Introduction
Glucose is an essential nutrient in the human body,
and its uptake via food results in energy production that is used
by various tissues, having been delivered via the general
circulation after it is absorbed in the intestine (1). The peak of the blood glucose level
(BGL) after a meal, as opposed to that after fasting, is usually
limited to 30–50 mg/dl, and this phenomenon is based on the net
balance between the rate of carbohydrate being absorbed from the
gastrointestinal tract and the rate at which it is taken up by the
liver and peripheral tissues (1).
Moreover, the disturbance of the net balance results in
uncontrolled glucose regulation and induces hyperglycemia, which is
widely recognized as a causal link between diabetes and
diabetes-related complications (2).
α-glucosidase is a key enzyme that plays a role in
glucose absorption in the gastrointestinal tract, and inhibition of
its activity induces the prevention of hyperglycemia (3). In addition, α-glucosidase inhibitors
such as acarbose, voglibose and miglitol have been used as
medicines for type 2 diabetes (1,4).
Furthermore, a similar effect is observed in natural resources. For
instance, vegetables in the daily diet potentially inhibit
α-glucosidase activity (5,6). Traditional natural products including
herbs and their phytochemicals are also known for their
α-glucosidase-inhibiting activities, and some of these products are
already sold as functional foods (7,8). Our
previous studies have focused on the biological activity and safety
of natural products, including food, traditional herbs, kampo and
their phytochemicals (9–12).
To identify the presence of an α-glucosidase
inhibitory effect in herbs, in which various functional effects
have been reported, the present study investigated the effects of
hot-water extracts of 26 types of herbs on α-glucosidase activity
in an in vitro assay. In addition, the effects of bearberry,
green tea and coltsfoot hot-water extracts on α-glucosidase
activity in vivo were evaluated according to the BGLs in
maltose- and glucose-load model rats.
Materials and methods
Materials
Acarbose, maltose, glycerol and Glucose CII-test
Wako kit were purchased from Wako Pure Chemical Industries, Ltd.,
unless otherwise stated. All herbs were purchased from various
companies (Table I). Intestinal
acetone powders from rat were purchased from Sigma-Aldrich (Merck
KGaA).
 | Table I.List of herb information. |
Table I.
List of herb information.
| Common name | Scientific
name | Family name | Site | Production
areas | Sales company | Yield (%) |
|---|
| Garlic | Allium
sativum |
Liliaceae | Bulb | Unknown | Mizkan Nakanos Co.,
Ltd. | 59.0 |
|
Burdocka | Arctium
lappa |
Asteraceae | Root | China | Connect Co., Ltd.
(https://www.enherb.jp/) | 50.3 |
| Bearberry | Arctostaphylos
uva-ursi |
Ericaceae | Leaf | Spain | Mono Co., Ltd. | 27.3 |
| Red rooibos | Aspalathus
linearis |
Leguminosae | Leaf | South Africa | Nasukogen HERB's
Co., Ltd. (http://www.n-park.jp/) | 10.7 |
| Green Tea | Camellia
Sinensis |
Theaceae | Leaf | Japan | Yamamoto nouen | 17.5 |
| Hempa | Cannabis
sativa |
Moraceae | Seed | China | Ohtsuyashop Co.,
Ltd. (http://ohtsuya.com/) | 9.2 |
| Cayenne pepper | Capsicum
annuum |
Solanaceae | Fruit | China | Ohtsuyashop Co.,
Ltd. (http://ohtsuya.com/) | 23.2 |
| Cinnamon | Cinnamomum
verum |
Lauraceae | Bark | China | SB foods Co., Ltd.
(https://www.sbfoods.co.jp/) | 11.2 |
| Bitter orange
peela | Citrus
aurantium |
Rutaceae | Hull | Paraguay | Yuwn, Inc.
(http://company.yuwn.com/) | 31.4 |
| Lemon
grassa | Cymbopogon
citratus | Poaceae | Leaf | India | Tree of life Co.,
Ltd. (https://www.treeoflife.co.jp/) | 8.5 |
| Heath | Erica
vulgaris |
Ericaceae | Leaf &
flower | Poland | Nasukogen HERB's
Co., Ltd. (http://www.n-park.jp/) | 6.7 |
| Eucalyptus | Eucalyptus
globulus |
Myrtaceae | Leaf | Australia | Hyakka-Saen. Co.,
Ltd. (http://www.hyakka-saen.com/) | 18.2 |
| Bladderwrack | Fucus
vesiculosus | Fucus | Thallus | Canada | NATURAL LIFE Co.,
Ltd. | 27.0 |
| Soybean | Glycine
max |
Leguminosae | Seed | Japan | Mitake Food
Manufacturing Co., Ltd. (http://official.mitake-shokuhin.co.jp/) | 29.5 |
| Maitake | Grifola
frondosa |
Polyporaceae | Fruiting body | Japan | Natural health Co.,
Ltd. | 24.3 |
| Maca | Lepidium
meyenii |
Brassicaceae | Tuber | Peru | Marukai Corporation
Co., Ltd. (http://www.marukai.co.jp/) | 28.1 |
| Melilot | Melilotus
officinalis |
Leguminosae | Terrestrial | Bulgaria | Connect Co., Ltd.
