Introduction
Toll-like receptors (TLRs), which have the most
extensive spectrum of pathogen recognition, detect invading
pathogens by recognizing pathogen-associated molecular patterns
(PAMPs) and damage-associated molecular pattern molecules (DAMPs)
(1,2). TLR4, a TLR family member, plays an
important role in innate immunity against allergy (3), obesity-associated metabolic disorders
(4), apoptosis (5), infectious diseases (6), and inflammatory bowel diseases
(7). Moreover, TLR4 is widely
expressed on the surface of immune cells, including macrophages,
neutrophils and lymphocytes (8).
Lipopolysaccharide (LPS), an endotoxin on the cell
wall of Gram-negative bacteria, is a major PAMP (1). On recognizing LPS, TLR4 interacts
with LPS via its cytosolic toll-interleukin (IL)-1 receptor (TIR)
domain (9). Furthermore, LPS binds
to LPS-binding protein (LBP) and CD14, which then transfers LPS to
the TLR4/myeloid differentiation protein-2 (MD2) complex, which
dimerizes and translocates to endosomes, triggering myeloid
differentiation primary response gene 88 (MyD88)-dependent and
MyD88-independent pathways (10).
Moreover, the two pathways can induce phosphorylation of
transcription factors, including nuclear factor κB (NF-κB),
activator protein 1 and interferon (IFN) regulatory factor 3
(IRF-3), to eventually promote the production of proinflammatory
cytokines, such as tumor necrosis factor (TNF)-α, IL-1, IL-6, IL-8,
IFN-β and IFN-γ (11–13). The inflammatory response to LPS
plays a key role in the defense against bacterial infections;
however, excessive host reaction to LPS causes severe inflammatory
conditions such as sepsis and fatal septic shock (14,15).
Therefore, regulation of TLR4-mediated signaling is critical for
maintaining the intensity of the immune response and treating
severe sepsis.
Monoclonal antibodies (mAbs) are a widely used
pharmacotherapeutic approach in the treatment of various
inflammatory diseases (16). Thus,
the aims of the present study were to prepare a novel human
monoclonal anti-TLR4 immunoglobulin G2 antibody by screening an
anti-TLR4 Fab fragment from a human Fab phage-display library, and
to examine whether human anti-TLR4 IgG2 decreases LPS-induced
immune responses. The present results suggest that the entire human
anti-TLR4 IgG2 antibody showed high affinity for TLR4 and
functioned well against LPS-induced inflammatory processes in mouse
macrophages.
Materials and methods
Reagents and mice
LPS used to stimulate inflammation responses was
obtained from Sigma-Aldrich (Merck KGaA). RPMI-1640 medium,
DMEM/F12 and FBS used for cell culture were purchased from Gibco
(Thermo Fisher Scientific, Inc.). Diagnostic ELISA kits for the
measurement of mouse TNF-α (cat. no. MTA00B), IL-6 (cat. no.
M6000B) and IFN-β (cat. no. MIFNB0) were obtained from R&D
Systems, Inc. C57BL/6J female mice (age, 6–8 weeks; weight, 20–25
g) were purchased from SLAC Laboratory Animal Company. The
following specific antibodies were obtained from Cell Signaling
Technology, Inc.: Anti-phosphorylated (p)-p38 (cat. no. 9215),
anti-p38 (cat. no. 8690), anti-p-p65 (cat. no. 3033), anti-p65
(cat. no. 8242), anti-p-JNK (cat. no. 4668), anti-JNK (cat. no.
9258), anti-p-ERK (cat. no. 4376), anti-ERK (cat. no. 4695),
anti-p-inhibitor of κB (IκB) (cat. no. 2859), anti-IκB (cat. no.
4812), anti-p-IRF-3 (cat. no. 29047), anti-IRF-3 (cat. no. 11904),
anti-p-IκB kinase (IKK) (cat. no. 2697), anti-IKK (cat. no. 8943)
and anti-β-actin (cat. no. 8457). All the animal experiments were
performed according to protocols that were approved by the Ethics
Committee of Huadong Medical Institute of Biotechniques.
Cells and cell culture
Mouse peritoneal macrophages (MPM) were isolated by
peritoneal lavage 3–4 days after intraperitoneal injection of mice
with 2 ml sterile 5% thioglycolate broth, as previously described
(17). MPM were cultured in
RPMI-1640 medium supplemented with 10% FBS and 1%
antibiotic-antimycotic (Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2.
Preparation of human anti-TLR4
IgG2
A phage-displayed library with >1013
phage clones was used to screen the human Fab against TLR4, as
previously described (18). A
total of seven rounds of screening with precoated recombinant TLR4
protein were performed to ensure the specificity of the binding
phage. VBASE2 database (vbase2.org/vbhelp.php) was used for analyzing the
sequence of Fab. Cloned anti-TLR4 Fab was selected to develop
complete human IgG2 via gene synthesis. The heavy (H) and light (L)
chains were cloned separately into the pMH3 vector
(AmProtein-China, Inc.). Recombinant IgG2 expression vectors
(pMH3-anti-TLR4-IgG2-H and pMH3-anti-TLR4-IgG2-L) were expressed in
Chinese hamster ovary (CHO) cells (American Type Culture
Collection) which were cultured in DMEM/F12 supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotic. Then, the cell culture media was
centrifuged at 200 × g for 5 min at 4°C, and the supernatant was
harvested after a transient transfection (Lipofectamine 2000;
Thermo Fisher Scientific, Inc.) for 6 days and purified with a
Protein A column (Cytiva). The purified protein was separated via
12% SDS-PAGE and visualized by staining with 0.1% Coomassie
brilliant blue R250 at room temperature for 1 h.
