Introduction
Allergic rhinitis (AR) refers to the non-infective
inflammatory disease of the nasal mucosa caused by the release of
IgE-mediated histamine following exposure to allergens by atopic
individuals, and involves a variety of immune active cells and
cytokines (1). The clinical
manifestations of AR are usually nasal congestion, rhinocnesmus,
rhinorrhea and sneezing. AR is the most common chronic refractory
disease reported in recent years in otorhinolaryngology, and it is
also one of the prioritized diseases for prevention and treatment
(2). It is mainly caused by
environmental changes, seasonal alternation, temperature change,
wind, precipitation, pollen and dust (3). The disease is not lethal, but the
nasal symptoms cause obvious discomfort, seriously affecting the
physical health of the patient, their ability to work and study,
and their quality of life. AR is difficult to control and leads to
numerous long-term complications that may cause more severe
diseases, such as nasopharyngeal carcinoma and tympanitis (4). Therefore, the identification of a
safe drug for the treatment of AR is currently a research priority
(2).
Bitongning drops are recognized in Volume 6 of the
Drug Standard of the Ministry of Health of the People's Republic of
China (5). The drops are made by
steam distillation of Flos magnoliae (FM) and Centipeda
minima (CM) to extract their volatile oil or aqua aromatica
(6). Bitongning drops can clear
nasal orifices, and therefore they are used for treating nasal
congestion, acute and chronic sinusitis, AR and colds caused by
wind chill (7–9). Pharmacological experiments have
indicated that the volatile oils of drugs used in Traditional
Chinese Medicine (TCM) have anti-inflammatory and anti-allergenic
effects, and they are currently used to treat acute and chronic
rhinitis, as well as AR. In particular, the volatile oils of FM and
CM serve important roles in the treatment of AR (10,11).
Due to the low and unstable extraction rate of volatile oil,
constituents such as eucalyptol and geraniene D were easy to
volatilize (12). The majority of
TCM volatile oils are used as a raw material only, and therefore
few previous studies have reported the clinical application of its
single preparation. The association between compatibility and
pharmacodynamics of FM and CM has been rarely studied. Our previous
study revealed that the combination of volatile oil from FM and CM
may effectively relieve local inflammatory cell infiltration and
cell necrosis of nasal mucosa in the nasal cavity of rats, thereby
decreasing the histamine content in blood and improving the
symptoms of AR (11). In
Bitongning drops, the active component is aromatic water. Although
aromatic water is mostly composed of volatile oil, it is a type of
solution that is nearly saturated or contains water. Compared with
pure volatile oil, the volatile oil content of aqua aromatica is
lower and extremely perishable, which is an impediment for its
production and storage on a large scale.
As a new drug carrier, microemulsions (ME) are
transparent or translucent, have low viscosity, and are isotropic
and thermodynamically stable systems. ME is spontaneously formed by
an oil phase, water phase, emulsifier and co-emulsifier in an
appropriate ratio (13,14). The particle size of ME ranges from
10–100 nm, and its characteristics include low viscosity, rapid
absorption and targeted drug release, which can increase the
solubility of volatile oil drugs, improve the bioavailability of
drugs and decrease side effects (15–17).
As the volatile oils of FM and CM are fat-soluble components, and
their absorption is poor and their bioavailability is low, the
common method of preparation results in poor absorption and low
bioavailability. Therefore, in the present study, the volatile oils
of FM and CM were prepared as an ME by phase conversion
emulsification, and its basic properties, including stability,
content and skin permeability were evaluated. The results led to
the generation of a novel type of FM and CM volatile oil ME
preparation with high stability, safety and loading capacity.
Materials and methods
Materials
FM volatile oil and CM volatile oil were produced by
the Key Laboratory of New Drugs and Chinese Medicine Foundation
Research, College of Pharmacy, Shaaxi University of Chinese
Medicine (7,20). Eucalyptol (purity >99.5%) was
purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd.
Hydrogenated castor oil polyoxyethylene ether (RH-40), castor oil
polyoxyethylene ether (EL-40), isopropyl myristate (IPM) and
isopropyl hexadecanoate (IPP) were purchased from Shanghai Yuanye
Bio-Technology Co., Ltd. Tween-80, Tween-20, glycerol,
1,2-propanediol, and oleic acid were purchased from Tianjin Tianli
Chemical Reagent Co., Ltd. Polyethylene glycol-400 (PEG-400) was
purchased Tianjin Kemiou Chemical Reagent Co., Ltd. Sudan III and
methylene blue were obtained from Shanghai Xinsheng Test Chemical
Technology Co., Ltd. Purified water, purchased from Wahaha Group
Co., Ltd., was used throughout the experiments. All other reagents
were of commercial analytical grade. A total of three
three-month-old female bullfrogs (150±20 g) were supplied by
Chengdu Dossy Experimental Animals Co., Ltd. Bullfrogs were housed
in a transparent box with a water depth of 20 cm at 25°C, 30–40%
humidity and a 12-h light day cycle, without food for 3 days.
Bullfrogs were sacrificed by marrow destruction, and the abdominal
skin and subcutaneous fat was removed. The present study was
approved by the Ethical Committee of Shaanxi University of Chinese
Medicine.
Screening of formula and preparation
factors of ME
Emulsifiers
Representing an important component in the
preparation of MEs, an emulsifier is a substance that can improve
the surface tension between various phases in emulsion, and can
form a uniform and stable dispersion system or emulsion (21). The hydrophilic and lipophilic
balance (HLB) value of the emulsifier is the basic metric used for
selecting a certain emulsifier (22). In the present study, RH-40, EL-40,
Tween-80, and Tween-20, emulsifiers with HLB values between 8–18
that can effectively improve the surface tension of oil and water,
were selected to investigate their effects on the formation of
volatile oil ME. The mixed phase with 1:1 ratio of IPM to FM-CM
volatile oil was used as the oil phase, and the preparation
temperature was 25°C. The different ratios of emulsifier to mixed
oil phase examined were 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8 and
1:9. ME was produced by stirring at constant temperature and
uniform speed, adding ultra-pure water drop by drop and measuring
the electrical conductivity. When the electrical conductivity of
the system reached the highest point, it was defined as being an
ME, and the dosage of emulsifier, mixed oil phase and water phase
which can form the ME group was recorded (14,23).