(https://www.enherb.jp/) | 18.1 |
| Arhat Fruit | Momordica
grosvenorii |
Cucurbitaceae | Fruit | China | NATURAL LIFE Co.,
Ltd. | 31.6 |
| Olive | Olea europaea | Oleaceae | Leaf | Japan | Hyakka-Saen. Co.,
Ltd. (http://www.hyakka-saen.com/) | 24.1 |
| American
ginsenga | Panax
quinquefolius | Araliaceae | Root | America | Kouyou
International Commercial Co., Ltd. (https://www.rakuten.co.jp/yanwo/) | 26.1 |
|
Psylliuma | Plantago Ovata | Plantaginaceae | Seed | Thailand | RST spices Shop
(https://rstspices.com/) | 1.8 |
| Blackcurrant | Ribes nigrum |
Grossulariaceae | Leaf | France | Purpurea
(http://www.purpurea.jp/) | 23.4 |
| White willow | Salix alba | Salicaceae | Bark | Poland | Connect Co., Ltd.
(https://www.enherb.jp/) | 11.5 |
| Pau d'arco | Tabebuia
avellanedae | Bignoniaceae | Bark | Brazil | TFI-Yokohama F1
Co., Ltd. | 10.7 |
| Coltsfoota | Tussilago
farfara | Asteraceae | Leaf | France | Aromafrance Co.,
Ltd. (https://www.aromafrance.net/) | 14.4 |
| Corn tea | Zea mays | Poaceae | Fruit/seed | Japan | Honjien Co., Ltd.
(https://www.honjien.co.jp/) | 8.6 |
Preparation of hot-water extract from
herbs
The extracts were prepared from herbs based on a
previously described method (9).
Briefly, 1 g each herb was decocted with 20 ml Milli-Q water at
100°C for 30 min. After the extracts were cooled and filtered, they
were used in animal experiments as described below. Furthermore, to
assess α-glucosidase activity, the extracts were evaporated using a
freeze dryer, after which the dried samples were weighed and
dissolved or suspended at a concentration of 100 mg/ml in Milli-Q
water and stored at −20°C until further use.
Measurement of α-glucosidase activity
in vitro
α-glucosidase activity was measured according to a
previously described method with modifications (13). Briefly, 10 µl of 72.5 mg/ml
intestinal acetone powder suspension in 50 mM Tris-HCl (pH 7.8)
containing 20% glycerol or Milli-Q water (blank), 5 µl of 5.17
µg/ml (8 µM) acarbose (final concentration: 0.52 µg/ml, as a
positive control), 26 types of herb extracts (Table I) [concentrations: 2.5, 5.0, 7.5
and 10 mg/ml (final concentrations: 250, 500, 750 and 1,000 µg/ml,
respectively)] and Milli-Q water (control or blank) and 27 µl
Milli-Q water were mixed in 1.5 ml tubes, and then pre-incubated in
a heat block at 37°C for 10 min. Subsequently, 8 µl of 500 mM
maltose monohydrate solution in Milli-Q water (final concentration:
80 mM) was added and incubated in a heat block at 37°C for 30 min.
The tubes were incubated at 100°C for 2 min to terminate the
reaction. To assess the amount of glucose production, a Glucose
CII-test Wako kit was used according to the manufacturer's protocol
with modifications. Samples were centrifuged at 20,400 × g for 5
min, and 2 µl supernatants and 100 µl chromogenic solution were
mixed and incubated in a 96-well plate at 37°C for 5 min.
Subsequently, the glucose levels of the samples were determined by
measuring the absorbance at 505 nm using a microtiter plate reader,
SpectraMax Pro M5e (Molecular Devices, LLC). The relative
α-glucosidase inhibitory activity (% of inhibition) was calculated
using the following equation:
% of inhibition=[(A505 control-A505
blank)-(A505 sample-A505
blank)]/(A505 control-A505 blank),
where A505 sample denotes the positive
control or herb extract samples.
The IC50 values were calculated from the
relative α-glucosidase inhibitory activity curve.
Animals
All experiments and the care and handling of animals
were approved by the Josai University Institutional Animal Care and
Use Committee. In total, 48 male Wistar rats (age, 5 weeks; weight,
140–160 g), were obtained from CLEA Japan, Inc. The rats were
housed in 16 cases, with three rats per cage. Animals were housed
in a 12-h light/dark cycle and maintained at a constant temperature
of 22±2°C and humidity of 55±10%. The rats were allowed 1 week to
adapt to the laboratory environment before the experiments and fed
laboratory pellet chow (CE-2; CLEA Japan, Inc.) and water ad
libitum. After the experiments had been completed, all rats
were euthanized via the intraperitoneal injection of sodium
pentobarbital (150 mg/kg).
