Introduction
In China, >100 million patients have diabetes
mellitus (DM) (1). Diabetic
nephropathy (DN), a main complication of DM, is a serious threat to
the life and health of Chinese patients and significantly increases
medical expenditure (2).
Pathological characteristics of DN include glomerular hypertrophy,
thickening of the glomerular and tubular basement membrane,
deposition of extracellular matrix and finally progression to
tubulointerstitial fibrosis and glomerulosclerosis (3). Although it is generally considered
that the changes associated with glomeruli serve a key role in the
pathogenesis of DN, tubulointerstitial injury may also be an
important marker of DN; tubular cells are one of the main targets
of high concentration glucose (HG)-induced injury (4). Tubulointerstitial injury is more
closely associated with the decline of renal function (5) and can more effectively predict DN
progression (6) compared with
glomeruli injury. However, the exact role of renal tubular injury
in the pathogenesis of DN remains unknown. Increasing evidence has
suggested that apoptosis is involved in renal tubular cell
damage-related DN (7–9), and endoplasmic reticulum (ER) stress
exerts a major role in cell death-related pathways (10,11).
Resveratrol (RSV) is a natural plant polyphenol with
anti-oxidative, anti-inflammatory and cytoprotective properties
(12). In addition to its
antioxidant effect or ability to activate AMP-activated protein
kinase or sirtuin 1 (SIRT1) genes (13), RSV has been reported to alleviate
the apoptosis of hepatocytes (14)
and cardiomyocytes (15), as well
as neuroinflammation in vasculitic peripheral neuropathy (16) via suppressing ER stress. A previous
study also revealed that RSV can attenuate the progression of DN
(17). However, to the best of our
knowledge, the renoprotective effects of RSV and its association
with attenuation of tubular cell injury by inhibiting ER
stress-induced apoptosis in DN are yet to be elucidated. The
present study performed in vivo and in vitro analyses
to determine whether inhibition of ER stress-induced apoptosis by
RSV could attenuate tubular cell injury in DN.
Materials and methods
Animals
Male db/db (C57BLKS/J-LepRdb/LepRdb) mice (average
age, 6 weeks; weight 30–33 g; n=20) and male db/m
(C57BLKS/J-LepRdb/+; average age, 6 weeks; weight 20–23 g; n=10)
were purchased from the National Mode Animal Centre of Nanjing
University. All mice were kept at a constant room temperature
(20±1°C) under a controlled 12-h light/12-h dark cycle in a
specific pathogen-free room and allowed to free access to rodent
chow and clean water. After adaptive feeding for 2 weeks, all mice
were randomly divided into the following groups (n=10/group): db/m
group, non-diabetic db/m mice; db/db group, non-treated db/db mice;
and db/db + RSV group, db/db mice administered with RSV by gavage.
RSV (Sigma-Aldrich; Merck KGaA) was dissolved in carboxymethyl
cellulose (0.5%; CMC; Sigma-Aldrich; Merck KGaA) and orally
administered via gavage tube at a dose of 40 mg/kg once a day for
12 weeks to mice in the db/db + RSV group. The mice in db/m and
db/db groups were administered 100 µl/10 g weight of 0.5% CMC. All
animal experimental protocols were ethically approved by the
Laboratory Animals Ethical Committee of Wannan Medical College
(approval no. LLSC-2020-057). According to the method reported
previously (18), mice were
anesthetized (fentanyl/medetomidine/midazolam; 0.05/5/0.5 mg/kg
body weight; intraperitoneal) at 20 weeks of age, punctured into
the abdominal aorta and exsanguinated.
Physical and biochemical analysis
The body weight (BW) of each mouse was measured and
blood glucose (BG) from the tail vein blood (50 µl) was tested with
a glucometer (Accu-Check Active; Roche Diagnostics GmbH) at both 8
and 20 weeks of age. The mice were placed in metabolic cages to
collect 24-h urine samples at 20 weeks of age. The urinary albumin
and urinary creatinine concentrations were determined using a mouse
albumin ELISA kit (cat. no. E99-134; Bethyl Laboratories, Inc.) and
mouse QuantiChrom™ Creatinine assay kit (cat. no. DICT-500;
BioAssay Systems), respectively, according to the manufacturer's
protocol.
Histology evaluation
Renal tissues from the mice were fixed for 24 h at
20°C in 10% neutral formalin, embedded in paraffin, manually
sectioned into 4-µm thick tissue sections and stained with
hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) and
Masson reagent as previously described (19,20).
