Introduction
Ischemic stroke remains a leading cause of mortality
or long-term disability. The goal of clinical treatment is to
restore the blood supply as soon as possible, allowing timely
supply of oxygen to ischemic brain tissue (1). However, blood reperfusion that occurs
after a long period of ischemia is likely to result in a higher
infarction volume and to, in turn, aggravate the initial injury,
which is the main cause of cerebral ischemia reperfusion (I/R)
injury (2). The mechanisms
involved in cerebral I/R is complicated and require comprehensive
understanding. To date, inflammatory response, free radical damage,
cytotoxicity, increased mitochondrial permeability and
intracellular calcium overload have been implicated to participate
in the occurrence or progression of cerebral I/R injury (3). Cerebral I/R injury is pathologically
characterized by the damage of brain tissues, infiltration of
leukocyte cells into the brain, influx of inflammatory cells,
excessive production of reactive oxygen species (ROS), degradation
of cytoskeletal protein and collapse of the blood-brain barrier
(4). Increased ROS production
following reperfusion will increase hemorrhagic infarction,
cerebral edema and infarct size (5). Inhibition of oxidative stress has
been demonstrated to protect against cerebral I/R injury (6,7).
Therefore, the use of safe and effective therapeutic agents with
antioxidant properties to interfere with oxidative damage provides
an encouraging treatment strategy.
Eupafolin is an active flavonoid component of
Eupatorium perfoliatum L., which is a traditional herbal
medicine from China and India, and has been widely used for
centuries to treat fever, malaria, infections and
inflammation-associated diseases (8). In recent years, eupafolin was
reported to exhibit anti-inflammatory, anti-oxidant and anti-tumor
cell proliferation effects (9).
For example, Zhang et al (10) indicated that eupafolin improved
acute renal injury and exhibited effective anti-oxidant and
anti-inflammatory activities via inhibiting reactive stress and
inactivating NF-κB, MAPK, ERK 1/2 and c-JNK signaling pathways
(10). Eupafolin ameliorated
cardiomyocyte autophagy via activation of the PI3K/AKT/mTOR
signaling pathway (11). Eupafolin
also protected against TNF-α-induced lung inflammation via
inhibiting NF-κB/p65 activation and also resulted in nuclear
translocation (12). In addition,
the suppressive effects of eupafolin on various tumor types,
including esophageal cancer, hepatocellular carcinoma, renal
carcinoma and prostate cancer has been extensively studied
(13–16). These data suggested the potential
value of eupafolin in treating cancer and inflammation-related
diseases. However, whether eupafolin may protect the brain against
I/R injury remains to be elucidated.
Toll-like receptor (TLR) is a type of transmembrane
protein that converts extracellular antigen information into cells
and triggers an inflammatory response. Toll-like receptor 4 (TLR4)
is the first discovered member of TLR, which serves a role in
immune defense and immune regulation by recognizing and binding
multiple endogenous and exogenous ligands. TLR4 transduces these
signals via the membrane and subsequently regulates the expression
inflammation mediators and cytokines (17). A previous study demonstrated that
TLR4 serves a role in I/R inflammation injury of the liver, lung
and heart, and induces apoptosis during cerebral I/R (18). NF-κB is a transcription factor that
may specifically bind promoters and enhancers of numerous genes and
thus participate in a variety of cellular functions, including
inflammation, cell proliferation and apoptosis. NF-κB is typically
inactivated in the cytoplasm due to the interaction of p65/p52 with
the inhibitory protein IκB. The subsequent activation of p65/p52
may be stimulated by pro-inflammatory cytokines, cellular stress,
DNA damaging agents and phosphorylation of κBs by the IκB kinase
complex (19). NF-κB also serves a
crucial role in the activation of I/R injury (20).
The present study investigated the protective
effects of eupafolin against cerebral I/R injury in rats and
investigated whether its action was dependent on blocking the
TLR4/NF-κB signaling pathway.
Materials and methods
Animals
Adult male Sprague-Dawley rats (7–8 week-old; n=48)
weighing 250–280 g were supplied by Nanjing Better Biotechnology
Co., Ltd, and were acclimatized for 1 week before experiments at
room temperature under a controlled 12/12 h light/dark cycle. All
rats received food and water ad libitum. The experimental
protocols involving rats were approved by the Animal Studies Ethics
Committee of the Shenzhen Hospital of Integrated Traditional
Chinese and Western Medicine.
