Introduction
Intracerebral hemorrhage (ICH), which accounts for
10–15% of all strokes, is associated with a high rate of mortality
and disability (1,2). In addition to the mass effect of
hematoma, which causes primary brain injury and potential
intracranial hypertension, secondary inflammation can affect the
outcome of patients with ICH (3,4).
Thus, inhibition of inflammation after ICH is important.
Microglia are considered crucial in the suppression
of inflammatory reactions after ICH, although only M2 microglia
have been shown to exert anti-inflammatory effects (5,6). It
was previously reported that M2 microglia can not only suppress
inflammation after ICH, but also inhibit neuroinflammation in
patients with Alzheimer's disease (AD) (7) and ischemic stroke (8). Moreover, activation of the peroxisome
proliferator-activated receptor γ (PPAR-γ) signaling pathway is
speculated to be the mechanism underlying these effects. When the
PPAR-γ signaling pathway is activated, more resting state microglia
are transformed to the M2 type (4). However, the molecular mechanism via
which M2 microglia exert their anti-inflammatory effects is not
fully understood.
The mTOR signal pathway has been revealed to have a
central role in cell metabolism, proliferation, differentiation and
development (9). In our previous
in vivo and in vitro studies, it was demonstrated
that the mTOR signaling pathway was crucial for protection against
ischemic injury, but could also exacerbate harmful inflammatory
reactions in ischemia-reperfusion injury (10–12).
Furthermore, in ICH studies, suppression of mTOR inhibits the
expression of inflammatory factors (13), although the underlying mechanism
remains to be elucidated. Thus, the role of mTOR in
neuroinflammation remains controversial.
Currently, the downstream factors of the PPAR-γ
signaling pathway that suppress inflammation in microglia remain
unknown. Although it has been reported that mTOR can regulate the
expression of inflammatory factors in the central nervous system
(14,15), few studies have elucidated the role
of the interaction between PPAR-γ and mTOR in neuroinflammatory
processes. It was hypothesized that PPAR-γ may inhibit certain
inflammatory factors, including interleukin (IL)-1β, NF-kB and
tumor necrosis factor (TNF)-α, via the mTOR signaling pathway, and
thus the aim of the present in vitro study was to examine
whether the activation of PPAR-γ can inhibit the expression of
neuroinflammatory cytokines via mTOR signaling.
Materials and methods
Cell line and cell culture
BV-2 cells, an immortalized murine microglial cell
line, were commercially obtained from the American Type Culture
Collection for use in the present study; BV-2 cells were cultured
according to manufacturer's instructions. Cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.; cat. no. C11995500CP)
supplemented with 10% (v/v) FBS (Biological Industries; cat. no.
04-001-1ACS) and 1% penicillin/streptomycin (Genom Biotech Pvt;
cat. no. GNM15140). All cells were maintained in a humidified
incubator with 95% air and 5% CO2 at 37°C. Cells were
seeded in 6-well culture plates at a density of 500,000 cells per
well.
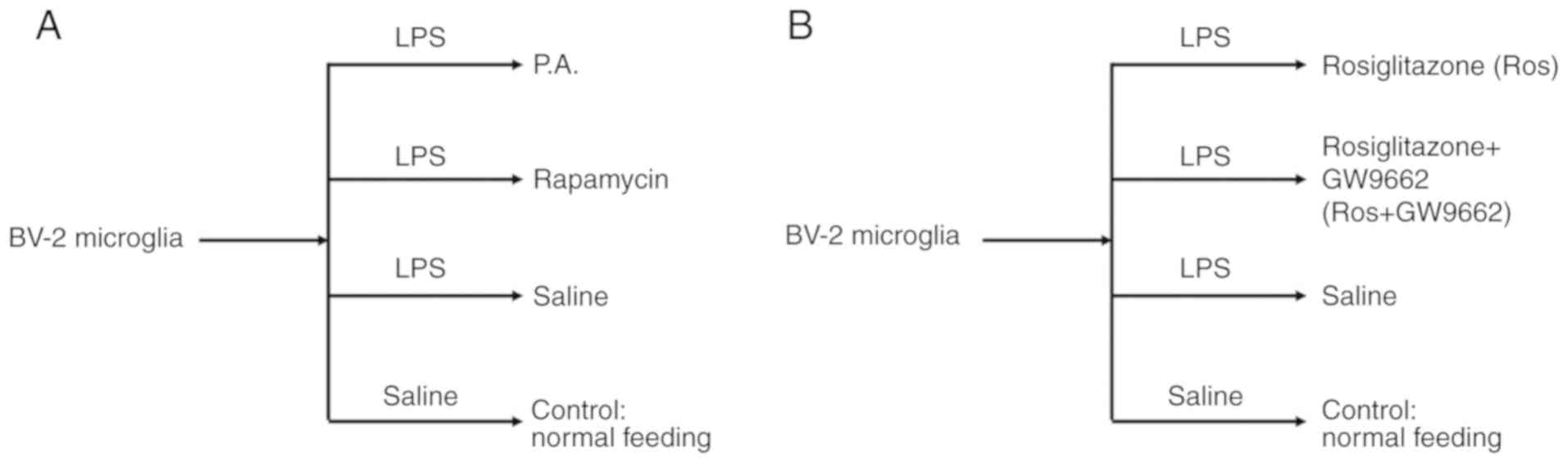
Study design and treatments
To elucidate the relationship between mTOR and the