The paraffin-embedded renal tissue sections were
analyzed using immunohistochemistry as described in our previous
study (19). Briefly, after
dewaxing and hydration, the slides were heated in sodium citrate
buffer (10 mM; pH 6) for 10 min at 100°C and incubated with
hydrogen peroxide (0.3%) for 10 min at room temperature. After
blocking with horse serum (Beyotime Institute of Biotechnology) at
37°C for 30 min, the sections were stained with primary monoclonal
antibodies against GRP78 (1:100; Cell Signaling Technology, Inc.;
cat. no. 3177) and CHOP (1:50; Cell Signaling Technology, Inc.;
cat. no. 2895) overnight at 4°C. After washing with Tris-buffered
saline containing 0.1% Tween-20 three times, anti-rabbit and
anti-mouse IgG labeled with horseradish peroxidase (Thermo Fisher
Scientific, Inc.; cat. nos. 31460 and 31430) were added and the
samples were incubated for 45 min at 20°C. Images were captured
using a light microscope (Nikon Corporation).
TUNEL staining
TUNEL staining was performed using the DeadEnd™
Colorimetric TUNEL system (Promega Corporation) as described
previously (19). Briefly, renal
paraffin sections were dewaxed, incubated with proteinase-K (1:500)
at 37°C for 10 min and reacted with the TUNEL reaction mixture
(balanced solution 98 µl + biotinylated nucleotide mix 1 µl + rTdT
1 µl) for 60 min at 37°C. The apoptotic cells were counted by two
independent pathologists under a light microscope in a blinded
manner, and the rate of apoptosis (%) was determined.
Cell culture experiments
Normal rat renal proximal tubular epithelial
(NRK-52E) cells were obtained from the Cell Resource Center of
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences. NRK-52E cells were maintained in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin, and incubated in
a 5% CO2 atmosphere at 37°C.
To test the effect of RSV on HG-induced ER stress,
NRK-52E cells were treated with FBS-free medium for 24 h until the
cells reached ~80% confluency. The cells were subsequently treated
with or without 20 µM RSV for 6 h at 37°C, before incubation with
normal concentration glucose (NG; 5.5 mM D-glucose), HG (30 mM
D-glucose) or high concentration mannitol (HM, 5.5 mM D-glucose
supplied with 24.5 mM D-mannitol; Sigma-Aldrich; Merck KGaA). All
tests were performed in triplicate wells and repeated three
times.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from NRK-52E cells using
TRizol® (Invitrogen; Thermo Fisher Scientifc, Inc.), and
the purity and concentration of the extracted RNA were assessed
with a spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific,
Inc.). RNA was reverse-transcribed with SuperScript III reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) using an
oligo dT primer according to the manufacturer's protocol. RT-qPCR
was performed in a StepOne Plus™ Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using a Quanti Nova™
SYBR® Green PCR kit (Qiagen GmbH). The thermocycling
conditions consisted of an initial denaturation at 95°C for 10 min
followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. The
following primers were used: i) GRP78-forward,
5′-GACTGGAATCCCTCCTGCTC-3′ and reverse, 5′-GGTCAGGCGGTTTTGGTC-3′;
ii) CHOP-forward, 5′-CACAAGCACCTCCCAAAGC-3′ and reverse,
5′-CTCTCATTCTCCTGCTCCTTCTC-3′; and iii) GAPDH-forward,
5′-ACTCCACGACATACTCAGCA-3′ and reverse, 5′-CATCAACGACCCCTCATT-3′.
mRNA expression levels were normalized to those of GAPDH in the
same cDNA sample. Relative quantification of gene expression was
performed using the 2−ΔΔCq method (21).
Western blotting
Tissues or cells were lysed with RIPA buffer
(Beyotime Institute of Biotechnology) containing protease and
phosphatase inhibitor cocktail, and the protein concentration was
measured using the bicinchoninic acid protein assay kit (Bio-Rad
Laboratories, Inc.). The protein samples (40 µg per lane for
tissue; 30 µg per lane for cells) were separated via SDS-PAGE on
10% gels and transferred to PVDF membranes, as described in our
previous study (19). After
blocking with PBST containing 3% BSA for 1 h at room temperature,
the membranes were incubated with the following primary antibodies
overnight at 4°C: i) GRP78 (1:1,000; Cell Signaling Technology,
Inc.; cat. no. 3177), CHOP (1:1,000; Cell Signaling Technology,
Inc.; cat. no. 2895); ii) cleaved caspase12 (1:1,000; ABclonal
Biotech Co., Ltd.; cat. no. A0217); and iii) β-actin (1:1,000;
Wuhan Boster Biological Technology, Ltd.; cat. no. BA2305).
