Introduction
Diabetes mellitus is one of the most common chronic
diseases in nearly all countries (1,2), in
2010, which collectively killed globally an estimated 12.9 million
people (3). According to the World
Health Organization Global Report, approximately 1.6 million people
worldwide died due to diabetes in 2016, and it is estimated that
425 million people are living with diabetes worldwide, this number
is predicted to rise to approximately 629 million globally in 2045
(4). Diabetic nephropathy (DN) is
one of the most significant complications of diabetes and the main
cause of end-stage renal failure (5), which seriously threatens the health
of patients and brings a heavy economic burden to the families and
society. Therefore, the treatment of DN has become a topic of
concern.
The exact etiology of DN is unknown; however,
various postulated mechanisms, including renal hemodynamic changes,
abnormal glucose metabolism, genetic factors, cytokines, oxidative
stress and other factors (6,7),
have been proposed. Recent research on the causes of DN has
demonstrated that DN is closely associated with apoptosis (8). The apoptosis of diabetic kidney
tissue cells is related podocytes and mesangial cells, especially
renal tubular epithelial cells are significantly associated with
apoptosis (9–11). The endoplasmic reticulum stress
(ERS) pathway is one of the apoptotic pathways. Studies have
demonstrated that DN initiates ERS-related ER-associated death
(ERAD) and induces apoptosis (12–14).
For example, the role of reactive oxygen species-mediated ERS has
been revealed in contrast-induced renal tubular cell apoptosis
(15). During the diabetic
process, ERS is induced by factors such as high sugar, excess
nutrients, increased free fatty acids and oxidative stress. These
persistent and unmotivated stimuli eventually cause irreversible
ERS, initiate ERAD, and amplify the cascade effects of apoptosis in
renal tubular epithelial cells (13). Qi et al have reported that
the regulation of the ERS inflammatory response attenuates DN in
diabetic rats (16). Therefore,
effective intervention against ERS may benefit the treatment of
DN.
Chromatin structural changes affect the regulation
of gene expression, and the acetylation of histones regulates gene
transcription (17). It has been
demonstrated that histone acetylation regulates the transcription
of ERS-related genes (18,19). Valproic acid (VPA) is a
representative drug of the non-selective histone deacetylase
inhibitors (HDACIs) and is clinically used as anticonvulsant and
sedative (16). Studies have
demonstrated that VPA reduces the expression of transforming growth
factor (TGF)-β in DN, thereby reducing renal fibrosis (20–23).
Studies have also demonstrated that VPA reduces mesenchymal
transition of NRK-52E cells (24,25).
Therefore, VPA mitigates DN; however, to the best of our knowledge,
the specific mechanisms are not fully elucidated.
Our previous study demonstrated significant
apoptosis of renal cell tissue and changes of ERS-related proteins
in renal tubular epithelial cells (26). A high concentration of glucose is
an ERS-inducing factor that mimics the natural, pathological
process of clinical DN. Therefore, in the present study, the renal
tubular epithelial cells NRK-52E were selected as the model cell
line to evaluate the impacts of high concentration of glucose on
ERS and apoptosis. To determine whether and how the effect of VPA
on DN is related to ERS-related apoptosis, the present study
investigated the regulatory mechanisms of VPA on histone
acetylation.
Materials and methods
Cell culture
The rat renal tubular epithelial cell line NRK-52E
(cat. no. ATCC® CRL-1571™) was purchased from the
American Type Culture Collection. The cells were cultured with
either high-glucose (HG; 25 mM glucose) DMEM (Gibco; Thermo Fisher
Scientific, Inc.) or low-glucose (5 mM glucose) DMEM. Culture
medium, containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), was supplemented with 100 U/ml penicillin
(Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). The culture flasks
(Beyotime Institute of Biotechnology) were placed in a humidified
incubator (Sanyo Electric Co., Ltd.) at 37°C with 5%
CO2. The culture medium was changed every 1–2 days, and
the cells were passaged every 2–3 days according to cell
confluence.
Titration of VPA and cell
treatment
NRK-52E cells were seeded in a 96-well plate (cat.
no. JY92-2D Beyotime Institute of Biotechnology) at a density of
1×104 cells/100 µl/well. The cells were cultured for 24
h to achieve a logarithmic growth phase and different
concentrations of VPA (0.1, 1, 10 and 100 nM) were administered.
Three replicate wells were set for each concentration, and three
negative control wells and blank control wells were also included.
