Introduction
Bisphenol A (BPA) is an industrial chemical used
widely in the manufacture of polycarbonate plastics and epoxy
resins, which are used in the production of food containers and
medical devices and are becoming the largest source of human
exposure to plastic (1). BPA
exposure is low but consistent across countries (2). BPA has been detected in amniotic
fluid, neonatal blood, placenta, cord blood and human breast milk,
demonstrating that this chemical might be passed on from mother to
fetus (3). The in vivo and
in vitro studies have demonstrated that BPA has
estrogen-like properties leading to reproductive and developmental
toxicity (4,5). Exposure to BPA during development is
concerning (6,7), yet the effect of BPA exposure during
pregnancy on reproductive health remains to be determined.
Soy isoflavones (SIFs) are naturally occurring
estrogen-like phytoestrogens that are abundant in various soy-based
foods and food supplements, such as soymilk, tofu, tempeh and
soy-based infant formula. Previous studies have suggested that SIFs
can interact with estrogen receptors (8,9) and,
similarly to BPA, trigger estrogen-dependent downstream effects.
Thus, the fetus can be simultaneously exposed to SIFs and BPA
during pregnancy. However, whether concomitant exposure to BPA and
SIFs can induce an additive or synergistic effect leading to
exacerbated toxicity is largely unknown.
The adverse effects of BPA are predominantly related
to its estrogenic activity, which may be involved in regulating
gonadotropin-releasing hormone and steroid receptor transcription
(10,11). Moreover, BPA has other effects such
as induction of inflammatory cytokines (12,13)
and oxidative stress (14,15), which are independent of its
estrogenic activity. Increasing evidence suggests that the
induction of oxidative damage in male reproductive tissues
represents another common response to exposure to environmental
toxicants (16–18). Imbalances in redox systems induce
oxidative damage, which in turn can negatively influence the
reproductive process. For instance, mitochondrial dysfunction is
detectable in clinically proven infertile men, and exposure to
environmental toxicants is a major factor in this context (19–21).
However, the relationship between BPA exposure, oxidative stress
and reproductive toxicity is still unclear.
The aim of the present study was to evaluate the
possible toxic effects of BPA and the synergistic actions of BPA
and SIFs exposure on the reproductive systems of murine F1
offspring. Accordingly, organ weights were recorded, and the
anogenital distance (AGD) was measured. Histopathological
examination of testes was also carried out. In addition, hormonal
status and oxidative stress in the F1 offspring were examined. The
present findings may provide insight into the mechanisms through
which BPA and SIFs might induce reproductive toxicity.
Materials and methods
Chemicals
BPA and diethylstilbestrol (DES) were purchased at
>99% purity from Sigma-Aldrich (Merck KGaA). BPA and DES were
first dissolved in 100% ethanol, then diluted in corn oil as
previously described (22,23), with a final ethanol concentration
in corn oil <1%. DES was used as a positive control to confirm
responsiveness of animals to estrogenic compounds. SIFs (>99%)
were purchased from Zhengzhou Linuo Biotechnology Co., Ltd., and
SIFs were prepared for suspension with corn oil.
Animals and experimental design
A total of 30 female and 10 male Kunming mice (4–5
weeks old, weighing 22–29 g) for each time-point were obtained from
the Laboratory Animal Center of Guilin Medical University. The mice
were housed in polycarbonate cages with sawdust bedding at a
controlled temperature (23±1°C) and 50–60% humidity under a 12-h
light/dark cycle. Food and tap water were available ad
libitum. Animals were acclimated to the laboratory environment
for 7 days before the start of the experiment. All animal
experiments in the present study were approved by The Animal Ethics
Committee of the Guilin Medical University (approval no.
GLMC201803066).
Female mice were randomly divided into 6 groups per
time-point and were placed in cages with male mice in a 2:1 ratio
overnight. Mating was confirmed by the presence of a vaginal plug.
The day the vaginal plug was observed was considered to be
gestation day (GD) 1. On GD 1, the females with vaginal plugs were
removed from males, weighed and individually caged. On GD 9 until
the birth of pups, the females were treated by gavage daily with:
i) A dose of 2, 20 or 200 mg/kg BPA alone (BPA2, BPA20 and BPA200
groups); ii) combination of 20 mg/kg BPA and 300 mg/kg SIFs (BPA20
+ SIF300 group); iii) 0.25 mg/kg DES (DES group), which was the
positive control; and iv) corn oil (control group). The
concentrations of BPA, SIFs and DES given by gavage were based on
previous studies (12,23). The gestational time, pup numbers
and the sex ratios of the pups were recorded (~30 pups per
time-point). The pups were weighed on postnatal day (PND) 0, 14 and
26. The AGD, defined as the distance between the anus and the
genital tubercle, was measured on PND 0, 7, 14, 21 and 26 using
calipers. The ratio of AGD to body weight was also calculated. The
offspring were euthanized on PND 7 or PND 26 and ~0.5–1.0 ml blood
samples were collected by cardiac puncture under anesthesia with
40–50 µl diethyl ether per mouse. Vital parameters and
disappearance of corneal and pain reflexes were monitored to ensure
the animals were fully anesthetized. Following blood collection,
mice were sacrificed by CO2 inhalation at 10–30% chamber
volume/min. Death was confirmed by cessation of the heartbeat and
breathing. The thymus, liver, spleen, heart, lung, kidney, brain,
testes and uterus from the offspring were carefully dissected free
of adhering fat and mesentery, then weighed.
Histological analysis of testes
tissue
The tissue blocks, which were 1×2×0.2 cm in size
were put into 10% formaldehyde solution at room temperature. The
tissue blocks were dehydrated with gradient alcohol (70, 80, 95 and
100% ethanol) and washed with xylene. Paraffin sections at a
thickness of 5 µm were mounted on a slide and treated with xylene
dewaxing twice (10 min each time) and gradient alcohol rehydration
(volume fraction of alcohol was 100, 95, 80, 70 and 0%). The
paraffin sections were kept in hematoxylin for 5–10 min at room
temperature, then the nuclei were stained. The paraffin-embedded
sections were put into a mixed solution of hydrochloric acid and
alcohol (70% hydrochloric acid volume fraction) for 30 sec, then
the paraffin-embedded section was hydrated (the non-specific
staining was differentiated to make the chromatin in the nucleus
more clear). At the same time, the paraffin-embedded section was
immediately put into the water, washed with water for blueing for
10–15 min, and then eosin staining was performed on the
paraffin-embedded section (to stain the cytoplasm) at room
temperature. Then, the sections were dehydrated with gradient
alcohol, washed with xylene and sealed with resin adhesive.