expression levels of inflammatory factors in microglia, including
TNF-α, IL-1β and NF-κB, phosphatidic acid (P.A.; Sigma-Aldrich;
Merck KGaA; 100 nM/well) and rapamycin (EMD Millipore; 100 nM/well)
(16) were used to activate or
inhibit mTOR signal pathway for 48 h with 95% air and 5%
CO2 at 37°C, respectively. A flow chart of the current
study design is presented in Fig.
1A.
Then, to examine whether mTOR could be regulated by
the PPAR-γ signaling pathway, rosiglitazone (a PPAR-γ activator;
Sigma-Aldrich; Merck KGaA; 50 mg/ml) and GW9662 (a PPAR-γ
inhibitor; Sigma-Aldrich; Merck KGaA; 10 mg/ml) (17) were selected to interfere with the
activation of PPAR-γ (Fig. 1B).
Cells were incubated with lipopolysaccharide (LPS; Sigma-Aldrich;
Merck KGaA; 10 mM/ml) for 24 h with 95% air and 5% CO2
at 37°C to induce an inflammatory injury as previously described
(18).
Protein preparation and western blot
analysis
Protein expression levels of PPAR-γ, mTOR,
phosphorylated (p)mTOR, ribosomal protein S6 kinase (S6K), pS6K,
Toll-like receptor (TLR) 4, NF-κB, TNF-α and IL-1β were determined
using western blotting as previously described (11). In brief, Cell pellets were
homogenized in 100 µl lysis buffer (cat. no. p0013; Beyotime
Institute of Biotechnology) supplemented with 1 mM
phenylmethylsulphonyl fluoride (Sigma-Aldrich; Merck KGaA). Then,
prepared protein (50 µg) in each lane was subjected to SDS-PAGE
using 4–15% Ready Gel (cat. no. L050505A2; Bio-Rad Laboratories,
Inc.) under 200 V for 45 min. Protein bands were transferred from
the gel to PVDF (EMD Millipore) membranes under 100 V for 2 h.
After blocking with with 5% non-fat milk in Tris-buffered saline
with 0.05% Tween-20 at 4°C overnight, the membrane was incubated
with primary antibodies overnight at 4°C, followed by Alexa Fluor
488 donkey anti-rabbit (cat. no. A-21206; 1:5,000; Invitrogen;
Thermo Fisher Scientific, Inc.) or anti-rat IgG secondary antibody
(cat. no. A-21210; 1:5,000; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature in a dark room. Then, membranes
were scanned using Typhoon trio (Cytiva), visualized using an
enhanced chemiluminescence kit (Cytiva) and the optical densities
of all protein bands were analyzed using IMAGEQUANT 5.2 software
(Cytiva). All samples were run on the same gel. Protein bands were
rearranged solely to ease comparison in figures. The manufacturers,
dilutions and cat. nos. of all primary antibodies used are listed
in Table SI.
Reverse transcription-quantitative PCR
(RT-qPCR)
qPCR was performed using SYBR Green QPCR system
(Qiagen, Inc.). The protocol of RT-qPCR was described in our
previous study (12). Total RNA
was extracted from cultured microglia cells using PrepEase RNA Spin
kit (Affymetrix; Thermo Fisher Scientific, Inc.; cat. no. 78767)
according to manufacturer's protocol. Isolated RNA (1 mg) was
reversed transcribed into cDNA using Verso cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.; cat. no. AB-1453/B) according to
manufacturer's protocol. RT-PCR analyses were performed as
described previously (12). GAPDH
was used as an internal standard. The primers used for qPCR are
listed in Table SII. Expression
of each gene transcript was calculated using the 2−ΔΔCq
method and normalize to GAPDH (12).