Following incubation, the PVDF membranes were washed with PBS
containing 0.1% Tween-20 and incubated with horseradish
peroxidase-conjugated goat anti-rabbit (1:4,000; cat. no. 31460) or
goat anti-mouse IgG secondary antibodies (1:4,000; cat. no. 31430)
(both Thermo Fisher Scientific, Inc.) at room temperature for 1 h.
Protein bands were visualized using electrochemiluminescence
western blotting detection reagent (Beyotime Institute of
Biotechnology) and scanned using a Bio-Rad Imaging system (version
2.0; Bio-Rad Laboratories, Inc.). ImageJ software (version 1.8.0;
National Institutes of Health) was used for analysis.
Cell apoptosis assay
FITC Annexin V Apoptosis Detection Kit I (BD
Biosciences; cat. no. 556547) was used to quantify the rate of
apoptosis (at early phase) according to the manufacturer's
protocols. Briefly, the harvested NRK-52E cells were resuspended in
100 µl binding buffer, followed by staining with 5 µl annexin
V-FITC and 1 µg/ml PI solutions in the dark at room temperature for
15 min. Data were acquired with a Gallios flow cytometer (Beckman
Coulter, Inc.) and analyzed using FlowJo software (version 10;
FlowJo LLC).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc.). Data are presented as the mean ± SEM. Multiple groups
was compared using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RSV attenuates renal injury and
improves renal morphology in diabetic db/db mice
The present study determined BG, BW, urine albumin
to creatinine ratio (UACR) and urine albumin excretion (UAE) of the
mice in each group at 8 and 20 weeks (Fig. 1). Compared with db/m mice, db/db
mice had significant increases in BW, BG, UACR and UAE at 8 and 20
weeks. However, after treatment with RSV, BW, UACR and UAE were
significantly decreased at 20 weeks. Although BG improved after RSV
treatment, the change was not significantly different.
The characteristic renal histopathological changes
were observed via H&E, PAS and Masson staining in diabetic
db/db mice at 20 weeks. Compared with the healthy kidney structure
in the db/m group, mesangial cell proliferation, accumulation and
expansion of focal mesangial matrix and tubulointerstitial fibrosis
were observed in the db/db group. These changes were notably
attenuated in the RSV-treated group compared with those in
non-treated db/db mice (Fig. 2).
These results indicated that RSV has beneficial effects in delaying
the development of DN.
RSV inhibits ER stress in the kidneys
of diabetic db/db mice
ER stress has been reported to be involved in the
pathogenesis of DN (22).
Therefore, the expression levels of GRP78, CHOP and cleaved caspase
12 were measured in the kidneys of db/db mice. The expression
levels of GRP78, CHOP and cleaved caspase 12 in the renal cortex of
db/db mice were significantly higher than those in non-diabetic
db/m mice. Treatment of db/db mice with RSV significantly
downregulated the expression levels of GRP78, CHOP and cleaved
caspase 12 (Fig. 3). The
expression levels of GRP78 and CHOP in the renal tubules of
diabetic db/db mice were significantly higher, compared with those
in non-diabetic db/m mice. Downregulation of GRP78 and CHOP in the
renal tubule of diabetic db/db mice after RSV treatment was also
demonstrated via immunohistochemistry (Fig. 4). Thus, the results suggested that
ER stress was induced in the kidneys of diabetic db/db mice,
particularly in the tubules, and was significantly inhibited by RSV
treatment.
RSV inhibits ER stress-induced
apoptosis in the kidneys of db/db mice
ER stress-induced apoptosis serves an important role
in cell death (23). The results
of TUNEL assay identified that the number of apoptotic renal cells
in db/db mice was higher compared with those in db/m mice, which
was significantly decreased by RSV treatment (Fig. 5). Furthermore, western blotting was
performed to confirm whether apoptosis was induced by ER stress.
The expression of cleaved caspase 12 was significantly increased in
the kidney tissues of db/db mice compared with db/m mice, which was
significantly decreased after RSV treatment (Fig. 3D). Therefore, RSV treatment may
alleviate ER stress-induced cell apoptosis.