Data input was analyzed using Origin 8.0 drawing software
(OriginLab Corporation) to generate pseudo-ternary phase
diagrams.
Co-emulsifier
According to the screening results, EL-40 was
selected as the emulsifier, the mixed phase of IPM and FM-CM
volatile oil at a ratio of 1:1 was selected as the oil phase, the
Km was 2:1, and the preparation temperature was 25°C. The effects
of the co-emulsifiers such as absolute ethyl alcohol, glycerol,
1,2-propanediol and PEG-400 on ME were investigated.
Oil phase
In the preparation of ME, the size and chain length
of the oil phase molecules are important for the formation of ME.
In the present study, IPM, IPP and oleic acid were selected as the
components of the oil phase. According to the screening results of
emulsifier and co-emulsifier, EL-40 was used as emulsifier, while
anhydrous ethanol was used as co-emulsifier; the Km value was 2:1
and the preparation temperature was 25°C. IPM, IPP, oleic acid and
the FM-CM volatile oil of FM-CM were mixed in a certain proportion
as the oil phase. The optimum oil phase for ME preparation was
screened.
Km value
In the preparation of MR, the Km value is the ratio
of emulsifier quality to co-emulsifier mass. The ability of ME
formation varies with Km value. According to the screening results
of the emulsifier, co-emulsifier and oil phase, EL-40 was used as
emulsifier, anhydrous ethanol as co-emulsifier, and IPM and FM-CM
volatile oil as mixed oil phase. The preparation temperature was
25°C, the effects of Km values of 1:1, 2:1, 3:1 and 4:1 on ME
formation were investigated.
Preparation temperature
Throughout the screening procedures of the above
prescription factors, the basic prescription was determined, and
the effects of different temperatures (25, 30, 40 and 50°C) on the
preparation of ME were investigated.
Characterization of ME
ME was characterized as described in previous
studies (15,24–27).
Appearance
The copper mesh of the carbon-plated support film
was placed on the sealing film, and a drop of sample (~30 µl) was
added to the support film. Following incubation for 5–10 min, the
excess solution was removed from the edge of the filter paper with
a pointed sheet of filter paper, and placed on the filter paper for
1 min and allowed to drain. Then, the dried supporting film was
placed on the sealing film, and a drop of uranyl acetate dye
solution was added for 90 sec. Subsequently, the excess dye
solution was removed with a pointed filter paper, clip it on the
filter paper and dried for 3 h. A JEM-1200EX transmission electron
microscope (JEOL, Ltd.) was used for observation, as described
previously (26).
Type identification
According to the principle of ‘similar miscibility’,
1 g/l of sudan III (an oil-soluble dye) and methylene blue (a
water-soluble dye) solution were added at room temperature, and
their diffusion rates in ME were directly observed by the naked eye
for 1 min to determine ME. When the ME was of the water in oil
type, the diffusion rate of Sudan III was increased compared with
that of methylene blue, and when the ME was of the oil in water
type, the diffusion rate of methylene blue was increased compared
with that of Sudan III.
pH and refractive index
Substances delivered by nasal administration must
have a suitable pH value, and the pH value of the ME in turn
affects the stability of the system. The pH and refractive index of
the freshly prepared ME were measured using a PHS-3C pH meter
(Shanghai Yidian Scientific Instruments Co., Ltd.) and WYA-2W Abbe
refractometer (Shanghai Instrument Electrophysical Optical
Instruments Co., Ltd.). At room temperature (25°C), ultra-pure
water was used as the control for calibration. In total, the pH
values of 3 batches of ME were determined, and the average value
was calculated.
Particle size and Zeta potential
In total, 3 batches of 1 ml MEs were selected, and
the particle size and zeta potential of the freshly prepared MEs
were determined by Malvern Zetasizer NanoZS90 instrument (Malvern
Instruments Ltd.). According to the basic definition of ME, the
particle size of ME should be between 10–100 nm (28). Zeta potential measurement is the
use of electrophoretic scattering to detect the potential of
suspended particles in a specific solution environment. Its purpose
is to detect the charged properties of the particle surface,
including electrical properties and potential level, in order to
predict the stability of the whole suspension system.
Physical stability
In total, 3 batches of the same ME quantity were
placed in a centrifuge tube in a 416 low-speed centrifuge (Gene
Company, Ltd.) and centrifuged at 1,890 × g for 30 min at 4°C.
Following centrifugation for 30 min, the appearance of ME was
observed.
Thermodynamic stability
A total of 3 batches of ME were subjected to a
heating-cooling cycle experiment. The 10 ml ME was placed in a
centrifuge tube. Following continuous heating-cooling cycles
between 40 and 4°C for 6 times, its appearance was observed.
In total, 3 batches of ME were subjected to a
freezing-thawing cycle experiment. The 10 ml ME was placed in a
centrifuge tube and frozen in the refrigerator at −20°C for 24 h.
Upon thawing at room temperature for 24 h, after 6 consecutive
cycles, its appearance was observed.
Detection of eucalyptol in ME
Preparation of reference
substance
The weight of the eucalyptol reference substance was
0.2541 g, the volume of anhydrous ether was set as 5 ml as the
reference substance solution, and the concentration of the
reference liquid was 50.82 mg/ml.
Preparation of samples
Weighed ME (1.0 g in 5 ml) was added to a brown
flask, followed by 5 ml of anhydrous ether. Following
demulsification with Eddy for 1 min, centrifugation at 1,890 × g,
at 4°C for 10 min and filtration of the supernatant through a 0.22
µm microporous filter membrane, the sample solution was
obtained.
Gas chromatography-mass spectrometry (GC-MS). An
Agilent 7890GC/5977MS (Agilent Technologies, Inc.) was used to
investigate the subsequent experimental methodology and the content
of Eucalyptol, according to the manufacturer's protocol. In
addition, the content of eucalyptol in ME was determined.