Histological examination was carried out under a Nikon Eclipse Ti-S
fluorescence microscope in a bright-field with light
(magnification, ×100 and ×400; Nikon Corporation).
Serum estrogen receptor and hormone
analysis
Blood samples were kept at 2–8°C for 12 h, then
centrifuged at 1,000 × g for 15 min at 4°C for serum collection.
The serum estrogen receptor (ESR; cat. no. ml260315),
follicle-stimulating hormone (FSH; cat. no. ml263000-3),
luteinizing hormone (LH; cat. no. ml063366-1) and testosterone (T;
cat. no. ml001948-1) levels were measured using ELISA kits
(Shanghai Meilian Biotechnology Co., Ltd.) according to the
manufacturer's instructions. Specifically, 40 µl dilution buffer
and 10 µl serum were added to antibody-coated 96-well microplates
and incubated at 37°C for 30 min. After washing, horseradish
peroxidase-labeled secondary antibody was added to each well. The
presence of enzyme complexes was detected by the addition of TMB
reagent. The measurable protein ranges for ESR, FSH, LH and T were
10–320 ng/l, 0.5–16 U/l, 70–2400 pg/ml and 8–240 nmol/l,
respectively.
Measurement of mitochondrial DNA
(mtDNA) copy number
DNA was extracted from whole blood using a
commercial kit (Tiangen Biotech Co., Ltd.). The relative mtDNA copy
number was measured using quantitative PCR with the SYBR-Green
Real-time PCR Master Mix (Toyobo Life Science). Relative mtDNA
levels were normalized to actin. The primers used were as follows:
Actin forward, 5′-AGCCATGTACGTAGCCATCCA-3′ and reverse,
5′-TCTCCGGAGTCCATCACCATG-3′; mitochondrial DNA-ND1 forward,
5′-CCATTTGCAGACGCCATAAA-3′ and reverse,
5′-GAGTGATAGGGTAGGTGCAATAA-3′. The thermocycling conditions
consisted of an initial denaturation step at 55°C for 10 min,
followed by 40 cycles at 95°C for 30 sec, 55°C for 30 sec, then
72°C for 60 sec. Relative mtDNA levels were calculated using the
2−ΔΔCq method (24),
where ΔCq=Cqactin-CqND1.
Measurement of serum malondialdehyde
(MDA) levels and superoxide dismutase (SOD) activity
Serum MDA levels were measured using an MDA
determination kit (cat. no. A003-1-2; Nanjing Jiancheng
Bioengineering Institute) based on the thiobarbituric acid
detection method for lipid peroxides. MDA in the serum reacts with
thiobarbituric acid, producing a color change, with maximum
absorbance detectable at a wavelength of 532 nm.
Inhibition of hydroxylamine oxidation by the
xanthine-xanthine oxidase system was assessed by measuring serum
SOD levels (25). This was carried
out using a SOD assay kit (cat. no. A001-1-2; Nanjing Jiancheng
Bioengineering Institute). Briefly, the reaction was initiated by
incubating serum with hypoxanthine, hydroxylamine and xanthine
oxidase at 37°C for 40 min. The reaction was terminated by adding
16% (v/v) acetic acid solution containing sulfanilic acid and
naphthyl ethylenediamine, and the absorbance was measured at 550 nm
to determine SOD activity.
Statistical analysis
Statistical analysis was conducted using Prism 5.0
software (GraphPad Software, Inc.). All data are presented as the
mean ± SEM or medians (n=5). Differences between groups were
analyzed using one-way ANOVA, followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Reproductive toxicity of F0 female
mice
All pregnant mice underwent normal parturition. The
reproductive and fetal findings are presented in Table I. No significant differences were
found in gestation length between groups.
 | Table I.Offspring number in the F1
generation. |
Table I.
Offspring number in the F1
generation.
|
|
|
| Pups/litter, n |
|---|
|
|
|
|
|
|---|
| Group | Gestation time,
days | P-value | Total | P-value | Females | P-value | Males | P-value |
|---|
| Control | 19.8±0.6 | N/A | 12.3±2.1 | N/A | 6.0±1.0 | N/A | 6.3±1.1 | N/A |
| BPA2 | 20.2±0.6 | 0.103 | 13.3±0.5 | 0.203 | 6.8±0.9 | 0.363 | 6.3±0.9 | 1.000 |
| BPA20 | 19.9±0.5 | 0.356 | 13.6±2.2 | 0.102 | 7.8±1.5 | 0.105 | 6.2±1.1 | 0.363 |
| BPA200 | 19.8±0.5 | 0.766 |
9.1±1.7a | 0.011 | 4.3±1.5 | 0.075 | 4.7±0.6 | 0.107 |
| BPA20 + SIF300 | 20.3±0.5 | 0.103 |
10.1±1.1b | 0.006 | 4.8±0.8 | 0.238 | 4.8±0.8 | 0.107 |
| DES | 19.8±0.3 | 0.360 |
10.3±2.1a | 0.018 | 4.0±1.0 | 0.049 | 6.3±1.2 | 1.000 |
Moreover, on PND 0, sex was determined by examining
external genitalia and was confirmed by autopsy at the end of the
experiment (Table I). The sex
distribution did not differ between the offspring in the control
group and any BPA or DES-treated group or BPA + SIF-treated group.
However, the number of live pups per litter in BPA200, BPA20 +
SIF300 and DES-treated groups were significantly reduced, compared
with the control. Besides, there were fewer females pups in the
BPA20 + SIF300 group compared with BPA20 alone (P<0.05).
Total body weight and relative organ
weight in F1 offspring
All body weights were recorded on PND 0, 14 and 26
(Fig. 1). On PND 0, the body
weight of all offspring in the BPA and DES-treated groups were
significantly greater than controls, except for the BPA200 group.