ELISA
ELISA was used to assay the affinity of human
anti-TLR4 IgG2. Briefly, 96-well plates were precoated with 50 ng
TLR4 antigen (R&D Systems, Inc.) per well in coating buffer (50
mM sodium carbonate buffer, pH 9.6) overnight at 4°C. After
blocking with 5% non-fat milk at room temperature for 1 h, 100 µl
human anti-TLR4 IgG2 at different concentrations was added to the
wells (1:2 serial dilution; 3 wells per dilution) for incubation at
37°C for 1 h. The plates were washed three times with 250 µl PBS
containing 0.1% Tween 20. Horseradish peroxidase (HRP)-conjugated
goat anti-human IgG (1:3,000; cat. no. AP113P; Sigma-Aldrich; Merck
KGaA) was then added. The absorbance values of the wells were
determined at 450 nm and analyzed by GraphPad Prism software
version 5.0 (GraphPad Software Inc.). The negative control
comprised PBS (10mM). The experiment was performed in
triplicate.
Affinity and kinetic assay of human
anti-hTLR4 IgG2
Affinity and kinetic assays of human anti-hTLR4 IgG2
were performed using a BLItz system (ForteBio, Inc.). TLR4 was
diluted to 50 ng/µl using PBS and then loaded to the biosensor
(cat. no. 18–5012; ForteBio, Inc.), while the anti-TLR4 IgG2 was
diluted in different concentrations (100–1,600 nM) . The
association time was 120 sec and the dissociation time was 120 sec.
Then, BLItz Pro 1.0 software (ForteBio, Inc.) was used to analyze
sensograms.
Fluorescence-activated cell
sorting
Each group of MPM (1×106 cells) was
pretreated with 5 ng/µl human anti-TLR4 IgG2 at 37°C for 1 h,
washed three times with PBS and probed with FITC-conjugated
anti-human IgG (1:1,000; cat. no. F9512; Sigma-Aldrich; Merk KGaA)
at 37°C for 1 h. A LSRII flow cytometer (BD Biosciences) was used
to detect the fluorescence intensity of cells after washing three
times with PBS.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
To optimize the pretreatment concentration, MPM were
cultured in 24-well plates (5×105 cells/well),
pretreated with human anti-TLR4 IgG2 at different concentrations
(1, 5 and 10 ng/µl) at 37°C for 2 h and then stimulated with 1
ng/µl LPS at 37°C for 4 h. The optimum concentration of human
anti-TLR4 IgG2 (5 ng/µl) was used for subsequent analyses. After
stimulation, total RNA was extracted using the RNAfast200 kit (cat.
no. 220010; ShanghaiFastagen Biotech Co., Ltd.) and RT RNA to cDNA
(37°C for 15 min) using the PrimeScript RT Master Mix kit (cat. no.
RR036A; Takara Bio, Inc.). RT-qPCR was performed using SYBR-Green I
kit (cat. no. DRR041A; Takara Bio, Inc.) under the following
conditions: Initial denaturation at 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec, 58°C for 10 sec and 72°C for 30 sec and
an end-up synthesis at 72°C for 30 sec. The relative expressions of
cytokines were normalized to those of β-actin, using the
2−ΔΔCq method (19).
The primers, as previously described, were shown in Table I (20).
 | Table I.Primers for mouse inflammatory
factors used in reverse transcription-quantitative PCR. |
Table I.
Primers for mouse inflammatory
factors used in reverse transcription-quantitative PCR.
