Introduction
Spinal cord injury (SCI) is a devastating disease
that often results in temporary and/or permanent functional
impairment below the injured level (1,2).
Currently, strategies used to treat SCI, such as surgery with
medical strategy, are supplemented with physical rehabilitation
and/or gene therapy. Although these remedies provide some benefits,
most patients with SCI are unable to achieve any substantial
functional recovery (3).
Considering that few satisfactory therapeutic strategies are
available for SCI treatment (4),
exploring new strategies for SCI is a cardinal public health
issue.
Cell transplantation therapy is associated with
immunomodulation, axon regeneration, neuroprotection, neuronal
myelin regeneration and relay formation, thus making it a promising
therapeutic strategy for SCI (5).
Studies have indicated that neural stem cell (NSC) transplantation
into lesions after SCI helps to regenerate injured tissue by
promoting axonal regeneration, spinal conductivity and functional
recovery (6–9). However, studies have also
demonstrated that limited numbers of transplanted NSCs survive for
the long-term in lesions (10–12).
Thus, developing new approaches to improve NSC survival after
engraftment is an important area of study when investigating
cell-based strategies.
The NSC preconditioning method is an emerging
approach that facilitates NSC survival and neuronal differentiation
following implantation. Recently, it was revealed that NSC
transplantation prior to hypoxia preconditioning promotes
functional recovery, by enhancing the secretion of neurotrophic and
growth factors [neurotrophin-3 (NT-3), glial cell line-derived
neurotrophic factor (GDNF) and brain-derived neurotrophic factor
(BDNF)] (2). The release of such
factors increases the number of residual neurons, particularly
5-hydroxytryptamine- and choline acetyltransferase-positive
neurons, and reduces the area positive for glial fibrillary acidic
protein at the epicenter of the injured spinal cord in rats
(2). Most recently, a previous
study indicated that 1 ng/ml high mobility group box-1 protein
(HMGB1) facilitates NSC proliferation and migration, via the
HMGB1/advanced glycation end products (RAGE) axis to enhance
filopodia formation in vitro (13). Hence, the effect of HMGB1
preconditioning prior to NSC transplantation on the functional
recovery of injured spinal cords in rats, and the potential
underlying mechanism need to be elucidated. The aim of the present
study was to develop a cell-based approach for the treatment of SCI
and to examine the role of HMGB1 in NSC activation.
Materials and methods
Animals
This study was approved by The Third Military
Medical University Ethics Committee (approval no. SYXK 2012-0002)
and followed the regulations of the China Laboratory Animal
Guidelines (RB/T 019-2019 Edition). Every effort was made to
minimize the number of animals used and their suffering. All
Sprague Dawley (SD) rats were maintained on a constant photoperiod
(12-h light/dark cycle), temperature (22-25°C) and moisture
(55–60%), and provided food and water ad libitum. A total of
60 male SD rats (51 rats were used for research and 9 rats died
during the experiment) and 8 embryonic day 14.5 (E14.5) SD rats
were used in the present study. All rats were sacrificed by
decapitation after anesthesia with 2% isoflurane/air mixture (1–2
l/min).
Primary NSC culture
A total of 8 E14.5 SD rats were employed to obtain
primary NSCs. Briefly, the neocortices of pups were obtained and
used for NSC culture after decapitation of E14.5 SD rats, as
previously described (14). Then,
the samples were washed twice in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) after incubation
in 0.25% trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc.) at
37°C for 30 min. Thereafter, the tissue samples were triturated
using a Pasteur pipette and passed through a 100-µm nylon cell
strainer (BD Falcon™; BD Biosciences) to collect the dissociated
cells in the suspension, after washing twice with DMEM. Cell
suspension was cultured in DMEM/F12 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with B27 (Gibco; Thermo Fisher
Scientific, Inc.), 20 ng/ml epidermal growth factor (PeproTech,
Inc.), 20 ng/ml fibroblast growth factor 2 (PeproTech, Inc.) and 1%
penicillin-streptomycin (vol/vol; Beyotime Institute of
Biotechnology) at 37°C in a humidified atmosphere with 5%
CO2, as recommended. For cell passaging, neurospheres
were harvested by centrifugation (100 × g) at room temperature for
5 min, dissociated in StemPro™ Accutase™ Cell Dissociation reagent
(Gibco; Thermo Fisher Scientific, Inc.) and cultured in medium as
aforementioned. For HMGB1 preconditioning, NSCs were incubated in 1
ng/ml HMGB1 for 24 h as previously described (13), and NSCs was washed twice with DMEM
before transplantation. NSCs at passage 3–5 were used for all
experiments in the present study.