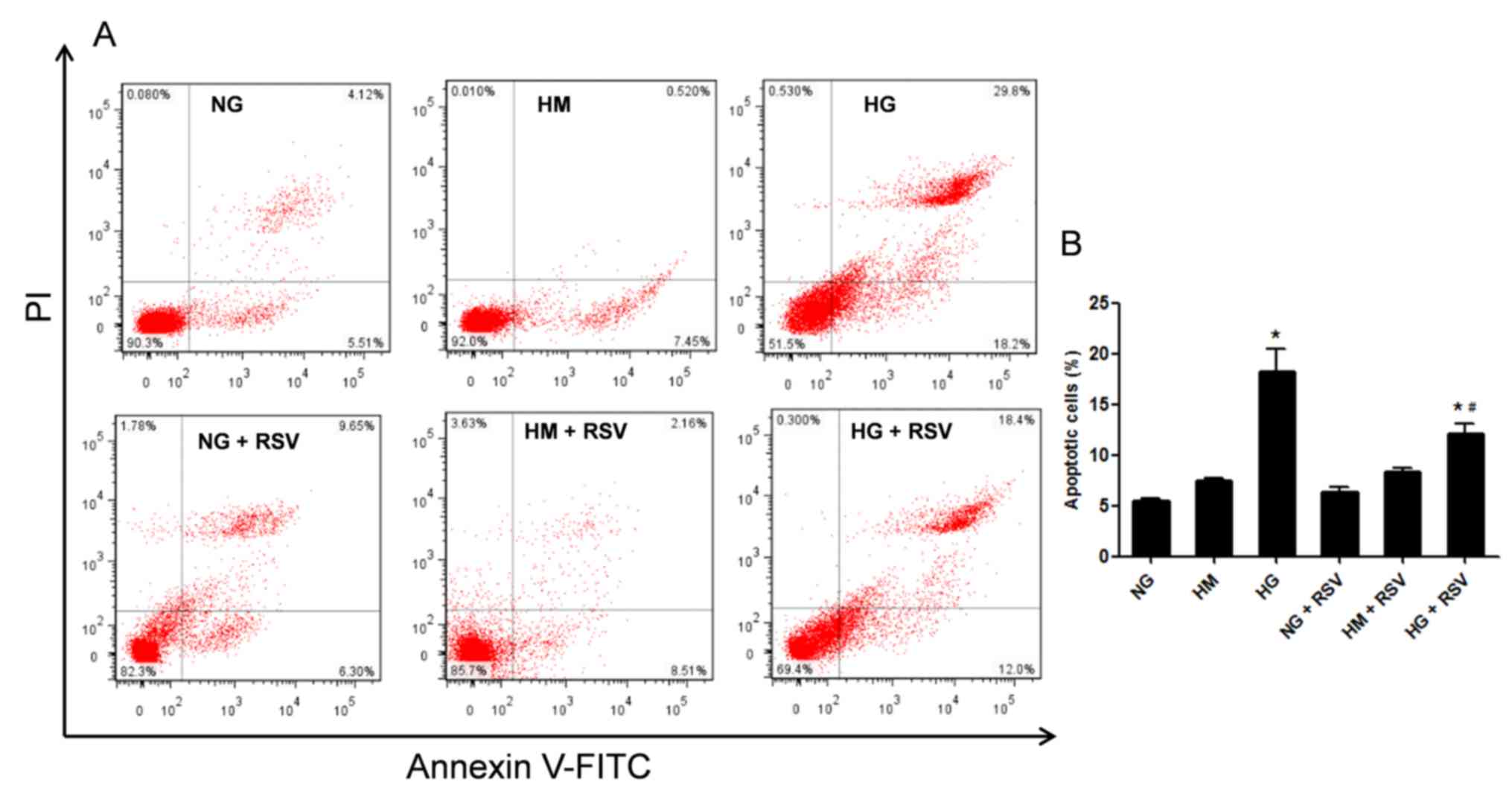
RSV inhibits HG-induced apoptosis in
NRK-52E cells
The effects of RSV in vitro were validated
using rat renal cells treated with HG. The results demonstrated
that HG-induced apoptosis of NRK-52E cells was significantly
inhibited by RSV treatment (Fig.
6). These data suggested that HG could induce apoptosis of
NRK-52E cells compared with normal concentration glucose, and that
RSV could alleviate apoptosis of NRK-52E cells induced by HG.