After culturing for 48 h, 10 µl MTT saline solution was added to
each well, and the culture was continued for 4 h. The absorbance
(A) value of each well was measured at a wavelength of 450 nm by a
microplate reader (Tecan Group Ltd.) to calculate the cell growth
inhibition rate. Cell growth viability rate (%) at different
concentrations=(A value of VPA group-A value of blank group)/(A
value of negative control group-A value of blank group) × 100%. The
selected concentration of the drug was 10 nM (Fig. 1). The cells in the logarithmic
growth phase were divided into four groups: i) Control: Cells were
cultured with low glucose (5 mM) DMEM; ii) Control+VPA: Cells were
cultured with low glucose (5 mM) DMEM supplemented with 10 nM VPA
(Sigma-Aldrich: Merck KGaA); iii) HG: Cells were cultured with HG
(25 mM) DMEM; and iv) HG+VPA: Cells were cultured with HG (25 mM)
DMEM supplemented with 10 nM VPA.
Total cellular protein extraction
Harvested cells were washed twice with pre-cooled
PBS, and transferred to a 1.5-ml EP tube. After centrifugation at
1,000 g/5 min at 4°C, the supernatant was discarded and 150 µl RIPA
protein lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM
Na2EDTA, 1 mM EDTA, 1% Triton, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 mM NaF, 1 µg/ml leupeptin and 1 mM
PMSF; Beyotime Institute of Biotechnology) supplemented with
cocktails of protease inhibitor and phosphatase inhibitor (Amresco)
were added to each EP tube. All the operations were performed on
ice to avoid protein degradation. The cells were subjected to
ultrasonication with an ultrasonic cell crusher (Ningbo Xinzhi
Technology Co., Ltd.) three times for 5 sec and then the cells were
allowed to stand at 4°C for 45 min. Next, the cells were
centrifuged at 1,000 × g for 15 min at 4°C, and the supernatant was
taken and dispensed in EP tubes. After the protein concentration
was measured with a Pierce BCA kit (Thermo Fisher Scientific Inc.),
every 100 µl protein sample was mixed with 25 µl of 5× loading
buffer. The mixture was boiled in water at 95°C for 10 min and then
incubated at 37°C for 10 min in a water bath (Shanghai Bo Xun
Industrial Co., Ltd.). The samples that were not immediately used
were stored at −20°C.
Western blot analysis
Protein lysates (30–50 µg) were resolved by 12% SDS
polyacrylamide gel electrophoresis (Beyotime Institute of
Biotechnology) and transferred onto immobilon-P transfer membranes
(EMD Millipore). The membranes were blocked with 5% non-fat milk
powder in buffer [10 mM Tris-HCl, (pH 7.6), 100 mM NaCl and 0.1%
Tween 20] for 2 h at room temperature and then incubated with the
desired primary antibody, including polyclonal rabbit anti-human
glycine-rich RNA-binding protein 78 (GRP78; cat. no. ab21685,
1:1,000), polyclonal rabbit anti-human caspase-12 (cat. no. ab4051,
1:500), rabbit polyclonal anti-caspase-3 (cat. no. ab13847, 1:500),
mouse anti-human monoclonal C/EBP homologous protein (CHOP; cat.
no. ab11419, 1:500), monoclonal rat anti-human B-cell lymphoma 2
(Bcl-2; cat. no. ab182858, 1:2,000), rabbit monoclonal
anti-Bcl-2-associated X protein (Bax; cat. no. ab32503, 1:3,000),
polyclonal rabbit anti-phosphorylated JNK (p-JNK; cat. no. ab4821,
1:1,000), monoclonal mouse anti-activating transcription factor 4
(ATF4; cat. no. ab23760, 1:1,000) or monoclonal mouse anti-β-actin
(1:1,000; cat. no. ab6276; all antibodies were obtained from Abcam)
overnight at 4°C, followed by incubation with a horseradish
peroxidase (HRP)-conjugated secondary antibody (Hangzhou HuaAn
Biotechnology Co., Ltd.) at a 1:2,000 dilution for 90 min at room
temperature. The immunoreactive bands were visualized with
3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA). The
representative bands were measured by a Tanon GIS gel imager system
(Tanon Science & Technology Co., Ltd.) and analyzed. The levels
of proteins were normalized to those of β-actin, and the ratios are
presented as the mean ± standard deviation of three independent
experiments. Protein levels were quantified by densitometry using
the Quantity One 1-D software (version 4.4.02; Bio-Rad
Laboratories, Inc.).