Surgical procedures
The surgical procedures were performed as previously
described (2,15). Briefly, 2-month old male SD rats
(weight, 220–250 g) were placed in a stereotaxic frame after
anesthesia with 2% isoflurane/air mixture (1–2 l/min). A 4-cm-long
incision was made in the skin along the midline of the back, and a
laminectomy was carried out to expose the thoracic 9–11 spinal
segments, leaving the dura intact. Then, the spinal cord was
compressed using a calibrated aneurysm clip, which provided 20
g/cm2 pressure. The clip was released after 60 sec.
Subsequently, the muscles and skin were sutured in separate layers
10 min after injection. Body temperature was maintained at 37±0.3°C
by a feedback-controlled heating pad during surgery. Their bladders
were emptied twice daily until they could do so themselves.
NSC transplantation
Following surgery, a total of 51 male SD rats (n=17
for each group) were randomly divided into three experimental
groups: i) Control group, DMEM only; ii) NSC group, DMEM and NSCs;
iii) HGMB1-NSC group, DMEM and HMGB1-preconditioned NSCs. The
control group received 3 µl DMEM, which was injected into the
middle of the lesions. The NSC group received 2×105
normal NSCs in 3 µl DMEM, whereas the HGMB1-NSC group received
2×105 NSCs preconditioned with 1 ng/ml HMGB1 in 3 µl
DMEM. To maximize the engraftment of the NSCs injected into the
spinal cord, the needle remained in the spinal cord for 10 min
after injection. All transplantation procedures were performed
under sterile condition.
Evaluation of behavioral and sensory
functions
The locomotor recovery in rats after SCI was
assessed according to the Basso, Beattie and Bresnahan (BBB) scale
(15). Prior to undergoing SCI,
rats (n=6 from each experimental group) were exposed to the testing
system for 3 days, and their bladders were emptied before testing.
For examination, rats were placed individually in an open field
with a non-slippery surface in a large Plexiglas® field
(150 cm long × 150 cm wide × 30 cm high) on days 1, 7, 14, 21 and
28 post-SCI. Locomotor recovery was assessed and scored by two
independent examiners blinded to the experimental groups.
Mechanical allodynia was measured by two independent
researchers blinded to the experimental conditions on days 1, 7,
14, 21 and 28 post-SCI using an automatic von Frey apparatus
(Dynamic Plantar Aesthesiometer; Ugo Basile SRL). Rats were placed
individually in a plastic cage with a wire net floor and were left
for 30 min to acclimate. The withdrawal response threshold (26 g)
was performed five times, and the values reported are the mean of
these five measures.
The thermal sensitivity of the plantar hind paws was
assessed by two independent investigators using the cold plate test
(Cold/Hot Plate Analgesia Meter; Columbus Instruments), which was
reported previously (16). The
temperature of the cold plate was maintained at 5°C and the cut-off
latency was 30 sec. The latency (in sec) to withdrawal from the
cold plate was recorded along with other behaviors indicating
attendance to the stimulus, including licking, looking at the
affected paw sniffing or attacking the stimulus. The remaining five
latencies were averaged for each rat.
Hematoxylin and eosin (H&E) and
Nissl staining
On day 21 post-SCI, rats (n=4 from each experimental
group) were anesthetized using 2% isoflurane/air mixture (1–2
L/min) and perfused through the ascending aorta with 0.9% saline
followed by 400 ml 4% paraformaldehyde in phosphate buffered
solution (PBS, 0.1 M, pH ~7.4). The T10 spinal cord segment (1 cm,
0.5 cm either side from the injury epicenter) containing the injury
epicenter and surrounding uninjured tissues were dissected,
post-fixed for 48 h in 4% paraformaldehyde at 4°C, embedded in
paraffin, and cut into 5-µm sections using a vibratome. Sections
were stained with a standard H&E or Nissl staining procedure
(17,18) and visualized using a light
microscope (Leica Microsystems GmbH). The sagittal plane sections
with every fifth interval were prepared. Five sections were
stained, analyzed, and the cross-sectional areas were calculated
and reported as the average of four independent measurements. All
measurements were made by a technician who was blinded to the
experiment groups.