On PND 14, male offspring had significantly larger weights in the
BPA2, BPA20 + SIF300 and DES groups, compared with the control. The
offspring exposed to the low dose of BPA appeared heavier than
those exposed to the higher dose of BPA. By contrast, female
offspring weight was significantly higher in all treated groups,
compared with the control. On PND 26, all offspring weighed more in
the all treated groups, compared with the control group, except for
the female offspring in the BPA200 group. A general trend observed
was that at higher BPA doses body weight was lower. In addition,
pups in the BPA20 + SIF300 group weighed more than pups in the
BPA20 group at each time-point.
The relative weights of the testes and uterus were
determined on PND 7 and 26 (Fig.
2). On PND 7, the relative weight of the testes or uterus did
not differ across groups, both in male and female offspring.
However, on PND 26, the relative weight of testes declined in a
dose-dependent manner in male offspring following treatment with
BPA. Moreover, the relative weight of the testes was significantly
lower for mice in the BPA20 + SIF300 group, compared with mice in
the BPA20 group. On PND 26, the relative uterus weight of the
female offspring was elevated only in the DES-treated group.
Furthermore, the relative weights of the thymus,
liver, spleen, heart, lung, kidney and brain were also obtained
both for male and female mice (Tables
SI and SII). The relative
brain weight decreased after treatment with BPA and DES. Lastly, in
all offspring on PND 26, the thymus and lung weight was higher in
the BPA and DES treatment groups.
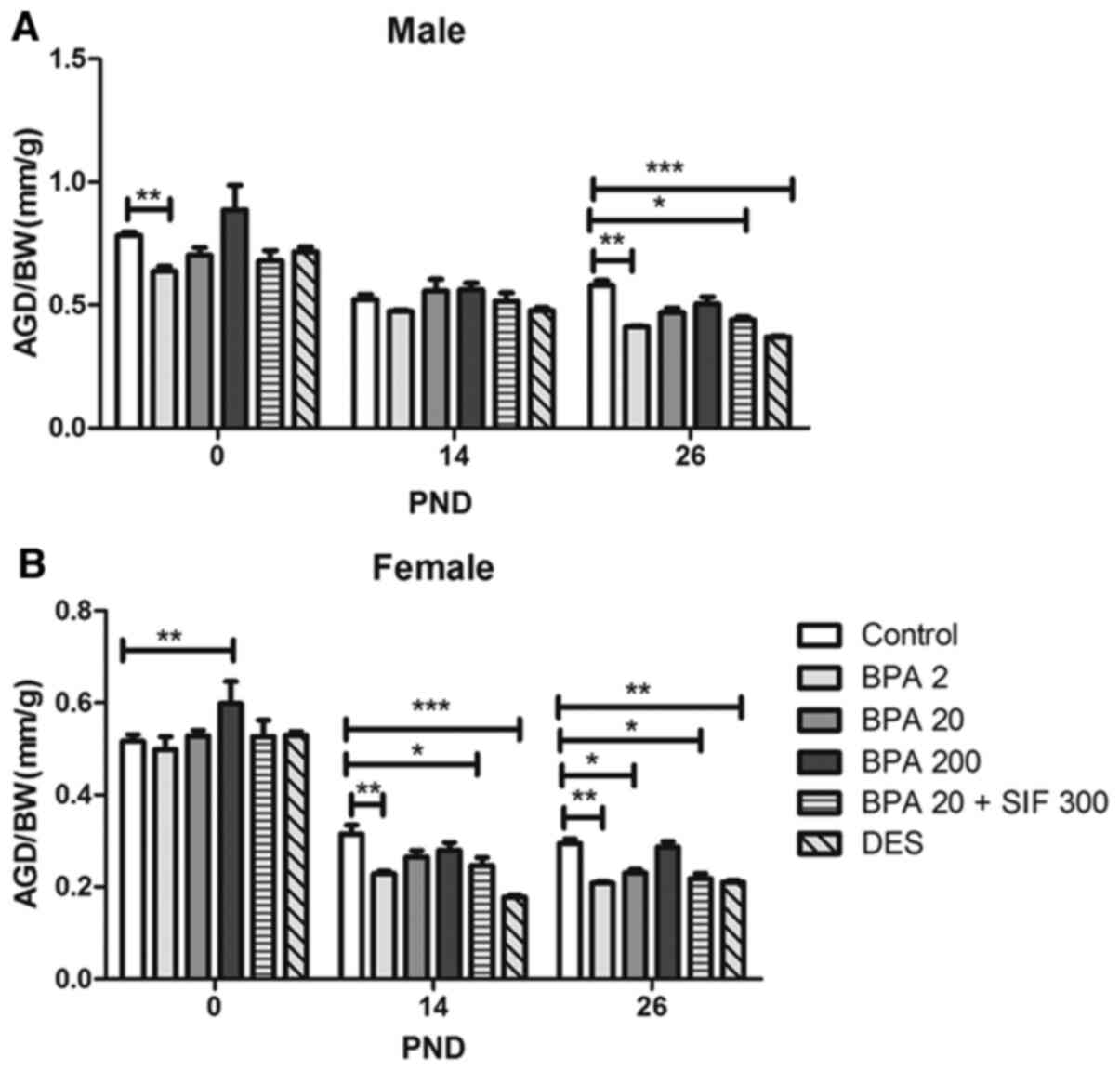
AGD in F1 offspring mice
AGD was measured on PND 0, 7, 14, 21 and 26.
Overall, exposure to BPA and DES resulted in a significant increase
in AGD at each time-point, both in male (Fig. S1) and female offspring (Fig. S2). From PND 14 to 26, the effect
of BPA was dose-dependent, as increasing BPA dose during pregnancy
was associated with longer AGD at these timepoints. However, there
was a significant difference in male of PND 7 (P<0.05; Fig. S1).
However, the AGD to body weight ratio displayed a
different trend (Fig. 3). On PND
26, this ratio decreased in male offspring in the BPA2, BPA20 +
SIF300 and DES groups, compared with the control group. Similarly,
the AGD to body weight ratio in female offspring was lower in the
BPA2 and DES-treated groups on PND 14 and 26. Co-exposure to BPA
and SIFs appeared to result in a reduction in AGD to total body
weight ratio, compared with BPA20, although this was not
statistically significant.