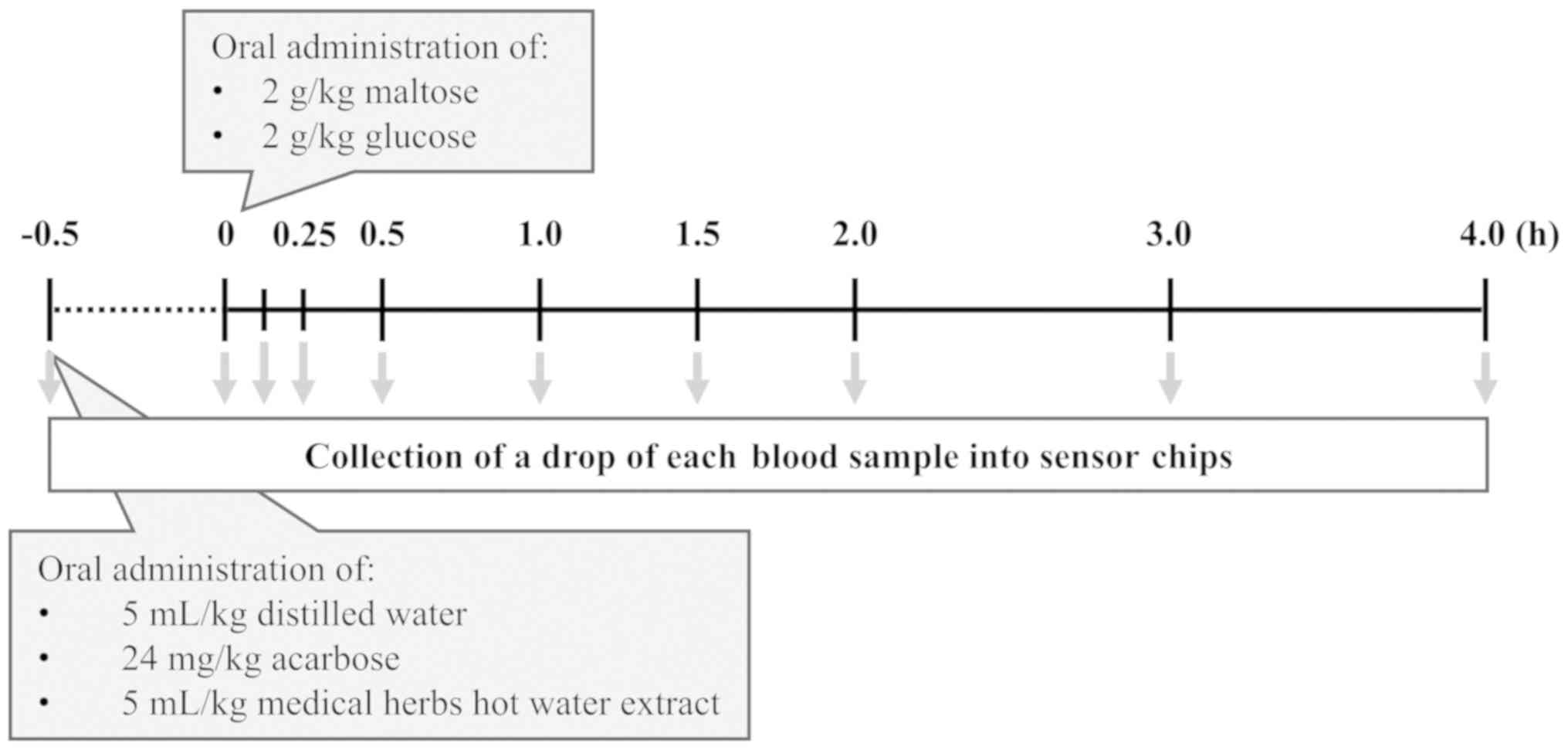
To investigate the antihyperglycemic effects based
on α-glucosidase inhibition in rats, an oral glucose tolerance test
(OGTT) was performed based on a previously published method with
modifications (14). The access to
pellet chow supplied to the rats was denied the night before the
experiment. The rats were then randomly divided into five groups:
Control (n=6); acarbose (24 mg/kg, n=6); bearberry (~13.7 mg/ml,
n=6); green tea (~8.8 mg/ml, n=6); and coltsfoot (~7.2 mg/ml, n=6).
All groups were orally administrated with distilled water
(control), each of three herb extracts or acarbose (5 ml/kg,
respectively) 0.5 h before oral administration of 2 g/kg maltose or
glucose. A diagram of the timeline of the experiment is presented
in Fig. 1.
Measurement of BGLs
Blood samples (~10 µl) were collected from all
groups into sensor chips (Breeze2 sensor; Panasonic Corporation)
sequentially at 0.083, 0.25, 0.5, 1.0, 1.5, 2.0, 3.0 and 4.0 h via
a small incision in the tail vein using a razor blade. Then, the
blood samples were measured using a glucometer (Breeze2; Bayer Thai
Co., Ltd.). The area under the curve (AUC) was calculated using
GraphPad Prism Ver 8.1.2 (GraphPad Software, Inc.) for subsequent
analysis.
Statistical analysis
Statistical analysis was performed with GraphPad
Prism Ver 8.1.2 (GraphPad Software, Inc.) using a one-way ANOVA
(in vitro studies) followed by Dunnett's test for multiple
comparisons and a repeated-measures one-way ANOVA or a
repeated-measures two-way ANOVA (in vivo studies) followed
by Bonferroni's multiple comparison test. P<0.05 was considered
to indicate a statistically significant difference. Data from the
animal experiments are presented as the mean ± SEM or mean ± 95% CI
(n=6, respectively), and the other data as the mean ± SD of three
separate experiments.