Immunostaining
For immunostaining, single NSCs
(1×105/ml) seeded in poly-L-ornithine (PLO)-pre-coated
confocal culture dishes or frozen sections (−20°C) from spinal
cords (n=4 from each experimental group) were fixed using 4%
paraformaldehyde in 0.01 M PBS at room temperature for 1 h. Then,
the samples were blocked with 5% v/v FBS supplemented with 0.5%
Triton X-100 (Beyotime Institute of Biotechnology) in 0.01 M PBS at
37°C for 30 min. Samples were incubated with rabbit monoclonal
anti-βIII-tubulin primary antibody (clone no. Tuj-1; 1:200; cat.
no. 5568; Cell Signaling Technology, Inc.) overnight at 4°C. Then,
samples were incubated with a Alexa Fluor® 555 or
488-conjugated secondary antibody (1:100; cat. nos. A0453 and
A0423; Beyotime Institute of Biotechnology) at room temperature for
2 h. Cell nuclei were counterstained with DAPI (Sigma-Aldrich;
Merck KGaA) for 10 min at room temperature. Samples were mounted
onto glass slides and images were captured using a confocal
microscope (LSM780; Carl Zeiss AG) and examined by Zen 2011
software (Carl Zeiss AG). The sagittal plane sections at every
fifth interval were prepared. Five sections were stained, analyzed,
and the cross-sectional areas were calculated and reported as the
average of four independent measurements. All measurements were
made by a technician who was blinded to the experiment groups.
Western blotting
The T10 spinal cord segments (n=3 from each
experimental group; 1 cm, 0.5 cm either side from the injury
epicenter) containing the injury epicenter and surrounding
uninjured tissues or NSCs after preconditioning with 1 ng/ml HMGB1
were isolated and collected immediately from rats following
decapitation on day 21 after SCI. The samples were homogenized with
RIPA (Beyotime Institute of Biotechnology) supplemented with
protease inhibitor cocktail (Roche Diagnostics). Then, the protein
concentration was measured using a BCA Protein Assay kit (Beyotime
Institute of Biotechnology). Proteins (40 µg/lane) were separated
by SDS-PAGE on a 10% gel under reducing conditions and
electroblotted to polyvinylidene difluoride membranes (Roche
Diagnostics). The membranes were blocked in 5% BSA (Beyotime
Institute of Biotechnology) in TBS with Tween-20 (TBST) at room
temperature for 2 h. Subsequently, the membranes were cut out at
different parts according to a pre-stained protein molecular ladder
(cat. no. 26616; Thermo Fisher Scientific, Inc.) to allow separate
detection of proteins migrating at the same distance, and were
incubated with anti-βIII-tubulin rabbit monoclonal antibody
(1:1,000; cat. no. 5568; Cell Signaling Technology, Inc.), anti-ERK
rabbit monoclonal antibody (1:1,000; cat. no. 4695; Cell Signaling
Technology, Inc.), anti-phosphorylated (p)-ERK rabbit polyclonal
antibody (1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.)
and anti-GAPDH mouse monoclonal antibody (1:1,000; cat. no. AF0006;
Beyotime Institute of Biotechnology) overnight at 4°C. Membranes
were washed 3 times with TBST, following which they were incubated
in the corresponding horseradish peroxidase-conjugated goat
anti-rabbit or goat anti-mouse secondary IgG antibody (1:5,000;
cat. nos. A0208 and A0216, respectively; Beyotime Institute of
Biotechnology) at room temperature for 2 h. Subsequently, bands
were visualized with a chemiluminescence reagent kit (Beyotime
Institute of Biotechnology) using a ChemiDoc™ XRS+ imaging system
(Bio-Rad Laboratories, Inc.), and semi-quantified using Image Lab
software (version 2.0.1; Bio-Rad Laboratories, Inc.). GAPDH was
used as the internal control to normalize the expression level of
each protein.