Total histone H4 acetylation
assay
The fast detection of total histone H4 acetylation
in NRK-52E cells was performed using the EpiQuik™ Total Histone H4
Acetylation Detection Fast kit (Colorimetric) (cat. no. P-4032;
Epigentek Group Inc.), as previously described.
Chromatin immunoprecipitation assay
(CHIP)
CHIP assays were performed using a CHIP assay kit
(EMD Millipore), according to the manufacturer's instructions.
Briefly, NRK-52E cells were treated as indicated, cross-linked with
formaldehyde and sonicated. Resulting cell lysates (input) were
immunoprecipitated with the anti-acetylated-histone H4 antibody
(cat. no. ab109463; Abcam). The precipitated protein-DNA complexes
(IP) were subjected to proteinase treatment. The following primers
were used: GRP78 forward, 5′-CTCGAGGAAGGGCATAAGAGCATCA-3′ and
reverse, 5′-CCGCTTCTCCTCAGGTTCCGGCTGT-3′; CHOP forward,
5′-ACTGACAACGACAAGACCCC-3′ and reverse, 5′-AGTCACAGCCAGTATCGAGC-3′;
and GAPDH forward, 5′-ACAACCTGGTCCTCAGTGTAGCC-3′ and reverse,
5′-AAGGTCATCCCAGAGCTGAACGG-3′.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean (SEM) of three independent experiments, each conducted in
triplicate. One-way analysis of variance (ANOVA) followed by a
Dunnett's multiple comparison test was performed to compare
differences between multiple groups. All comparisons were performed
with GraphPad Prism (version 5, GraphPad Software Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
VPA attenuates HG-induced ERS in
NRK-52E cells
First, a titration of VPA concentrations by MTT
assays was performed to select the optimal concentration of VPA for
treating NRK-52E cells. Following 48 h of culture, the cells
treated with 100 nM VPA had a significantly decreased viability
compared with the control cells under normal glucose conditions
(Fig. 1A; P<0.05). The
viability of cells cultured with the HG medium significantly
increased slightly with the addition of 10 nM VPA (P<0.05), but
significantly decreased with the addition of 100 nM VPA (P<0.05;
Fig. 1B). Therefore, the optimal
working concentration of VPA was determined to be 10 nM.
To investigate the effects of VPA on ERS induced by
HG in NRK-52E cells, the expression levels of the ERS-related
proteins GRP78, ATF4, CHOP and caspase-12 in NRK-52E cells cultured
under HG conditions and treated with 10 nM VPA were determined.
Compared with the control group, the expression of the GRP78
protein in the HG group was significantly increased (P<0.05),
while supplementation of VPA further increased the protein levels
of GRP78 (P<0.05; Fig. 2A). The
expression of ATF4 was also significantly increased after
stimulation with HG for 48 h, while supplementation of VPA (the
HG+VPA group) significantly reversed this effect (P<0.05;
Fig. 2A). A similar trend of CHOP
or caspase-12 expression was obtained (Fig. 2A), and similar to ATF4, VPA was
demonstrated to significantly reduce the expression levels of CHOP
and caspase-12 (P<0.05). By contrast, VPA at 10 nM did not
affect the expression of these molecules under the low glucose
condition. These results suggested that VPA attenuated the
HG-induced ERS in NRK-52E cells.
 | Figure 2.Western blotting revealed that VPA
attenuates ER stress-induced apoptosis in NRK-52E cell upon high
glucose treatment. NRK-52E cells were treated as indicated for 48
h, and cell lysates were collected to determine the protein levels
of (A) ER stress-related proteins (GRP78, ATF4, CHOP, caspase-12
and p-JNK) and (B) apoptosis-related molecules (Bax, cleaved
caspase-3 and Bcl-2) by western blot analyses. *P<0.05,
**P<0.01, ***P<0.001. VPA, valproic acid; GPR78, ATF4,
activating transcription factor 4; CHOP, C/EBP homologous protein;
HG, high glucose; p-, phosphorylated. |
VPA attenuates apoptosis of NRK-52E cells cultured
under the HG condition. Compared with the control group, HG
stimulation also significantly enhanced the ratio of p-JNK/JNK in
NRK-52E cells, and VPA treatment significantly attenuated the
increase of p-JNK/JNK upon HG culture (Fig. 2A). Since JNK activation is reported
to be involved in ER stress-induced cell death, and since both
caspase-12 and CHOP are key ERS-related apoptotic proteins, the
present study hypothesized that VPA may affect ERS-induced
apoptosis. To observe the effect of VPA on the apoptosis of NRK-52E
cells induced by HG, western blotting was performed to detect the
expression levels of apoptosis-related proteins, such as Bax,
cleaved-caspase-3 and Bcl-2 (Fig.