Results
Dose-dependent inhibitory effects of
acarbose on α-glucosidase activity
To assess the effects of acarbose on α-glucosidase
inhibitory activity and to calculate the IC50 value of
acarbose, α-glucosidase activity was assessed in vitro. It
was demonstrated that α-glucosidase inhibitory activity
significantly increased in a dose-dependent manner (Fig. 2A). Furthermore, the IC50
value was 0.52 µg/ml (95% CI, 0.4356-0.6047;
R2=0.9528).
Inhibitory effect of herb extracts on
α-glucosidase activity
To investigate the α-glucosidase inhibitory effect
of the 26 types of 1,000 µg/ml herb extracts, α-glucosidase
activity was assessed in vitro (Fig. 2B). Overall, >50% inhibition of
α-glucosidase activity was significantly demonstrated in 0.52 µg/ml
acarbose, which served as the positive control, and in 1,000 µg/ml
coltsfoot, green tea and bearberry extracts. Moreover, significant
increases in the inhibition of α-glucosidase were observed in 1,000
µg/ml olive (P<0.01), white willow (P<0.01) and red rooibos
extracts (P<0.05). However, significant decreases were observed
in 1,000 µg/ml hemp, cayenne pepper and bitter orange peel extracts
(P<0.01; Fig. 2B).
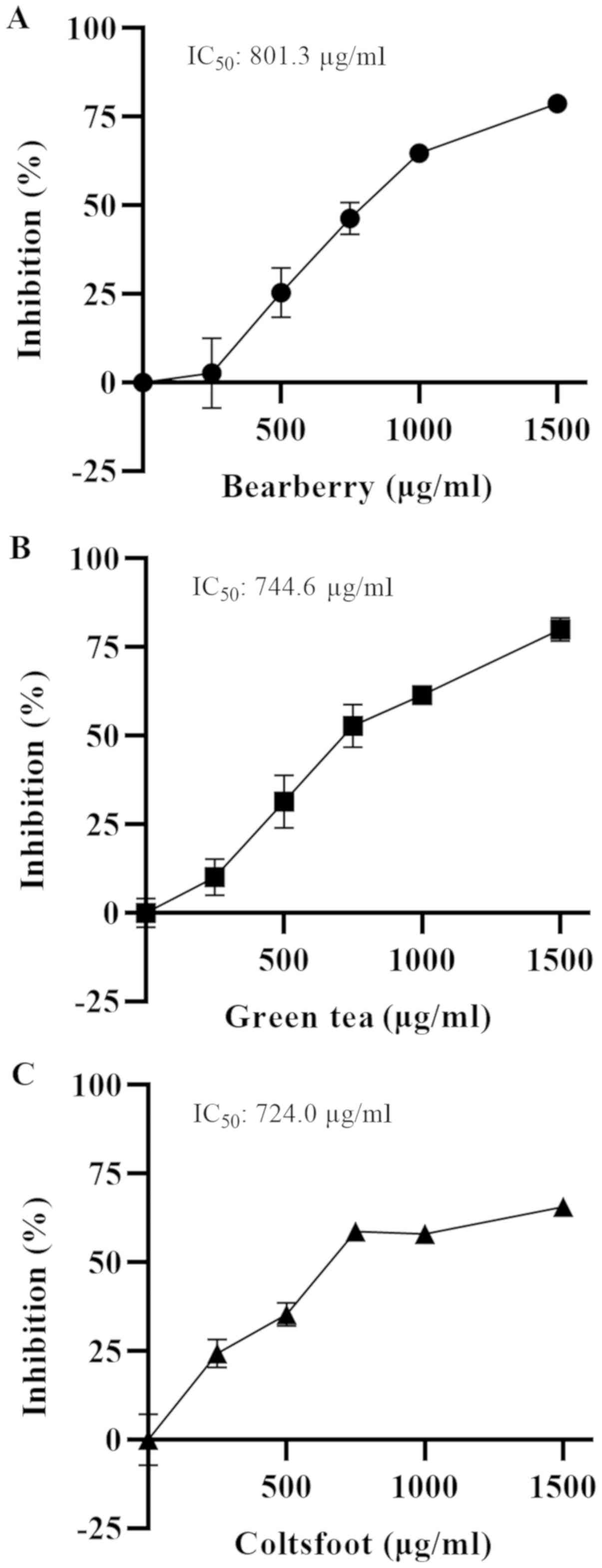
To calculate the IC50 values of the three
herb extracts, the dose-dependent inhibition of α-glucosidase
activity was assessed (Fig. 3).
The IC50 values were 801.3 (bearberry; 95% CI,
751.9-854.0; R2=0.9739; Fig. 3A), 744.6 (green tea; 95% CI,
696.7-795.1, R2=0.9767; Fig. 3B) and 724.0 µg/ml (coltsfoot; 95%
CI, 641.9-818.1; R2=0.9538; Fig. 3C).