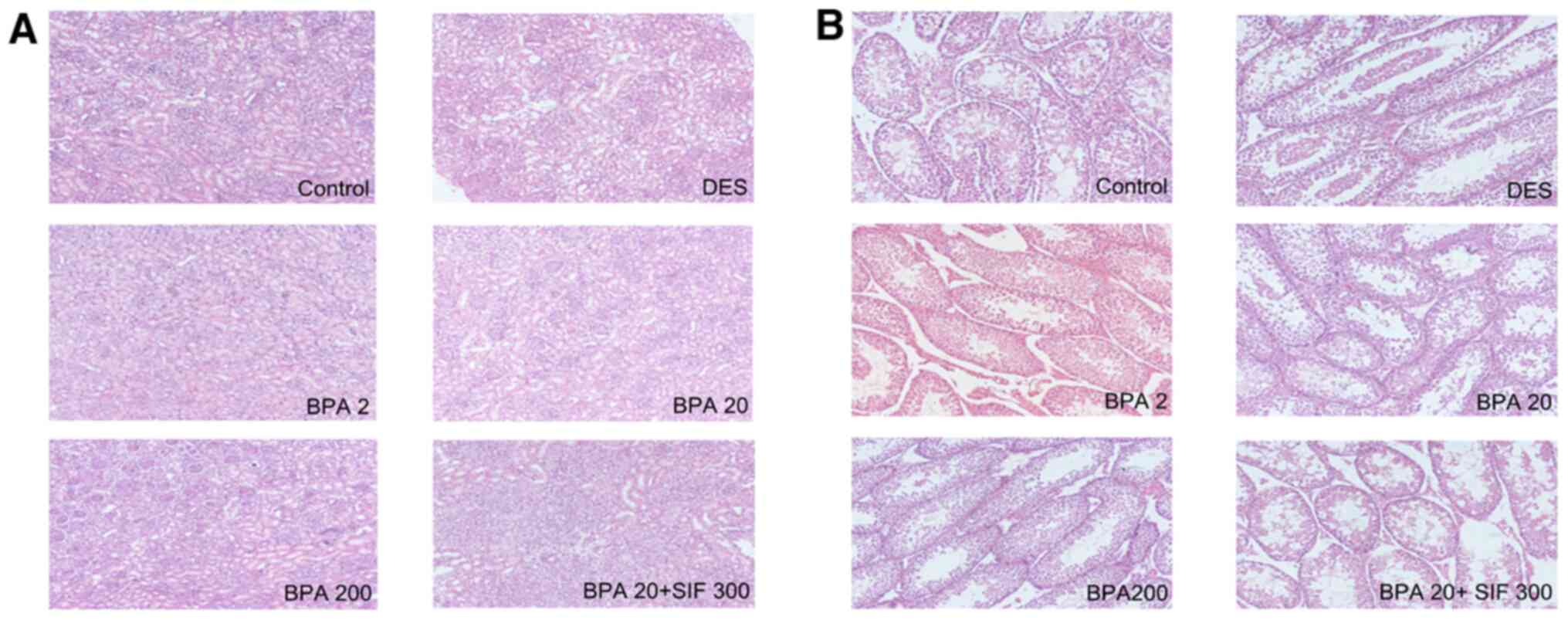
Histological analysis of testes
tissue
On PND 7, histological examination of the testes in
the control group indicated growing seminiferous tubules, with the
interstitial space being relatively large and predominantly filled
with mesenchymal cells. By contrast, in the DES group, growth of
the tubules was defective and the interstitial mesenchymal tissue
was loosely organized (Fig. 4A).
Upon exposure to BPA and SIFs, structural disturbance of testes was
also observed. On PND 26, the architecture of the seminiferous
tubules in the control group offspring was normal, with regularly
arranged rows and complete set of germinal epithelia (Fig. 4B). Furthermore, while the diameter
of the tubules increased, the interstitial space appeared to
decrease. However, in the DES group, there was tubular degeneration
and loss of cellular architecture in spermatogenic series.
Sloughing of seminiferous epithelium and spermatogenic cells into
the lumen of the seminiferous tubules was also observed in the
DES-exposed group. Testicular lesions in male offspring progressed
with increasing doses of BPA. Moreover, a higher degree of damage
was observed in the BPA20 + SIF300 offspring compared with groups
exposed to BPA only.
Serum ESR and hormonal analysis
Serum ESR, FSH, LH and T levels were determined on
PND 26. In male offspring, ESR levels significantly decreased in
the BPA20 + SIF300 and DES groups, compared with the control group.
Moreover, co-exposure to BPA and SIFs significantly decreased serum
T levels, compared with BPA20. No significant differences were
noted in FSH and LH levels across the groups (Fig. 5A).