RSV attenuates HG-induced ER stress in
NRK-52E cells
The role of ER stress in inducing apoptosis of
NRK-52E cells treated with HG was subsequently investigated.
Compared with NG, HG upregulated the expression of ER
stress-related proteins GRP78 and CHOP, while HM did not
effectively activate ER stress (Fig.
7A). Furthermore, pretreating NRK-52E cells with RSV at 20 µM
for 6 h significantly inhibited the upregulation of GRP78 and CHOP
expression levels in NRK-52E cells exposed to HG. Consistent with
the western blotting results, the gene expression levels of GRP78
and CHOP in NRK-52E cells cultured with HG were upregulated, which
was inhibited by RSV (Fig. 7B).
Collectively, it was indicated that ER stress was induced in
HG-stimulated NRK-52E cells and RSV effectively suppressed ER
stress.
Discussion
The present results demonstrated that RSV
administration can delay the development of DN, as indicated by
decreases in UACR and UAE, improvements in the renal histopathology
of db/db mice and inhibition of ER stress-induced apoptosis.
Moreover, RSV inhibited HG-induced ER stress and reduced the rate
of apoptosis in tubular cells in vitro. Thus, the present
results suggested that RSV reduced HG-induced apoptosis in renal
tubular cells by suppressing ER stress.
Renal tubular cells are direct targets of enhanced
glucose levels in patients with diabetes, and renal tubular injury
can precede microalbuminuria (24). HG stimulates renal proximal tubular
cells and promotes the production of various cytokines, growth
factors, reactive oxygen species (ROS) and matrix proteins
(24), which in turn results in
tubular hypertrophy and tubular basement membrane thickening
(25). Furthermore, HG-induced ROS
production results in apoptosis of pancreatic β-cells by targeting
SIRT1 (26), an enzyme that
regulates antioxidant-related genes. RSV has been revealed to
attenuate several types of renal injury, including DN, drug-induced
renal damage, obstructive nephrology, ischemia-reperfusion and
sepsis-induced renal damage, in animal models via its antioxidant
effect or SIRT1 activation (13).
A previous study reported the effect of antioxidants on ER stress
(27), which prompted the present
study to evaluate the inhibitory role of RSV on ER stress in
HG-induced tubular cell injury in DN.
The present findings are in line with those of a
recent report by Yuan et al (28), who found that RSV treatment
improved diabetes-induced renal damage and decreased ER
stress-related markers in streptozotocin-induced diabetic rats. The
current study identified that the expression levels of GRP78, CHOP
and caspase12 were downregulated in the kidney cortex of diabetic
db/db mice administered RSV. Under physiological conditions, GRP78,
an important ER chaperone, forms a complex with three transmembrane
proteins to maintain the inactive state of ER (29). GRP78 is also a key regulatory
factor in unfolded protein response (29). CHOP, a transcription factor, is
involved in ER stress-induced cell apoptosis; therefore, it is
considered a proapoptotic protein (30). CHOP has been reported to be
upregulated during ER stress and is closely associated with the
onset of ER stress-induced apoptosis (31). Moreover, CHOP deletion protects
cells against ER stress-induced injury (30). The present results demonstrated a
downregulation of CHOP in RSV-treated mice, which was accompanied
by decreased apoptosis of renal cells, particularly tubular cells.
Caspase12 has been shown to be specifically localized on the ER
(32,33) in rodents and is considered a
specific sign of ER stress-induced apoptosis (33,34).
During the ER stress-induced apoptosis cascade, caspase12 is
cleaved and subsequently activated to induce cell death (35). The present results indicated that
RSV treatment can alleviate ER stress-induced renal cell apoptosis
by modulating GRP78, CHOP and caspase12 gene expression levels.
The effect of RSV on lowering blood glucose levels
in db/db mice remains controversial. Previous studies have shown
that RSV administration decreases blood glucose levels in db/db
mice (36,37), whereas recent reports demonstrated
no such effect (38,39). The present study found that RSV
lowered blood glucose levels in db/db mice compared with those in
non-treated db/db mice; however, the difference was not
significant. Therefore, lowering of blood glucose levels may not
account for the renoprotective effects of RSV in diabetic mice.
Furthermore, in vitro experimental results demonstrated that
RSV effectively protected NRK-52E cells against HG-induced
apoptosis, suggesting that the renoprotective effects of RSV do not
occur via lowering of blood sugar.