The animals were randomly assigned to six groups
(n=8 for each group): Control, model, 10 mg/kg eupafolin (purity
>98%; Shanghai Rongbia Biological Technology Co., Ltd.), 20
mg/kg eupafolin, 50 mg/kg eupafolin and 20 mg/kg nimodipine
(MedChemExpress). The control group underwent sham surgery.
Eupafolin and nimodipine were intragastrically (i.g.) administered
into the rats once a day for 7 consecutive days. The control and
model groups were administered 200 µl normal saline i.g. For the
eupafolin + lipopolysaccharide (LPS) group, the animals were
intraperitoneally injected with 50 µg/kg LPS (21) at the same time that they received
eupafolin 20 mg/kg for the last time.
Establishment of a cerebral I/R
model
One hour after the last administration, the focal
cerebral I/R rat model was prepared using the middle cerebral
artery occlusion (MCAO) method, as previously reported with slight
modifications (22). In brief,
following weighing, the rats were anesthetized with 1%
pentobarbital (50 mg/kg; i.p) and fixed in the supine position on a
heated operating table with the body temperature maintained at
37±0.5°C. Following skin incision, the left common carotid artery,
external carotid artery and internal carotid artery were carefully
exposed and dissected away from the adjacent nerve. The left middle
cerebral artery was occluded by inserting a 3.5-mm monofilament
suture through the internal carotid artery from the external
carotid artery. Following ischemia for 1.5 h, the suture was gently
withdrawn allowing reperfusion. At 24 h post-reperfusion, various
indexes were measured (23). The
animals in the sham-operated group were anesthetized with 1%
pentobarbital (50 mg/kg; i.p) prior to being subjected to the same
surgical procedure as the model group but without occlusion of the
middle cerebral artery (24).
Neurological score
The neurological deficit was scored in each mouse 24
h after reperfusion in a blinded manner by two independent
investigators according to the 3-point scoring system of Bederson
et al (25): No
neurological symptoms=0; forelimb flexion and no other
abnormality=1; decreased resistance to lateral push (and forelimb
flexion) without circling=2; same behavior as grade 2, with
circling toward the paretic side=3.
Measurement of brain edema
To evaluate brain edema, the brain water content was
measured using the standard wet-dry method. After 24 h of
reperfusion, the rats were decapitated under deep anesthesia and
the brains were carefully removed. The wet weight was obtained
immediately by weighing the ischemic hemispheres, and the tissues
were dried at 100°C for 24 h to determine the dry weight. The
degree of brain edema was calculated using the following equation:
Water content=(wet weight-dry weight)/wet weight ×100%; brain
index=wet weight/body weight ×100%.
Measurement of infract volume
Brain infarction size was evaluated by the
2,3,5-triphenyltetrazolium chloride (TTC) staining method (26) 24 h after I/R. Brains were carefully
removed and maintained at −20°C for 10 min. Brain tissues were then
sliced into consecutive 2-mm-thick coronal sections and immersed in
2% TTC solution (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. TTC
stains non-infarcted regions with a deep red pigment, while the
infarcted brain area appears white. The infarct area of each
section was photographed and image analysis software (NIH Image
version 1.63; National Institutes of Health) was applied to measure
the infarcted area.
Measurement of oxidative stress
The fresh brain tissues of rats at 24 h after
cerebral I/R were collected and the superoxide dismutase (SOD; cat.
no. ab65354), malondialdehyde levels (MDA; cat. no. ab118970) and
lactate dehydrogenase (LDH; cat. no. ab102526) activities in the
brain tissues were determined using the commercial kits (Abcam).
