Introduction
Ischemia/reperfusion (I/R) injury in the kidney is a
severe clinical condition associated with acute inflammation and
progressive deterioration of renal function (1). However, the mechanisms underlying
renal I/R injury remain unclear. Previous studies have demonstrated
that oxidative stress, apoptosis, and inflammation are involved in
I/R injury of the kidney (2–5). To
develop effective prevention measures, it is necessary to
understand the pathological mechanisms of renal I/R damage. Thus,
the present study aimed to examine potential mechanisms that could
prevent renal I/R injury.
Nobiletin (NOB; 3′,4′,5,6,7,8-hexamethoxyflavone) is
a dietary polymethoxylated flavonoid isolated from citrus fruits
with protective effects on I/R injury (6–8). Liu
et al (6) indicated that
NOB could attenuate I/R injury in H9c2 cardiomyocytes by activating
the Akt/GSK-3β signaling pathway. Zheng et al (8) suggested that NOB protected cerebral
neurons from I/R-induced injury through the MAPK pathway.
Nevertheless, the exact roles of NOB in I/R injury of the kidney
remain to be determined.
Endoplasmic reticulum stress (ERS) is considered a
driving force of acute renal failure (ARF) induced by I/R (9). Increasing evidence indicates that
persistent activation of ERS and subsequent elevation of apoptotic
cascades are key to the pathogenesis of renal I/R injury (10–13).
Additionally, a previous study confirmed that apoptosis occurs in
the early phase of ischemia and can be worsened by reperfusion
(13). Although the benefits of
inhibition of ERS-induced apoptosis against renal I/R injury are
well-known, the potential mechanisms of ERS-related apoptosis still
remain to be investigated (14).
Moreover, therapeutic approaches targeting ERS-related renal
apoptosis remain to be studied.
The PI3K/AKT pathway affects several biological
processes, including cellular proliferation and organ growth
(15–17). Recent evidence demonstrates that
the PI3K/AKT pathway can ameliorate I/R injury in the kidney
(18), liver (19), myocardium (20) and brain (21,22).
Wei et al (23) suggested
that propofol could attenuate renal I/R injury by modulating
PI3K/AKT signaling and partially reduce apoptosis and secretion of
pro-inflammatory cytokines (23).
The present study aimed to determine whether NOB
pre-treatment could attenuate renal I/R injury in vivo and
to verify the hypothesis that the protective effects of NOB are
related to activation of the PI3K/AKT pathway.
Materials and methods
Experimental animals
A total of 30 C57BL6/J male mice aged 6–8 weeks
(15–20 g) were provided by Vital River Laboratories. All animals
were housed and maintained in a temperature- (20±5°C) and a
humidity-controlled (40–70%) animal facility adhering to a 12-h
light/dark cycle, in nondirectional airflow cages. Mice were
provided with water and rodent chow ad libitum. The present
study was approved by Jingmen No. 2 People's Hospital Laboratory
Animals Ethics Committee (approval no. 4237668). All experiments
adhered to the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (the 8th Edition,
NRC 2011).
Renal I/R model and treatment
NOB was obtained from Sigma-Aldrich (Merck KGaA;
purity, >99%). All mice were anesthetized with intraperitoneal
(i.p.) injection of 40 mg/kg pentobarbital sodium. Laparotomy
followed by nephrectomy on the right side were then performed.
Subsequently, the left kidney was clamped by a non-traumatic vessel
forceps for 45 min to establish a model of ischemia, followed by
reperfusion for 24 h. A total of 30 mice were divided into six
groups (n=5 in each group). Except for two mice that died of
unexplained death after I/R injury surgery, all mice were
sacrificed by cervical dislocation following anesthesia with 40
mg/kg i.p. pentobarbital sodium. The groups were as follows: i) in
the sham group, the right kidney was removed; ii) in the I/R group,
a vessel forceps was used to occlude the vessels of the left kidney
for 45 min, followed by reperfusion for 24 h; ii) in the I/R + NOB
groups, mice received NOB (25, 50, or 100 mg/kg) i.p. injection for
7 consecutive days, followed by the same surgical procedures as the
I/R group. The duration of the experiment was 9 days.
Evaluation of the kidney function
Renal function was evaluated by determination of
blood urea nitrogen (BUN) and serum creatinine (Cr) levels. At the
24th h post reperfusion, blood was taken from the tail vein and
centrifuged to collect serum. BUN and SCr were measured using ELISA
kits (cat. nos. SJH-033595 and C011-1-1; T&T Scientific
Corporation).
Evaluation of renal injury
The morphological changes of renal tubules were
observed, and the degree of renal injury was determined using
hematoxylin and eosin H&E staining. The kidney was isolated,
washed, fixed in 4% paraformaldehyde and embedded with paraffin.