| Gene name | Primers
(5′-3′) |
|---|
| TNF-α | F:
GACGTGGAACTGGCAGAAGAG |
|
| R:
TTGGTGGTTTGTGAGTGTGAG |
| IFN-β | F:
CAGCTCCAAGAAAGGACGAAC |
|
| R:
GGCAGTGTAACTCTTCTGCAT |
| IL-6 | F:
TAGTCCTTCCTACCCCAATTTCC |
|
| R:
TTGGTCCTTAGCCACTCCTTC |
| β-actin | F:
AGTGTGACGTTGACATCCGT |
|
| R:
GCAGCTCAGTAACAGTCCGC |
Western blot analysis
In order to analyze the inhibitory effect of human
anti-TLR4 IgG2 on TLR4 signal transduction, western blotting
analysis of phosphorylation levels of the NF-κB, mitogen-activated
protein kinase (MAPK) and IRF-3 pathways was performed as
previously described (20). The
mouse macrophages, cultured in 6-well plates (106 cells
per well), were pre-incubated with the human anti-TLR4 IgG2 (5
ng/µl) at 37°C for 2 h and induced with LPS (1 ng/µl) at 37°C for
0, 30 or 60 min. Cells were lysed in a RIPA lysis buffer
supplemented with protein inhibitor cocktail (Sigma-Aldrich; Merck
KGaA). Lysates were mixed and centrifuged at 12,000 × g for 15 min
at 4°C. A total of 30 µg protein/lane was then loaded onto an
SDS-PAGE gel (12% resolving gel) and electrotransferred onto a
nitrocellulose membrane after determining the protein concentration
by BCA protein assay kit (cat. no. 23225; Thermo Fisher Scientific
Inc.). After blocking with 5% non-fat milk in TBST (0.1% Tween 20)
at 37°C for 1 h, the membranes were incubated with primary
antibodies (anti-p-p38, anti-p38, anti-p-p65, anti-p65, anti-p-JNK,
anti-JNK, anti-p-ERK, anti-ERK, anti-p-IκB, anti-IκB, anti-p-IRF3,
anti-IRF3, anti-p-IKK, anti-IKK and anti-β-actin antibody) diluted
at 1:1,000 with 5% non-fat milk in TBST overnight at 4°C. Membranes
were then washed three times with TBST and probed with
HRP-conjugated secondary antibodies (1:2,000; cat. no. 7074; Cell
Signaling Technology, Inc.) in 5% non-fat milk in TBST at room
temperature for 1 h. Then, the bands were visualized by an enhanced
chemiluminescence kit (cat. no. 1805001; Tanon Science and
Technology Co., Ltd.). The relative protein expression levels were
analyzed using Gel-Pro-analyzer software 4.0 (Media Cybernetics,
Inc.).
In vivo neutralization assay
To determine the protective efficacy of human
anti-TLR4 IgG2 in the cecal ligation and puncture (CLP) model
(21), female C57BL/6 mice (n=39)
were randomly divided into three experimental groups with 13 mice
each. All mice were housed in 12:12 h light/dark cycle at 22°C and
free access to food and water. Mice in Group L + A (L, LPS; A,
human anti-TLR4 IgG2) were passively immunized with 15 µg/g body
weight anti-TLR4 IgG2 2 h before exposure to 15 µg/g body weight
LPS to assess its prophylactic potential. Mice in Group L were
injected with 15 µg/g body weight LPS, and mice in Group A were
injected with 15 µg/g body weight human anti-TLR4 IgG2. For this
experiment, human anti-TLR4 IgG2 was injected intravenously,
whereas LPS was injected peritoneally. Animals were observed
continuously for the first 4 h, at 8 h and throughout the next few
days, which was followed by forth-daily assessment for 1 week.
Statistical analysis
Multiple comparisons were determined by one-way
ANOVA with the Tukey's multiple comparison test. Comparisons
between two groups were analyzed by independent samples t-test. The