The brain tissues were harvested, washed with PBS and homogenized
using RIPA lysis buffer supplemented with PMSF protease inhibitor
(both from Abcam). After being centrifuged at 13,000 × g and 4°C
for 10 min to remove insoluble material, the supernatants were
collected and incubated with corresponding reaction mix according
to the manufacturer's protocols. The optical density was measured
(OD of 450 nm for SOD and LDH; OD of 532 nm for MDA) to calculate
the relative level of SOD, MDA and LDH. The relative MDA levels, as
well as SOD and LDH activities was expressed as the value dividing
by the OD of the control group after normalization to the standard
curve.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of TNF-α, IL-1β and IL-6 in the
serum of rats at 24 h after cerebral I/R were measured in strict
accordance with the manufacturer's protocols provided by the ELISA
kits (Abcam) for TNF-α (cat. no. ab236712), IL-1β (cat. no.
ab255730) and IL-6 (cat. no. ab234570). In brief, blood samples
were collected into a serum separator tube. Following clot
formation, samples were centrifuged at 2,000 × g and 4°C for 10 min
to collect serum. Samples were incubated with antibodies (included
in the kits) targeting TNF-α, IL-1β and IL-6 at room temperature
for 1 h. Following washing with the supplied wash buffer, TMB
development solution was added and incubated at room temperature
for 10 min. The stop solution was added and the absorbance at a
wavelength of 450 nm was detected using a microplate reader (Thermo
Fisher Scientific, Inc.).
TUNEL staining
A TUNEL assay (Beyotime Institute of Biotechnology)
was used according to the manufacturer's protocols to assess
neuronal apoptosis in brain tissues. In brief, isolated brains were
fixed in 4% paraformaldehyde at room temperature for 10 min and cut
into sections of 20-µm thickness, followed by paraffin embedding.
Following dewaxing with xylene, sections were incubated with
protease K for 30 min and subsequently washed with
phosphate-buffered saline (PBS). Subsequently, 50 µl TUNEL reaction
mixture was added and incubated for 1 h at 37°C. The sections were
then washed with PBS and incubated for 30 min following the
addition of 50 µl confining liquid. The nuclei were stained with
DAPI at room temperature for 5 min and TUNEL-positive cells were
observed under a DMi8 fluorescence microscope (Leica Microsystems
GmbH). Three fields of view were examined (magnification,
×200).
Western blotting
The ischemic side of the cerebral cortex was
dissected to extract total protein using RIPA buffer (Applygen
Technologies Inc.) and quantified using a BCA assay. Following
quantification, equal amount of proteins (8 µg) were separated by
10% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes (EMD Millipore). Following blocking with 5% skimmed milk
at room temperature for 2 h, the membranes were incubated with the
following primary antibodies at 4°C overnight (all Abcam): Bcl-2
(cat. no. ab32124; dilution, 1:1,000), Bax (cat. no. ab32503;
dilution, 1:5,000), pro-caspase 3 (cat. no. ab32499; dilution,
1:10,000), cleaved-caspase 3 (cat. no. ab32024; dilution, 1:500),
myeloid differentiation factor 88 (MyD88; cat. no. ab133739;
dilution, 1:10,000), TNF receptor-associated factor 6 (TRAF6; cat.
no. ab33915; dilution, 1:10,000), TGF-β-activated kinase 1 (TAK1;
cat. no. ab109526; dilution, 1:1,000), IKKα (cat. no. ab32041;
dilution, 1:10,000), p65 (cat. no. ab16502; dilution, 1:5,000),
phosphorylated (p)-IKKα (cat. no. ab38515; dilution, 1:1,000),
p-p65 (cat. no. ab183559; dilution, 1:1,000) and GAPDH (cat. no.
ab8245; dilution, 1: 10,000). The antibodies were detected using
horseradish peroxidase-conjugated IgG (Abcam; goat anti-rabbit,
cat. no. ab7090; dilution, 1:10,000) at room temperature for 2 h
and visualized using enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS (version 22.0, IBM Corp.) was used to perform the paired T
test between two groups and one-way analysis of variance, followed
by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of eupafolin on the
neurological defect score, brain edema and infract volume in rats
subjected to cerebral I/R
The alteration in neurological functions and
phenotypes was first investigated. The effects of eupafolin on the
neurological defect scores at 24 h post-reperfusion in the control,
model, eupafolin (10, 20 and 50 mg/kg) and nimodipine (20 mg/kg)
groups are presented in Table I.
Rats in the control group did not exhibit neurological deficits,
with scores of 0. However, in the model group, there were clear
signs of neurological deficits, including forelimb flexion,
decreased resistance to lateral push and circling toward the
paretic side, with significantly higher neurological defect scores
compared with the control group, indicating the occurrence of
cerebral I/R injury. Eupafolin at any dose significantly decreased
the neurological defect scores compared with the model group,
similar to that in the nimodipine group.
 | Table I.Effect of eupafolin on the
neurological deficit score. |
Table I.