The samples were cut into sections with a thickness of 3 µm. After
dewaxing with xylene, the sections were sequentially incubated for
5 min in water and alcohol of different concentrations. H&E
staining was then carried out at room temperature with hematoxylin
dye solution for 5 min, followed by rinsing with tap water/for 5
min and incubation with eosin solution for 2 min. Sections were
observed under a light microscope (magnification, ×400).
Quantitative analysis was conducted using Image-Pro Plus (version
6.0; National Institutes of Health) to assess capsule area, tubular
injury scores, and glomerular tuft area as mentioned previously
(24).
Detection of apoptosis by flow
cytometry
Single-cell suspensions were prepared from fresh
tissue. The cells were then washed twice with PBS, centrifuged
(4°C, 1,000 × g, 5 min) and collected. Subsequently, the cells were
resuspended in 500 µl binding buffer, then with 10 µl Annexin
V-FITC and 5 µl PI. The samples were then incubated at room
temperature for 5 min in the dark, then acquired on a BD FACSCanto
flow cytometer (BD Biosciences). The flow cytometry data was
analyzed using BD FACSDiva (BD Biosiences). In the flow cytometry
dotplots, the Annexin V+PI+ quadrant
represents cells in late apoptosis, while the Annexin
V+PI− quadrant shows cells in early
apoptosis.
Reverse transcription-quantitative
(RT-q) PCR
mRNA levels were determined by RT-qPCR, as
previously described (3,16). Extraction of total RNA from the
animals kidney was carried out using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) after the indicated
treatments. cDNA was obtained from mRNA by reverse transcription
(at 42°C for 60 min, 70°C for 5 min and 4°C for 15 min
preservation) using a commercial Reverse Transcription cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.). RT-qPCR was
conducted with SYBR-Green Master Mix kit (Thermo Fisher Scientific,
Inc.) using the 7500 system ABI Prism system (Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows: i)
2 min at 50°C; ii) 95°C for 10 min; and iii) 40 cycles at 95°C for
30 sec, and 60°C for 30 sec. The mRNA expression levels of C/EBP
homologous protein (CHOP), glucose-regulated protein of 78 kDa
(GRP78), and caspase-12 were normalized to those of GAPDH.
The following primers were used: i) CHOP-reverse,
5′-CTTCAGCAAGCTGTGCCACT-3′; ii) CHOP-forward,
5′-TAGCTTGGCTGACTGAGGAGC-3′; iii) GRP78-reverse,
5′-GCAAACTTCTCGGCGTCATT-3′; iv) GRP78-forward,
5′-GATAATCAGCCCACCGTAACAAT-3′; v) caspase-12-reverse,
5′-CCTTCCTTCTCCATCACTGGA-3′; vi) caspase-12-forward,
5′-CATTGCCAATTCCGACAAAC-3′; vii) GAPDH-reverse,
5′-TTTGAGGGTGCAGCGAACTT-3′; and viii) GAPDH-forward,
5′-ACAGCAACAGGGTGGTGGAC-3′.
Western blot analysis
Renal samples were homogenized on ice with an IKA
homogenizer (12,000 × g; 15 min at 4°C), then lysed in RIPA lysis
buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40 (v/v), 1
mg/ml SDS, 5 mg/ml hyodeoxycholic acid sodium, 1 mM PMSF, 10 mg/ml
aprotinin, 10 mg/ml leupeptin and 10 mg/ml pepstatin A]. SDS-PAGE
(10%) was used to separate the proteins (~40 µg), which were then
transferred onto polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked with 5% non-fat
milk for 2 h at room temperature. Protein strips were then
incubated with a primary antibody overnight at 4°C and then washed
with 5% bovine serum albumin (BSA; Gibco; Thermo Fisher Scientific,
Inc.) in PBS/0.1% Tween-20 and incubated with the secondary
antibody for 2 h at room temperature. The protein strip was
developed with a EZ-ECL kit (Biological Industries) and the protein
quantity was analyzed using ImageJ software (version 6.0; National
Institutes of Health). GAPDH was used as an endogenous control. The
primary antibodies used in the study were as follows: anti-GAPDH
(mouse; 1:1,000; cat. no. sc-47724; Santa Cruz Biotechnology);
anti-AKT (rabbit; 1:500; cat. no. 9139; Cell Signaling Technology);
anti-phosphorylated (p)-AKT (rabbit; 1:1,000; cat. no. 9145; Cell
Signaling Technology); anti-PI3K (rabbit; 1:800; cat. no. 3230S;
Cell Signaling Technology); anti-p-PI3K (rabbit; 1:600; cat. no.
3776S; Cell Signaling Technology). The secondary antibodies
included horseradish peroxidase (HRP)-conjugated goat anti-mouse
IgG (1:3,000; cat. no. SA00001-1; ProteinTech Group, Inc.) and
HRP-conjugated goat anti-rabbit IgG (1:3,000; cat. no. SA00001-2;
ProteinTech Group, Inc.).