2B). Compared with the control group, HG stimulation in NRK-52E
cells significantly enhanced the expression of the
apoptosis-promoting factors Bax and cleaved caspase-3, but reduced
the expression of the apoptosis-inhibiting factor Bcl-2 (Fig. 2B). In addition, VPA exhibited no
effects on the expression of these factors in cells cultured under
low glucose condition, whereas VPA treatment in cells under HG
condition significantly attenuated the effects of HG-induced
changes in protein levels of these molecules (Fig. 2B). Therefore, these results
demonstrated that VPA is an antagonistic factor against HG-induced
apoptosis in NRK-52E cells.
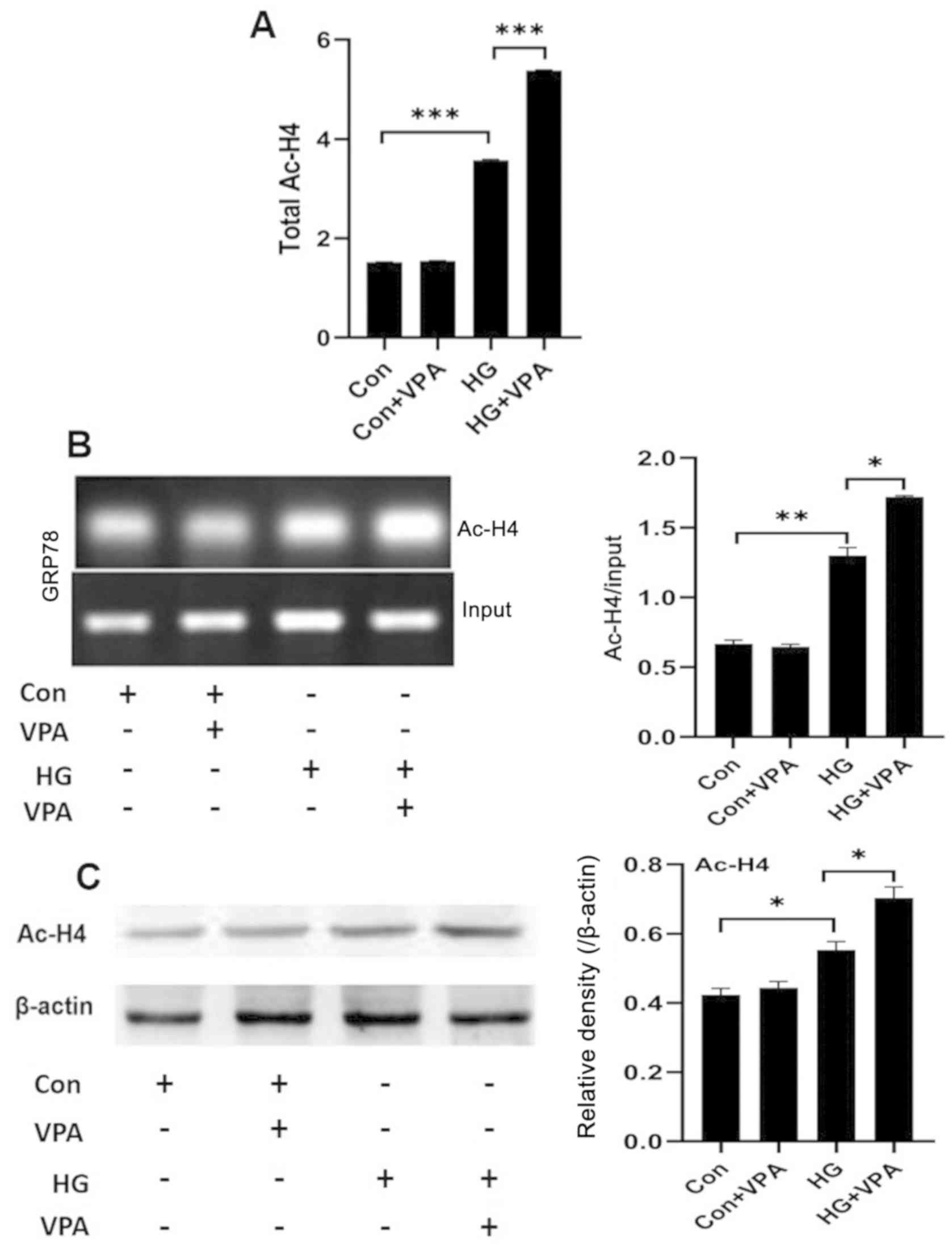
Investigating the role of VPA in histone acetylation
on HG-induced ERS and apoptosis in NRK-52E cells. VPA inhibits
histone deacetylase and promotes histone acetylation, which is
involved in the transcriptional activity of genes (27). Thus, the role of VPA was evaluated
by investigating whether and how it affected histone acetylation in
NRK-52E cells under the HG (25 mmol/l) and low-glucose (5 mmol/l)
culture conditions. First, a colorimetric assay was used to
determine the total acetylation levels of histone H4 in NRK-52E
cells. Compared with the control group, the total histone H4
acetylation level in the HG group was significantly higher, while
the treatment of HG in combination with 10 nM VPA (the HG+VPA
group) further increased the total histone H4 acetylation levels
(Fig. 3A and C). Next, CHIP assays
were performed to measure the histone H4 acetylation levels in the
promoter region of the ERS key protein GRP78. The acetylation level
of histone H4 in the GRP78 promoter region was significantly
increased in the HG group compared with that of the control group
and was further increased in the HG+VPA group (Fig. 3B). Taken together, VPA exhibits a
role of broad enhancement on histone H4 acetylation in NRK-52E
cells.
Discussion
As a major chronic complication of diabetes, DN
gains more and more attention as the number of DN cases increases
as the incidence of diabetes increases. A total of 30–40% of