Effect of eupafolin on the
neurological deficit score.
| Group | Neurological
deficit scores (range, 0–3) |
|---|
| Control | 0.00±0.00 |
| Model |
2.63±0.51a |
| Eupafolin (10
mg/kg) |
2.13±0.31b |
| Eupafolin (20
mg/kg) |
1.10±0.43c |
| Eupafolin (50
mg/kg) |
0.85±0.17c |
| Nimodipine |
1.13±0.24c |
The results of the water content and brain index
studies were consistent with the trend observed for the
neurological defect scores. As shown in Table II, the brain water content and
brain index of the rats were increased in the model group, while
those in the low, moderate and high-dose eupafolin and nimodipine
groups were significantly lower those in the model group.
 | Table II.Effect of eupafolin on brain
edema. |
Table II.
Effect of eupafolin on brain
edema.
| Group | Brain water
content, % | Brain index, % |
|---|
| Control | 82.58±0.44 | 0.52±0.02 |
| Model |
84.10±0.51a |
0.72±0.04a |
| Eupafolin (10
mg/kg) |
83.41±0.41b |
0.68±0.02b |
| Eupafolin (20
mg/kg) |
82.90±0.43c |
0.60±0.03d |
| Eupafolin (50
mg/kg) |
82.60±0.38c |
0.57±0.01d |
| Nimodipine |
82.94±0.34c |
0.63±0.03d |
The cerebral infarct volumes were assessed using TTC
staining. As shown in Fig. 1, no
infarction (white staining) was found in the control group.
Compared with the control group, cerebral I/R injury induced
significant infarction in the model group. However, treatment with
eupafolin at 10, 20 and 50 mg/kg markedly decreased the infarct
volume of brain tissues. Nimodipine exerted similar effects in
terms of decreasing the infarct volumes.
Eupafolin decreases oxidative stress
and inflammation in brain tissues and serum of rats subjected to
cerebral I/R
Subsequently, oxidative stress was examined in
ischemic brain tissues, which is considered the initial step of
cerebral I/R injury. SOD is an important antioxidant enzyme, while
MDA and LDH activities reflect oxidative damage. Therefore, SOD,
MDA and LDH contents were investigated (Fig. 2A-C). Compared with the control
group, SOD activity was significantly decreased, while MDA and LDH
content was increased in the model group. Treatment with eupafolin
effectively increased SOD activity and decreased MDA and LDH
content, which was similar to the results in the nimodipine
group.
 | Figure 2.Levels of oxidative stress and
inflammation in rats from control, model, 10 mg/kg eupafolin, 20
mg/kg eupafolin, 50 mg/kg eupafolin and nimodipine groups. The
activities of (A) SOD, (B) MDA and (C) LDH in the brain tissues of
rats at 24 h after cerebral I/R. The concentrations of (D) TNF-α,
(E) IL-1β and (F) IL-6 in the serum of rats at 24 h after cerebral
I/R. ***P<0.001 vs. control. #P<0.05,
##P<0.01 and ###P<0.001 vs. model. SOD,
superoxide dismutase; MDA, malondialdehyde; LDH, lactate
dehydrogenase; I/R, ischemia/reperfusion. |
To investigate the effects of eupafolin on
inflammation, the generation of pro-inflammatory cytokines
including TNF-α, IL-1β and IL-6 were assessed (Fig. 2D-F). Cerebral I/R injury
significantly increased the concentration of TNF-α, IL-1β and IL-6
in the serum of model rats. However, this increase was reversed by
eupafolin or nimodipine treatment. The aforementioned findings
suggested that, similar to nimodipine, eupafolin attenuated
oxidative stress and inflammation induced by cerebral I/R
injury.