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis. All data are presented as the mean ± SD. The experiments
were repeated three times. Multiple comparisons was performed with
one-way ANOVA followed by Tukey's post hoc test. Paired student's
t-test was performed for comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
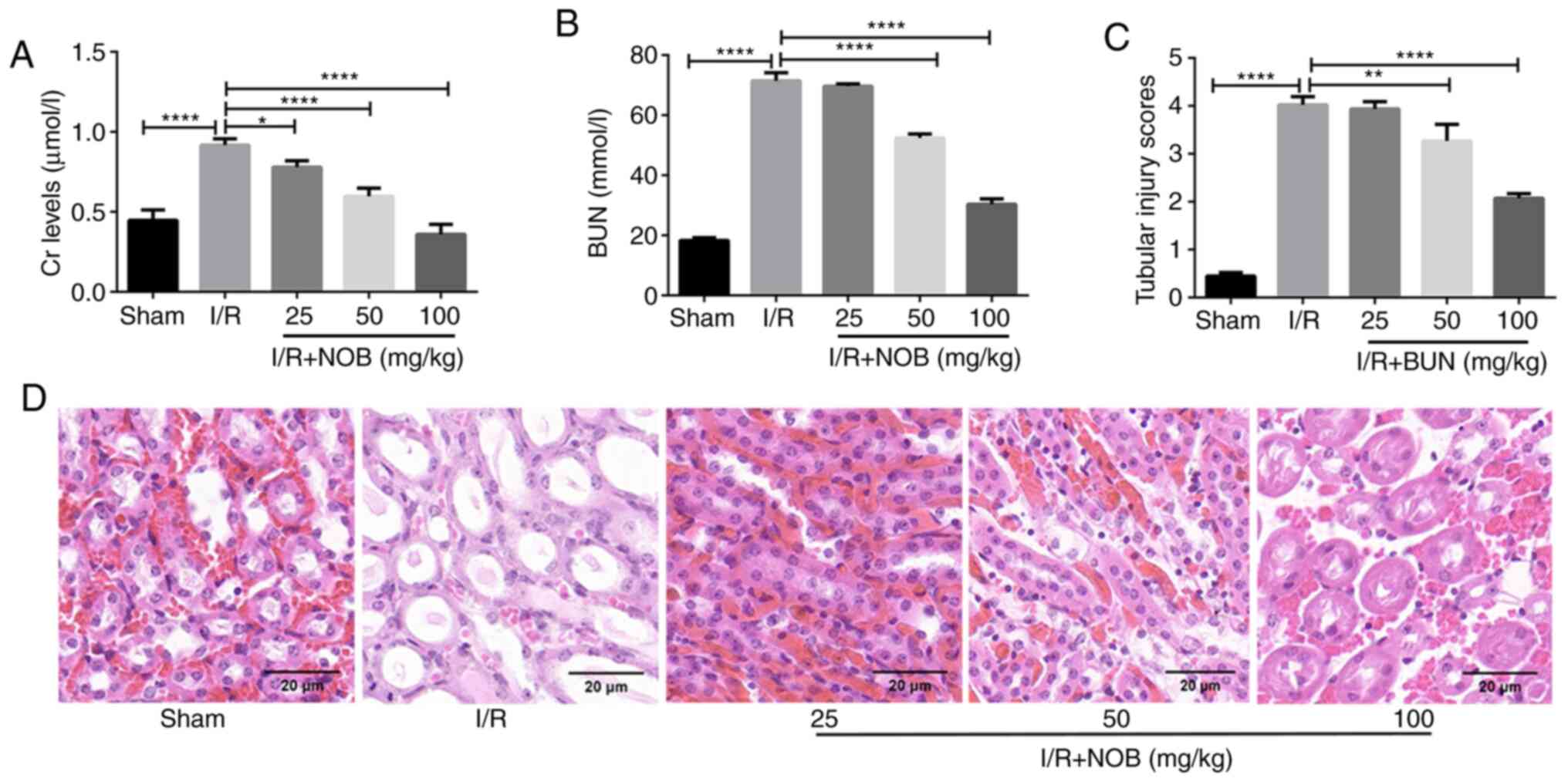
NOB pre-treatment reduces renal damage
caused by I/R injury
To investigate the role of NOB in renal damage, an
in vivo model of renal I/R was established. The levels of Cr
and BUN were measured as markers of renal injury. Compared with the
sham group, the levels of Cr and BUN were significantly increased
in the I/R group, indicating that the kidney suffered damage
following I/R injury. In contrast, Cr and BUN levels after NOB
treatment (25, 50 and 100 mg/kg) were significantly decreased,
compared with the I/R group. NOB decreased Cr and BUN levels in a
dose-dependent manner (Fig. 1A and
B). In addition, NOB concentrations significantly reduced BUN
levels starting at 50 mg/kg, compared with the I/R group.
H&E staining was performed using kidney tissues
from each group, and renal tubular damage was observed (Fig. 1D). In comparison with the sham
group, renal tubular injury was most apparent in the I/R group.