Different mechanisms, such as the RAGE/p22phox/NF-κB
pathway (40), oxidative
stress/TRAF3 interacting protein 2/NF-κB pathway (41) and TGF-β1/Smad2/3 signaling pathway
(42), have been reported to be
associated with HG-induced tubular damage. The involvement of ER
stress in tubular injury was found to be mainly due to acute kidney
injury (43,44). However, in our previous study, it
was demonstrated that tauroursodeoxycholic acid, an effective
inhibitor of ER stress, attenuated renal tubular injury in a mouse
model of type 2 diabetes by suppressing the ER stress signaling
pathway (19). In agreement with
these previous findings, the present results indicated that tubular
cell injury in DN was associated with ER stress. Thus, suppressing
HG-induced ER stress and associated apoptosis using RSV treatment
may reduce the death of tubular cells.
The present study demonstrated that RSV can inhibit
diabetes-induced ER stress in vivo, which was consistent
with the finding of a previous study in which RSV treatment
inhibited tunicamycin-induced ER stress in vivo (45), indicating a direct inhibitory
effect of RSV. HG activates NADPH oxidase, resulting in the
production of ROS, which in turn induces ER stress (46). In such cases, suppression of ER
stress via RSV may be attributed to its indirect inhibitory effects
on oxidative stress. Future studies will further examine the
relevant mechanisms in the role of RSV in DN via experiments, such
as animal models with gene knockouts, as well as cell models and
rescue experiments.
In conclusion, the present study indicated that RSV
exerted renoprotective effects in vivo, reduced HG-induced
tubular cell apoptosis in vitro and suppressed ER stress.
Furthermore, the current study provides evidence for the clinical
application of RSV in preventing the development of DN.
Acknowledgements
Not applicable.
Funding
This work was supported by the Funding of Yijishan
Hospital (grant no. KY24560348), the Scientific Research Project of
Wannan Medical College (grant no. WK2019F15). Anhui Provincial
Natural Science Foundation (grant no. 1908085MH248), the Overseas
Visiting and Training Program for the Outstanding Young Talents in
the Colleges of Anhui Province (grant no. gxgwfx2018054) and
National Training Programs of Innovation and Entrepreneurship for
Undergraduates (grant nos. 201810368004 and 201910368019).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JZ, GFX and GDW designed the study. JZ, XJD, MRD and
CYY performed the experiments. YW and MJYW helped to feed and
prepare animals, and also performed some of the biochemical kit
measurements. JZ, XL and GFX analyzed the data. JZ, XJD, GFX and
GDW wrote and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Laboratory Animals Ethical Committee of Wannan Medical College
(approval no. LLSC-2020-057).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DN
|
diabetic nephropathy
|
|
RSV
|
resveratrol
|
|
ER
|
endoplasmic reticulum
|
|
DM
|
diabetes mellitus
|
|
CMC
|
carboxymethyl cellulose sodium
salt
|
|
BG
|
blood glucose
|
|
BW
|
body weight
|
|
NG
|
normal glucose
|
|
UACR
|
urine albumin to creatinine ratio
|
|
UAE
|
urine albumin excretion
|
|
ROS
|
reactive oxygen species
|
|
GRP78
|
glucose-regulated protein 78 kD
|
|
CHOP
|
C/EBP-homologous protein
|
References
|
1
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao W, Yip CW and Chen W: Complications of
diabetes in China: Health system and economic implications. BMC
Public Health. 19:2692019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pourghasem M, Shafi H and Babazadeh Z:
Histological changes of kidney in diabetic nephropathy. Caspian J
Intern Med. 6:120–127. 2015.PubMed/NCBI
|
|
4
|
Jenkin KA, McAinch AJ, Zhang Y, Kelly DJ
and Hryciw DH: Elevated cannabinoid receptor 1 and G
protein-coupled receptor 55 expression in proximal tubule cells and
whole kidney exposed to diabetic conditions. Clin Exp Pharmacol
Physiol. 42:256–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao L, Wang M, Yang S, Liu F and Sun L: A
glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling
in diabetic nephropathy. Biomed Res Int. 2013:9870642013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gilbert RE and Cooper ME: The
tubulointerstitium in progressive diabetic kidney disease: More
than an aftermath of glomerular injury? Kidney Int. 56:1627–1637.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ju Y, Su Y, Chen Q, Ma K, Ji T, Wang Z and
Li W and Li W: Protective effects of Astragaloside IV on
endoplasmic reticulum stress-induced renal tubular epithelial cells
apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother.