Chromatographic conditions
The chromatographic column used in the present study
was a HP-5 quartz capillary column (30 m × 0.25 mm, 0.25 µm). The
temperature of the injection port was 250°C. The initial
temperature of the oven was 55°C (held for 2 min), with an increase
of 8°C/min until it reached 80°C (held for 0 min), followed by an
increase of 6°C/min until reaching 160°C (held for 2 min), and then
an increase of 8°C/min until reaching 200°C (held for 0 min), with
an increase of 3°C/min until reaching a final temperature of 250°C
(held for 3 min). The total run time was 42.25 min. The sample was
injected at 40:1 with high-purity helium as carrier gas and a
constant flow rate of 1 ml/min.
Mass spectrometry conditions
The ion source was an EI source, the multiplication
voltage was 1.5 kV, the electron energy was 70 EV, the mass
scanning range was 28–555 m/z, and the solvent delay was 3 min.
Methodological investigation
The eucalyptol reference solution was prepared by
pouring 25, 50, 100, 200, 400 or 800 µl of eucalyptol solution into
brown volumetric bottles. Then anhydrous ether was added to a final
volume of 10 ml to prepare reference solutions with concentrations
of 0.12705, 0.2541, 0.5082, 1.0164, 2.0328 and 4.0656 mg/ml were
prepared. Linear regression was performed using the concentration
and peak area as abscissa and longitudinal coordinates with
Microsoft Excel 2016 (Microsoft Corporation).
The same sample solution was injected repeatedly 6
times according to the above conditions. The relative standard
deviation (RSD) of the peak area of eucalyptol was determined, and
the precision of the method was investigated. In total, 6 samples
were prepared in parallel with the same batch of sample solutions.
According to the aforementioned conditions, the RSD of the peak
area of eucalyptol was calculated, and the experiment method was
repeated six times to investigate the repeatability. The same batch
of ME solution was prepared according to the treatment method of
the test sample, and the peak area of eucalyptol was determined at
0, 1, 2, 4, 6, 8, 10, 12 and 24 h. The peak area of eucalyptol RSD
was calculated to investigate the stability of the ME within 24 h.
A total of 9 samples of 0.5000 g ME were precisely weighed and
divided into 3 groups. Eucalyptol reference solution was added
according to the known levels of 50, 100 and 150%, respectively.
Following mixing, the samples were analyzed according to the above
chromatographic conditions, and the recovery and RSD value were
calculated.
Determination of eucalyptol
In total, 3 batches of samples were combined into a
sample solution according to the treatment method of the sample
solution, and the determination was performed according to the
above chromatographic conditions.
Percutaneous permeability test
A marrow-destroying needle was inserted into the
foramen magnum of the bullfrog, the spinal cord was transected left
and right, and the brain was destroyed. The myelocytic needle was
then withdrawn from the foramen magnum and inserted into the spinal
canal to destroy the spinal cord. Death was confirmed by checking
for complete relaxation of the limbs muscles. The abdominal skin of
bullfrog was removed, and the fat and subcutaneous tissues were
stripped, washed repeatedly with normal saline and fixed on the
diffusion interface of the diffusion chamber, while the dermis was
fixed to the reception tank. As the receptor fluid, 20% ethanol
saline (Kunshan Hechuang Ultrasonic Instruments Co., Ltd) was used,
which was injected into the reception tank following ultrasonic
cleaning to remove bubbles. The temperature of the circulating
water bath was 37±0.2°C, and a constant speed of 350 rpm was
employed to drain the bubbles in the receiving solution. Following
balancing of the water bath for 30 min, 2.0 g ME and 2.0 g oil
solution were placed into the supply tank. At 0.5, 1, 2, 4, 6, 8,
10, 12 and 24 h, 1 ml was sampled, and the same volume of blank
receptor fluid was added to the reception tank and the bubbles were
excreted (25,29–32).
The ME solution was extracted with anhydrous ether 3 times, and the
supernatant was collected and combined. The supernatant was
centrifuged for 10 min in a high-speed centrifuge (335 × g, 4°C),
1.5 ml supernatant was extracted with a disposable syringe and
passed through a filter membrane of 0.22 µm, and the receiving
solution at each time-point was obtained. The content of eucalyptol
was determined according to the above chromatographic conditions,
and the data were recorded. The cumulative transdermal volume was
calculated according to the following formula:
Qn=(V x Cn+∑i-1n-1x Vi x Ci)/A
where Qn is the cumulative permeability per unit
area at the time n, A is the effective transdermal area
(superficial area =1.54 cm2 and diameter =1.4 cm), Cn is
the concentration of the drug measured at time n, V is the volume
of the receiving pool (15 ml), and Vi is the volume of each sample.
Cumulative transmittance was calculated using the formula Ln=Qn/W,
where Ln is the cumulative transmittance and W is the content of
eucalyptol in the sample.
Results
Screening of prescription and preparation
factors of ME
Emulsifiers
The data from the present study were collated to
draw a pseudo-ternary phase diagram, as shown in Fig. 1, where the area of ME accounts for
SRH-40 =0.0283, SEL-40 =0.0298 and
STween-80 =0.0287. The Tween-20 group could not form ME,
and therefore the pseudo-ternary phase diagram could not be
generated. The proportion of ME area in the EL-40 group was the
largest. Therefore, EL-40 was selected as the emulsifier of FM-CM
volatile oil ME.
Co-emulsifiers
A co-emulsifier can adjust the HLB value of the
emulsifier and form smaller droplets, and an auxiliary emulsifier
can improve the formation of an ME. The results of pseudo-ternary
phase diagram in Fig. 2 indicate
that the area of ME was Sanhydrous ethanol =0.0782,
S1,2-propanediol =0.0417, Sglycerol =0.0414
and SPEG-400 =0.0522. Anhydrous ethanol had the largest
proportion of ME area; thus, it was selected as the co-emulsifier
of FM-CM volatile oil ME.
Oil phase
The results of pseudo-ternary phase diagram showed
that the proportion of ME area was SIPP =0.0530,
SIPM =0.0766 and Soleic acid =0.0441. As the
proportion of ME area was the largest in the IPM group, this was
selected as the oil phase of FM-CM volatile oil ME. The results are
presented in Fig. 3.
Km values
Different Km values have different effects on the
formation of ME. According to the results of the pseudo-ternary
phase diagram in Fig. 4, the area
of ME was S1:1 =0.0450, S2:1 =0.0766,
S3:1 =0.0540 and S4:1 =0.0398. When Km was
2:1, the ME area was the largest; thus, 2:1 was determined to be
the best Km value.