survival analysis was performed by Kaplan-Meier estimates. Data are
presented as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction and expression of human
anti-TLR4 IgG2
Following seven rounds of affinity panning, 11 of
the 60 candidate phage clones showed a strong positive signal.
After inspecting the sequence in the VBASE2 database, a κ-type
light chain was identified. It was found that the variable heavy
(VH) and variable light (VL) chains were amplified up to ~350 bp
each (Fig. 1A), and the
synthetized H and L chains with constant regions of human IgG2
(Fig. 1B) were each separately
inserted into the eukaryotic expression vector pMH3. Moreover,
eukaryotic expression vectors harboring human anti-TLR4 IgG2
(pMH3-anti-hTLR4-IgG2-H and pMH3-anti-hTLR4-IgG2-L) were
successfully constructed (Fig.
1C).
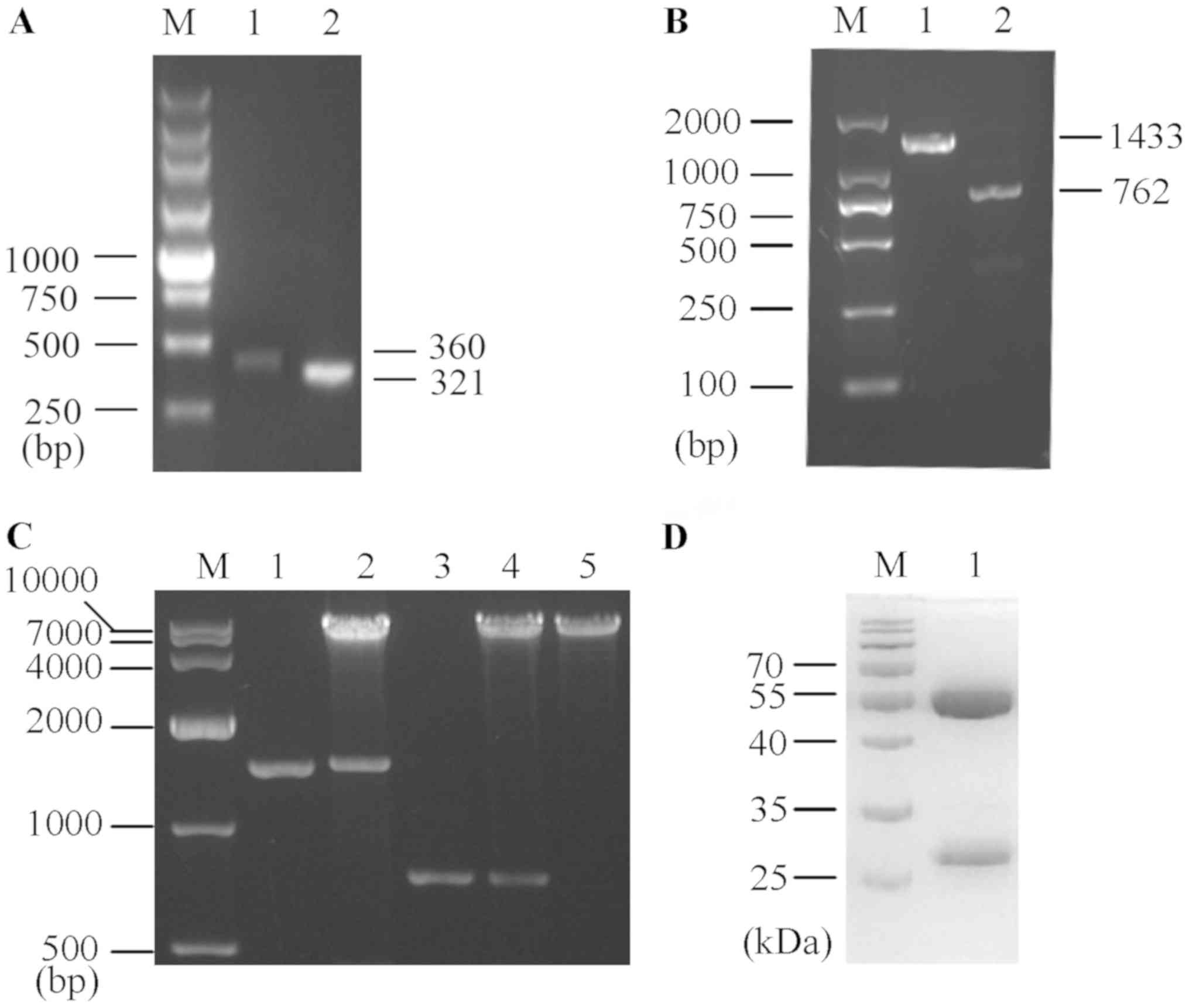 | Figure 1.Preparation of human anti-TLR4 IgG2.
(A) Anti-TLR4 IgG2-VH and anti-TLR4 IgG2-VL variable regions were
screened from a human Fab phage library. M, 250 bp DNA ladder
Marker. Lane 1, anti-TLR4 IgG2-VH variable region (360 bp). Lane 2,
anti-TLR4 IgG2-VL variable region (321 bp). (B) Amplification
products of the synthetized anti-TLR4 IgG2-H and anti-TLR4 IgG2-L
chains. M, DL2000 DNA Marker. Lane 1, anti-TLR4 IgG2-H (1,433 bp).
Lane 2, anti-TLR4 IgG2-L (762 bp). (C) The two recombinant
eukaryotic expression vectors (pMH3-anti-hTLR4-IgG2-H and
pMH3-anti-hTLR4-IgG2-L) were double-digested with EcoR I and
Not I. M, DL10000 DNA Marker. Lane 1, anti-TLR4 IgG2-H. Lane
2, double-digested pMH3-anti-hTLR4-IgG2-H. Lane 3, anti-TLR4
IgG2-L. Lane 4, double-digested pMH3-anti-hTLR4-IgG2-L. Lane 5,
Linearized pMH3. (D) SDS-PAGE identified the purify of human
anti-TLR4 IgG2. M, A protein Marker. Lane 1, Purified human
anti-TLR4 IgG2. TLR4, Toll-like receptor 4; L, light chain; H,
heavy chain. |
The recombinant expression vector was transfected
into CHO cells, which were cultured for 6 days. Cells were
centrifuged and the supernatant was purified with a Protein A
affinity column and examined via SDS-PAGE, followed by Coomassie
Brilliant Blue staining (Fig. 1D).
It was identified that the purification efficiency was ~95% with
2.5 mg/ml human anti-TLR4 IgG2.
Verification of specific binding of
human anti-TLR4 IgG2 to TLR4
The present study determined whether human anti-TLR4
IgG2 can specifically and selectively bind to human TLR4 using
ELISA. Gradient dilutions of human anti-TLR4 IgG2 were prepared for
ELISA and it was found that this antibody can specifically bind to
TLR4 in a dose-dependent manner (Fig.
2A). The antigen-antibody affinity constant was assessed to
analyze the binding affinity of human anti-TLR4 IgG2 to TLR4. Data
obtained from BLItz system analysis had an equilibrium dissociation
constant (KD) of 8.713×10−10 M (Fig. 2B), indicating that human anti-TLR4
IgG2 selectively and effectively bound to TLR4.
The binding ability of human anti-TLR4 IgG2 was
further assessed via flow cytometric analysis of TLR4-positive MPM.
Compared with the untreated control group, specific binding of the
human anti-TLR4 IgG2 to TLR4 reached ~66% (Fig. 2C), suggesting that human anti-TLR4
IgG2 effectively binds to TLR4 on the mouse cell surface.