109:84–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibusawa R, Yamada E, Okada S, Nakajima
Y, Bastie CC, Maeshima A, Kaira K and Yamada M: Dapagliflozin
rescues endoplasmic reticulum stress-mediated cell death. Sci Rep.
9:98872019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Zhu X, Zhang J and Shi J:
MicroRNA-25 inhibits high glucose-induced apoptosis in renal
tubular epithelial cells via PTEN/AKT pathway. Biomed Pharmacother.
96:471–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian J, Mo J, Xu L, Zhang R, Qiao Y, Liu
B, Jiang L, Ma S and Shi G: Scoulerine promotes cell viability
reduction and apoptosis by activating ROS-dependent endoplasmic
reticulum stress in colorectal cancer cells. Chem Biol Interact.
327:1091842020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang M, Wang H, Liu Z, Lin L, Wang L, Xie
M, Li D, Zhang J and Zhang R: Endoplasmic reticulum
stress-dependent activation of iNOS/NO-NF-KB signaling and NLRP3
inflammasome contributes to endothelial inflammation and apoptosis
associated with microgravity. FASEB J. 2020.(Epub ahead of print).
View Article : Google Scholar
|
|
12
|
Malhotra A, Bath S and Elbarbry F: An
organ system approach to explore the antioxidative,
anti-inflammatory, and cytoprotective actions of resveratrol. Oxid
Med Cell Longev. 2015:8039712015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitada M and Koya D: Renal protective
effects of resveratrol. Oxid Med Cell Longev. 2013:5680932013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu LQ, Fan ZQ, Tang YF and Ke ZJ: The
resveratrol attenuates ethanol-induced hepatocyte apoptosis via
inhibiting ER-related caspase-12 activation and PDE activity in
vitro. Alcohol Clin Exp Res. 38:683–693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan PT, Lin HY, Chuang CW, Wang PK, Wan
HC, Lee MC and Kao MC: Resveratrol alleviates nuclear
factor-KB-mediated neuroinflammation in vasculitic peripheral
neuropathy induced by ischaemia-reperfusion via suppressing
endoplasmic reticulum stress. Clin Exp Pharmacol Physiol.
46:770–779. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan C, Xu W, Huang Y, Li M, Shen Y, You H
and Liang X: HRD1-mediated IGF-1R ubiquitination contributes to
renal protection of resveratrol in db/db mice. Mol Endocrinol.
30:600–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spegel P, Chawade A, Nielsen S, Kjellbom P
and Rutzler M: Deletion of glycerol channel aquaporin-9 (Aqp9)
impairs long-term blood glucose control in C57BL/6 leptin
receptor-deficient (db/db) obese mice. Physiol Rep. 3:e125382015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Fan Y, Zeng C, He L and Wang N:
Tauroursodeoxycholic acid attenuates renal tubular injury in a
mouse model of type 2 diabetes. Nutrients. 8:5892016. View Article : Google Scholar
|
|
20
|
Zhang J, Cao P, Gui J, Wang X, Han J, Wang
Y and Wang G: Arctigenin ameliorates renal impairment and inhibits
endoplasmic reticulum stress in diabetic db/db mice. Life Sci.
223:194–201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan Y, Lee K, Wang N and He JC: The role
of endoplasmic reticulum stress in diabetic nephropathy. Curr Diab
Rep. 17:172017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morcos M, Sayed AA, Bierhaus A, Yard B,
Waldherr R, Merz W, Kloeting I, Schleicher E, Mentz S, Abd el Baki
RF, et al: Activation of tubular epithelial cells in diabetic
nephropathy. Diabetes. 51:3532–3544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szablewski L: Distribution of glucose
transporters in renal diseases. J Biomed Sci. 24:642017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin N, Li XY, Zhang HM, Yang Z and Su Q:
MicroRNA-199a-5p mediates high glucose-induced reactive oxygen
species production and apoptosis in INS-1 pancreatic β-cells by
targeting SIRT1. Eur Rev Med Pharmacol Sci. 21:1091–1098.