NSC differentiation
For differentiation, NSCs (1×105/ml) were
seeded in 10 µg/ml PLO-pre-coated confocal culture dishes and
incubated in DMEM/F12 medium supplemented with B27 and 1% GlutaMAX™
with or without 1 ng/ml HMGB1for 10 days at 37°C with 5%
CO2, as recommended. At the end of the initial 10-day
culture, the ERK antagonist U0126 (10 µM; cat. no. S1901; Beyotime
Institute of Biotechnology) was dissolved in DMSO and added to the
differentiation medium for 24 h at 37°C in a humidified atmosphere
with 5% CO2. Half of the volume of culture medium was
changed every 3 days.
Statistical analysis
All data are presented as the mean ± SEM, and
statistical analyses were performed using SPSS version 19.0 (IBM
Corp.). Two-tailed Student t-tests were used for comparisons
between two groups. Multiple comparisons were performed using ANOVA
followed by Tukey's post hoc test for multiple pair-wise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Transplantation of
HMGB1-preconditioned NSCs promotes functional recovery after SCI in
rats
To investigate the effect of transplanting
HMGB1-preconditioned NSCs on functional recovery after SCI in rats,
BBB scores, mechanical hypersensitivity and cold plate tests were
performed on days 1, 7, 14, 21 and 28 post-SCI in the three groups:
Control, NSCs and HMGB1-NSCs. The results indicated that rats in
the HMGB1-NSC group showed the highest improvement in locomotor
recovery compared with the control and NSC groups on days 14, 21
and 28 (Fig. 1A). Meanwhile, in
the NSC group the outcome of locomotor recovery was improved
compared with the control group on days 21 and 28 (Fig. 1A). Additionally, the score for
mechanical hypersensitivity was the highest in the HMGB1-NSC group
compared with the control and NSC groups on days 14, 21 and 28
(Fig. 1B). Additionally, the
mechanical stimulation score was higher in the NSC group compared
with the control group on days 14, 21 and 28 (Fig. 1B). In addition, the thermal
withdrawal latency score was highest in the HMGB1-NSC group
compared with the control and NSC groups on days 14, 21 and 28
(Fig. 1C). Furthermore, this score
was higher in the NSC group compared with the control group on days
21 and 28 (Fig. 1C). Collectively,
based on these data related to functional recovery, the time point
of day 21 was significant for all three groups. Therefore, we
performed the sampling measurements on day 21.
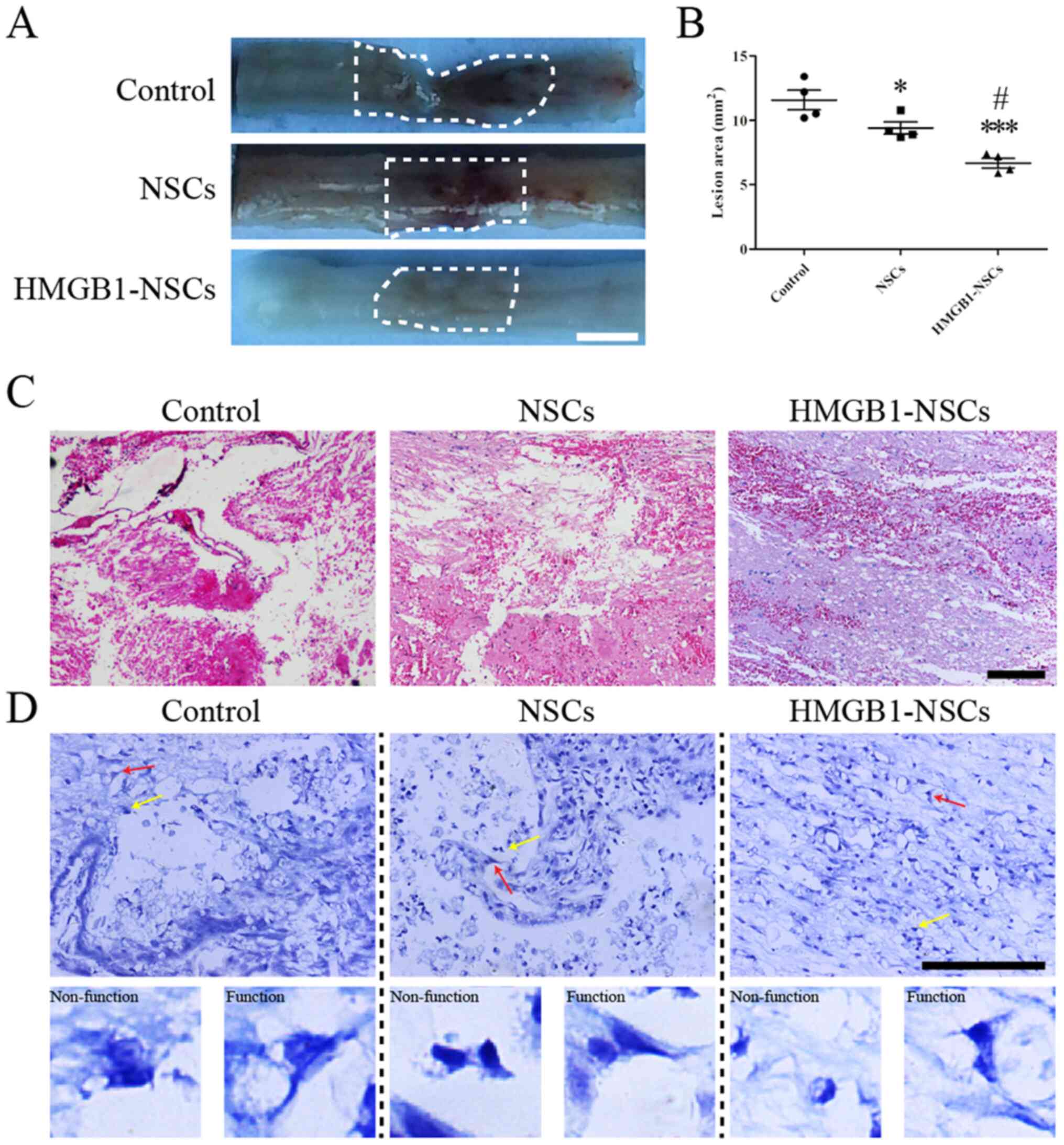
Transplantation of