Human anti-TLR4 IgG2 inhibits
LPS-induced production of inflammatory cytokines in vitro
To determine the optimal human anti-TLR4 IgG2
concentration that can inhibit LPS-stimulated MPM, the optimal
concentrations of human anti-TLR4 IgG2 and LPS were examined. LPS
was used at concentrations ranging from 0.01–1 ng/µl, and it was
demonstrated that the mRNA expression levels of TNF-α and IFN-β
were increased in a concentration-dependent manner compared with
the L + A group (Fig. S1A and B).
In addition, it was found that 1 ng/µl LPS induced significant
inflammation, on which the human anti-TLR4 IgG2 showed a higher
inhibition efficiency. Furthermore, compared with 1 ng/µl human
anti-TLR4 IgG2, a concentration of 5 ng/µl showed improved
inhibition efficiency on IFN-β and TNF-α mRNA expression levels
(Fig. 3). However, 10 ng/µl human
anti-TLR4 IgG2 did not have a higher performance compared with the
concentration of 5 ng/µl. Moreover, treatment with 5 ng/µl human
anti-TLR4 IgG2 reduced TNF-α, IFN-β and IL-6 expression levels by
approximately 50, 90 and 40%, respectively, compared with levels
after LPS treatment (Fig. 3).
Therefore, human anti-TLR4 IgG2 and LPS concentrations of 5 and 1
ng/µl, respectively, were used in the subsequent experiments.
 | Figure 3.Inhibitory effects of different
concentrations of human anti-TLR4 IgG2 on LPS-induced macrophages.
Macrophages were preincubated with different concentrations (1, 5
and 10 ng/µl) of human anti-TLR4 IgG2 for 2 h, and then induced
with 1 ng/µl LPS for 4 h. mRNA expression levels of (A)
TNF-α, (B) IFN-β and (C) IL-6 were determined
via reverse transcription-quantitative PCR and normalized to that
of the internal control, β-actin. The optimal concentration
of human anti-TLR4 IgG2 was 5 ng/µl. All experiments were performed
independently ≥3 times. Data are presented as the mean ± SD. N=3.
*P<0.05, **P<0.01, ***P<0.001 vs. LPS control. LPS,
lipopolysaccharide; TLR4, Toll-like receptor 4; L, LPS; A, human
anti-TLR4 IgG2; IL, interleukin; IFN-β, interferon-β; TNF-α,
tumor necrosis factor-α. |
To assess whether human anti-TLR4 IgG2 reduces the
production of LPS-induced inflammatory cytokines, the mRNA
expression levels of TNF-α, IFN-β and IL-6 were examined at
different time points. MPM were pretreated with 5 ng/µl human
anti-TLR4 IgG2 for 2 h and induced with 1 ng/µl LPS for 2, 4 and 8
h. It was demonstrated that TNF-α, IFN-β and IL-6 were
significantly upregulated in LPS-induced groups compared with those
in the untreated controls; however, these were significantly
downregulated after pretreatment with human anti-TLR4 IgG2
(Fig. 4). Furthermore, the
inhibition of human anti-TLR4 IgG2 was most potent at 4 h and was
50–60%.
 | Figure 4.Human anti-TLR4 IgG2 inhibits
LPS-induced production of inflammatory cytokines in mouse
macrophages. (A) TNF-α, (B) IFN-β and (C) IL-6
expression levels were quantified via reverse
transcription-quantitative PCR and normalized to the internal
control, β-actin. All experiments were performed
independently ≥3 times. Data are presented as the mean ± SD. N=3.
*P<0.05, **P<0.01, vs. LPS control. LPS, lipopolysaccharide;
TLR4, Toll-like receptor 4; L, LPS; A, human anti-TLR4 IgG2; IL,
interleukin; IFN-β, interferon-β; TNF-α, tumor necrosis
factor-α. |
Human anti-TLR4 IgG2 inhibits
phosphorylation levels of TLR4 signaling after LPS stimulation
To investigate the inhibition of human anti-TLR4
IgG2 on LPS-induced TLR4 signaling, western blotting was used to
analyze the phosphorylation of the NF-κB, MAPK and IRF-3 signaling
pathways, which are downstream effectors of the TLR4 pathway. After
treatment with LPS, the phosphorylation of p65, p38, JNK, ERK, IκB,
IKK and IRF-3 increased, but decreased after pretreatment with 5
ng/µl human anti-TLR4 IgG2 (Figs.
5–7). Thus, these results
indicated that human anti-TLR4 IgG2 inhibited LPS-induced
inflammatory responses in MPM by blocking TLR4.
 | Figure 5.Human anti-TLR4 IgG2 activates the
MAPK signal pathway after LPS stimulation. Cells were pretreated
with human anti-TLR4 IgG2 (5 ng/µl) for 2 h and further incubated
in the presence or absence of LPS (1 ng/µl) for 0, 30 and 60 min.
After immunoblotting, phospho-specific antibodies were used to
probe the regions containing p-ERK1/2, p-JNK1/2 and p-p38. β-actin
was used as the internal loading control. *P<0.05, **P<0.01,
***P<0.001 vs. LPS group. LPS, lipopolysaccharide; TLR4,
Toll-like receptor 4; L, LPS; A, human anti-TLR4 IgG2; p-,
phosphorylated. |
Human anti-TLR4 IgG2 protects mice
from LPS-induced sepsis in vivo
An in vivo protection assay was carried out
in the CLP model. After LPS administration, the mice treated with
PBS in the control group died within 35 h. Moreover, in the Group L
+ A receiving the antibody, human anti-TLR4 IgG2 protected mice
from LPS challenge with a survival rate of 40% and significantly
increased the survival time, compared with the Group L (Fig. 8). Furthermore, the present study
examined the serum levels of inflammatory factors by ELISA. It was
identified that treatment with human anti-TLR4 IgG2 reduced
LPS-initiated inflammatory responses, as it reduced TNF-α, IFN-β
and IL-6 levels by ~80, 75 and 60% at 4 h after LPS injection,
respectively (Fig. S2).
