mediated Cytotoxicity in MCF7 Cells. BMC Cancer. 19:3562019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bihari C, Rastogi A, Shasthry SM, Bajpai
M, Bhadoria AS, Rajesh S, Mukund A, Kumar A and Sarin SK: Platelets
contribute to growth and metastasis in hepatocellular carcinoma.
APMIS. 124:776–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin J, Niu X, Zou L, Li L, Li S, Han J,
Zhang P, Song J and Xiao F: AFP mRNA level in enriched circulating
tumor cells from hepatocellular carcinoma patient blood samples is
a pivotal predictive marker for metastasis. Cancer Lett. 378:33–37.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang SL, Liu LP, Yang S, Liu L, Ren JW,
Fang X, Chen GG and Lai PB: Preoperative serum α-fetoprotein and
prognosis after hepatectomy for hepatocellular carcinoma. Br J
Surg. 103:716–724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pang Q, Qu K, Bi JB, Liu SS, Zhang JY,
Song SD, Lin T, Xu XS, Wan Y, Tai MH, et al: Thrombocytopenia for
prediction of hepatocellular carcinoma recurrence: Systematic
review and meta-analysis. World J Gastroenterol. 21:7895–7906.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim HK, Lee KR, Yang JH, Yoo SJ, Lee SW,
Jang HJ, Park SJ, Moon YS, Park JW and Kim CM: Plasma levels of
D-dimer and soluble fibrin polymer in patients with hepatocellular
carcinoma: A possible predictor of tumor thrombosis. Thromb Res.
109:125–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu LR, Li J, Chen P, Jiang Q and Tang XP:
Clinical significance of plasma fibrinogen and D-dimer in
predicting the chemotherapy efficacy and prognosis for small cell
lung cancer patients. Clin Transl Oncol. 18:178–188. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Desch A, Gebhardt C, Utikal J and
Schneider SW: D-dimers in malignant melanoma: Association with
prognosis and dynamic variation in disease progress. Int J Cancer.
140:914–921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang T, Zhu J, Zhao L, Mai K, Ye J, Huang
S and Zhao Y: Lymphocyte to monocyte ratio and neutrophil to
lymphocyte ratio are superior inflammation-based predictors of
recurrence in patients with hepatocellular carcinoma after hepatic
resection. J Surg Oncol. 115:718–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu SJ, Lin YX, Ye H, Li FY, Xiong XZ and
Cheng NS: Lymphocyte to monocyte ratio and prognostic nutritional
index predict survival outcomes of hepatitis B virus-associated
hepatocellular carcinoma patients after curative hepatectomy. J
Surg Oncol. 114:202–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zubkova E, Semenkova L, Dudich E, Dudich
I, Parfyonova Y and Menshikov M: Alpha-fetoprotein contributes to
THP-1 cell invasion and chemotaxis via protein kinase and
Gi-protein-dependent pathways. Mol Cell Biochem. 379:283–293. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH,
Wang H, Cai SH, Yang X, Xie D, Zhang CZ and Yun JP: HBx-mediated
decrease of AIM2 contributes to hepatocellular carcinoma
metastasis. Mol Oncol. 11:1225–1240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Z, Guo H, Gao F, et al: Fibrinogen and
D-dimer levels elevate in advanced hepatocellular carcinoma: High
pretreatment fibrinogen levels predict poor outcomes. Hepatol Res.
47:1108–1117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hong YF, Chen ZH, Wei L, Ma XK, Li X, Wen
JY, Wang TT, Cai XR, Wu DH, Chen J, et al: Identification of the
prognostic value of lymphocyte-to-monocyte ratio in patients with
HBV-associated advanced hepatocellular carcinoma. Oncol Lett.
14:2089–2096. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Meng W, Bai B, Bai Z, Li Y, Yue P, Li X
and Qiao L: The immunosuppression role of alpha-fetoprotein in
human hepatocellular carcinoma. Discov Med. 21:489–494.
2016.PubMed/NCBI
|
|
56
|
Sun L, Xu G, Liao W, Yang H, Xu H, Du S,
Zhao H, Lu X, Sang X and Mao Y: Clinicopathologic and prognostic
significance of regulatory T cells in patients with hepatocellular
carcinoma: A meta-analysis. Oncotarget. 8:39658–39672. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Strauss L, Bergmann C and Whiteside TL:
Human circulating Cd4+Cd25 high foxp3+
regulatory T cells kill autologous Cd8+ but Not
Cd4+ responder cells by fas-mediated apoptosis. J
Immunol. 182:1469–1480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tembhre MK, Parihar AS, Sharma VK, Sharma
A, Chattopadhyay P and Gupta S: Alteration in regulatory T cells
and programmed cell death 1-expressing regulatory T cells in active
generalized vitiligo and their clinical correlation. Br J Dermatol.
172:940–950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang
JY, Yang YP, Tien P and Wang FS: PD-1 and PD-L1 upregulation
promotes CD8(+) T-cell apoptosis and postoperative recurrence in
hepatocellular carcinoma patients. Int J Cancer. 128:887–896. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu KS, Fan XQ, Zhang L, Wen QN, Feng JH,
Chen FC, Luo JM and Sun WB: Effects of recombinant human
interleukin-10 on Treg cells, IL-10 and TGF-β in transplantation of
rabbit skin. Mol Med Rep. 9:639–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen L, Zhang L, Zhu Z, He W, Gao L, Zhang
W, Liu J and Huang A: Effects of IL-10- and FasL-overexpressing
dendritic cells on liver transplantation tolerance in a heterotopic
liver transplantation rat model. Immunol Cell Biol. 97:714–725.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sawant DV, Yano H, Chikina M, Zhang Q,
Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, et al: Adaptive
plasticity of IL-10 + and IL-35 + T
reg cells cooperatively promotes tumor T cell
exhaustion. Nat Immunol. 20:724–735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Guo-he L, Jun W, Shu-hong L, et al: The
relationship between TGF-1 expression and Treg infiltration in
liver Cancer tissue and its clinical significance. Chin J Cancer.
29:442–447. 2010.
|
|
64
|
Lee JS, Scandiuzzi L, Ray A, Wei J,
Hofmeyer KA, Abadi YM, Loke P, Lin J, Yuan J, Serreze DV, et al:
B7× in the periphery abrogates pancreas-specific damage mediated by
self-reactive CD8 T cells. J Immunol. 189:4165–4174. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tur-Kaspa R, Shaul Y, Moore DD, Burk RD,
Okret S, Poellinger L and Shafritz DA: The glucocorticoid receptor
recognizes a specific nucleotide sequence in hepatitis B virus DNA
causing increased activity of the HBV enhancer. Virology.
167:630–633. 1988. View Article : Google Scholar : PubMed/NCBI
|