Renal tubular injury was attenuated after different doses of NOB
treatment. Tubular injury scores we also evaluated in order to
obtain more objective and reliable assessment of renal injury
(Fig. 1C). A concentration of 25
mg/kg NOB did not relieve renal tubular damage. Nevertheless, NOB
concentrations ≥50 mg/kg significantly reduced tubular injury
scores, compared with the I/R group. These results indicated that
NOB could reduce Cr and BUN levels, as well as renal damage
following I/R injury.
NOB suppresses ERS-related apoptosis
in renal I/R injury
I/R kidney injury is closely related ERS-induced
apoptosis (25), and NOB is
reported to be involved in the regulation of ERS (26). GRP78 is an ER chaperone that plays
an essential role in apoptosis during renal I/R injury (27). Additionally, ERS contributes to
apoptosis through CHOP-dependent signaling pathways (28). To explore whether the protective
effects of NOB on renal I/R injury are associated with ERS, flow
cytometry was used to evaluate apoptosis. Moreover, mRNA expression
levels of the ERS-related markers GRP78, CHOP, and caspase-12 were
determined by RT-qPCR. GRP78, CHOP, and caspase-12 mRNA levels in
the I/R group significantly increased, in comparison with the sham
group. By contrast, NOB could mitigate ERS by significantly
decreasing the mRNA expression levels of these molecules, compared
with the I/R group (Fig.
2A-C).
 | Figure 2.NOB inhibits ERS-associated
apoptosis. (A) GRP78 mRNA levels. (B) CHOP mRNA levels. (C)
Caspase-12 mRNA levels. n=3. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. (D) Flow cytometry pattern for
apoptosis. Annexin V+PI+ and Annexin
V+PI− cells were considered apoptotic (late
apoptotic and early apoptotic, respectively). NOB, nobiletin; ERS,
endoplasmic reticulum stress; I/R, ischemia/reperfusion; CHOP,
C/EBP homologous protein; FITC, fluorescein isothiocyanate; PI,
propidium iodide. |
Flow cytometry results are shown in Fig. 2D. Early apoptosis and late
apoptosis were increased in the I/R group, compared with the
control group. Late apoptosis gradually decreased with increasing
NOB concentrations. Moreover, while 25 mg/kg NOB did not markedly
inhibit the increase of early apoptosis caused by I/R, when NOB
concentrations reached 50 mg/kg, early apoptosis rates decreased.
Thus, NOB suppressed ERS induced by I/R and apoptosis in kidney.
Based on these results, 50 mg/kg NOB was used in all subsequent
experiments.
NOB promotes the PI3K/AKT pathway in
renal I/R injury
The PI3K/AKT pathway is known to promote survival
and reduce apoptosis following renal I/R injury (29). In order to determine if the
PI3K/AKT pathway participates in the amelioration of renal I/R
injury mediated by NOB, the protein levels of molecules involved in
the PI3K/AKT pathway were measured by western blot analysis
(Fig. 3). The levels of
phosphorylated AKT and PI3K were significantly decreased in the I/R
group, compared with the sham group. Notably, p-PI3K/AKT levels
were significantly increased following NOB treatment, compared with
the I/R group. Therefore, NOB could ameliorate renal I/R injury by
activating the PI3K/AKT pathway.
Inhibition of PI3K/AKT reverses the
effects of NOB on I/R injury and increases the levels of
ERS-associated markers following renal I/R insult
In order to further examine the role of the PI3K/AKT
in the amelioration of renal I/R injury, mice were treated with
LY294002, a specific PI3K/AKT inhibitor, following renal I/R
injury. The effect of NOB on kidney damage were inhibited following
treatment with LY294002, as indicated by elevated levels of Cr and
BUN, as well as GRP78, CHOP, and caspase-12 expression (Fig. 4A-F), and enhanced tubular injury
(Fig. 4C and G) in the I/R + NOB +
LY294002 group, compared with the I/R + NOB group. Thus, NOB could
ameliorate kidney damage caused by ERS in renal I/R injury by
activating the PI3K/AKT pathway.
 | Figure 4.inhibition of the PI3K/AKT pathway
reverses the effects of NOB. (A) Cr levels in serum (µmol/l). (B)
BUN levels in serum (mmol/l). (C) Tubular injury scores. (D) GRP78
mRNA levels. (E) CHOP mRNA levels. (F) Capase-12 mRNA levels. (G)
H&E staining of renal tissues. Magnification, ×200. Scale bar,
20 µm. n=3. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. NOB, nobiletin; Cr, creatinine; I/R,
ischemia/reperfusion; BUN, blood urea nitrogen; CHOP, C/EBP
homologous protein. |
Discussion
Renal I/R injury is one of the leading causes of
acute kidney injury and has a high mortality rate (30). In previous studies, it was proposed
that the anti-apoptotic, antioxidant, and anti-inflammatory
properties of NOB could protect against I/R insult (7,10).