Effects of bearberry, green tea and
coltsfoot extracts on BGLs in rats
All rats were healthy before the experiments, and
adverse events were not observed during the experiments. It was
revealed that treatment with 2 g/kg maltose caused temporary
hyperglycemia in the rats (maltose control group), as indicated by
a significant increase in BGLs at 0.25 (P<0.01), 0.5 (P<0.01)
and 1.0 h (P<0.05; Fig. 4A-D).
BGLs upon administration of 5 ml/kg bearberry extract were
significantly lower compared with the levels in the maltose control
group at 0.083 (P<0.05) and 0.5 h (P<0.01; Fig. 4A). Moreover, BGLs after
administration of 5 ml/kg green tea extract were significantly
lower compared with the levels in the maltose control group at 0.5
h (P<0.01; Fig. 4B). In
addition, BGLs after administration of 5 ml/kg coltsfoot extract
were significantly lower compared with the levels in the maltose
control group at 0.25 (P<0.01) and 0.5 h (P<0.01; Fig. 4C). After administration of 5 ml/kg
acarbose (24 mg/kg, positive control group) BGLs were significantly
lower compared with the levels in the maltose control group at 0.25
(P<0.01), 0.5 (P<0.01) and 1.5 h (P<0.05; Fig. 4D). Furthermore, the AUC of the
acarbose group, compared with the maltose control group, was
significantly decreased (P<0.05), but no significant differences
were observed in each of the three herb extracts (Fig. 4E).
It was demonstrated that treatment with 2 g/kg
glucose caused temporary hyperglycemia in the rats serving as the
glucose control group, as indicated by a significant increase in
BGLs at 0.083 (P<0.05), 0.25 (P<0.01), 0.5 (P<0.01), 1.0
(P<0.01) and 1.5 h (P<0.01; Fig.
5A-D). BGLs after the administration of 5 ml/kg bearberry
(Fig. 5A) and green tea extracts
(Fig. 5B) were not significantly
different compared with the levels of each time-point in the
glucose control group. Moreover, BGLs upon administration of 5
ml/kg coltsfoot extract were significantly lower compared with the
levels of each time-point in the glucose control group at 0.25
(P<0.05) and 0.5 h (P<0.05; Fig.
5C). It was indicated that the AUCs in each of the three herb
extracts were not significantly different compared with those in
the glucose control group (Fig.
5D).
Discussion
In the present study, it was demonstrated that
acarbose, an α-glucosidase inhibitor, significantly inhibited
α-glucosidase activity in vitro, indicating that acarbose
can be used as a positive control in the measurement of this
activity in vitro. In total, 6/26 herb extracts, olive,
white willow, rooibos, bearberry, green tea and coltsfoot,
significantly inhibited α-glucosidase activity in vitro.
However, 3/26 herb extracts, hemp, cayenne pepper and bitter orange
peel, induced significant α-glucosidase activation. Although the
mechanisms underlying the effects of these herbs are unknown, it
was speculated that the inherent values of the herb extracts in the
measurement of absorbance had an effect on the measurement of
α-glucosidase activity.
Olive leaf (Olea europea) is widely
recognized as a natural resource with potential beneficial effects
(15). Previous studies have
reported that 80% aqueous ethanol extract of olive significantly
inhibited BGLs in starch-intubated into fasting healthy or
streptozotocin (STZ)-injected diabetic Sprague-Dawley rats, and
treatment with olive extract was associated with a beneficial
hypoglycemic effect in patients with diabetes (16). In addition, hot water and 98%
ethanol extract was revealed to inhibit α-amylase activity, and
olive powder and its phytochemicals reduced BGLs in GK/Jcl rats
(known as type 2 diabetic rats) orally treated with starch
(17). Furthermore, hydroxytyrosol
and oleuropein, phytochemicals in olive extracts were revealed to
significantly inhibit α-glucosidase activity (18). Thus, both these previous results
and those of the present study indicate that olive extract may
induce a decrease in BGLs via an α-glucosidase inhibitory
effect.
Tea produced from rooibos (Aspalathus
lineari) leaf is generally known as ‘rooibos tea’ or ‘rooibos
tisane’ (19). Moreover, rooibos
is classified as red or green depending on the presence or absence
of fermentation treatment (19).
Pharmacologically, rooibos is traditionally used in the treatment
of asthma, colic, eczema, headache, nausea and mild depression; it
has also been used as an antihypertensive, immune stimulant,
laxative, sedative and spasmolytic agent, as well as for the
treatment of atherosclerosis and diabetes (19). A previous in vitro study
reported that 65, 75 or 85°C aqueous extracts from red (fermented)
rooibos significantly inhibited α-glucosidase activity (20). However, an in vivo study by
Ulicná et al (21) revealed
that hot aqueous extracts from fermented rooibos did not
significantly affect plasma glucose, glycated hemoglobin and
fructosamine levels in STZ-induced diabetic Wistar rats; similar
results were also reported by Ayeleso et al (22). Therefore, these studies and the
present results indicated that the hot-water extract of red rooibos
may not affect BGLs via α-glucosidase inhibitory effects in
vivo.
White willow, the white bark of Salix alba,
is known to have an anti-inflammatory effect resulting from the
suppression of prostaglandin synthesis by salicin, the main
component of white willow (23).