Preparation temperature
By generating a pseudo-ternary phase diagram, the
area of ME was S25°C =0.0766, S30°C =0.0696,
S40°C =0.0650 and S50°C =0.0613, suggesting
that 25°C was the best preparation temperature. The results are
shown in Fig. 5.
Preparation of optimal prescription
ME
According to the above screening results, the best
prescription of FM-CM volatile oil ME was as follows: Th emulsifier
was EL-40; the co-emulsifier was anhydrous ethanol; the Km was 2:1;
and IPM and FM-CM volatile oil were used as mixed oil phase at 1:1.
The mixed emulsifier accounted for 25.81%; the mixed oil phase
accounted for 2.81%; and the aqueous phase accounted for 71.38% of
the total ME system. The preparation temperature was 25°C. The
water representing 71.38% of the system was slowly added to form
clear and transparent ME droplets.
Characterization
Morphological observation
The results of the observation of the appearance and
morphology of the ME are presented in Fig. 6. The ME was a sphere with a round
appearance and uniform particle size distribution.
Identification of ME type
The methylene blue diffusion rate of the ME prepared
in the present study was markedly increased compared with that of
Sudan III, which indicates that it was an O/W ME (Fig. 7).
pH and refractive index
The pH value of ME was 5.67±0.01 and the refractive
index was 1.4198±0.0011 nd at 25°C.
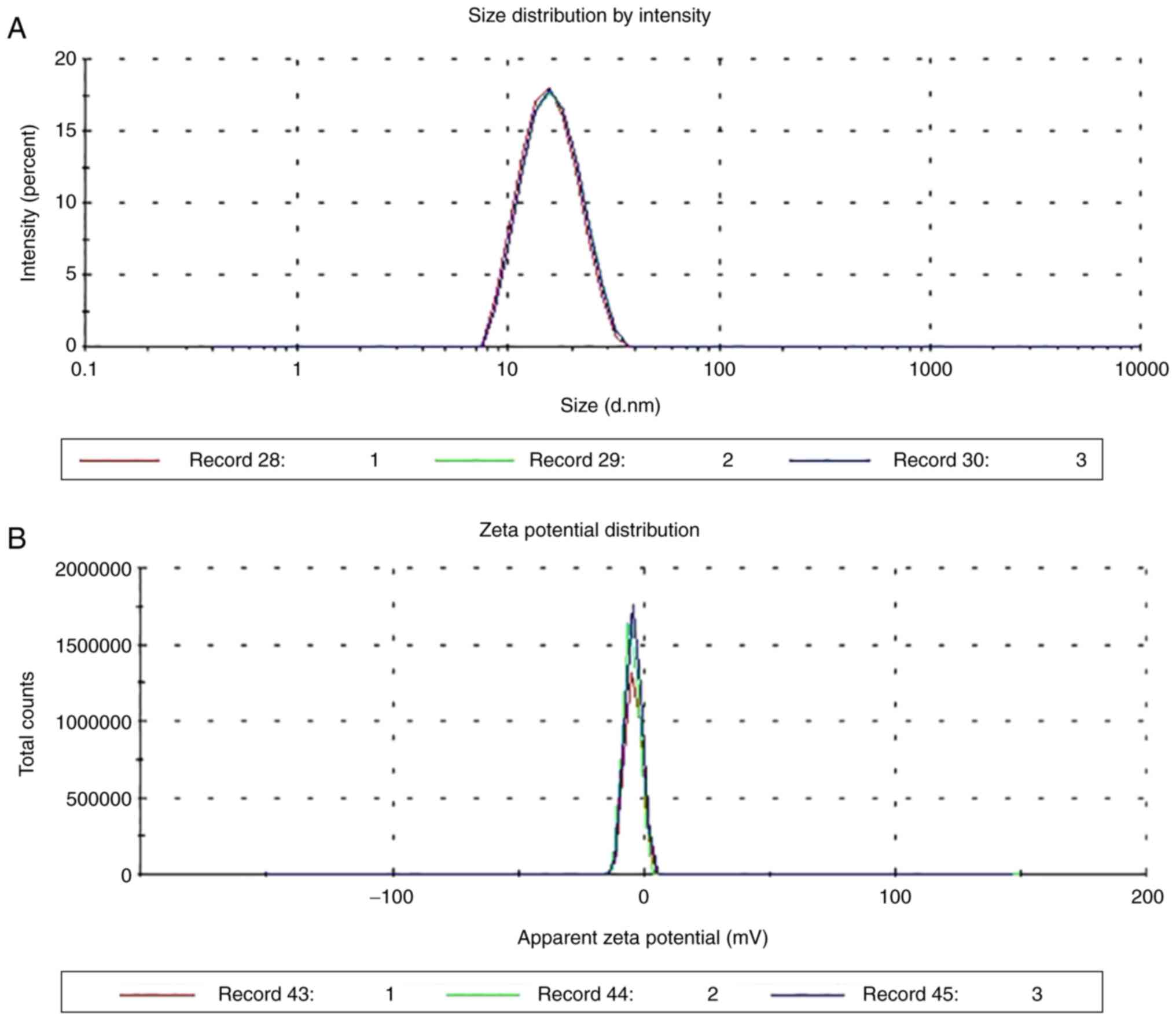
Particle size and Zeta potential
As shown in Fig. 8,
the particle size distribution of ME was uniform and the average
particle size was 14.62±0.4576 nm. The mean polydispersity index
value was 0.0747±0.0265 (n=3), and when there was only one peak in
the range of 10–100 nm, the average potential was −4.06±0.0702 mV
(n=3). These results indicated that the ME met the requirement of
particle size, and the system was stable.