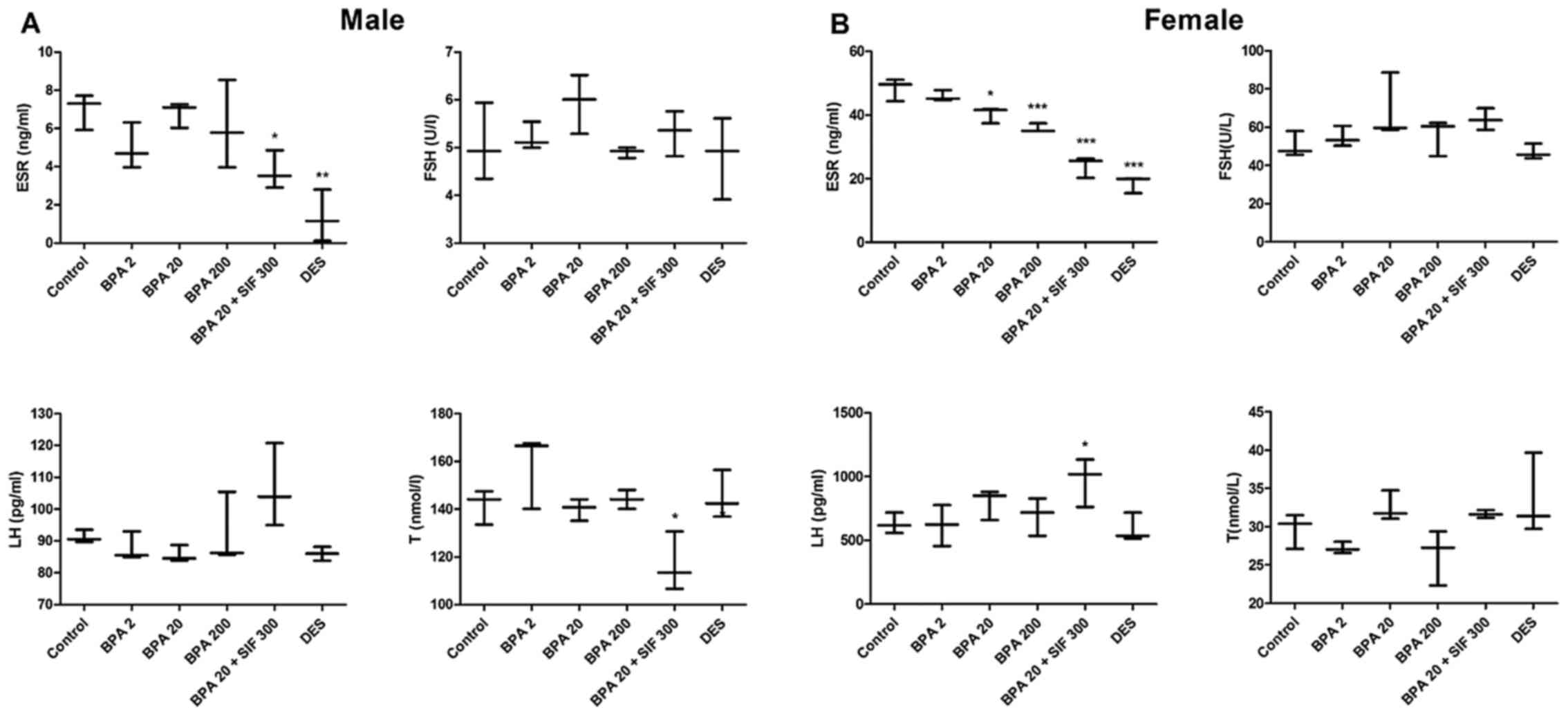 | Figure 5.Effects of BPA and SIFs on serum
levels of ESR, FSH, LH and T. Serum levels of the indicated groups
in (A) male and (B) female pups on PND 26. Data are presented as
the medians. *P<0.05, **P<0.01, ***P<0.001 vs.
control. BPA, bisphenol A; SIFs, soy isoflavone; DES,
diethylstilbestrol; PND, postnatal day; ESR, estrogen receptor;
FSH, follicle-stimulating hormone; LH, luteinizing hormone; T,
testosterone. |
In female offspring, ESR levels followed a
dose-dependent decline in the offspring from BPA-treated mice and
were lowest in the DES group, compared with the control. Female
offspring from the BPA20 + SIF300 groups displayed lower levels of
ESR, compared with BPA20. In addition, a significant increase in LH
levels was only observed in female offspring from mice exposed to
BPA and SIFs. There was no statistically significant difference in
FSH and T levels among the groups (Fig. 5B).
Oxidative stress parameters
MtDNA damage is related to increased oxidative
stress and inflammation (26,27).
Thus, on PND 26, the relative mtDNA copy number in whole blood was
evaluated in the offspring. The mtDNA copy numbers of the ND1 gene
significantly increased by 322.2% in the BPA200 group, compared
with the control group (Fig.
6A).
MDA is one of the most frequently used indicators of
lipid peroxidation (28). The
serum levels of MDA significantly increased in the BPA200 group in
comparison with the control group (Fig. 6B).
SOD is a key antioxidant enzyme that is essential
for the control of free radical production (25). SOD activity in the BPA200 and the
DES group decreased significantly, compared with the control group
(Fig. 6C).
No differences in mtDNA copy number, MDA levels or
SOD activity were observed between the BPA20 and BPA20 + SIF300
groups.
Discussion
EDCs are a structurally diverse class of synthetic
and natural compounds that alter endocrine and hormonal functions.
Exposure to EDCs often occurs in combination with several types of
diet and can result in adverse health outcomes, such as
reproductive damage, developmental impairment and cancer (29–33).
However, the effects of co-exposure to EDCs are poorly understood.
In the present study, the combined effects of two types of EDC, BPA
and SIFs, on the reproductive system were evaluated in mouse
offspring that were exposed gestationally. BPA exposure increased
the body weight of pups and decreased the AGD to body weight ratio,
especially in low-dose exposure groups. Moreover, decreased weight
and histopathological changes were identified in the testes of male
offspring. These BPA and SIFs-induced adverse effects were found to
be accompanied by serum hormonal alterations, which have an impact
on the reproductive process. Moreover, co-exposure to BPA and SIFs
aggravated these changes, compared with BPA alone. However, SIFs
exposure alone was not evaluated in the present study, which
represents an important limitation of the present findings.
EDCs contribute to the progression of metabolic
disorders, including obesity and diabetes (34). Children are hypothesized to be more
sensitive to EDCs, as they take up more calories per body surface
area and have higher minute ventilation (35). Previous animal studies suggested
that prenatal and/or neonatal exposure to low doses of BPA led to
an increase in body weight in the offspring (36,37).
The present findings were consistent with this trend.
Epidemiological studies also suggested that BPA is associated with
obesity in adults (38–41). However, the lowest concentration of
BPA causing adverse effects has been difficult to find, which
indicates an urgent need for reevaluation of BPA safety.
Human exposure to EDCs is frequent, persistent and
usually occurs in combination with other chemicals, leading to
unpredictable combined effects (42). Exposure to BPA and SIFs in
particular is common during pregnancy. A previous study
demonstrated that SIFs displayed numerous biological properties,
including antitumor activity, osteoporosis prevention and increase
of cognitive function (43).
However, there is growing concern regarding their safety, based
largely on their estrogen-like properties. Co-exposure to BPA and
SIFs has been reported to influence certain aspects of growth,
weight gain and puberty, suggesting that BPA and SIFs may interact
with each other, leading to these adverse outcomes. For example, in
a previous study, the anxiogenic phenotype induced by BPA exposure
during development could be mitigated by a soy-rich diet (44). Moreover, a soy-rich diet was
demonstrated to modulate the effects of BPA on meiotic processes in
periovulatory oocytes (45).