In the present study, NOB could relieve I/R injury in the kidney.
The levels of BUN and serum Cr were used as indicators of renal
injury (31). Compared with the
sham group, serum Cr and BUN levels were increased in the I/R
group, indicating that renal function was critically impaired. In
addition, NOB significantly alleviated renal apoptosis and
inhibited protein expression of ERS-related molecules, such as
caspase-12, CHOP and GRP78. Furthermore, using a specific PI3K/AKT
inhibitor, it was demonstrated that NOB could exert a protective
effect by activating PI3K/AKT signaling. These findings indicated
that NOB could ameliorate I/R-induced and ERS-related apoptosis
mainly through the PI3K/AKT-dependent pathway.
Ample evidence has suggested that NOB appears to be
beneficial in kidney disease. Wei et al (32) suggested that NOB elicited cell
cycle arrest at the G0/G1 phase, which
suppressed renal cancer cell proliferation and increased cellular
apoptosis. Malik et al (33) demonstrated that NOB pretreatment
could preserve kidney function and reduce oxidative stress by
inhibiting DNA damage and the activation of apoptosis-related
pathways. Furthermore, NOB ccould also attenuate tubular injury as
seen in histological examination (33). However, whether NOB is involved in
renal I/R injury remains unknown, and the underlying mechanisms are
largely unclear. Recently, accumulating evidence has suggested that
NOB is an important mediator in the initiation and development of
ERS and apoptotis (34). Moreover,
NOB regulates ERS progression and might be used as a therapeutic
strategy to inhibit ERS-related renal apoptosis and cell death
(35). Thus, we hypothesize that
NOB could exert protective effects on renal tubules and ameliorate
ERS-related apoptosis in renal I/R injury. The present study
provides evidence that NOB has protective effects on renal I/R
injury.
It is well-known that the most effective therapy for
acute kidney injury is to successfully restore reperfusion in the
ischemic region in an early stage (26,36,37).
Nevertheless, the accompanied I/R injury largely decreases the
benefits of this approach. Although an increasing number of studies
have attached importance to the pathological mechanisms of kidney
I/R injury, a satisfactory strategy to avoid the adverse effects of
reperfusion still remains to be explored. Apoptosis is considered a
key mechanism of renal I/R injury, in which ERS-elicited apoptosis
cascade played a pivotal role (38,39).
It has been demonstrated that I/R injury in the kidney causes
severe ERS due to increased GRP78 levels (1). Misfolded and unfolded proteins bind
with GRP78 competitively, leading to depolymerization between
ER-transmembrane transducers and GRP78 (40). CHOP, a member of the C/EBP family,
is a specific transcription factor involved in ERS-induced
apoptosis. The levels of CHOP are relatively low under normal
conditions, but can be markedly increased following prolonged ERS
(26). The results of the present
study are consistent with a previous study (14) and indicate that I/R contributes to
sustained ERS and severe renal injury, as evidenced the increased
levels of caspase-12,CHOP and GRP78, as well as apoptotic rates.
However, NOB reduced the expression levels of these proteins and
attenuated the severity of I/R injury. Therefore, the protective
effects of NOB on the kidney are mediated, at least in part, by the
inhibition of ERS and apoptosis.
The potential molecular mechanisms related to the
protective roles of NOB against renal I/R injury and ERS-related
apoptosis were also examined. PI3K and its downstream target
serine/threonine kinase AKT belong to a conserved family of
signaling molecules (26,41,42).
PI3K/AKT pathway activation is an endogenous regulatory mechanism
that promotes cell survival in response to harmful external
stimuli. Notably, numerous studies suggest that the PI3K/AKT
pathway plays a pivotal role in the pathological process of renal
I/R injury, in which inhibition of ERS-related apoptosis is an
important mechanism (43,44). Zhang et al (26) have reported that PI3K/AKT signaling
inhibits I/R injury by suppressing ERS, thus reducing GRP78, CHOP,
and caspase-12 levels and apoptosis. Moreover, NOB can promote the
activation of PI3K/AKT signaling pathway (45,46).
Li et al (45) suggested
that NOB could reduce the apoptosis induced by ERS in during
oxygen-glucose deprivation/reoxygenation injury by activating the
PI3K/AKT pathway in PC12 cells. Consistent with previous findings,
the present study confirmed that pretreatment with NOB inhibited
apoptosis resulting from renal I/R injury and upregulated AKT and
PI3K phosphorylation. To confirm this, a specific inhibitor of
PI3K/AKT signaling, LY294002, was also added at the onset of I/R.
The protective effect of NOB and inhibition of ERS-related
apoptosis were significantly reversed in the presence of LY294002,
confirming that NOB administration could attenuate I/R injury
through the PI3K/AKT pathway. However, the relationship between NOB
and the PI3K/AKT pathway remain controversial. Indeed, several
studies demonstrated that the anticancer effect of NOB was mediated
by the PI3K/AKT pathway (46).