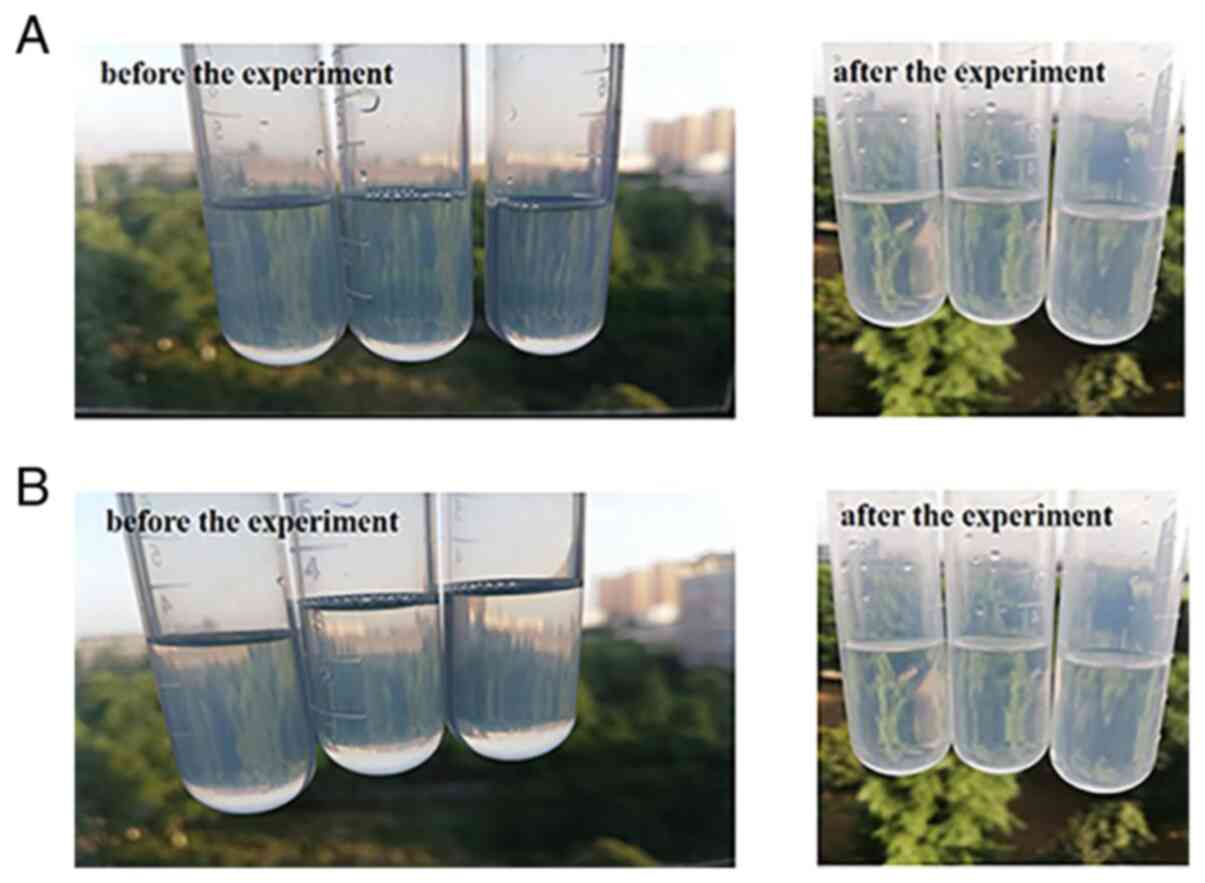
Physical stability
The appearance of the 3 batches of ME following
high-speed centrifugation for 30 min remained uniform, clear, and
transparent, and there was no stratification observed,
demonstrating that the centrifugal stability of ME was good
(Fig. 9A).
Thermodynamic stability
After 6 heating-cooling and freezing-thawing cycles,
the 3 batches of ME remained clear and transparent, and there was
no stratification, indicating that the thermodynamic stability of
ME was good (Fig. 9B).
Methodological investigation
The calibration curve of the peak area and
concentrations for eucalyptol was linear, ranging from
0.12705–4.0656 mg/ml. The calibration curve was Y=37,4632X +
2×108 (R2=0.9992), where Y represents the
eucalyptol peak area and X represents the concentration of
eucalyptus reference substance. The chromatograms of the reference
substance and the sample solution are shown in Fig. 10. In the precision experiment, the
RSD of the eucalyptol peak area was 1.36%, demonstrating that the
precision of this method was good. In the repeatability experiment,
the RSD of the eucalyptol peak area was 2.20%, indicating that this
method has good repeatability. In the stability experiment, the RSD
of the eucalyptol peak area was 1.93%, indicating that the sample
remained stable for 24 h. In the sample recovery experiment, the
mean recovery rate was 99.27%, and the RSD value was 2.91%. These
results showed that the accuracy of the experimental observations
was high.
Determination of eucalyptol
concentration
According to the results of sample content
determination, the mean content of eucalyptol in the ME was 2.57
mg/g, as shown in Table I.
 | Table I.Eucalyptol content in
microemulsions. |
Table I.
Eucalyptol content in
microemulsions.
| Sample number | Sampling quantity,
g | A1 | A2 | Ā | Eucalyptol content,
mg/g | Average content,
mg/g | RSD, % |
|---|
| 1 | 1.0167 | 412958743 | 383474502 | 398216622.5 | 2.60 | 2.57 | 1.19 |
| 2 | 1.0012 | 381962376 | 405573769 | 393768072.5 | 2.54 |
|
|
| 3 | 1.0032 | 398652865 | 391974721 | 395313793 | 2.56 |
|
|
Percutaneous permeability test
The curve was generated by considering the
cumulative permeability Q24 (µg/cm2) as the
longitudinal coordinate and the sampling time t as the abscissa.
Linear regression analysis of the obtained curve was performed. The
linear slope represents the steady penetration rate Jss
(µg/cm2/h). The results are shown in Table II, Fig. 11 and Table III.
 | Table II.Qn and Ln per unit area of ME at each
time-point (n=3). |
Table II.
Qn and Ln per unit area of ME at each
time-point (n=3).
|
| Eucalyptol Qn,
µg/cm2 | Ln, % |
|---|
|
|
|
|
|---|
| Time-point, h | ME group | Oil solution
group | ME group | Oil solution
group |
|---|
| 0.5 | 54.2507±0.4277 | 21.3442±1.0544 | 1.06 | 0.42 |
| 1 | 85.4878±3.2467 | 31.9255±3.0570 | 1.66 | 0.62 |
| 2 |
101.6378±1.1280 | 36.3763±1.2314 | 1.98 | 0.71 |
| 4 |
120.9467±1.5481 | 39.6531±0.4615 | 2.35 | 0.77 |
| 6 |
133.4428±1.4452 | 42.5442±0.3600 | 2.60 | 0.83 |
| 8 |
145.0037±3.2474 | 45.3398±0.3371 | 2.82 | 0.88 |
| 10 |
160.0303±0.4325 | 50.6580±1.5446 | 3.11 | 0.99 |
| 12 |
173.7156±3.5513 | 60.7943±0.3699 | 3.38 | 1.18 |
| 24 |
301.0797±2.8009 | 73.3491±2.1919 | 5.86 | 1.43 |
 | Table III.Comparison of transdermal permeation
parameters between ME and oil solution groups (n=3). |
Table III.