HMGB1-preconditioned NSCs enhances histological benefit after SCI
in rats
To investigate why functional recovery was evidently
improved in rats receiving transplantation of NSCs preconditioned
with 1 ng/ml HMGB1, H&E and Nissl staining were performed to
explore histological changes on day 21 post-SCI. The data showed
that the lesion area in the HMGB1-NSC group was significantly
decreased compared with the NSC and control groups (Fig. 2A and B). The lesion region in the
NSC group was smaller compared with the control group (Fig. 2A and B). Subsequently, H&E
staining showed the fewest number of basophilic nuclei and the
smallest areas of spared tissue on day 21 post-SCI (Fig. 2C). Moreover, functional Nissl
bodies were visible in the HMGB1-NSC group (Fig. 2D).
Transplantation of
HMGB1-preconditioned NSCs facilitates neuronal survival post-SCI in
rats
Furthermore, immunostaining was performed to
evaluate the number of residual neurons at the epicenter of the
injured spinal cord at day 21 post-SCI. The images indicated that
the βIII-tubulin+ area in the HMGB1-NSC group was larger
compared with the NSC group (Fig. 3A
and B). Similarly, the βIII-tubulin+ area in the NSC
group was larger compared with the control group (Fig. 3A and B). Next, βIII-tubulin
expression was determined via western blotting and the results were
consistent with the results obtained from immunostaining (Fig. 3C and D).
ERK signaling pathway plays a role in
NSC differentiation induced by HMGB1
To further elucidate how HMGB1-preconditioned NSC
transplantation facilitates residual neuronal survival in
vivo, NSCs were incubated in differentiation medium with or
without 1 ng/ml HMGB1 for 10 days in vitro. NSCs treated
with 1 ng/ml HMGB1 promoted neuronal differentiation, as indicated
by the increase in the neuronal marker βIII-tubulin (Fig. 4A and B). Next, the results were
confirmed by western blotting (Fig. 4C
and D). Furthermore, to uncover the potential mechanism
underlying the ability of HMGB1 treatment to promote neuronal
differentiation, the expression of p-ERK was determined by
immunoblotting. Treatment with HMGB1 led to a significant increase
in p-ERK expression in NSCs (Fig. 5A
and B). In addition, a specific ERK antagonist, 10 µM U0126,
was used to determine the role of ERK signaling in HMGB1-mediated
NSC differentiation. Although the frequency of
βIII-tubulin+ cells in the HMGB1-NSC group was higher
compared with the NSC group, U0126 reversed this effect (Fig. 5C and D).