patients with diabetes eventually develop DN (28); therefore, how to effectively treat
DN has become a focus of concern. Studies have highlighted the
crucial roles of ERS and apoptosis in the pathogenesis of DN
(12–14). In the present study, this notion is
further strengthened by the findings that HG-stimulated NRK-52E
renal tubular epithelial cells underwent ERS and that the HDACI VAP
attenuated the effects of HF, at least partially through the
regulation on the acetylation of histone H4.
Various studies have demonstrated that DN is closely
related to the apoptosis-associated ERS pathway, and ERS has become
one of the pathogenic processes of DN (29,30).
Diabetes hyperglycemia stimulates ERS in renal tubular epithelial
cells or podocytes, and causes apoptosis (9,31,32).
Our previous study demonstrated increased expression of the
ERS-related protein GRP78 and increased expression of ERS-related
apoptosis proteins CHOP and caspase-12 in DN rat kidneys compared
to wild type rats (26). Renal
apoptotic cells were mainly found in renal tubular epithelial cells
(33). Knockout of the ERS-related
protein GRP78 in mice leads to phenotypes that include renal
tubular atrophy, interstitial fibrosis and glomerular sclerosis
(34). Our previous study also
demonstrated that ERS and apoptosis of DN rats mainly occurred in
the renal tubules (26);
accordingly, the present study investigated the effects of HG on
ERS of the NRK-52E renal tubular epithelial cells. The results of
the present study demonstrated that compared with the control group
the expression of the ERS-related protein GRP78 was increased, and
the expression of the ERS-related apoptosis proteins CHOP and
caspase-12 was also increased when NRK-52E cells were cultured
under HG. This suggested that HG activates the ERS-related
apoptosis pathways in NRK-52E cells. Consistent with other relevant
research findings, the results of the present study suggested that
ERS-induced apoptosis may be one of the causes of kidney failure.
Thus, we hypothesize that reducing ERS-related apoptosis may
attenuate the development of DN.