Statistical analysis
All experiments were independently performed ≥3
times. Continuous variables are presented as the mean ± standard
deviations or as median (interquartile range). For group
comparisons, the one-ANOVA was used for continuous variables with a
normal distribution, and a one-ANOVA followed by Dunnett's post hoc
test was used for continuous variables with skewed distributions.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS 23.0
(SPSS, Inc.) and MedCalc statistical software (version 15.2.2;
MedCalc Software bvba).
Results
Suppression of mTOR signaling reduces
the expression of TNF-α and IL-1β
To examine whether inflammatory reactions are
exacerbated or alleviated by mTOR, P.A. (a mTOR agonist) and
rapamycin (a mTOR antagonist) were used to modulate the activation
of mTOR, and then the expression levels of PPAR-γ, mTOR
signaling-related proteins and inflammatory cytokines were
evaluated. The expression levels of mTOR and S6K were similar
between the groups, but the expression levels of pmTOR (P=0.039)
and pS6K (P=0.0073) were increased by P.A. treatment compared with
rapamycin, saline or control groups (Fig. 2A); this indicated that the mTOR
signaling pathway was successfully activated. It was found that
PPAR-γ expression was not significantly different between the
groups (P=0.0839). In addition, the protein expression levels of
TNF-α (P=0.028) and IL-1β (P=0.0082) were significantly lower in
the rapamycin vs. P.A. group, but there was no difference in the
expression of NF-κB among the P.A., rapamycin and saline groups.
These results suggested that some neuroinflammatory factors,
including TNF-α and IL-1β, can be suppressed when the mTOR
signaling pathway is inhibited.
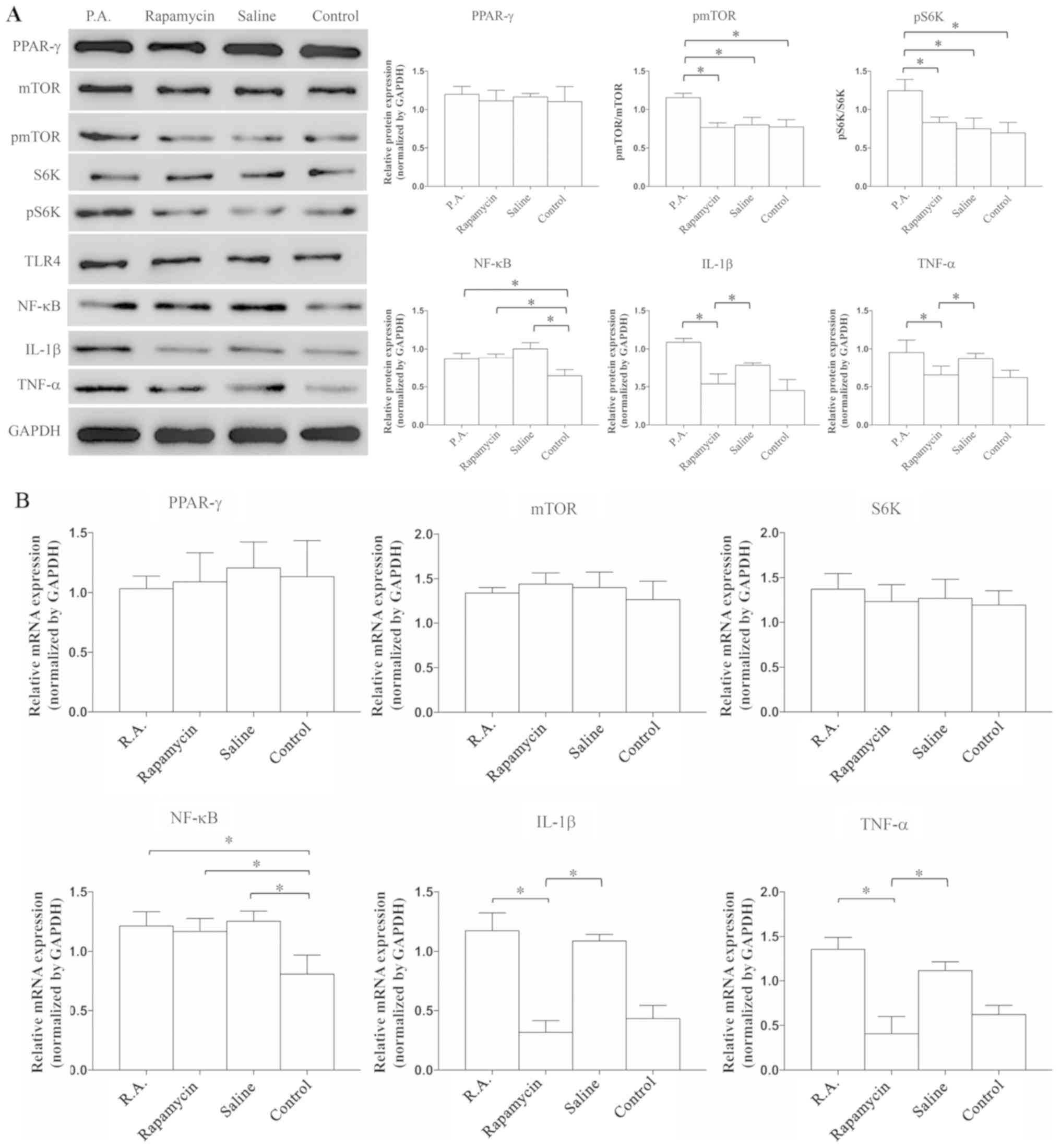 | Figure 2.mTOR suppression is able to reduce
the expression levels of TNF-α and IL-1β. Expression levels were
detected by (A) western blot analysis and (B) reverse
transcription-quantitative PCR. Expression levels of PPAR-γ, mTOR,
S6K and TLR4 were similar within the four groups. The expression of