2017.PubMed/NCBI
|
|
27
|
Malhotra JD, Miao H, Zhang K, Wolfson A,
Pennathur S, Pipe SW and Kaufman RJ: Antioxidants reduce
endoplasmic reticulum stress and improve protein secretion. Proc
Natl Acad Sci USA. 105:18525–18530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan D, Liu XM, Fang Z, Du LL, Chang J and
Lin SH: Protective effect of resveratrol on kidney in rats with
diabetic nephropathy and its effect on endoplasmic reticulum
stress. Eur Rev Med Pharmacol Sci. 22:1485–1493. 2018.PubMed/NCBI
|
|
29
|
Cunard R and Sharma K: The endoplasmic
reticulum stress response and diabetic kidney disease. Am J Physiol
Renal Physiol. 300:F1054–F1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marciniak SJ, Yun CY, Oyadomari S, Novoa
I, Zhang Y, Jungreis R, Nagata K, Harding HP and Ron D: CHOP
induces death by promoting protein synthesis and oxidation in the
stressed endoplasmic reticulum. Genes Dev. 18:3066–3077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jing G, Wang JJ and Zhang SX: ER stress
and apoptosis: A new mechanism for retinal cell death. Exp Diabetes
Res. 2012:5895892012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
N Y Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hitomi J, Katayama T, Taniguchi M, Honda
A, Imaizumi K and Tohyama M: Apoptosis induced by endoplasmic
reticulum stress depends on activation of caspase-3 via caspase-12.
Neurosci Lett. 357:127–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang CC, Chang CY, Wu YT, Huang JP, Yen
TH and Hung LM: Resveratrol retards progression of diabetic
nephropathy through modulations of oxidative stress,
proinflammatory cytokines, and AMP-activated protein kinase. J
Biomed Sci. 18:472011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kitada M, Kume S, Imaizumi N and Koya D:
Resveratrol improves oxidative stress and protects against diabetic
nephropathy through normalization of Mn-SOD dysfunction in
AMPK/SIRT1-independent pathway. Diabetes. 60:634–643. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park HS, Lim JH, Kim MY, Kim Y, Hong YA,
Choi SR, Chung S, Kim HW, Choi BS, Kim YS, et al: Resveratrol
increases AdipoR1 and AdipoR2 expression in type 2 diabetic
nephropathy. J Transl Med. 14:1762016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu XH, Ding DF, Yong HJ, Dong CL, You N,
Ye XL, Pan ML, Ma JH, You Q and Lu YB: Resveratrol
transcriptionally regulates miRNA-18a-5p expression ameliorating
diabetic nephropathy via increasing autophagy. Eur Rev Med
Pharmacol Sci. 21:4952–4965. 2017.PubMed/NCBI
|
|
40
|
Yang PY, Li PC and Feng B: Protective
effects of gliclazide on high glucose and AGEs-induced damage of
glomerular mesangial cells and renal tubular epithelial cells via
inhibiting RAGE-p22phox-NF-kB pathway. Eur Rev Med Pharmacol Sci.
23:9099–9107. 2019.PubMed/NCBI
|
|
41
|
Das NA, Carpenter AJ, Belenchia A, Aroor
AR, Noda M, Siebenlist U, Chandrasekar B and DeMarco VG:
Empagliflozin reduces high glucose-induced oxidative stress and
miR-21-dependent TRAF3IP2 induction and RECK suppression, and
inhibits human renal proximal tubular epithelial cell migration and
epithelial-to-mesenchymal transition. Cell Signal. 68:1095062020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Zhang X, Mao Y, Liang L, Liu L,
Peng W, Liu H, Xiao Y, Zhang Y, Zhang F, et al: Smad2 and Smad3
play antagonistic roles in high glucose-induced renal tubular
fibrosis via the regulation of SnoN. Exp Mol Pathol.
113:1043752020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferre S, Deng Y, Huen SC, Lu CY, Scherer
PE, Igarashi P and Moe OW: Renal tubular cell spliced X-box binding
protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney
injury and inflammation. Kidney Int. 96:1359–1373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang Z, Guo F, Xia Z, Liang Y, Lei S, Tan
Z, Ma L and Fu P: Activation of GPR120 by TUG891 ameliorated
cisplatin-induced acute kidney injury via repressing ER stress and
apoptosis. Biomed Pharmacother. 126:1100562020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li C, Wang L, Huang K and Zheng L:
Endoplasmic reticulum stress in retinal vascular degeneration:
Protective role of resveratrol. Invest Ophthalmol Vis Sci.
53:3241–3249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ozgur R, Uzilday B, Sekmen AH and Turkan
I: The effects of induced production of reactive oxygen species in
organelles on endoplasmic reticulum stress and on the unfolded
protein response in arabidopsis. Ann Bot. 116:541–553. 2015.
View Article : Google Scholar : PubMed/NCBI
|