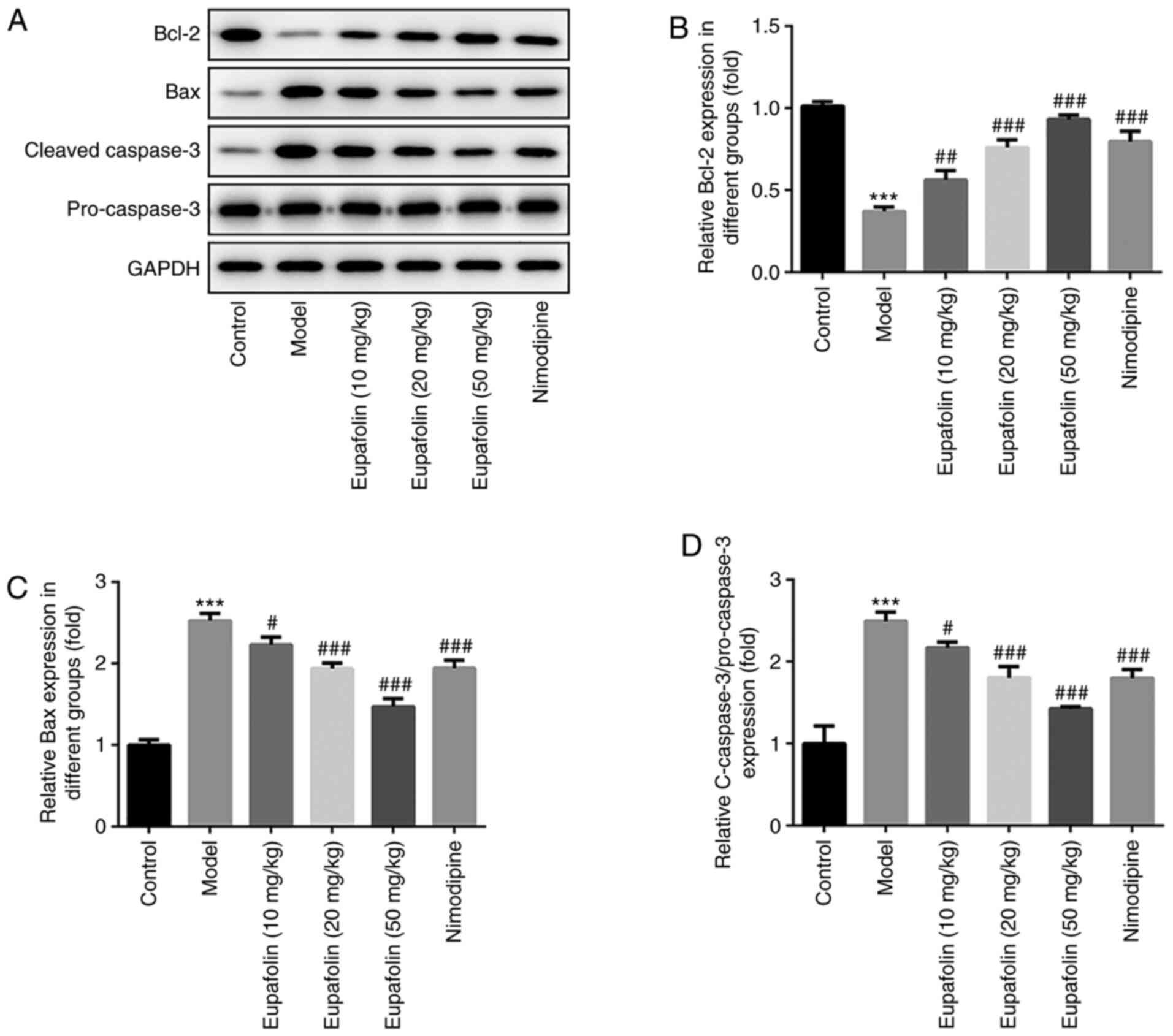
Eupafolin prevents brain cell
apoptosis in rats subjected to cerebral I/R
Next, the effects of eupafolin on cell apoptosis
were observed by TUNEL staining and western blotting. As shown in
Fig. 3, the number of
TUNEL-positive cells in the brain tissues of the model group was
significantly increased, compared with the control group. These
effects were significantly impaired by eupafolin or nimodipine
treatment. Furthermore, western blotting was utilized to detect the
expression of proteins associated with apoptosis. The results
revealed that cerebral I/R lead to an enhancement in Bax and
caspase-3 expression, but a decline in the anti-apoptotic protein
Bcl-2 expression (Fig. 4).
However, eupafolin and nimodipine partially reversed the levels of
these proteins, compared with the model group. Consistent with
TUNEL staining, these results demonstrated that eupafolin may
prevent cerebral I/R-induced cell apoptosis in the brain tissues of
rats.