NF-κB was significantly lower in the control group compared with
the other groups. When administrated with P.A., the protein
expression levels of pmTOR and pS6K were significantly increased.
Neuroinflammatory factors, IL-1β and TNF-α, were significantly
suppressed when treated by rapamycin. N=8-10 in ≥3 independent
experiments. *P<0.05. TNF, tumor necrosis factor; IL,
interleukin; P.A., phosphatidic acid; p, phosphorylated; TLR4,
Toll-like receptor 4; S6K, ribosomal protein S6 kinase; PPAR-γ,
proliferators-activated receptors-γ. |
RT-qPCR was used to detect the mRNA expression
levels of related proteins and inflammatory factors. The mRNA
expression levels of TNF-α (P=0.0097) and IL-1β (P=0.0053) were
significantly decreased in the rapamycin and control groups, but
the expression of NF-κB was similar among P.A, rapamycin and saline
groups (Fig. 2B). The RT-qPCR
results were in line with those of the western blot analysis.
Activation of PPAR-γ can prevent the
expression of TNF-α and IL-1β via mTOR inhibition
After applying rosiglitazone, the expression of
PPAR-γ was significantly increased (P=0.0047), while that of pmTOR
(P=0.0041) and pS6K (P=0.0013) was significantly decreased compared
with rosiglitazone+GW9662, saline or control groups; however, these
effects were abrogated by GW9662 treatment (Fig. 3A). These findings indicated that
the mTOR signaling pathway can be suppressed by the activation of
PPAR-γ. Combined with the previous results demonstrating that the
expression of PPAR-γ is stable regardless of whether mTOR is
activated or inhibited (Fig. 2A),
it was speculated that mTOR signaling likely occurs downstream of
PPAR-γ. Moreover, as detected by western blotting, the protein
expression levels of TNF-α and IL-1β were significantly reduced
when rosiglitazone was administered (P=0.0086 and P=0.0073,
respectively; Fig. 3A), and such
anti-inflammatory ability was prevented by GW9662. However, the
expression of NF-κB was not significantly different vs. the group
without PPAR-γ activation.