Discussion
The present study verified the hypothesis that
transplantation of HMGB1-preconditioned NSCs facilitated the
functional recovery of SCI in rats, as indicated by the decreased
lesion area and increased neuronal survival. Meanwhile, the results
also indicated that treatment with 1 ng/ml HMGB1 enhanced NSC
differentiation into neurons in vitro, and the ERK signaling
pathway played a role in this process. Mechanistically, the present
results indicated that the HMGB1 preconditioning method is a
feasible approach for stem cell replacement therapy.
HMGB1 has been demonstrated to help facilitate
angiogenesis and neurogenesis after central nervous system injury.
HMGB1 was first reported on in 1973 as a non-histone chromosomal
protein (19) and is commonly
known as a danger signal- or damage-associated molecular pattern
(20). Recently, a study revealed
that transplantation of NSCs prior to anti-HMGB1 antibody
administration alleviated blood-spinal cord barrier disruption and
edema formation, and increased the number of neurites from spared
axons and survival of host neurons, resulting in functional
recovery (21). Meanwhile, a study
also indicated that the HMGB1/RAGE signaling pathway played a
central role in facilitating endogenous NSC differentiation into
mature neurons (22). Furthermore,
a previous study demonstrated that 1 ng/ml HMGB1 not only promoted
NSC proliferation, but also facilitated NSC migration in
vitro (13). The present study
confirmed that HMGB1 promoted NSC differentiation into neurons
after SCI in rats, which is consistent with previous studies
(22,23). In addition, another study reported
that HMGB1 promoted angiogenesis and neurogenesis in the late phase
of intracerebral hemorrhage by upregulating vascular endothelial
growth factor and nerve growth factor (24).
It has been proposed that the HMGB1/RAGE axis may
play a significant role in promoting NSC differentiation into
neurons following HMGB1 treatment. A previous study demonstrated
that 1 ng/ml HMGB1 activated RAGE to augment NSC proliferation and
migration (13). Differentiation
is one of the main features of NSCs. In the present study, the
results also indicated that the HMGB1/RAGE signaling pathway
increased the number of residual neurons at the epicenter of the
injured spinal cord to promote functional recovery. Meanwhile, the
present results also demonstrated that ERK expression in NSCs was
upregulated after HMGB1 treatment, which is consistent with a
previous finding that 1 ng/ml HMGB1 enhanced neurulation via the
MAPK signaling pathway (25).
Moreover, a previous study also reported that the HMGB1/RAGE/NF-κB
signaling pathway played an essential role in promoting
differentiation of hippocampal neural progenitor cells into neurons
in Alzheimer's disease (26).
Whether the RAGE/NF-κB axis is involved in the process of
HMGB1-facilitated NSC differentiation needs further investigation
in the future. Meanwhile, the present data indicated that
engraftment of HMGB1-preconditioned NSCs increased the number of
neurons at the epicenter and facilitated neuronal differentiation
following SCI. The increased number of neurons might partly derive
from transplanted NSCs. In addition, the engrafted NSCs might also
promote the survival of local neurons due to the secretion of
neurotrophic and growth factors (NT-3, GDNF and BDNF) from
engrafted NSCs, as described in a previous study (2). Herein, our next study will focus on
determining the proportion of local surviving neurons and those
differentiation from engrafted NSCs at the epicenter of the injured
spinal cord.
However, there are limitations of the present study
that need to be investigated or improved upon in future studies,
these include: i) The method of administrating HMGB1-preconditioned
NSCs, such as via intravenous injection; ii) the ideal time point
of HMGB1-preconditioned NSC engraftment; iii) the transfection rate
of NSCs/HMGB1-preconditioned NSCs injected into the spinal cord;
iv) axon formation between caudal and rostral sides of the injury
site; v) a more appropriate time point for the sampling
measurements (immunostaining and western blotting), instead of day
21; vi) the use of a non-model control that the present study
lacked; vii) more NSC-specific markers and differentiation markers
should be analyzed via immunostaining to provide more evidence for
HMGB1-facilitaed NSC differentiation. However, the present study
provides evidence that the use of HMGB1 is a feasible approach to
optimize cell replacement therapy when transplanting NSCs after
SCI.