Comparison of transdermal permeation
parameters between ME and oil solution groups (n=3).
| Group | Cumulative
permeability - time equation | Steady penetration
rate Jss, µg/cm2/h | Cumulative
permeability, µg/cm2 |
|---|
| ME group | Q=9.4349t+70.971
(R2=0.9758) | 9.4349 | 301.0800±2.80 |
| Oil solution
group | Q=2.0082t+29.604
(R2=0.9119) | 2.0082 | 73.3491±2.19 |
These results indicated that the cumulative
permeabilities of eucalyptol in the ME and oil solution were
301.0800±2.80 and 73.3491±2.19 µg/cm2, respectively, and
the steady state penetration rates were 9.4349 and 2.0082
µg/cm2/h, respectively. The cumulative permeability of
the ME was increased 4.10-fold compared with that of the oil
solution, and the steady penetration rate was increased 4.70-fold
compared with that of the oil solution, which indicated that ME
could effectively promote the transdermal absorption of the
drugs.
Discussion
Bitongning drops serve an important role in the
clinical treatment of AR due to their active ingredient, the
aromatic water of FM and CM, and previous studies have shown that
their therapeutic effect on AR is due to their volatile oil
components (33–37). The aim of the present study was to
increase the drug concentration in a standard AR treatment method
by replacing traditional aromatic water with volatile oil. Volatile
oils have an important therapeutic effect in numerous TCM
treatments, and are an indispensable functional ingredient
(38). According to the theory of
TCM, volatile oils function as ‘aromatic strings’. An aromatic
string is called ‘Fangxiangzouchuan’ in TCM. It can be interpreted
as ‘the fragrance of the medicine can be dispersed everywhere’, the
curative effect is exact (39).
However, volatile oils are a fat-soluble component with poor water
solubility that can be highly irritable. In the preliminary
experiments in the present study, the best compatibility ratio of
the volatile oils from FM and CM in the treatment of AR was
screened, and the results revealed that the best proportion was 7:1
FM: CM. In the present study, an ME was generated to improve
bioavailability, to resolve the issues of poor absorption and low
solubility normally associated with volatile oils, and to decrease
drug toxicity, irritation, and adverse reactions.
The ME was prepared by water titration with
different emulsifiers, co-emulsifiers, oil phases, Km values, and
preparation temperatures. The area of the ME was mapped using a
pseudo-ternary phase diagram, and the particle size was combined
with the size of the ME The best formula for the preparation of ME
was determined by centrifugal stability and other factors. When the
Km value increased from 1.0 to 2.0, the area of ME increased, which
was primarily due to the increase of emulsifiers ratio, emulsifiers
can effectively reduce oil/water interfacial tension and decrease
the interfacial film tension formed by ME, thereby enhancing the
emulsification ability to form a larger ME area. When studying Km,
it has been observed that when the Km value increased from 2.0 to
4.0, possibly due to the increase of emulsifiers to a certain
value, an increase in viscosity and expansion of the gel region and
double continuous zone were observed, which led to the decrease of
the formation area of ME (40).
When examining preparation temperature, the area of ME formation
was observed to decrease with the increase in preparation
temperature, since volatile oil was used as part of the mixed oil
phase. It was hypothesized that this may have been caused by the
increase in volatilization of the drug with the increase in
temperature. Therefore, 25°C was selected as the most suitable
temperature for ME preparation. However, this hypothesis requires
further experimental verification. The ME was identified as an O/W
ME, and its particle size, pH value and refractive index all met
the requirements of an ME. It is generally considered that the
absolute value of Zeta potential in a stable-dispersion system
should be >30 mV, and the larger the absolute value is, the more
stable the system is (41).
However, the absolute value of Zeta potential in the present study
was <30 mV. It has been reported that the Zeta potential of ME
may be low (41), which does not
indicate that ME is unstable. Instead, the primary reason for this
is that the ME is an uncharged system. The emulsifier EL-40 in ME
is a non-ionic solubilizer, which can be used in the emulsification
of vegetable, animal, and mineral oils. It can also effectively
promote the transdermal absorption of drugs and decrease skin
irritation (42). The
co-emulsifier anhydrous ethanol may decrease the polarity of the
aqueous phase and surface tension, thus compensating for the poor
fluidity of the oil-water interface film when a emulsifier is used
alone, and when combined with emulsifiers, the preparation of ME is
improved (43,44). Previous studies reported that
adding the volatile oil of TCM to the oil phase of ME could improve
transdermal permeation (45–47).
In the present study, according to preliminary experiments, the
drug with mass ratio of 1:1 and IPM were used as mixed oil phase to
promote drug absorption. During the in vitro transdermal
comparison between ME and oil solution in the present study, it was
identified that the cumulative and steady permeabilities of ME were
significantly increased compared with those of the oil solution
group, indicating that ME may effectively promote the transdermal
absorption of volatile oil. These results provide a reference for
follow-up development and research of ME.
In the present study, the preparation method and
quality of ME were examined systematically. It was identified that
the in vitro transdermal performance of ME was significantly
increased compared with that of the oil solutions, and that its
properties were stable. In order to further demonstrate the good
bioavailability of ME, in vivo pharmacokinetic studies or
cell experiments will be conducted in the future. Notably, in the
preparation process of ME, the specific formula used and
preparation process factors have important effects on the formation
of ME. Consequently, the selection of the appropriate emulsifier,
co-emulsifier, oil phase, Km, and preparation temperature are
important.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Chinese Medicine Pharmaceutical Key Discipline of Shaanxi Province
(grant no. 303061107), Natural Science Foundation of China (grant
no. 81703720), Key R&D Program of Shaanxi Province (grant nos.