The regulation of expression of ERS-related
apoptotic proteins is related to chromatin structure, in addition
to the regulation of genomic DNA levels (35). Since the acetylation of histones is
related to chromatin remodeling, it is also involved in the
regulation of gene expression. It has been demonstrated that
histone acetylation may be involved in the regulation of
ERS-related protein expression (36), and HDACIs are closely associated
with cell proliferation, migration and apoptosis (37). VPA is a non-selective HDACI; a
recent study demonstrated that it reduces proteinuria in a rat
model of renal ischemia-reperfusion injury, thereby alleviating
renal damage, and its mechanism may be related to the regulation of
TGF-β (38). VPA also alleviates
DN renal fibrosis (39). The
results of the present study demonstrated that compared with those
of the control group, the expression levels of the ERS-related
protein GRP78, which is an anti-apoptotic protein, was further
increased after HG-induced NRK-52E cells were administered VPA,
whereas the expression levels of ERS-related pro-apoptotic proteins
ATF4, CHOP, caspase-12 and p-JNK were reduced. In addition, VPA
also reduced the expression of other pro-apoptotic related proteins
such as Bax and cleaved-caspase-3, and increased the expression of
the anti-apoptotic protein Bcl-2. In corroboration with the results
of the present study, in a model of myocardial ischemia-reperfusion
injury, trichostatin A (another type of HDACI) was demonstrated to
increase the expression of GRP78 and reduce the expression of the
pro-apoptotic proteins CHOP and caspase-12 (40,41).
Thus, VPA may impede the development of DN by attenuating
ERS-related apoptosis.
The present study also investigated the possible
mechanisms of the regulation of ERS-related protein expression by
VPA. Zhang et al (42)
demonstrated that VPA increases the expression of the ERS-related
protein GRP78 by increasing the acetylation level of histone H3,
and reduce the expression of CHOP and caspase-12, thereby reducing
ischemia-reperfusion-associated retinal injury. By contrast, the
results of the present study demonstrated that VPA further
increased the total acetylation level of histone H4. This may be
due to the existence of relatively more acetylation sites of
histone H4 than that of histone H3 (43). In addition, it has been revealed
that HDACIs regulate the promoter of GRP78 in cancer cells and
promote the transcription of GRP78 (44). Baumeister et al (45) demonstrated that histone
acetyltransferase p300 binds to the GRP78 promoter to promote GRP78
transcription, and Bown et al (46) also demonstrated that VPA regulates
the transcription of ERS-related proteins. These studies suggest
that histone acetylation may be involved in the transcriptional
regulation of GRP78. Accordingly, the CHIP method was performed to
detect the specific H4 acetylation on the GRP78 gene, and the
results demonstrated that the histone H4 acetylation levels of the
GRP78 gene promoter were increased. This suggested that VPA may
regulate the transcription of the ERS-related gene GRP78 and
increases the ability of cells to resist apoptosis. Unfortunately,
the present study did not detect altered levels of histone H4
acetylation at the promoters of CHOP and caspase-12 genes despite
repeated attempts. This may be due to the inability of histone
acetylation to regulate the transcription of all genes. Although
HDACIs work in various tissues, they only regulate the
transcription of ~20% of genes (47–49).
HDACIs may be selective for the regulation of particular genes, and
the underlying mechanisms require a large and complex panel to be
explored in future research.
In summary, the results of the present study
revealed that the HDACI VPA attenuated HG-induced ERS and
ERS-related apoptosis in NRK-52E cells, which is at least partially
associated with VPA-regulated histone H4 acetylation; thus HDACIs
are promising therapeutics for mitigating DN.
Acknowledgements
Not applicable.
Funding
This work was supported, in part, by The Health
Science and Technology Foundation of Jilin Provincial Health
Commission (grant no. 2018J080 to XS), Jin Province Natural Science
Education Foundation (grant no. JJKH20200055kJ to XS) and Natural
Science Foundation of Jilin Province (grant no. 20200201354JC to
XS).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS conceived and coordinated the study, designed,
performed and analyzed the experiments, and wrote the paper. YS,
SL, YX and DZ performed the data collection, data analysis and
revised the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forouhi NG and Wareham NJ: Epidemiology of
diabetes. Medicine (Abingdon). 42:698–702. 2014.PubMed/NCBI
|
|
2
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
WHO: Global Report on Diabetes. World
Health Organization. 2016.
|
|
5
|
Tesch GH: Diabetic nephropathy-is this an
immune disorder? Clin Sci (Lond). 131:2183–2199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Z and Cooper ME: Pathogenesis of
diabetic nephropathy. J Diabetes Investig. 2:243–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao B, Li H, Liu J, Han P, Zhang C, Bai
H, Yuan X, Wang X, Li L, Ma H, et al: MicroRNA-23b targets Ras
GTPase-activating protein SH3 domain-binding protein 2 to alleviate
fibrosis and albuminuria in diabetic nephropathy. J Am Soc Nephrol.