Collectively, these results were consistent with those from the
in vitro inhibition assay.
Discussion
Excessive host responses to LPS can lead to systemic
inflammatory conditions, including sepsis and fatal septic shock
(22–24). The mortality rate of severe sepsis
can reach 30–50% worldwide, possibly due to the lack of efficient
therapies (22–24). Previous findings have confirmed the
role of inflammatory pathways stimulated by the interaction between
TLR4 and LPS (13); therefore,
blocking of LPS-TLR4 signaling is important.
The present study extracted an anti-TLR4 Fab from a
human phage library and transformed it to IgG2 with an affinity of
8.713×10−10 M, which has stronger affinity to TLR4
compared with the Fab region alone. The eukaryotic expression
vector pMH3-hTLR4-IgG2 was successfully constructed and using a
BLItz system and ELISA, the specific binding ability of the human
anti-TLR4 IgG2 to TLR4 was assessed. The present results indicated
that the H and L chains of human anti-TLR4 IgG2 were efficiently
assembled into the active form, and the approaches to prepare mAb
did not change the specificity of the human anti-TLR4 IgG2.
LPS increases the secretion of numerous inflammatory
cytokines by activating the phosphorylation of the TLR4-mediated
NF-κB, MAPK and IRF3 pathways, and also elevates the production of
T proinflammatory cytokines (11–13,25).
Thus, the present study evaluated human anti-TLR4 IgG2 by measuring
TNF-α, IFN-β and IL-6 levels, which are involved in the MyD88
pathway, after LPS stimulation. The results showed that human
anti-TLR4 IgG2 at 5 ng/µl was sufficient for blocking TLR4 on the
surface of MPM, and did not show a higher inhibitory effect at an
increased concentration (10 ng/µl). Moreover, RT-qPCR results
identified a significant increase in LPS-induced production of
TNF-α, IFN-β and IL-6 mRNA expression levels, but this production
decreased after pretreatment with human anti-TLR4 IgG2. Western
blotting results demonstrated that LPS-stimulated phosphorylation
of p65, p38, JNK, ERK, IκBα, IKKα/β and IRF3. However, these
results were reversed by preincubation with human anti-TLR4 IgG2,
which was consistent with the decreased expression levels of
proinflammatory cytokines. In addition, given the high homology
between mice and humans, an in vivo neutralization assay as
performed using the mouse CLP model in which LPS was injected
peritoneally. However, as the half-life of antibody is short, mice
were immunized via intravenous injection to increase the absorption
rate. It was found that human anti-TLR4 IgG2 efficiently protected
mice from LPS challenge with a survival rate of 40% and inhibited
LPS-induced sepsis in mice by decreasing serum levels of
proinflammatory cytokines. Thus, it was speculated that human
anti-TLR4 IgG2 could rescue mice from severe sepsis. However, while
this mouse model is used for several purposes, such as for
investigating pathogenic mechanisms and evaluating new therapeutic
approaches (26–28), individual gene activation in humans
may not necessarily predicted by the ortholog in the corresponding
mouse model (29), which is a
limitation to the neutralization assay. In addition, the
experiments performed in mouse macrophages do not completely mimic
human inflammatory responses (29–32).
Thus, further studies are required to assess the inhibitory effects
of human anti-TLR4 IgG2 for the treatment of infection-associated
immune dysfunction in humans.
Previous studies have been aimed at evaluating TLR4
inhibition by targeted small-molecule compounds or antibodies for
the therapy of multiple inflammatory responses (33–37).
In addition to the human anti-TLR4 IgG2 designed in the present
study, several mAbs against TLR4 have been reported, which can be
divided into two categories. Firstly, agonistic mAbs, such as UT12
and Sa15-21, which induce NF-κB activation and protect mice from
subsequent lethal LPS challenges (36,37);
this phenomenon is called LPS tolerance. However, UT12 is
significantly distinguished from Sa15-21 as the latter enhances
LPS-induced TNF-α production, while Sa15-21 alone induces minimal
TNF-α production (36). The other
category is antagonistic mAbs, such as MTS510, which inhibit
LPS-induced NF-κB activation in TLR4-expressing cells (34). The human anti-TLR4 IgG2 designed in
the present study belongs to the second category. Moreover, there
are two advantages in the application of the human anti-TLR4 IgG2.
Firstly, previous reported antibodies are mouse mAbs or humanized
murine mAbs (20,34,36–38),
and these antibodies contain some non-human components that could
cause antigen-reactive responses. Furthermore, the human anti-TLR4
IgG2 used in the present study was a complete human antibody,
without any potential to elicit mAb production in humans. Moreover,
human anti-TLR4 IgG2 was produced using a eukaryotic expression
system that had post-translational modification capabilities, while
also eliminating the effect of Escherichia coli endotoxin,
which is prevalent in anti-TLR4-Fab produced in prokaryotic
expression systems (20,38).