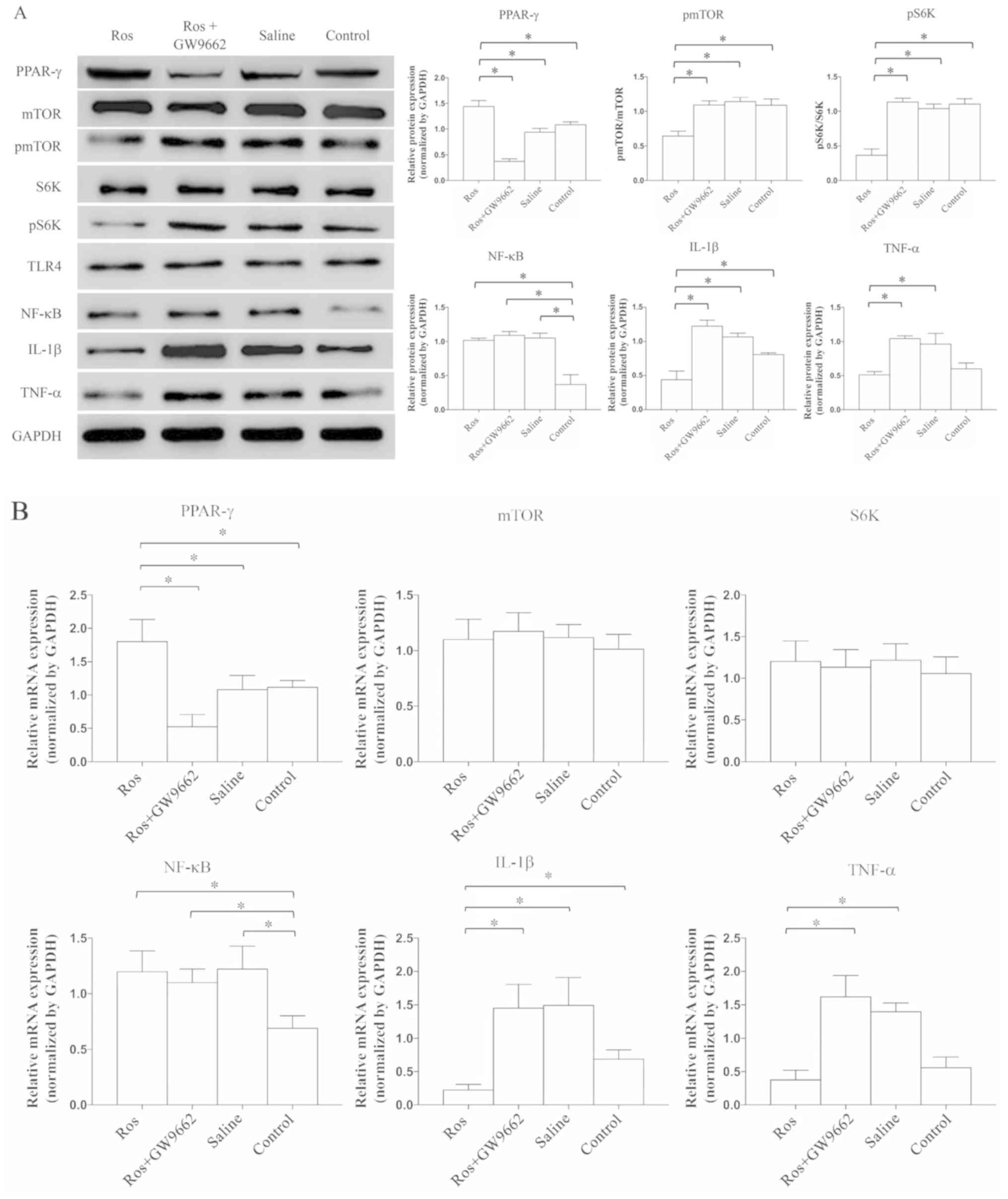 | Figure 3.Activation of PPAR-γ can reduce the
expression of TNF-α and IL-1β via inhibiting mTOR. Expression
levels were detected by (A) western blot analysis and (B) reverse
transcription-quantitative PCR. Expression levels of mTOR, S6K and
TLR4 were similar. The expression of NF-κB was significantly lower
in the control group compared with the other groups. When
administrated with rosiglitazone, the expression levels of pmTOR
and pS6K were significantly reduced. Moreover, the expression
levels of IL-1β and TNF-α were significantly suppressed. N=8-10 in
≥3 independent experiments. *P<0.05. TNF, tumor necrosis factor;
IL, interleukin; p, phosphorylated; TLR4, Toll-like receptor 4;
S6K, ribosomal protein S6 kinase; PPAR-γ, proliferators-activated
receptors-γ; Ros, rosiglitazone. |
Next, RT-qPCR was performed to evaluate the mRNA
expression levels of related cytokines and proteins (Fig. 3B). The mRNA expression of PPAR-γ
was significantly increased in the presence of the PPAR-γ agonist
(P=0.0093), while the mRNA expression levels of mTOR and S6K were
stable. In addition, the mRNA expression levels of TNF-α (P=0.028)
and IL-1β (P=0.031) were significantly suppressed in the
rosiglitazone group, and such inhibition was abrogated by treatment
with a PPAR-γ antagonist (Fig.