27:2597–2608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anil Kumar P, Welsh GI, Saleem MA and
Menon RK: Molecular and cellular events mediating glomerular
podocyte dysfunction and depletion in diabetes mellitus. Front
Endocrinol (Lausanne). 5:1512014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Habib SL: Diabetes and renal tubular cell
apoptosis. World J Diabetes. 4:27–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo C, Li Y, Zhang R, Zhang Y, Zhao J, Yao
J, Sun J, Dong J and Liao L: Protective effect of salidroside
against diabetic kidney disease through inhibiting BIM-mediated
apoptosis of proximal renal tubular cells in rats. Front Pharmacol.
9:14332018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao X, Liu G, Shen H, Gao B, Li X, Fu J,
Zhou J and Ji Q: Liraglutide inhibits autophagy and apoptosis
induced by high glucose through GLP-1R in renal tubular epithelial
cells. Int J Mol Med. 35:684–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Y, Lee K, Wang N and He JC: The role
of endoplasmic reticulum stress in diabetic nephropathy. Curr Diab
Rep. 17:172017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cunard R and Sharma K: The endoplasmic
reticulum stress response and diabetic kidney disease. Am J Physiol
Renal Physiol. 300:F1054–F1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cameron NE: Role of endoplasmic reticulum
stress in diabetic neuropathy. Diabetes. 62:696–697. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Yang D, Yang D, Jia R and Ding G:
Role of reactive oxygen species-mediated endoplasmic reticulum
stress in contrast-induced renal tubular cell apoptosis. Nephron
Exp Nephrol. 128:30–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi W, Mu J, Luo ZF, Zeng W, Guo YH, Pang
Q, Ye ZL, Liu L, Yuan FH and Feng B: Attenuation of diabetic
nephropathy in diabetes rats induced by streptozotocin by
regulating the endoplasmic reticulum stress inflammatory response.
Metabolism. 60:594–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verdone L, Agricola E, Caserta M and Di
Mauro E: Histone acetylation in gene regulation. Brief Funct
Genomic Proteomic. 5:209–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Tsai YH and Tseng SH: HDAC
inhibitors and RECK modulate endoplasmic reticulum stress in tumor
cells. Int J Mol Sci. 18:2582017. View Article : Google Scholar
|
|
19
|
Donati G, Imbriano C and Mantovani R:
Dynamic recruitment of transcription factors and epigenetic changes
on the ER stress response gene promoters. Nucleic Acids Res.
34:3116–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Beneden K, Geers C, Pauwels M,
Mannaerts I, Wissing KM, Van den Branden C and van Grunsven LA:
Comparison of trichostatin A and valproic acid treatment regimens
in a mouse model of kidney fibrosis. Toxicol Appl Pharmacol.
271:276–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan S, Jena G and Tikoo K: Sodium
valproate ameliorates diabetes-induced fibrosis and renal damage by
the inhibition of histone deacetylases in diabetic rat. Exp Mol
Pathol. 98:230–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kochar DK, Rawat N, Agrawal RP, Vyas A,
Beniwal R, Kochar SK and Garg P: Sodium valproate for painful
diabetic neuropathy: A randomized double-blind placebo-controlled
study. QJM. 97:33–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan S, Bhat ZR and Jena G: Role of
autophagy and histone deacetylases in diabetic nephropathy: Current
status and future perspectives. Genes Dis. 3:211–219. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Beneden K, Geers C, Pauwels M,
Mannaerts I, Verbeelen D, van Grunsven LA and Van den Branden C:
Valproic acid attenuates proteinuria and kidney injury. J Am Soc
Nephrol. 22:1863–1875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H
and Lee HB: Histone deacetylase-2 is a key regulator of diabetes-
and transforming growth factor-beta1-induced renal injury. Am J
Physiol Renal Physiol. 297:F729–F739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun XY, Qin HJ, Zhang Z, Xu Y, Yang XC,
Zhao DM, Li XN and Sun L: Valproate attenuates diabetic nephropathy
through inhibition of endoplasmic reticulum stressinduced
apoptosis. Mol Med Rep. 13:661–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wade PA: Transcriptional control at
regulatory checkpoints by histone deacetylases: Molecular
connections between cancer and chromatin. Hum Mol Genet.
10:693–698. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu R: The real culprit behind diabetic
nephropathy: Impaired renal autoregulation? Physiol Rep.