However, there are few experiments on the hyperglycemic and/or
α-glucosidase inhibitory effects of the white willow. The present
results indicated that the white willow extract inhibited
α-glucosidase activity in vitro; however, more detailed
in vivo experiments are required in the future.
Bearberry (Arctostaphylos uva-ursi) is one of
the most commonly used antimicrobial botanicals for the treatment
of urinary tract infections (24);
however, only a few studies have been conducted to investigate its
hyperglycemic and/or α-glucosidase inhibitory effects. Huerta et
al (25) reported that 80°C
aqueous extracts of bearberry leaves inhibited α-amylase and
α-glucosidase activity in vitro. Moreover, the present
results demonstrated that bearberry extracts had ≥50% inhibition of
α-glucosidase activity (IC50=801.3 µg/ml). However,
Swanston-Flatt et al (26)
revealed that the use of standard powdered diets containing
powdered bearberry ad libitum did not induce a reduction of
the increasing BGLs in STZ-injected adult male mice. In contrast,
in the present study, administration of bearberry extract reduced
the increasing BGLs until 0.5 h in maltose-treated rats, but not in
glucose-treated rats. Furthermore, the administration of bearberry
extract in rats did not reveal any effect on AUCs, an indicator of
glucose absorption. These differences may be because the present
study used extracts different from those used by Swanston-Flatt
et al (26). Therefore, the
details of this result may become more evident in future studies
using an animal model of diabetes. However, it can be speculated
that bearberry can inhibit α-glucosidase activity, resulting in
antihyperglycemic activity.
Camellia sinensis leaf is used to produce
various types of teas, such as green tea, black tea and oolong tea;
in particular, green tea is one of the most widely consumed
beverages in the world (27).
Previous studies on green tea, including those focusing on its
antihyperglycemic, α-glucosidase inhibitory and antidiabetic
activities, have been reported in vitro, in vivo and in
clinical research (28–33). Furthermore, the present results
demonstrated that each extract of green tea extract had ≥50%
inhibition (IC50=744.6 µg/ml), and administration of
green tea extract reduced the increasing BGLs at 0.5 h in
maltose-treated rats, but not in glucose-treated rats. However, the
administration of green tea extract in rats had no effect on AUCs.
Moreover, similar results have been revealed by Nishiumi et
al (34); OGTT in mice when
fed a control diet or high-fat diet with water, hot-water extracts
of green tea or black tea demonstrated a in reduction the BGLs.
Furthermore, the effect on AUCs was not found in control diet-fed
mice, but was reduced in high-fat diet-fed mice (34). In addition, the intake of their
extracts prevented the impairment of glucose transporter type
4-dependent glucose transport in muscle in high-fat diet-fed mice.
Yang et al (35) revealed
that weight reduction, alleviation of metabolic syndrome and risk
reduction in diabetes were only observed in individuals who consume
≥3–4 cups of tea (600–900 mg tea catechins) daily. Thus, based on
all these results it was speculated that green tea may inhibit
α-glucosidase activity, resulting in antihyperglycemic activity.
Furthermore, the various effects of green tea may reduce the risk
of diabetes.
Numerous parts of coltsfoot (Tussilago
farfara), including the flower buds, flowers, leaves and roots,
have been traditionally used for the treatment of several
conditions, such as colds, asthma, influenza, gastroenteritis
diarrhea, metabolic stimulation and wounds in China, North Africa,
Siberia and Europe (36).
Moreover, Uysal et al (37)
reported that methanolic extracts from the leaves of coltsfoot
exhibited significant α-glucosidase activity. In addition, Gao
et al (38) and Sun et
al (39) revealed that
methanolic extracts from the flower buds of coltsfoot exhibited
maltase inhibitory activity, with maltose as a substrate. However,
the effects of hot-water extract obtained from the leaves of
coltsfoot remain unknown. The present results demonstrated that
coltsfoot extract had ≥50% inhibition (IC50=724.0
µg/ml), and administration of coltsfoot extract reduced the
increasing BGLs until 0.5 h, not only in maltose-treated rats, but
also in glucose-treated rats. However, the administration of
coltsfoot extract in rats had no effect on AUCs. These results
indicated that the reducing effect of these extracts on increasing
BGLs results not only from the inhibition of α-glucosidase
activity, but also from the absorption of dietary glucose. However,
the effect of the absorption of dietary glucose requires further
examination in future experiments, such as increasing BGLs without
intestinal absorption.
In conclusion, the present results revealed that the
hot-water extracts from olive, white willow, red rooibos,
bearberry, green tea and coltsfoot inhibited α-glucosidase activity
in vitro. Moreover, bearberry, green tea and coltsfoot
extracts suppressed BGL increase following maltose administration,
due to their α-glucosidase inhibitory effects. Furthermore, it was
demonstrated that coltsfoot extract exerted inhibitory effects on
glucose absorption, as well as α-glucosidase activity.