In summary, the present study indicated that
transplantation of NSCs preconditioned with 1 ng/ml HMGB1 could
significantly improve functional recovery by decreasing injured
spinal cord atrophy and increasing the number of
βIII-tubulin+ cells at the epicenter of the injured
spinal cord, which provides a possible strategy for NSC
transplantation in the treatment of SCI.
Acknowledgements
The authors would like to thank Dr Hongfei Ge
(Department of Neurosurgery, Southwest Hospital, Third Military
Medical University, Chongiqng, China) for his technical
support.
Funding
This work was supported by The National Natural
Science Foundation of China (grant no. 81972068).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MYL and JHZ participated in the design of the study.
XX, LZ and XY performed the experiments. XXC, ZFC, CXW and YX
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Third Military
Medical University Ethics Committee (approval no. SYXK 2012-0002)
and followed the regulations of the China Laboratory Animal
Guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li X, Liu D, Xiao Z, Zhao Y, Han S, Chen B
and Dai J: Scaffold-facilitated locomotor improvement post complete
spinal cord injury: Motor axon regeneration versus endogenous
neuronal relay formation. Biomaterials. 197:20–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan WL, Liu P, Wang G, Pu JG, Xue X and
Zhao JH: Transplantation of hypoxic preconditioned neural stem
cells benefits functional recovery via enhancing neurotrophic
secretion after spinal cord injury in rats. J Cell Biochem.
119:4339–4351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vismara I, Papa S, Rossi F, Forloni G and
Veglianese P: Current options for cell therapy in spinal cord
injury. Trends Mol Med. 23:831–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Upadhyay G, Shankar S and Srivastava RK:
Stem cells in neurological disorders: Emerging therapy with
stunning hopes. Mol Neurobiol. 52:610–625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Assinck P, Duncan GJ, Hilton BJ, Plemel JR
and Tetzlaff W: Cell transplantation therapy for spinal cord
injury. Nat Neurosci. 20:637–647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin H, Zhang YT, Yang Y, Wen LY, Wang JH,
Xu HY, Lai BQ, Feng B, Che MT, Qiu XC, et al: Electroacupuncture
facilitates the integration of neural stem cell-derived neural
network with transected rat spinal cord. Stem Cell Reports.
12:274–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yousefifard M, Rahimi-Movaghar V,
Nasirinezhad F, Baikpour M, Safari S, Saadat S, Moghadas Jafari A,
Asady H, Razavi Tousi SMT and Hosseini M: Neural stem/progenitor
cell transplantation for spinal cord injury treatment; A systematic
review and meta-analysis. Neuroscience. 322:377–397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karova K, Wainwright JV, Machova-Urdzikova
L, Pisal RV, Schmidt M, Jendelova P and Jhanwar-Uniyal M:
Transplantation of neural precursors generated from spinal
progenitor cells reduces inflammation in spinal cord injury via
NF-βB pathway inhibition. J Neuroinflammation. 16:122019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kojima K, Miyoshi H, Nagoshi N, Kohyama J,
Itakura G, Kawabata S, Ozaki M, Iida T, Sugai K, Ito S, et al:
Selective ablation of tumorigenic cells following human induced
pluripotent stem cell-derived neural stem/progenitor cell
transplantation in spinal cord injury. Stem Cells Transl Med.
8:260–270. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hooshmand MJ, Sontag CJ, Uchida N, Tamaki
S, Anderson AJ and Cummings BJ: Analysis of host-mediated repair
mechanisms after human CNS-stem cell transplantation for spinal
cord injury: Correlation of engraftment with recovery. PLoS One.