2019SF-290 and 2018SF-314). Key discipline of traditional Chinese
Medicine Pharmaceutical Engineering of Shaanxi Provincial
Administration of traditional Chinese Medicine (grant no.
132018004), Discipline Innovation team Project of Shaanxi
University of Chinese Medicine (grant no. 2019-YL11), Shaanxi
Provincial Department of Education Project (grant no. 18JS026), and
the Shaanxi Provincial Administration of traditional Chinese
Medicine Project (grant no. JPC056).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YL performed the experiments and data analysis, and
wrote the manuscript. JZ performed the experiment and participated
in the analysis of the experimental data. YS conceptualized the
study and analyzed the experimental data. JT and YW performed
experiments. XZ, DG and MY conceptualized the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
Shaanxi University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lam HY, Tergaonkar V and Ahn KS:
Mechanisms of allergen-specific immunotherapy for allergic rhinitis
and food allergies. Biosci Rep. Apr 30–2020.(Epub ahead of print).
doi: 10.1042/BSR20200256. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitsias DI, Dimou MV, Lakoumentas J,
Alevizopoulos K, Sousa-Pinto B, Fonseca JA, Bousquet J and
Papadopoulos NG: Effect of nasal irrigation on allergic rhinitis
control in children; complementarity between CARAT and MASK
outcomes. Clin Transl Allergy. 10:92020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rawls M, Thiele J, Adams DE, Steacy LM and
Ellis AK: Clinical symptoms and biomarkers of Bermuda grass-induced
allergic rhinitis using the nasal allergen challenge model. Ann
Allergy Asthma Immunol. 124:608–615.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang GA: Efficacy of desloratadine citrate
disodium combined with mometasone furoate in the treatment of
allergic rhinitis and its effect on patients' complications.
Chinese Journal of Rational Drug Use. 16:179–181. 2019.(In
Chinese).
|
|
5
|
Yang CS, Xu J and Zhang YY: Determination
of 1,8-cineole in bitongning spray by gas chromatography. Guizhou
Med J. 35:929–930. 2011.(In Chinese).
|
|
6
|
Li XB and Wang P: Determination of
volatile oil content in bitongning drops by GC method. Heilongjiang
Med J. 24:16–17. 2011.
|
|
7
|
Cai XZ: Rudimentary research on
identification of Herba Centipedae and screening of its
active material anti-Allergic Rhinitis. Southern Medical
University; 2008, (In Chinese).
|
|
8
|
Lin YC and Gao M: Research progress on
chemical constituents and pharmacology of Centipeda minima.
J Zhejiang Chin Med Univ. 35:303–304. 2011.
|
|
9
|
Yang XX and Zhuang ZQ: Research progress
on chemical constituents and pharmacological action of Flos
magnoliae. Chin Tradit Herbal Drugs. 7:490–492. 1998.
|
|
10
|
Yuan JM, Huang XY and Zhou YT: Preparation
of Flos magnoliae-Herba Centipedae thermosensitive nasal in
situ gels. Zhong Cheng Yao. 40:2, 656–662. 2018.
|
|
11
|
Liang YL, Zhang XF and Zou JB:
Pharmacology mechanism of and for treating allergic rhinitis based
on pharmacology network. Drug Dev Ind Pharm. 45:547–555. 2019.
View Article : Google Scholar
|
|
12
|
Okińczyc P, Szumny A, Szperlik J, Kulma A,
Franiczek R, Żbikowska B, Krzyżanowska B and Sroka Z: Profile of
polyphenolic and essential oil composition of polish propolis,
black poplar and Aspens buds. Molecules. 23:E12622018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katdare A, Khunt D, Thakkar S, Polaka SN
and Misra M: Comparative evaluation of fish oil and butter oil in
modulating delivery of galantamine hydrobromide to brain via
intranasal route: Pharmacokinetic and oxidative stress studies.
Drug Deliv Transl Res. 10:1136–1146. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitsou E, Pletsa V, Sotiroudis GT, Panine
P, Zoumpanioti M and Xenakis A: Development of a microemulsion for
encapsulation and delivery of gallic acid. The role of chitosan.
Colloids Surf B Biointerfaces. 190:1109742020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu T, Ge SM, Deng LH, Wei MY, Xu YH and Wu
CB: Preparation of pseudolaric acid B microemulsion and its
transdermal penetration. Chin Tradit Herbal Drugs. 43:683–689.
2012.
|
|
16
|
Lu SS, Qian YH and Xu BB: Application of
microemulsification technology in transdermal penetration of
volatile oil from Chinese material medical. Sci Technol Vis.
25:237–239. 2018.
|
|
17
|
Wu JY, Li YJ, Han M, Hu XB, Yang L, Wang
JM and Xiang DX: A microemulsion of puerarin-phospholipid complex
for improving bioavailability: Preparation, in vitro and in vivo
evaluations. Drug Dev Ind Pharm. 44:1336–1341, 336–341. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Cheng G, Hu R, Chen S, Lu W, Gao
S, Xia H, Wang B, Sun C, Nie X, et al: A nasal temperature and pH
dual-responsive in situ gel delivery system based on microemulsion
of huperzine A: Formulation, evaluation, and in vivo
pharmacokinetic study. AAPS PharmSciTech. 20:3012019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mishra R, Prabhavalkar KS and Bhatt LK:
Preparation, optimization, and evaluation of Zaltoprofen-loaded
microemulsion and microemulsion-based gel for transdermal delivery.
J Liposome Res. 26:297–306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai H, Ma KY and Li HL: Study on
extraction technology of volatile oil from Flos magnoliae.
Jiujiang Xueyuan Xuebao Ziran Kexue Ban. 32:8–12. 2017.(In
Chinese).
|
|
21
|
Cho YH, Kim S, Bae EK, Mok CK and Park J:
Formulation of a cosurfactant-free O/W microemulsion using nonionic
surfactant mixtures. J Food Sci. 73:E115–E121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shah A, Thool P, Sorathiya K, Prajapati H,
Dalrymple D and Serajuddin AT: Effect of different polysorbates on
development of self-microemulsifying drug delivery systems using
medium chain lipids. Drug Dev Ind Pharm. 44:215–223. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song WJ and Gu W: Pharmacology study and
prospect of aromatic Chinese herbs. Zhonghua Zhongyiyao Zazhi.