Previous findings have characterized the
three-dimensional structures of LPS receptors (39–42).
It has also been reported that the LBP binds firstly to LPS and
presents it to CD14, and then CD14 transfers LPS to the TLR4/MD2
complex to form the M-shaped TLR4/MD2/LPS complex dimer (40). The TIR domains of TLR4 are located
in close spatial contiguity upon dimer formation, activating
downstream signaling molecules and promoting the secretion of
inflammatory cytokines (40,41).
Therefore, it was speculated that the human anti-TLR4 IgG2 may
prevent the interaction of MD2 with TLR4, thus blocking LPS-induced
TLR4 signal pathway transduction. However, the protective mechanism
of the human anti-TLR4 IgG2 requires further investigation.
In conclusion, the present study established a full
human anti-TLR4 IgG2 that bound specifically to TLR4 with high
affinity, inhibited the TLR4/MAPKs/NF-κB signaling pathway and
reduced the production of downstream inflammatory mediators, such
as TNF-α, IL-6 and IFN-β. Therefore, the specific blockade of TLR4
activation by the human anti-TLR4 IgG2 may be promising in the
treatment of infection-associated diseases in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by research grants from the
National Key R&D Program of China (grant no. 2018YFC1200603),
the National Natural Science Foundation of China (grant no.
31701181) and the Jiangsu Social Development Project (grant no.
BE2018617).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW performed most of the experiments, analyzed the
data and wrote the manuscript. DG helped with the partial plasmid
construction. FZ and TZ helped with the preparation of experimental
samples. CY and QC helped with experimental operations. MW helped
with experiment design. JZ and XZ designed the research and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments in this study were performed
according to the protocols approved by the Ethics Committee of
Huadong Medical Institute of Biotechniques.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
West AP, Koblansky AA and Ghosh S:
Recognition and signaling by toll-like receptors. Annu Rev Cell Dev
Biol. 22:409–437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Neill LA, Bryant CE and Doyle SL:
Therapeutic targeting of Toll-like receptors for infectious and
inflammatory diseases and cancer. Pharmacol Rev. 61:177–197. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fairweather D and Frisanchokiss S: Mast
cells and inflammatory heart disease: Potential drug targets.
Cardiovasc Hematol Disord Drug Targets. 8:80–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Laat MA, Gruntmeir KJ, Pollitt CC,
Mcgowan CM, Sillence MN and Lacombe VA: Hyperinsulinemia
down-regulates TLR4 expression in the mammalian heart. Front
Endocrinol (Lausanne). 5:1202014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gay NJ, Symmons MF, Monique G and Bryant
CE: Assembly and localization of Toll-like receptor signalling
complexes. Nat Rev Immunol. 14:546–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Huang LF, Wen C, Yang ZC and Chen
CY: Roles of cardiac mast cells and Toll-like receptor 4 in viral
myocarditis among mice. Zhongguo Dang Dai Er Ke Za Zhi. 15:896–902.
2013.(In Chinese). PubMed/NCBI
|
|
7
|
Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W,
Deng C, Fan C, Di S, Sun Y and Yi W: The emerging role of Toll-like
receptor 4 in myocardial inflammation. Cell Death Dis. 7:e22342016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sternberg EM: Neural regulation of innate
immunity: A coordinated nonspecific host response to pathogens. Nat
Rev Immunol. 6:318–328. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beutler B, Du X and Poltorak A:
Identification of Toll-like receptor 4 (Tlr4) as the sole conduit
for LPS signal transduction: Genetic and evolutionary studies. J
Endotoxin Res. 7:277–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan Y and Kagan JC: A cross-disciplinary
perspective on the innate immune responses to bacterial
lipopolysaccharide. Mol Cell. 54:212–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Husebye H, Halaas Ø, Stenmark H, Tunheim
G, Sandanger Ø, Bogen B, Brech A, Latz E and Espevik T: Endocytic
pathways regulate Toll-like receptor 4 signaling and link innate
and adaptive immunity. EMBO J. 25:683–692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kagan JC, Su T, Horng T, Chow A, Akira S
and Medzhitov R: TRAM couples endocytosis of Toll-like receptor 4
to the induction of interferon-beta. Nat Immunol. 9:361–368. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu YC, Yeh W and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Annane D, Bellissant E and Cavaillon JM:
Septic shock. Lancet. 365:63–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hotchkiss RS and Karl IE: The
Pathophysiology and Treatment of Sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaplon H and Reichert JM: Antibodies to
watch in 2019. MAbs. 11:219–238. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park J and Rikihisa Y: Inhibition of
ehrlichia risticii infection in murine peritoneal macrophages by
gamma interferon, a calcium ionophore, and concanavalin A. Infect
Immun. 59:3418–3423. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiao Y, Zhao P, Zhu J, Grabinski T, Feng
Z, Guan X, Skinner RS, Gross MD, Hay RV, Tachibana H and Cao B:
Construction of human naïve Fab library and characterization of
anti-met Fab fragment generated from the library. Mol Biotechnol.