Collectively, the present results may facilitate the understanding
of the novel functionality in traditional herbs, which will aid in
the prevention of disease onset and progression in hyperglycemia
and type 2 diabetes, among other diseases. However, to assess the
effective use of the present findings, the active substance(s) need
to be identified and the improving effects of hyperglycemia should
be examined using a diabetic animal model.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Japan Herb
Association (grant no. FY2016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS conceived this study, and with HK had full access
to all of the data in the study and took responsibility for the
integrity of the data and the accuracy of the data analysis. All
authors contributed to the design of the study. NT and SE acquired
data. HK, NT, SY and YH analyzed and interpreted the data. HK and
NT wrote the draft of the manuscript, and KS and YH revised it
critically for important intellectual content. All authors
participated in the preparation of this manuscript, revised it
critically for important intellectual content and approved the
version submitted for publication.
Ethics approval and consent to
participate
All experiments and the care and handling of animals
were approved by the Josai University Institutional Animal Care and
Use Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BGL
|
blood glucose level
|
|
AUC
|
area under the curve
|
References
|
1
|
Lebovitz HE: Alpha-glucosidase inhibitors.
Endocrinol Metab Clin North Am. 26:3525–551. 1997. View Article : Google Scholar
|
|
2
|
Rolo AP and Palmeira CM: Diabetes and
mitochondrial function: Role of hyperglycemia and oxidative stress.
Toxicol Appl Pharmacol. 212:167–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Puls W, Keup U, Krause HP, Thomas G and
Hoffmeister F: Glucosidase inhibition. A new approach to the
treatment of diabetes, obesity, and hyperlipoproteinaemia.
Naturwissenschaften. 64:536–537. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z and Ma S: Recent advances in
synthetic α-Glucosidase inhibitors. ChemMedChem. 12:819–829. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawada Y, Miura M and Gomyo T: Inhibitory
effect of vegetables, fruits and herbs on α-glucosidase in an
immobilized enzyme assay system. Food Sci Technol Res. 12:275–277.
2006. View Article : Google Scholar
|
|
6
|
Kim JS, Kwon CS, Son KH and Kim JI:
Alpha-glucosidase inhibitory activities of some wild vegetable
extracts. J Food Sci Nutr. 5:174–176. 2000.
|
|
7
|
Morikawa T, Akaki J, Ninomiya K, Kinouchi
E, Tanabe G, Pongpiriyadacha Y, Yoshikawa M and Muraoka O:
Salacinol and related analogs: New leads for type 2 diabetes
therapeutic candidates from the Thai traditional natural medicine
Salacia Chinensis. Nutrients. 7:1480–1493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benalla W, Bellahcen S and Bnouham M:
Antidiabetic medicinal plants as a source of alpha glucosidase
inhibitors. Curr Diabetes Rev. 6:247–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kikuchi H, Kogure S, Arai R, Saino K,
Ohkubo A, Tsuda T and Sunaga K: Rosehip inhibits xanthine oxidase
activity and reduces serum urate levels in a mouse model of
hyperuricemia. Biomed Rep. 6:539–544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohkubo A, Chida T, Kikuchi H, Tsuda T and
Sunaga K: Effects of tomato juice on the pharmacokinetics of
CYP3A4-substrate drugs. Asian J Pharm Sci. 12:464–469. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sunaga K, Ohkawa K, Nakamura K, Ohkubo A,
Harada S and Tsuda T: Mechanism-based inhibition of recombinant
human cytochrome P450 3A4 by tomato juice extract. Biol Pharm Bull.
35:329–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kikuchi H, Yuan B, Hu X and Okazaki M:
Chemopreventive and anticancer activity of flavonoids and its
possibility for clinical use by combining with conventional
chemotherapeutic agents. Am J Cancer Res. 9:1517–1535.
2019.PubMed/NCBI
|
|
13
|
Oki T, Matsui T and Osajima Y: Inhibitory
effect of alpha-glucosidase inhibitors varies according to its
origin. J Agric Food Chem. 47:550–553. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serra-Barcellona C, Habib NC, Honoré SM,
Sánchez SS and Genta SB: Enhydrin regulates postprandial
hyperglycemia in diabetic rats by inhibition of α-glucosidase
activity. Plant Foods Hum Nutr. 72:156–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El SN and Karakaya S: Olive tree (Olea
europaea) leaves: Potential beneficial effects on human health.
Nutr Rev. 67:632–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wainstein J, Ganz T, Boaz M, Bar Dayan Y,
Dolev E, Kerem Z and Madar Z: Olive leaf extract as a hypoglycemic
agent in both human diabetic subjects and in rats. J Med Food.
15:605–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komaki E, Yamaguchi S, Maru I, Kinoshita
M, Kakehi K, Ohta Y and Tsukada Y: Identification of anti-α-amylase
components from olive leaf extracts. Food Sci Technol Res. 9:35–39.
2003. View Article : Google Scholar
|
|
18
|
Hadrich F, Bouallagui Z, Junkyu H, Isoda H
and Sayadi S: The α-glucosidase and α-amylase enzyme inhibitory of
hydroxytyrosol and oleuropein. J Oleo Sci. 64:835–843. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McKay DL and Blumberg JB: A review of the
bioactivity of South African herbal teas: Rooibos (Aspalathus
linearis) and honeybush (Cyclopia intermedia). Phytother Res.