These studies are inconsistent with a previous report suggesting
that NOB markedly represses migration and proliferation of
non-renal tubular cells by inhibiting AKT signaling (26). These discrepancies might be
partially explained by the fact that the role of NOB in the
activation of the PI3K/AKT pathway could be cell- and
stimulus-dependent.
One of the limitations of the study is that
expression of CHOP, caspase-12 and GRP78 were not detected at the
protein level. In addition, one of the classic methods for
detecting apoptosis, TUNEL staining is not used, although it can
accurately reflect the typical biochemical and morphological
characteristics of apoptosis cells. In conclusion, this study
demonstrated that NOB pretreatment alleviated renal I/R injury by
attenuating ERS-associated apoptosis, which was dependent on the
PI3K/AKT pathway. However, several other signaling pathways, such
as MAPK and NF-κB signaling are related to the pathophysiological
mechanisms of I/R injury in the kidney. Whether these signaling
pathways are also associated with the protective effects of NOB
against renal I/R injury remains to be determined. Altogether, the
present findings indicate that NOB may represent a potential
therapeutic option for renal I/R injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and WZ designed the study. QD and LZ acquired and
analyzed the data. WZ drafted and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Jingmen No. 2
People's Hospital Laboratory Animals Ethics Committee (approval no.
4237668).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aboutaleb N, Jamali H, Abolhasani M and
Pazoki TH: Lavender oil (lavandula angustifolia) attenuates
renal ischemia/reperfusion injury in rats through suppression of
inflammation, oxidative stress and apoptosis. Biomed Pharmacother.
110:9–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong DJ, Wang L, Yang YY, Zhang JJ and Liu
XH: Diabetes aggravates renal ischemia and reperfusion injury in
rats by exacerbating oxidative stress, inflammation, and apoptosis.
Ren Fail. 41:750–761. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kar F, Hacioglu C, Senturk H, Donmez DB
and Kanbak G: The role of oxidative stress, renal inflammation, and
apoptosis in post ischemic reperfusion injury of kidney tissue: The
protective effect of dose-dependent boric acid administration. Biol
Trace Elem Res. 195:150–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Z, Zhao K, Han P, Qi X, Zhang W and Niu
T: Octreotide ameliorates renal ischemia/reperfusion injury via
antioxidation and anti-inflammation. Transplant Proc. 49:1916–1922.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hardi P, Nagy T, Fazekas G, Arató E,
Menyhei G, Sétáló G Jr, Vecsernyés M, Pintér O, Takács I, Bohonyi N
and Jancsó G: Sodium pentosan polysulfate reduced renal
ischemia-reperfusion-induced oxidative stress and inflammatory
responses in an experimental animal model. J Vasc Res. 53:230–242.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Zhang H, Li Y and Lu X: Nobiletin
suppresses oxidative stress and apoptosis in H9c2 cardiomyocytes
following hypoxia/reoxygenation injury. Eur J Pharmacol. 854:48–53.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang T, Wang F, Yu L and Li Z: Nobiletin
alleviates cerebral ischemic-reperfusion injury via MAPK signaling
pathway. Am J Transl Res. 11:5967–5977. 2019.PubMed/NCBI
|
|
8
|
Zheng Y, Bu J, Yu L, Chen J and Liu H:
Nobiletin improves propofol-induced neuroprotection via regulating
Akt/mTOR and TLR 4/NF-κB signaling in ischemic brain injury in
rats. Biomed Pharmacother. 91:494–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Xu L, Zhang S, Wang D, Dong G, Chen
H, Li X, Shu C and Wang R: STF-083010, an inhibitor of XBP1
splicing, attenuates acute renal failure in rats by suppressing
endoplasmic reticulum stress-induced apoptosis and inflammation.
Exp Anim. 67:373–382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Li H, Zheng H, Zhai M, Lu F, Dong
S, Fang T and Zhang W: CaSR activates PKCδ to induce cardiomyocyte
apoptosis via ER stress-associated apoptotic pathways during
ischemia/reperfusion. Int J Mol Med. 44:1117–1126. 2019.PubMed/NCBI
|
|
11
|
Chi X, Jiang Y, Chen Y, Yang F, Cai Q, Pan
F, Lv L and Zhang X: Suppression of microRNA27a protects against
liver ischemia/reperfusion injury by targeting PPARγ and inhibiting
endoplasmic reticulum stress. Mol Med Rep. 20:4003–4012.