4:e58712009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Babu H, Cheung G, Kettenmann H, Palmer TD
and Kempermann G: Enriched monolayer precursor cell cultures from
micro-dissected adult mouse dentate gyrus yield functional granule
cell-like neurons. PLoS One. 2:e3882007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ronaghi M, Erceg S, Moreno-Manzano V and
Stojkovic M: Challenges of stem cell therapy for spinal cord
injury: Human embryonic stem cells, endogenous neural stem cells,
or induced pluripotent stem cells? Stem Cells. 28:93–99.
2010.PubMed/NCBI
|
|
13
|
Xue X, Chen X, Fan W, Wang G, Zhang L,
Chen Z, Liu P, Liu M and Zhao J: High-mobility group box 1
facilitates migration of neural stem cells via receptor for
advanced glycation end products signaling pathway. Sci Rep.
8:45132018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge H, Tan L, Wu P, Yin Y, Liu X, Meng H,
Cui G, Wu N, Lin J, Hu R and Feng H: Poly-L-ornithine promotes
preferred differentiation of neural stem/progenitor cells via ERK
signalling pathway. Sci Rep. 5:155352015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jurga AM, Rojewska E, Piotrowska A, Makuch
W, Pilat D, Przewlocka B and Mika J: Blockade of toll-like
receptors (TLR2, TLR4) attenuates pain and potentiates
buprenorphine analgesia in a rat neuropathic pain model. Neural
Plast. 2016:52387302016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu R, Zhou J, Luo C, Lin J, Wang X, Li X,
Bian X, Li Y, Wan Q, Yu Y and Feng H: Glial scar and
neuroregeneration: Histological, functional, and magnetic resonance
imaging analysis in chronic spinal cord injury. J Neurosurg Spine.
13:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Zhou J, Gu L and Zuo Y: The change
of HCN1/HCN2 mRNA expression in peripheral nerve after chronic
constriction injury induced neuropathy followed by pulsed
electromagnetic field therapy. Oncotarget. 8:1110–1116. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paudel YN, Semple BD, Jones NC, Othman I
and Shaikh MF: High mobility group box 1 (HMGB1) as a novel
frontier in epileptogenesis: From pathogenesis to therapeutic
approaches. J Neurochem. 151:542–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hei Y, Chen R, Yi X, Long Q, Gao D and Liu
W: HMGB1 neutralization attenuates hippocampal neuronal death and
cognitive impairment in rats with chronic cerebral hypoperfusion
via suppressing inflammatory responses and oxidative stress.
Neuroscience. 383:150–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uezono N, Zhu Y, Fujimoto Y, Yasui T,
Matsuda T, Nakajo M, Abematsu M, Setoguchi T, Mori S, Takahashi HK,
et al: Prior treatment with anti-high mobility group box-1 antibody
boosts human neural stem cell transplantation-mediated functional
recovery after spinal cord injury. Stem Cells. 36:737–750. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Mei X, Cao Y, Liu C, Zhao Z, Guo
Z, Bi Y, Shen Z, Yuan Y, Guo Y, et al: HMGB1/advanced glycation end
products (RAGE) does not aggravate inflammation but promote
endogenous neural stem cells differentiation in spinal cord injury.
Sci Rep. 7:103322017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tirone M, Tran NL, Ceriotti C, Gorzanelli
A, Canepari M, Bottinelli R, Raucci A, Di Maggio S, Santiago C,
Mellado M, et al: High mobility group box 1 orchestrates tissue
regeneration via CXCR4. J Exp Med. 215:303–318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lei C, Lin S, Zhang C, Tao W, Dong W, Hao
Z, Liu M and Wu B: Effects of high-mobility group box1 on cerebral
angiogenesis and neurogenesis after intracerebral hemorrhage.
Neuroscience. 229:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Yu L, Zhang T, Wang L, Leng Z,
Guan Y and Wang X: HMGB1 enhances embryonic neural stem cell
proliferation by activating the MAPK signaling pathway. Biotechnol
Lett. 36:1631–1639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meneghini V, Bortolotto V, Francese MT,
Dellarole A, Carraro L, Terzieva S and Grilli M: High-mobility
group box-1 protein and β-amyloid oligomers promote neuronal
differentiation of adult hippocampal neural progenitors via
receptor for advanced glycation end products/nuclear factor-κB
axis: Relevance for Alzheimer's disease. J Neurosci. 33:6047–6059.
2013. View Article : Google Scholar : PubMed/NCBI
|