Furthermore, previous studies have suggested that co-exposure of
BPA and SIFs might have an additive effect on the reproductive
system. For instance, Do et al (40) demonstrated that high isoflavone
content and BPA had a synergistic effect on the induction of
uterine peroxidase. Moreover, BPA and SIFs have been implicated in
ESR-mediated transcriptional transactivation (46). Consistent with these previous
findings, co-exposure to BPA and SIFs potentiated the reproductive
toxicity of BPA in the present study. The present results also
indicated that SIFs could have potential adverse effects in early
life, particularly when combined with other EDCs, such as BPA.
Thus, the use of EDCs requires accurate risk assessment. Moreover,
understanding the underlying mechanisms through which these
interactions between BPA and SIFs occur is also critical for
addressing public health concerns.
The present study also suggested that the toxicity
of BPA and SIFs during development, as well as their combined
effects, may be mediated via the ESR. The ESR serves important
roles in differentiation and maintenance of the reproductive
system, as evidenced by the abnormal shape of the reproductive
organs perinatally exposed to the synthetic estrogen
diethylstilbestrol (47–50). The ESR regulates gene expression
and proliferation in epithelial and stromal cells. Both BPA and
SIFs have been reported to bind to the ESR, leading to different
protein and mRNAt changes (51–55).
FSH, LH and T are essential for the development and
function of the reproductive system. LH and FSH are secreted by the
anterior pituitary gland in response to hypothalamic
gonadotropin-releasing hormone. In men, LH stimulates T release
from Leydig cells in the testes. T is also an essential factor in
normal spermatogenesis (56).
Moreover, an increase in T levels during puberty promotes
development of the sexual organ and enables semen production
(57). In the present study,
co-exposure to BPA and SIFs led to a decrease in serum ESR and T,
compared with offspring exposed to BPA only, demonstrating a
synergistic effect on ESR expression and T release. These observed
hormonal changes could account for the smaller AGD to body weight
ratio and relative testes weight following BPA and SIFs
co-exposure. Histopathological examination also demonstrated
spermatid damage and degeneration in a dose-dependent manner
following treatment with BPA, which was exacerbated following BPA
and SIFs co-exposure. Therefore, the possible synergetic effects of
BPA and SIFs on the reproductive system could be attributable to
changes in ESR and testosterone levels.
In addition to hormonal regulation, alterations in
the redox system have also been implicated in the regulation of
reproductive processes in both animals and humans (58,59).
SOD enzymes participate in the removal of O2−
and regulate intracellular O2− levels. In
semen samples from patients with infertility with high
O2− levels, prolonged inhibition of sperm
mitochondrial function could inhibit sperm motility (60,61).
Since mitochondria regulate energy metabolism and reactive oxygen
species release in response to extracellular stimuli, mitochondrial
constituents, including mtDNA, are particularly susceptible to
oxidative damage (62). A previous
study demonstrated that mtDNA copy numbers (mitochondrial genome as
a whole) are critical to fertilization outcomes and can serve as an
important marker of oocyte quality (63). The extent of oxidative damage can
be assessed by measuring MDA levels, one of the final products of
lipid peroxidation. Increased MDA levels are associated with
decreased sperm motility (64). In
the present study, both the mtDNA copy number and MDA levels
significantly increased following gestational co-exposure to
BPA200. SOD activity diminished with exposure to high-dose BPA. By
contrast, there were no differences between combined BPA and SIFs
exposure, and BPA alone. Thus, high doses of BPA alone can lead to
dysregulation of reproductive function via oxidative damage in F1
offspring, which indicated that the changes in oxidative
stress-related parameters were not due to the synergistic effect of
BPA and SIFs.
In conclusion, the present study revealed that
co-exposure to BPA and SIFs could have a synergic effect on the
reproductive system. The interaction between BPA and SIFs could be
mediated by regulation of ESR and hormone release. These results
may aid in the development of precise prevention strategies and
treatment of BPA exposure.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant no.
81460446,81860580), The Guangxi Natural Science Foundation (grant
nos. 2015GXNSFDA139021 and 2018GXNSFAA294095), and The National
Guangxi College Students Innovation and Entrepreneurship Training
Program (grant no. 201810601030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM, YLi, YZ and PY conceived the methodology; LY,
YLi and SW validated and formally analyzed the data; JM, YLu, YN
and YH performed the experiments; HZ conducted the data curation.
HZ and YLu prepared the original draft. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments in the present study were
approved by The Animal Ethics Committee of the Guilin Medical
University (approval no. GLMC201803066).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
vom Saal FS, Akingbemi BT, Belcher SM,
Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ Jr,
Hauser R, Heindel JJ, et al: Chapel Hill bisphenol A expert panel
consensus statement: Integration of mechanisms, effects in animals
and potential to impact human health at current levels of exposure.
Reprod Toxicol. 24:131–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mustieles V, Williams PL, Fernandez MF,
Mínguez-Alarcón L, Ford JB, Calafat AM, Hauser R and Messerlian C:
Environment and Reproductive Health (EARTH) St: Maternal and
paternal preconception exposure to bisphenols and size at birth.
Hum Reprod. 33:1528–1537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vandenberg LN, Hauser R, Marcus M, Olea N
and Welshons WV: Human exposure to bisphenol A (BPA). Reprod
Toxicol. 24:139–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapin RE, Adams J, Boekelheide K, Gray LE
Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM,
Selevan SG, et al: NTP-CERHR expert panel report on the
reproductive and developmental toxicity of bisphenol A. Birth
Defects Res B Dev Reprod Toxicol. 83:157–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samuelsen M, Olsen C, Holme JA,
Meussen-Elholm E, Bergmann A and Hongslo JK: Estrogen-like
properties of brominated analogs of bisphenol A in the MCF-7 human
breast cancer cell line. Cell Biol Toxicol. 17:139–151. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rutkowska A and Rachoń D: Bisphenol A
(BPA) and its potential role in the pathogenesis of the polycystic
ovary syndrome (PCOS). Gynecol Endocrinol. 30:260–265. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barrett ES and Sobolewski M: Polycystic
ovary syndrome: Do endocrine-disrupting chemicals play a role?
Semin Reprod Med. 32:166–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faqi AS, Johnson WD, Morrissey RL and
McCormick DL: Reproductive toxicity assessment of chronic dietary
exposure to soy isoflavones in male rats. Reprod Toxicol.
18:605–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Zhang X, Zhang W and Song Y: Effects
of lactational exposure to soy isoflavones on steroid receptor
expression in neonate rat ovaries. Wei Sheng Yan Jiu. 36:564–567.