3B). Furthermore, in either treatment, it was suggested that
the expression of TLR4 was similar among the four groups (P=0.0831;
Figs. 2A, 3A and 4). Collectively, the results suggested
that the expression levels of neuroinflammatory cytokines can be
suppressed via the PPAR-γ and mTOR signaling pathways.
Discussion
In this in vitro study, it was found that
PPAR-γ activation inhibited the expression levels of TNF-α and
IL-1β via the suppression of mTOR signaling in microglia, but NF-κB
expression was not significantly affected. Therefore, the present
study identified a possible anti-inflammation mechanism of
microglia.
Since neuroinflammatory reactions have both
protective and detrimental effects in the brain, these are
considered a double-edged sword (19). Neuroinflammation is a crucial
factor not only in the outcome of patients with AD (7), but also in acute neurological
insults, including ICH (4) and
traumatic brain injury (3).
Microglia, which are the brain's resident innate immune cells, were
identified in the present study as a key component of the
neuroinflammatory response. Mechanistically, M2 microglia release
cytokines to reduce inflammation, and migrate to damaged tissue to
participate in regenerative processes (6). However, the mechanism via which
PPAR-γ activation mediates the suppression of inflammatory
cytokines in microglia remains unknown.
The specific function of mTOR in neurons is
controversial. Previous studies have reported that mTOR activation
may be harmful, and can exacerbate neuroinflammation after neuronal
injury in rats (14,20); however, other studies suggested
that mTOR has positive effects in ischemic rats (13,21).
Along with these opposing findings, the present results suggested
that mTOR may be regulated by different signaling pathways,
proteins or mRNAs depending on the disease state.
In the present study, P.A. and rapamycin were used
to test the aforementioned hypothesis that PPAR-γ may inhibit
certain inflammatory factors, including IL-1β, NF-κB and TNF-α, via
the mTOR signalling pathway. It was demonstrated that the
expression of PPAR-γ was similar between the P.A. and rapamycin
groups, which indicated that the mTOR signaling pathway was located
downstream of PPAR-γ and may be involved in the regulation of
inflammatory cytokines. When PPAR-γ was activated by rosiglitazone,
the expression levels of mTOR and S6K were similar between the
groups, but those of pmTOR and pS6K were significantly decreased;
this suggested that mTOR signaling can be inhibited by PPAR-γ
activation, and such inhibition can be abrogated with GW9662.
Moreover, the results indicated that PPAR-γ can inhibit the
expression levels of IL-1β and TNF-α via the suppression of the
mTOR signaling pathway.
It was previously shown that PPAR-γ activation could
suppress the expression of NF-κB (22,23).
However, in the present study, the expression of NF-κB was not
significantly different between the treatment groups. A possible
explanation for this finding is that the current study did not
manipulate the expression of the TLR4. The present results
suggested that the expression of TLR4 was similar among the four
groups.
TLRs are responsible for detecting microbial
pathogens and generating innate immune responses (24). TLR4 is a membrane receptor for
various substances, including LPS and heme (23,24).
It has also been reported that activation of TLR4 leads to the
transformation of a larger proportion of resting state microglia
into the M1 type, which promotes inflammation (25). In addition, harmful
neuroinflammatory reactions can be exacerbated by the activation of
TLR4 (26). Moreover, the
expression of NF-κB can be positively regulated by TLR4, but
negatively regulated by PPAR-γ (27,28).
The present results suggested that inflammatory factors were
inhibited by PPAR-γ via mTOR suppression; thus, the relationship
between TLR4 and mTOR may be crucial in the regulation of
neuroinflammation.
The interaction between TLR4 and mTOR has been
investigated in a previous studies (24,25,28),
especially in microglia, and it has been shown that dietary
L-arginine attenuates intestinal mucosal disruption via the
inhibition of the TLR4 and mTOR pathways (29). Furthermore, activation of mTOR
plays an essential role in TLR4-triggered neutrophil and macrophage
activation (30). A recent study
also revealed that mTOR-dependent autophagy regulates gut
inflammatory responses via the upstream TLR4/myeloid
differentiation primary response 88/mitogen-activated protein
kinase signaling and the downstream NF-κB pathway (31). The current findings suggested that
PPAR-γ activation can suppress certain neuroinflammatory factors
via mTOR. However, it is necessary to further elucidate the
relationship between mTOR and TLR4 to increase the understanding of
the anti-inflammation ability of microglia.
There were several limitations to the present study.