31:41–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai B, Wang M, Zhu X, Xu J, Zheng W, Zhang
Y, Zheng F, Feng Z and Zhu J: The Fab fragment of a humanized
anti-Toll like receptor 4 (TLR4) monoclonal antibody reduces the
lipopolysaccharide response via TLR4 in mouse macrophage. Int J Mol
Sci. 16:25502–25515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hubbard WJ, Choudhry M, Schwacha MG, Kerby
JD, Rue LW III, Bland KI and Chaudry IH: Cecal ligation and
puncture. Shock. 24 (Suppl 1):S52–S57. 2005. View Article : Google Scholar
|
|
22
|
Bryant CE, Spring DR, Gangloff M and Gay
NJ: The molecular basis of the host response to lipopolysaccharide.
Nat Rev Microbiol. 8:8–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller SI, Ernst RK and Bader MW: LPS,
TLR4 and infectious disease diversity. Nat Rev Microbiol. 3:36–46.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosadini CV and Kagan JC: Early innate
immune responses to bacterial LPS. Curr Opin Immunol. 44:14–19.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lauren A, Brown AG, Samuel P and Elovitz
MA: Lipopolysaccharide induces cytokine production and decreases
extravillous trophoblast invasion through a mitogen-activated
protein kinase-mediated pathway: Possible mechanisms of first
trimester placental dysfunction. Hum Reprod. 27:61–72. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davis MM: A prescription for human
immunology. Immunity. 29:835–838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayday AC and Peakman M: The habitual,
diverse and surmountable obstacles to human immunology research.
Nat Immunol. 9:575–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woodcock J and Woosley R: The FDA critical
path initiative and its influence on new drug development. Annu Rev
Med. 59:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seok J, Warren HS, Cuenca AG, Mindrinos
MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy
L, et al: Genomic responses in mouse models poorly mimic human
inflammatory diseases. Proc Natl Acad Sci USA. 110:3507–3512. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mitka M: Drug for severe sepsis is
withdrawn from market, fails to reduce mortality. JAMA.
306:2439–2440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mestas J and Hughes CC: Of mice and not
men: Differences between mouse and human immunology. J Immunol.
172:2731–2738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warren HS, Fitting C, Hoff E, Adib-Conquy
M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J,
Hayden D and Cavaillon JM: Resilience to bacterial infection:
Difference between species could be due to proteins in serum. J
Infect Dis. 201:223–232. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Y, Chen S, Cao Y, Zhou P, Chen Z and
Cheng K: Discovery of novel small molecule TLR4 inhibitors as
potent anti-inflammatory agents. Eur J Med Chem. 154:253–266. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andresen L, Theodorou K, Grünewald S,
Czech-Zechmeister B, Könnecke B, Lühder F and Trendelenburg G:
Evaluation of the therapeutic potential of Anti-TLR4-antibody
MTS510 in experimental stroke and significance of different routes
of application. PLoS One. 11:e1484282016. View Article : Google Scholar
|
|
35
|
Neal MD, Jia H, Eyer B, Good M, Guerriero
CJ, Sodhi CP, Afrazi A, Prindle T Jr, Ma C, Branca M, et al:
Discovery and validation of a new class of small molecule Toll-like
receptor 4 (TLR4) inhibitors. PLoS One. 8:e657792013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohta S, Bahrun U, Shimazu R, Matsushita H,
Fukudome K and Kimoto M: Induction of long-term lipopolysaccharide
tolerance by an agonistic monoclonal antibody to the toll-like
receptor 4/MD-2 complex. Clin Vaccine Immunol. 13:1131–1136. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akashi-Takamura S, Furuta T, Takahashi K,
Tanimura N, Tanimura N, Kusumoto Y, Kobayashi T, Saitoh S, Adachi
Y, Doi T and Miyake K: Agonistic antibody to TLR4/MD-2 protects
mice from acute lethal hepatitis induced by TNF-alpha. J Immunol.
176:4244–4251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang M, Zheng W, Zhu X, Xu J, Cai B, Zhang
Y, Zheng F, Zhou L, Yang Z, Zhang X, et al: A human anti-Toll like
receptor 4 Fab fragment inhibits lipopolysaccharide-induced
pro-inflammatory cytokines production in macrophages. PLoS One.
11:e1468562016.
|
|
39
|
Park BS, Song DH, Kim HM, Choi BS, Lee H
and Lee JO: The structural basis of lipopolysaccharide recognition
by the TLR4-MD-2 complex. Nature. 458:1191–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eckert JK, Kim YJ, Kim JI, Gürtler K, Oh
DY, Sur S, Lundvall L, Hamann L, van der Ploeg A, Pickkers P, et
al: The crystal structure of lipopolysaccharide binding protein
reveals the location of a frequent mutation that impairs innate
immunity. Immunity. 39:647–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim JI, Lee CJ, Jin MS, Lee CH, Paik SG,
Lee H and Lee JO: Crystal structure of CD14 and its implications
for lipopolysaccharide signaling. J Biol Chem. 280:11347–11351.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim SJ and Kim HM: Dynamic
lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and
CD14. BMB Rep. 50:55–57. 2017. View Article : Google Scholar : PubMed/NCBI
|





