2007.(In Chinese). PubMed/NCBI
|
|
10
|
Guan L, Huang Y and Chen ZY: Developmental
and reproductive toxicity of soybean isoflavones to immature SD
rats. Biomed Environ Sci. 21:197–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Veiga-Lopez A, Beckett EM, Abi Salloum B,
Ye W and Padmanabhan V: Developmental programming: Prenatal BPA
treatment disrupts timing of LH surge and ovarian follicular wave
dynamics in adult sheep. Toxicol Appl Pharmacol. 279:119–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu B, Chen QF, Liu ZP, Xu HF, Zhang XP,
Xiang Q, Zhang WZ, Cui WM, Zhang X and Li N: Estrogen receptor α
and β expressions in hypothalamus-pituitary-ovary axis in rats
exposed lactationally to soy isoflavones and bisphenol A. Biomed
Environ Sci. 23:357–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogo FM, de Lion Siervo GEM,
Staurengo-Ferrari L, de Oliveira Mendes L, Luchetta NR, Vieira HR,
Fattori V, Verri WA Jr, Scarano WR and Fernandes GSA: Bisphenol A
exposure impairs epididymal development during the peripubertal
period of rats: Inflammatory profile and tissue Changes. Basic Clin
Pharmacol Toxicol. 122:262–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savastano S, Tarantino G, D'Esposito V,
Passaretti F, Cabaro S, Liotti A, Liguoro D, Perruolo G, Ariemma F,
Finelli C, et al: Bisphenol-A plasma levels are related to
inflammatory markers, visceral obesity and insulin-resistance: A
cross-sectional study on adult male population. J Transl Med.
13:1692015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tiwari D and Vanage G: Bisphenol A induces
oxidative stress in bone marrow cells, lymphocytes, and
reproductive organs of holtzman rats. Int J Toxicol. 36:142–152.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
D'Cruz SC, Jubendradass R and Mathur PP:
Bisphenol A induces oxidative stress and decreases levels of
insulin receptor substrate 2 and glucose transporter 8 in rat
testis. Reprod Sci. 19:163–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abarikwu SO, Adesiyan AC, Oyeloja TO,
Oyeyemi MO and Farombi EO: Changes in sperm characteristics and
induction of oxidative stress in the testis and epididymis of
experimental rats by a herbicide, atrazine. Arch Environ Contam
Toxicol. 58:874–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saradha B and Mathur PP: Effect of
environmental contaminants on male reproduction. Environ Toxicol
Pharmacol. 21:34–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mathur PP and D'Cruz SC: The effect of
environmental contaminants on testicular function. Asian J Androl.
13:585–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghiasvand T, Goodarzi MT, Shafiee G,
Zamani A, Karimi J, Ghorbani M and Amiri I: Association between
seminal plasma neopterin and oxidative stress in male infertility:
A case-control study. Int J Reprod Biomed (Yazd). 16:93–100. 2018.
View Article : Google Scholar
|
|
21
|
Bisht S, Faiq M, Tolahunase M and Dada R:
Oxidative stress and male infertility. Nat Rev Urol. 14:470–485.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naher ZU, Ali M, Biswas SK, Mollah FH,
Fatima P, Hossain MM and Arslan MI: Effect of oxidative stress in
male infertility. Mymensingh Med J. 22:136–142. 2013.PubMed/NCBI
|
|
23
|
Wang W, Hafner KS and Flaws JA: In utero
bisphenol A exposure disrupts germ cell nest breakdown and reduces
fertility with age in the mouse. Toxicol Appl Pharmacol.
276:157–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ziv-Gal A, Wang W, Zhou C and Flaws JA:
The effects of in utero bisphenol A exposure on reproductive
capacity in several generations of mice. Toxicol Appl Pharmacol.
284:354–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Husain K, Dube SN, Sugendran K, Singh R,
Das Gupta S and Somani SM: Effect of topically applied sulphur
mustard on antioxidant enzymes in blood cells and body tissues of
rats. J Appl Toxicol. 16:245–248. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
López-Armada MJ, Riveiro-Naveira RR,
Vaamonde-García C and Valcárcel-Ares MN: Mitochondrial dysfunction
and the inflammatory response. Mitochondrion. 13:106–118. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nikolova G, Karamalakova Y and Gadjeva V:
Reducing oxidative toxicity of L-dopa in combination with two
different antioxidants: An essential oil isolated from Rosa
damascena mill., and vitamin C. Toxicol Rep. 6:267–271. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ide T, Tsutsui H, Hayashidani S, Kang D,
Suematsu N, Nakamura K, Utsumi H, Hamasaki N and Takeshita A:
Mitochondrial DNA damage and dysfunction associated with oxidative
stress in failing hearts after myocardial infarction. Circ Res.
88:529–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matés JM: Effects of antioxidant enzymes
in the molecular control of reactive oxygen species toxicology.
Toxicology. 153:83–104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao J, Echelberger R, Liu M, Sluzas E,
McCaffrey K, Buckley B and Patisaul HB: Soy but not bisphenol A
(BPA) or the phytoestrogen genistin alters developmental weight
gain and food intake in pregnant rats and their offspring. Reprod
Toxicol. 58:282–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maqbool F, Mostafalou S, Bahadar H and
Abdollahi M: Review of endocrine disorders associated with
environmental toxicants and possible involved mechanisms. Life Sci.
145:265–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abaci A, Demir K, Bober E and Buyukgebiz
A: Endocrine disrupters-with special emphasis on sexual
development. Pediatr Endocrinol Rev. 6:464–475. 2009.PubMed/NCBI
|
|
34
|
Nohynek GJ, Borgert CJ, Dietrich D and
Rozman KK: Endocrine disruption: Fact or urban legend? Toxicol
Lett. 223:295–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Legler J, Fletcher T, Govarts E, Porta M,
Blumberg B, Heindel JJ and Trasande L: Obesity, diabetes, and
associated costs of exposure to endocrine-disrupting chemicals in
the European Union. J Clin Endocrinol Metab. 100:1278–1288. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Webb E, Moon J, Dyrszka L, Rodriguez B,
Cox C, Patisaul H, Bushkin S and London E: Neurodevelopmental and
neurological effects of chemicals associated with unconventional
oil and natural gas operations and their potential effects on
infants and children. Rev Environ Health. 33:3–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rubin BS, Murray MK, Damassa DA, King JC
and Soto AM: Perinatal exposure to low doses of bisphenol A affects
body weight, patterns of estrous cyclicity, and plasma LH levels.