2019.PubMed/NCBI
|
|
12
|
Yan B, Liu S, Li X, Zhong Y, Tong F and
Yang S: Preconditioning with endoplasmic reticulum stress
alleviated heart ischemia/reperfusion injury via modulating
IRE1/ATF6/RACK1/PERK and PGC-1α in diabetes mellitus. Biomed
Pharmacother. 118:1094072019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Liu S and Chen G: Aggravation of
cerebral ischemia/reperfusion injury by peroxisome
proliferator-activated receptor-gamma deficiency via endoplasmic
reticulum stress. Med Sci Monit. 25:7518–7526. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Wang L, Weng X, Chen H, Du Y, Diao
C, Chen Z and Liu X: Inhibition of Brd4 alleviates renal
ischemia/reperfusion injury-induced apoptosis and endoplasmic
reticulum stress by blocking FoxO4-mediated oxidative stress. Redox
Biol. 24:1011952019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Z, Wang Y and Gai Y: Effects of
MiR-21 on proliferation and apoptosis of fibroblast-like
synoviocytes in rheumatoid arthritis through PTEN/PI3K/AKT
signaling pathway. Panminerva Med. Oct 24–2019.(Online ahead of
print). PubMed/NCBI
|
|
16
|
Li C, Zhan Y, M X, Fang H and Gai X: B7-H4
facilitates proliferation and metastasis of colorectal carcinoma
cell through PI3K/Akt/mTOR signaling pathway. Clin Exp Med.
20:79–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun W, Hu S, Hu J, Yang S, Hu B, Qiu J,
Gan X, Liu H, Li L and Wang J: miR-365 inhibits duck myoblast
proliferation by targeting IGF-via PI3K/Akt pathway. Biosci Rep.
39:BSR201902952019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amini-Khoei H, Saghaei E, Mobini GR,
Sabzevary-Ghahfarokhi M, Ahmadi R, Bagheri N and Mokhtari T:
Possible involvement of PI3K/AKT/mTOR signaling pathway in the
protective effect of selegiline (deprenyl) against memory
impairment following ischemia reperfusion in rat. Neuropeptides.
77:1019422019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li TF, Ma J, Han XW, Jia YX, Yuan HF, Shui
SF, Guo D and Yan L: Chrysin ameliorates cerebral
ischemia/reperfusion (I/R) injury in rats by regulating the
PI3K/Akt/mTOR pathway. Neurochem Int. 129:1044962019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao Y, Li H, Pi Y, Li Z and Jin S:
Cardioprotective effect of IGF-1 against myocardial
ischemia/reperfusion injury through activation of PI3K/Akt pathway
in rats in vivo. J Int Med Res. 47:3886–3897. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian Z, Tang C and Wang Z: Neuroprotective
effect of ginkgetin in experimental cerebral ischemia/reperfusion
via apoptosis inhibition and PI3K/Akt/mTOR signaling pathway
activation. J Cell Biochem. 120:18487–18495. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan L, Fan L, Li Q, Cui W, Wang X and
Zhang Z: Inhibition of miR-181b-5p protects cardiomyocytes against
ischemia/reperfusion injury by targeting AKT3 and PI3KR3. J Cell
Biochem. 120:19647–19659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei Q, Zhao J, Zhou X, Yu L, Liu Z and
Chang Y: Propofol can suppress renal ischemia-reperfusion injury
through the activation of PI3K/AKT/mTOR signal pathway. Gene.
708:14–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dominguez JH, Liu Y, Gao H, Dominguez JM
II, Xie D and Kelly KJ: Renal tubular cell-derived extracellular
vesicles accelerate the recovery of established renal ischemia
reperfusion injury. J Am Soc Nephrol. 28:3533–3544. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang C, Hub Y, Gaoc J, Jiangd J, Shib S,
Wanga J, Genga Q, Liange X and Chaia X: Dexmedetomidine
pretreatment attenuates myocardial ischemia reperfusion induced
acute kidney injury and endoplasmic reticulum stress in human and
rat. Life Sci. 257:1180042020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang BF, Jiang H, Chen J, Guo X, Li Y, Hu
Q and Yang S: Nobiletin ameliorates myocardial ischemia and
reperfusion injury by attenuating endoplasmic reticulum
stress-associated apoptosis through regulation of the PI3K/AKT
signal pathway. Int Immunopharmacol. 73:98–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ardic S, Gumrukcu A, Cekic OG, Erdem M,
Reis Kose GD, Demir S, Kose B, Yulug E, Mentese A and Turedi S: The
value of endoplasmic reticulum stress markers (GRP78 and CHOP) in
the diagnosis of acute mesenteric ischemia. Am J Emerg Med.
37:596–602. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guoa Y, Guoa R, Sua Y, Fua J, Wanga S,
Konga Y, Wua C, Wanga J, Tanc C, Mod C and Zhaoa B: The
PERK/eIF2α/ATF4/CHOP pathway plays a role in regulating
monocrotaline-induced endoplasmic reticulum stress in rat liver.
Res Vet Sci. 130:237–239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hana F, Gaoa Y, Dinga C, Xiaa X, Wanga Y,
Xuea W, Dinga X, Zhenga J and Tiana P: Knockdown of NLRC5
attenuates renal I/R injury in vitro through the activation of
PI3K/Akt signaling pathway. Biomed Pharmacothe. 103:222–227. 2018.