First, an in vivo study is required to assess the in
vitro results. Thus, future studies will modulate the
expression of S6K (downstream of mTOR) to further validate the
hypothesized molecular mechanism involving the interaction between
mTOR and PPAR-γ. This will include determining whether PPAR-γ
directly regulated or mediated mTOR, pNF-κB and p65 nuclear
translocation. In addition, the relationship between mTOR and TLR4
requires further investigation.
In conclusion, the mTOR signaling pathway may be
located downstream of PPAR-γ. Furthermore, neuroinflammatory
reactions may be inhibited by the activation of PPAR-γ via the
suppression of mTOR signaling in microglia.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science
Foundation of China (grant nos. 81701206, 81671200, 81870968,
81571111 and 81870909).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was designed by JLZ, JH and RX. The
experiments were performed and data were collected by JLZ, CW, XX,
YHD, BT, GC and QY, and the data were analyzed by ZYD, CW, YRS and
JY. The manuscript was written by JLZ and RX, and was revised and
approved by all authors. The manuscript was finally proofread and
approved by JH and RX.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICH
|
intracerebral hemorrhage
|
|
PPAR-γ
|
peroxisome proliferator-activated
receptor-γ
|
References
|
1
|
An SJ, Kim TJ and Yoon BW: Epidemiology,
risk factors, and clinical features of intracerebral hemorrhage: An
update. J Stroke. 19:3559–10. 2017. View Article : Google Scholar
|
|
2
|
Weimar C and Kleine-Borgmann J:
Epidemiology, prognosis and prevention of non-traumatic
intracerebral hemorrhage. Curr Pharm Des. 23:2193–2196. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng H, Chen C, Zhang J and Hu Z:
Mechanism and therapy of brain edema after intracerebral
hemorrhage. Cerebrovasc Dis. 42:155–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao Z, Tu S and Shao A:
Pathophysiological mechanisms and potential therapeutic targets in
intracerebral hemorrhage. Front Pharmacol. 10:10792019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Z, Zhang Z, Lu H, Yang Q, Wu H and
Wang J: Microglial polarization and inflammatory mediators after
intracerebral hemorrhage. Mol Neurobiol. 54:1874–1886. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang CF, Wan J, Li Q, Renfroe SC, Heller
NM and Wang J: Alternative activation-skewed microglia/macrophages
promote hematoma resolution in experimental intracerebral
hemorrhage. Neurobiol Dis. 103:54–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hemonnot AL, Hua J, Ulmann L and Hirbec H:
Microglia in Alzheimer disease: Well-known targets and new
opportunities. Front Aging Neurosci. 11:2332019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanazawa M, Ninomiya I, Hatakeyama M,
Takahashi T and Shimohata T: Microglia and monocytes/macrophages
polarization reveal novel therapeutic mechanism against stroke. Int
J Mol Sci. 18:21352017. View Article : Google Scholar
|
|
9
|
Jewell JL and Guan KL: Nutrient signaling
to mTOR and cell growth. Trends Biochem Sci. 38:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Gu S, Ling Y, Shen C, Cao X and Xie
R: p53 inhibition provides a pivotal protective effect against
ischemia-reperfusion injury in vitro via mTOR signaling. Brain Res.
1605:31–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie R, Wang P, Cheng M, Sapolsky R, Ji X
and Zhao H: Mammalian target of rapamycin cell signaling pathway
contributes to the protective effects of ischemic postconditioning
against stroke. Stroke. 45:2769–2776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie R, Cheng M, Li M, Xiong X, Daadi M,
Sapolsky RM and Zhao H: Akt isoforms differentially protect against
stroke-induced neuronal injury by regulating mTOR activities. J
Cereb Blood Flow Metab. 33:1875–1885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang JP and Zhang MY: Role for target of
rapamycin (mTOR) signal pathway in regulating neuronal injury after
intracerebral hemorrhage. Cell Physiol Biochem. 41:145–153. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Q, Gao L, Huang L, Ruan L, Yang J,
Huang W, Li Z, Zhang Y, Jin K and Zhuge Q: Inhibition of mammalian
target of rapamycin improves neurobehavioral deficit and modulates
immune response after intracerebral hemorrhage in rat. J
Neuroinflammation. 11:442014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Durocher M, Ander BP, Jickling G, Hamade
F, Hull H, Knepp B, Liu DZ, Zhan X, Tran A, Cheng X, et al:
Inflammatory, regulatory, and autophagy co-expression modules and
hub genes underlie the peripheral immune response to human
intracerebral hemorrhage. J Neuroinflammation. 16:562019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie R, He WQ, Shen M, Shou XF, Wang YF,
Bao WM and Zhao Y: Specific inhibition of mTOR pathway induces
anti-proliferative effect and decreases the hormone secretion in
cultured pituitary adenoma cells. J Neurooncol. 125:79–89. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seargent JM, Yates EA and Gill JH: GW9662,
a potent antagonist of PPARgamma, inhibits growth of breast tumour
cells and promotes the anticancer effects of the PPARgamma agonist
rosiglitazone, independently of PPARgamma activation. Br J
Pharmacol. 143:933–937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Armartmuntree N, Murata M, Techasen A,
Yongvanit P, Loilome W, Namwat N, Pairojkul C, Sakonsinsiri C,
Pinlaor S and Thanan R: Prolonged oxidative stress down-regulates
Early B cell factor 1 with inhibition of its tumor suppressive
function against cholangiocarcinoma genesis. Redox Biol.