5:e131382017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gardner BM and Walter P: Unfolded proteins
are Ire1-activating ligands that directly induce the unfolded
protein response. Science. 333:1891–1894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Y and Hendershot LM: The unfolding tale
of the unfolded protein response. Cell. 107:827–830. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai H, Liu Q and Liu B: Research progress
on mechanism of podocyte depletion in diabetic nephropathy. J
Diabetes Res. 2017:26152862017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W
and Duan H: Role of endoplasmic reticulum stress in apoptosis of
differentiated mouse podocytes induced by high glucose. Int J Mol
Med. 33:809–816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Y, Cui H, Xia Y and Gan H:
RIPK3-mediated necroptosis and apoptosis contributes to renal
tubular cell progressive loss and chronic kidney disease
progression in rats. PLoS One. 11:e01567292016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo S, Mao C, Lee B and Lee AS: GRP78/BiP
is required for cell proliferation and protecting the inner cell
mass from apoptosis during early mouse embryonic development. Mol
Cell Biol. 26:5688–5697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schram AW, Baas R, Jansen PW, Riss A, Tora
L, Vermeulen M and Timmers HT: A dual role for SAGA-associated
factor 29 (SGF29) in ER stress survival by coordination of both
histone H3 acetylation and histone H3 lysine-4 trimethylation. PLoS
One. 8:e700352013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ngwa CJ, Kiesow MJ, Papst O, Orchard LM,
Filarsky M, Rosinski AN, Voss TS, Llinás M and Pradel G:
Transcriptional profiling defines histone acetylation as a
regulator of gene expression during human-to-mosquito transmission
of the Malaria parasite plasmodium falciparum. Front Cell Infect
Microbiol. 7:3202017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J and Zhong Q: Histone deacetylase
inhibitors and cell death. Cell Mol Life Sci. 71:3885–3901. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Alam A, Soo AP, George AJT and Ma
D: Ischemia-reperfusion injury reduces long term renal graft
survival: Mechanism and beyond. EBioMedicine. 28:31–42. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seet LF, Toh LZ, Finger SN, Chu SW,
Stefanovic B and Wong TT: Valproic acid suppresses collagen by
selective regulation of Smads in conjunctival fibrosis. J Mol Med
(Berl). 94:321–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng J, Li S and Chen H: Tanshinone IIA
ameliorates apoptosis of cardiomyocytes induced by endoplasmic
reticulum stress. Exp Biol Med (Maywood). 241:2042–2048. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Y, Tang Y, Xiang Y, Xie YQ, Huang XH
and Zhang YC: Shengmai injection improved doxorubicin-induced
cardiomyopathy by alleviating myocardial endoplasmic reticulum
stress and caspase-12 dependent apoptosis. Biomed Res Int.
2015:9526712015.PubMed/NCBI
|
|
42
|
Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv
X and Wu X: Valproate protects the retina from endoplasmic
reticulum stress-induced apoptosis after ischemia-reperfusion
injury. Neurosci Lett. 504:88–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Spotswood HT and Turner BM: An
increasingly complex code. J Clin Invest. 110:577–582. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baumeister P, Dong D, Fu Y and Lee AS:
Transcriptional induction of GRP78/BiP by histone deacetylase
inhibitors and resistance to histone deacetylase inhibitor-induced
apoptosis. Mol Cancer Ther. 8:1086–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baumeister P, Luo S, Skarnes WC, Sui G,
Seto E, Shi Y and Lee AS: Endoplasmic reticulum stress induction of
the Grp78/BiP promoter: Activating mechanisms mediated by YY1 and
its interactive chromatin modifiers. Mol Cell Biol. 25:4529–4540.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bown CD, Wang JF, Chen B and Young LT:
Regulation of ER stress proteins by valproate: Therapeutic
implications. Bipolar Disord. 4:145–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bush EW and McKinsey TA: Protein
acetylation in the cardiorenal axis: The promise of histone
deacetylase inhibitors. Circ Res. 106:272–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bannister AJ and Kouzarides T: Regulation
of chromatin by histone modifications. Cell Res. 21:381–395. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Choudhary C, Kumar C, Gnad F, Nielsen ML,
Rehman M, Walther TC, Olsen JV and Mann M: Lysine acetylation
targets protein complexes and co-regulates major cellular
functions. Science. 325:834–840. 2009. View Article : Google Scholar : PubMed/NCBI
|