View Article : Google Scholar
|
|
30
|
Park WS, Park MS, Kang SW, Jin SA, Jeon Y,
Hwang J and Kim SK: Hesperidin shows protective effects on renal
function in ischemia-induced acute kidney injury (sprague-dawley
rats). Transplant Proc. 51:2838–2841. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Peng C, Zhang Z, Shi J, Lin Y, Gu
L, Ma X and Li H: Intravenous infusion of ulinastatin attenuates
acute kidney injury after cold ischemia/reperfusion. Int Urol
Nephrol. 51:1873–1881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei D, Zhang G, Zhu Z, Zheng Y, Yan F, Pan
C, Wang Z, Li X, Wang F, Meng P, et al: Nobiletin inhibits cell
viability via the SRC/AKT/STAT3/YY1AP1 pathway in human renal
carcinoma cells. Front Pharmacol. 10:6902019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malik S, Bhatia J, Suchal K, Gamad N,
Dinda AK, Gupta YK and Arya DS: Nobiletin ameliorates
cisplatin-induced acute kidney injury due to its anti-oxidant,
anti-inflammatory and anti-apoptotic effects. Exp Toxicol Pathol.
67:427–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo J, Cui Y, Liu Q, Yang Y, Li Y, Weng L,
Tang B, Jin P, Li XJ, Yang S and Li S: Piperine ameliorates SCA17
neuropathology by reducing ER stress. Mol Neurodegener. 13:42018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hammad AS, Ravindran S, Khalil A and
Munusamy S: Structure-activity relationship of piperine and its
synthetic amide analogs for therapeutic potential to prevent
experimentally induced ER stress in vitro. Cell Stress Chaperones.
22:417–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng G, Lian C, Yang P, Zheng M, Ren H and
Wang H: E3-ubiquitin ligase TRIM6 aggravates myocardial
ischemia/reperfusion injury via promoting STAT1-dependent
cardiomyocyte apoptosis. Aging (Albany NY). 11:3536–3550. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruan Z, Wang S, Yu W and Deng F: LncRNA
MALAT1 aggravates inflammation response through regulating PTGS2 by
targeting miR-26b in myocardial ischemia-reperfusion injury. Int J
Cardiol. 288:1222019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu R, Wang W and Yang S: Cryptotanshinone
inhibits hypoxia/reoxygenation-induced oxidative stress and
apoptosis in renal tubular epithelial cells. J Cell Biochem.
120:13354–13360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding C, Han F, Xiang H, Wang Y, Dou M, Xia
X, Li Y, Zheng J, Ding X, Xue W and Tian P: Role of prostaglandin
E2 receptor 4 in the modulation of apoptosis and mitophagy during
ischemia/reperfusion injury in the kidney. Mol Med Rep.
20:3337–3346. 2019.PubMed/NCBI
|
|
40
|
Park SH, Gong JH, Choi YJ, Kang MK, Kim YH
and Kang YH: Kaempferol inhibits endoplasmic reticulum
stress-associated mucus hypersecretion in airway epithelial cells
and ovalbumin-sensitized mice. PLoS One. 10:e1435262015. View Article : Google Scholar
|
|
41
|
Shu Z, Yang Y, Yang L, Jiang H, Yu X and
Wang Y: Cardioprotective effects of dihydroquercetin against
ischemia reperfusion injury by inhibiting oxidative stress and
endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt
pathway. Food Funct. 10:203–215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen D, Chen R, Zhang L, Rao Z, Ruan Y, Li
L, Chu M and Zhang Y: Sulodexide attenuates endoplasmic reticulum
stress induced by myocardial ischaemia/reperfusion by activating
the PI3K/Akt pathway. J Cell Mol Med. 23:5063–507. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tian X, Ji Y, Liang Y, Zhang J, Guan L and
Wang C: LINC00520 targeting miR-27b-3p regulates OSMR expression
level to promote acute kidney injury development through the
PI3K/AKT signaling pathway. J Cell Physiol. 234:14221–14233. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu Y, Wang W, Jin K, Zhu Q, Lin H, Xie M
and Wang D: Perillyl alcohol protects human renal tubular
epithelial cells from hypoxia/reoxygenation injury via inhibition
of ROS, endoplasmic reticulum stress and activation of
PI3K/Akt/eNOS pathway. Biomed Pharmacother. 95:662–669. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li ZR, Yang L, Zhen J, Zhao Y and Lu ZN:
Nobiletin protects PC12 cells from ERS-induced apoptosis in OGD/R
injury via activation of the PI3K/AKT pathway. Exp Ther Med.
16:1470–1476. 2018.PubMed/NCBI
|
|
46
|
Shi MD, Liao YC, Shih YW and Tsai LY:
Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways
in HGF-treated liver cancer HepG2 cells. Phytomedicine. 20:743–752.
2013. View Article : Google Scholar : PubMed/NCBI
|