Environ Health Perspect. 109:675–680. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Angle BM, Do RP, Ponzi D, Stahlhut RW,
Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P,
Parmigiani S, et al: Metabolic disruption in male mice due to fetal
exposure to low but not high doses of bisphenol A (BPA): Evidence
for effects on body weight, food intake, adipocytes, leptin,
adiponectin, insulin and glucose regulation. Reprod Toxicol.
42:256–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hao M, Ding L, Xuan L, Wang T, Li M, Zhao
Z, Lu J, Xu Y, Chen Y, Wang W, et al: Urinary bisphenol A
concentration and the risk of central obesity in Chinese adults: A
prospective study. J Diabetes. 10:442–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Do MT, Chang VC, Mendez MA and de Groh M:
Urinary bisphenol A and obesity in adults: Results from the
Canadian health measures survey. Health Promot Chronic Dis Prev
Can. 37:403–412. 2017.(In English, French). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carwile JL and Michels KB: Urinary
bisphenol A and obesity: NHANES 2003–2006. Environ Res.
111:825–830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ribeiro E, Ladeira C and Viegas S: EDCs
mixtures: A stealthy hazard for human health? Toxics. 5:52017.
View Article : Google Scholar
|
|
43
|
Wang Q, Ge X, Tian X, Zhang Y, Zhang J and
Zhang P: Soy isoflavone: The multipurpose phytochemical (Review).
Biomed Rep. 1:697–701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patisaul HB, Sullivan AW, Radford ME,
Walker DM, Adewale HB, Winnik B, Coughlin JL, Buckley B and Gore
AC: Anxiogenic effects of developmental bisphenol A exposure are
associated with gene expression changes in the juvenile rat
amygdala and mitigated by soy. PLoS One. 7:e438902012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Muhlhauser A, Susiarjo M, Rubio C,
Griswold J, Gorence G, Hassold T and Hunt PA: Bisphenol A effects
on the growing mouse oocyte are influenced by diet. Biol Reprod.
80:1066–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wade MG, Lee A, McMahon A, Cooke G and
Curran I: The influence of dietary isoflavone on the uterotrophic
response in juvenile rats. Food Chem Toxicol. 41:1517–1525. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katchy A, Pinto C, Jonsson P, Nguyen-Vu T,
Pandelova M, Riu A, Schramm KW, Samarov D, Gustafsson JÅ, Bondesson
M and Williams C: Coexposure to phytoestrogens and bisphenol a
mimics estrogenic effects in an additive manner. Toxicol Sci.
138:21–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Couse JF and Korach KS: Estrogen
receptor-alpha mediates the detrimental effects of neonatal
diethylstilbestrol (DES) exposure in the murine reproductive tract.
Toxicology. 205:55–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Couse JF, Dixon D, Yates M, Moore AB, Ma
L, Maas R and Korach KS: Estrogen receptor-alpha knockout mice
exhibit resistance to the developmental effects of neonatal
diethylstilbestrol exposure on the female reproductive tract. Dev
Biol. 238:224–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dupont S, Krust A, Gansmuller A, Dierich
A, Chambon P and Mark M: Effect of single and compound knockouts of
estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse
reproductive phenotypes. Development. 127:4277–4291.
2000.PubMed/NCBI
|
|
51
|
Newbold R: Cellular and molecular effects
of developmental exposure to diethylstilbestrol: Implications for
other environmental estrogens. Environ Health Perspect. 103 (Suppl
7):S83–S87. 1995. View Article : Google Scholar
|
|
52
|
Aloisi AM, Della Seta D, Ceccarelli I and
Farabollini F: Bisphenol-A differently affects estrogen
receptors-alpha in estrous-cycling and lactating female rats.
Neurosci Lett. 310:49–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matthews JB, Twomey K and Zacharewski TR:
In vitro and in vivo interactions of bisphenol A and its
metabolite, bisphenol A glucuronide, with estrogen receptors alpha
and beta. Chem Res Toxicol. 14:149–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Setchell KD: Soy isoflavones-benefits and
risks from nature's selective estrogen receptor modulators (SERMs).
J Am Coll Nutr. 20 (Suppl 5):S354–S383. 2001. View Article : Google Scholar
|
|
55
|
Setchell KD: Phytoestrogens: The
biochemistry, physiology, and implications for human health of soy
isoflavones. Am J Clin Nutr. 68 (Suppl 6):S1333–S1346. 1998.
View Article : Google Scholar
|
|
56
|
Arisha AH and Moustafa A: Potential
inhibitory effect of swimming exercise on the Kisspeptin-GnRH
signaling pathway in male rats. Theriogenology. 133:87–96. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huirne JA and Lambalk CB:
Gonadotropin-releas-ing-hormone-receptor antagonists. Lancet.
358:1793–1803. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sikka SC: Relative impact of oxidative
stress on male reproductive function. Curr Med Chem. 8:851–862.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Agarwal A, Gupta S and Sharma RK: Role of
oxidative stress in female reproduction. Reprod Biol Endocrinol.
3:282005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Armstrong JS, Rajasekaran M, Chamulitrat
W, Gatti P, Hellstrom WJ and Sikka SC: Characterization of reactive
oxygen species induced effects on human spermatozoa movement and
energy metabolism. Free Radic Biol Med. 26:869–880. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Aitken RJ, Buckingham D, West K, Wu FC,
Zikopoulos K and Richardson DW: Differential contribution of
leucocytes and spermatozoa to the generation of reactive oxygen
species in the ejaculates of oligozoospermic patients and fertile
donors. J Reprod Fertil. 94:451–462. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nissanka N and Moraes CT: Mitochondrial
DNA damage and reactive oxygen species in neurodegenerative
disease. FEBS Lett. 592:728–742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Santos TA, El Shourbagy S and St John JC:
Mitochondrial content reflects oocyte variability and fertilization
outcome. Fertil Steril. 85:584–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Agarwal A and Prabakaran SA: Mechanism,
measurement, and prevention of oxidative stress in male
reproductive physiology. Indian J Exp Biol. 43:963–974.
2005.PubMed/NCBI
|