14:637–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fourrier C, Remus-Borel J, Greenhalgh AD,
Guichardant M, Bernoud-Hubac N, Lagarde M, Joffre C and Layé S:
Docosahexaenoic acid-containing choline phospholipid modulates
LPS-induced neuroinflammation in vivo and in microglia in vitro. J
Neuroinflammation. 14:1702017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li D, Liu F, Yang T, Jin T, Zhang H, Luo X
and Wang M: Rapamycin protects against neuronal death and improves
neurological function with modulation of microglia after
experimental intracerebral hemorrhage in rats. Cell Mol Biol
(Noisy-le-grand). 62:67–75. 2016.PubMed/NCBI
|
|
21
|
Gao X, Chen W, Li J, Shen C, Zhou P, Che
X, Li X and Xie R: The protective effect of alpha-lipoic acid
against brain ischemia and reperfusion injury via mTOR signaling
pathway in rats. Neurosci Lett. 671:108–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu
XF, Zhou QG, Chen YY, Guo AZ and Hu CM: Indirubin inhibits
LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB
and MAPK signaling pathways. Inflammation. 40:1–12. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu AH, Wu YT and Wang YP: MicroRNA-129-5p
inhibits the development of autoimmune encephalomyelitis-related
epilepsy by targeting HMGB1 through the TLR4/NF-κB signaling
pathway. Brain Res Bull. 132:139–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JQ, Szodoray P and Zeher M: Toll-like
receptor pathways in autoimmune diseases. Clin Rev Allergy Immunol.
50:1–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X and
Fu J: Curcumin inhibits LPS-induced neuroinflammation by promoting
microglial M2 polarization via TREM2/TLR4/ NF-κB pathways in BV2
cells. Mol Immunol. 116:29–37. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi H, Wang XL, Quan HF, Yan L, Pei XY,
Wang R and Peng XD: Effects of betaine on LPS-stimulated activation
of microglial M1/M2 phenotypes by suppressing TLR4/NF-κB pathways
in N9 cells. Molecules. 24:3672019. View Article : Google Scholar
|
|
27
|
Kutsenko NL, Vesnina LE and Kaidashev IP:
Pioglitazone, an activator of PPAR-gamma, reduces the expression of
kB nuclear factor and inhibits apoptosis in mononuclear cells of
peripheral blood in vitro. Fiziol Zh. 58:33–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang JS, Xiao WW, Zhong YS, Li XD, Du SX,
Xie P, Zheng GZ and Han JM: Galectin-3 deficiency protects
lipopolysaccharide-induced chondrocytes injury via regulation of
TLR4 and PPAR-gamma-mediated NF-κB signaling pathway. J Cell
Biochem. 120:10195–10204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan J, Applegate TJ, Liu S, Guo Y and
Eicher SD: Supplemental dietary L-arginine attenuates intestinal
mucosal disruption during a coccidial vaccine challenge in broiler
chickens. Br J Nutr. 112:1098–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu M, Kang X, Xue H and Yin H: Toll-like
receptor 4 is up-regulated by mTOR activation during THP-1
macrophage foam cells formation. Acta Biochim Biophys Sin
(Shanghai). 43:940–947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang
C, Ge W, Wu J, Du P and Chen Y: Boosting mTOR-dependent autophagy
via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB
pathway quenches intestinal inflammation and oxidative stress
injury. EBioMedicine. 35:345–360. 2018. View Article : Google Scholar : PubMed/NCBI
|