32:2, 609–611. 2017.
|
|
24
|
Yang XY and Yi L: Preparation and in vitro
transdermal study of zolmitriptan-diclofenac microemulsion. China
Pharmacy. 28:1841–1844. 2017.
|
|
25
|
Golmohammadzadeh S, Farhadian N, Biriaee
A, Dehghani F and Khameneh B: Preparation, characterization and in
vitro evaluation of microemulsion of raloxifene hydrochloride. Drug
Dev Ind Pharm. 43:1619–1625, 619–625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kotmakçı M, Akbaba H, Erel G, Ertan G and
Kantarcı G: Improved method for solid lipid nanoparticle
preparation based on hot microemulsions: Preparation,
characterization, cytotoxicity, and hemocompatibility evaluation.
AAPS PharmSciTech. 18:1355–1365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akram A, Rasul A, Waqas MK, Irfan M,
Khalid SH, Aamir MN, Murtaza G, Ur Rehman K, Iqbal M and Khan BA:
Development, characterization and evaluation of in-vitro
anti-inflammatory activity of ginger extract based micro emulsion.
Pak J Pharm Sci. 32:1327–1332. 2019.PubMed/NCBI
|
|
28
|
Lee J, Lee Y, Kim J, Yoon M and Choi YW:
Formulation of microemulsion systems for transdermal delivery of
aceclofenac. Arch Pharm Res. 28:1097–1102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Golwala P, Rathod S, Patil R, Joshi A, Ray
D, Aswal VK, Bahadur P and Tiwari S: Effect of cosurfactant
addition on phase behavior and microstructure of a water dilutable
microemulsion. Colloids Surf B Biointerfaces. 186:1107362020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang JJ, Cui SF and Tang XL: Study on
preparation technology and in vitro transdermal permeability of
compound clove microemulsion. Zhong Yao Cai. 608–611. 2007.
|
|
31
|
Liu X, Zhang ZH and Chen Y: Preparation of
colchicine microemulsion and its transdermal permeation in vitro.
Chin Tradit Herbal Drugs. 42:963–967. 2011.
|
|
32
|
Yang F, Zhou J, Hu X, Yu SK, Liu C, Pan R,
Chang Q, Liu X and Liao Y: Preparation and evaluation of
self-microemulsions for improved bioavailability of ginsenoside-Rh1
and Rh2. Drug Deliv Transl Res. 7:731–737. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Xu H, Wu S, Ju B, Zhu D, Yan Y,
Wang M and Hu J: Preparation and evaluation of microemulsion based
transdermal delivery of Cistanche tubulosa phenylethanoid
glycosides. Mol Med Rep. 15:109–116. 2017. View Article : Google Scholar
|
|
34
|
Zhai XY: Effect of volatile oil from
magnolia on Th cells of Guinea pigs with allergic rhinitis. Shaanxi
J Tradit Chin Med. 31:116–118. 2010.
|
|
35
|
Liu ZG, Yu HM, Wen SL and Liu YL:
Histopathological study on allergic rhinitis treated with
Centipeda minima. Zhongguo Zhong Yao Za Zhi. 30:292–294.
2005.(In Chinese). PubMed/NCBI
|
|
36
|
Wu M, Zhang J and Zhang X: Clinical
observation of Flos magnoliae volatile oil nano-liposome
nasal drops in treating pediatric allergic rhinitis. Zhong guo
Zhong Xi Yi Jie He Za Zhi. 29:740–742. 2009.
|
|
37
|
Jia XS and Zhang YY: Research progress on
essential oils of Cenpiteda minima (L.) A. Br. Aschers.
Shandong Chem Ind. 47:66–68. 2018.
|
|
38
|
Guan Z, Ma XZ and Lv GY: Effects of VOM bp
on IL-12, IFN-γ and histamine levels of rats with allergic
rhinitis. Pharmacol Clin Chin Materia Med. 27:70–72. 2011.
|
|
39
|
Wang YQ, Yang YZ, Wu ZF, Xiong YK and Yang
M: Traditional function and modern research progress on volatile
oil in Chinese materia medica. Chin Tradit Herbal Drugs.
49:455–461. 2018.
|
|
40
|
Das S, Lee SH, Chia VD, Chow PS, Macbeath
C, Liu Y and Shlieout G: Development of microemulsion based topical
ivermectin formulations: Pre-formulation and formulation studies.
Colloids Surf B Biointerfaces. 189:1108232020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li SL, Duan Q, Zhao ZD, Xia L, Liu WH,
Wang XG and Shen XZ: Preparation and quality evaluation of Acori
Tatarinowii Rhizoma-volatile oil microemulsion. Chin Tradit Herbal
Drugs. 50(1): 935–941. 2019.(In Chinese).
|
|
42
|
Xu WT: Study on the regularity of the
formation of microemulsion by nonionic surfactans. PhD
dissertation. Jiangnan University; 2009, (In Chinese).
|
|
43
|
Paulo BB, Alvim ID, Reineccius G and Prata
AS: Performance of oil-in-water emulsions stabilized by different
types of surface-active components. Colloids Surf B Biointerfaces.
190:1109392020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su J, Wu CH and Jiang F: Preparation of
Paeonol self-microemulsion transdermal delivery system with
peppermint oil as carrier. Zhongguo Shiyan Fangjixue Zazhi.
23:11–16. 2017.(In Chinese).
|
|
45
|
Yuan ZZ, Yin SY and Jin Y: Preparation and
quality evaluation of Eugenia oleifera oil bano-emulsion.
Lishizhen Med Materia Med Res. 28:620–622. 2017.
|
|
46
|
Chen JJ, Xia J, Song J, Luo MF and Huang
Z: Effect of Eugenol in the microemulsion as an oil excipient and
enhancer on percutaneous absorption of Baicalin. Xiandai Shengwu
Yixue Jinzhan. 16:410–413. 2016.(In Chinese).
|
|
47
|
Lu SS, Zhao YR, Yao JH, Ren L, Li Y and
Chen J: Preparation technology on essential oil from Chinese
materia medica as penetration enhancers. Chin Tradit Herbal Drugs.
49:2477–2481. 2018.(In Chinese).
|