21:1–16. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santos JS, Deolindo CTP, Esmerino LA,
Genovese MI, Fujita A, Marques MB, Rosso ND, Daguer H, Valese AC
and Granato D: Effects of time and extraction temperature on
phenolic composition and functional properties of red rooibos
(Aspalathus linearis). Food Res Int. 89:476–487. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ulicná O, Vancová O, Bozek P, Cársky J,
Sebeková K, Boor P, Nakano M and Greksák M: Rooibos tea
(Aspalathus linearis) partially prevents oxidative stress in
streptozotocin-induced diabetic rats. Physiol Res. 55:157–164.
2006.PubMed/NCBI
|
|
22
|
Ayeleso AO, Oguntibeju OO and Brooks NL:
Impact of co-administration of red palm oil (Elaeis guineensis
Arecaceae) and rooibos (Aspalathus linearis Fabaceae) on
glycaemic parameters, liver function and key glycolytic enzymes in
diabetic rats. Trop J Pharm Res. 14:1613–1619. 2015.
|
|
23
|
Shara M and Stohs SJ: Efficacy and safety
of white willow bark (Salix alba) extracts. Phytother Res.
29:1112–1116. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Head KA: Natural approaches to prevention
and treatment of infections of the lower urinary tract. Altern Med
Rev. 13:227–244. 2008.PubMed/NCBI
|
|
25
|
Huerta V, Mihalik K, Maitin V, Crixell SH
and Vattem DA: Effect of Central/South American medicinal plants on
energy harvesting ability of the mammalian GI tract. J Med Plant
Res. 1:38–49. 2007.
|
|
26
|
Swanston-Flatt SK, Day C, Bailey CJ and
Flatt PR: Evaluation of traditional plant treatments for diabetes:
Studies in streptozotocin diabetic mice. Acta Diabetol Lat.
26:51–55. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boehm K, Borrelli F, Ernst E, Habacher G,
Hung SK, Milazzo S and Horneber M: Green tea (Camellia
sinensis) for the prevention of cancer. Cochrane Database Syst
Rev. 2009:CD0050042009.
|
|
28
|
Yang X and Kong F: Evaluation of the in
vitro α-glucosidase inhibitory activity of green tea polyphenols
and different tea types. J Sci Food Agric. 96:777–782. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Snoussi C, Ducroc R, Hamdaoui MH, Dhaouadi
K, Abaidi H, Cluzeaud F, Nazaret C, Le Gall M and Bado A: Green tea
decoction improves glucose tolerance and reduces weight gain of
rats fed normal and high-fat diet. J Nutr Biochem. 25:557–564.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chacko SM, Thambi PT, Kuttan R and
Nishigaki I: Beneficial effects of green tea: A literature review.
Chin Med. 5:132010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh J, Jo SH, Kim JS, Ha KS, Lee JY, Choi
HY, Yu SY, Kwon YI and Kim YC: Selected tea and tea pomace extracts
inhibit intestinal α-glucosidase activity in vitro and postprandial
hyperglycemia in vivo. Int J Mol Sci. 16:8811–8825. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Venables MC, Hulston CJ, Cox HR and
Jeukendrup AE: Green tea extract ingestion, fat oxidation, and
glucose tolerance in healthy humans. Am J Clin Nutr. 87:778–784.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsuneki H, Ishizuka M, Terasawa M, Wu JB,
Sasaoka T and Kimura I: Effect of green tea on blood glucose levels
and serum proteomic patterns in diabetic (db/db) mice and on
glucose metabolism in healthy humans. BMC Pharmacol. 4:182004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishiumi S, Bessyo H, Kubo M, Aoki Y,
Tanaka A, Yoshida K and Ashida H: Green and black tea suppress
hyperglycemia and insulin resistance by retaining the expression of
glucose transporter 4 in muscle of high-fat diet-fed C57BL/6J mice.
J Agric Food Chem. 58:12916–12923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang CS, Wang H and Sheridan ZP: Studies
on prevention of obesity, metabolic syndrome, diabetes,
cardiovascular diseases and cancer by tea. J Food Drug Anal.
26:1–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roeder E: Medicinal plants in Europe
containing pyrrolizidine alkaloids. Pharmazie. 50:83–98.
1995.PubMed/NCBI
|
|
37
|
Uysal S, Senkardes I, Mollica A, Zengin G,
Bulut G, Dogan A, Glamočlija J, Soković M, Lobine D and
Mahomoodally FM: Biologically active compounds from two members of
the Asteraceae family: Tragopogon dubius Scop. and
Tussilago farfara L. J Biomol Struct Dyn. 37:3269–3281.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao H, Huang YN, Gao B, Xu PY, Inagaki C
and Kawabata J: α-Glucosidase inhibitory effect by the flower buds
of Tussilago farfara L. Food Chem. 106:1195–1201. 2008.
View Article : Google Scholar
|
|
39
|
Sun J, Yu JH, Zhang JS, Song XQ, Bao J and
Zhang H: Chromane enantiomers from the flower buds of Tussilago
farfara L. and assignments of their absolute configurations.
Chem Biodivers. 16:e18005812019. View Article : Google Scholar : PubMed/NCBI
|