Eupafolin inhibits the activation of
the TLR4/NF-κB signaling pathway
Finally, to underline the possible mechanism of
eupafolin protection against cerebral I/R injury, the expression of
proteins involved in TLR4/NF-κB signaling, including MyD88, TRAF6,
TAK1, IKKα and p65, in brain tissues were examined. As shown in
Fig. 5, in the model group, the
expression of MyD88, TRAF6, TAK1, p-IKKα and p65 was significantly
higher than that in the control group, suggesting the activation of
TLR4 signaling and nuclear translocation of the NF-κB complex.
However, eupafolin (10, 20 and 50 mg/kg) or nimodipine (20 mg/kg)
significantly inhibited the expression of these proteins.
 | Figure 5.Expression of proteins involved in
TLR4/NF-κB in brain tissues of rats from control, model, 10 mg/kg
eupafolin, 20 mg/kg eupafolin, 50 mg/kg eupafolin and nimodipine
groups. (A) Representative western blot bands for detecting MyD88,
TRAF6, TAK1, p-IKKα/IKKα and p-p65/p65. GAPDH was used as loading
control. (B) Densitometric quantification of protein expression.
***P<0.001 vs. control. #P<0.05,
##P<0.01 and ###P<0.001 vs. model.
MyD88, myeloid differentiation factor 88; TRAF6, TNF receptor
associated factor 6; TAK1, TGF-activated kinase 1; p,
phosphorylated. |
To verify whether the protective effects of
eupafolin rely on blocking TLR4 signaling, animals that were
treated with 20 mg/kg eupafolin were injected with LPS, which is
the agonist of TLR4 (21).
Table III and Fig. 6 demonstrate that, compared with the
eupafolin groups, following MCAO and reperfusion, animals in the
eupafolin + LPS group exhibited markedly higher neurological
deficit scores, brain water content and brain indexes (Table III), as well as larger infract
volume (Fig. 6).
 | Table III.The neurological deficit score and
brain edema evaluation in the absence or presence of TLR4
agonist-LPS. |
Table III.
The neurological deficit score and
brain edema evaluation in the absence or presence of TLR4
agonist-LPS.
| Group | Neurological
deficit scores (range, 0–3) | Brain water
content, % | Brain index, % |
|---|
| Eupafolin (20
mg/kg) | 1.15±0.31 | 83.04±0.53 | 0.62±0.02 |
| Eupafolin +
LPS |
2.09±0.47b |
83.84±0.66a |
0.69±0.04b |
Discussion
In the present study, treatment of rats subjected to
cerebral I/R with eupafolin not only decreased the neurological
deficit score, brain edema and cerebral infarct size, but also
weakened oxidative stress, inflammation and cell apoptosis. This
protection by eupafolin against cerebral I/R injury was accompanied
by the downregulation of MyD88, TRAF6, TAK1, p-IKKα and p-p65.
These results indicated that TLR4/NF-κB pathways are inactivated in
this process, implying that the TLR4/NF-κB pathways are involved in
the neuroprotective effects of eupafolin.
Previous studies have shown that inflammation,
oxidative stress and apoptosis are the three dominating mechanisms
underlying the detrimental process of cerebral I/R injury, which
was proven to be the second most common lethal factor and the
leading cause of adult neurological disabilities worldwide
(3,4,27,28).
In addition, inflammation is one of the central preventive
mechanisms against cerebral I/R injury (3,4).
Therefore, inhibiting neuronal inflammation is a pivotal target for
the treatment of ischemic stroke. To date, in preclinical animal
studies, >700 drugs have shown beneficial effects in cerebral
ischemia, but the results are far from satisfactory (29). Eupafolin is a potent
anti-inflammatory, antioxidant and antitumor agent, extracted from
the traditional herb E. perfoliatum. Although eupafolin has
been used as a traditional medicine for treating
inflammatory-related diseases in China and India, the
pharmacological research on it has only begun in the past ten
years. Published reports have mainly focused on in vitro
studies, and are lacking evidence from in vivo studies for
clinical therapy (30). Jiang
et al (14) demonstrated
that eupafolin at 60 mg/kg significantly inhibited tumor growth and
tumor angiogenesis in a hepatocellular carcinoma xenograft model.
The present study investigated the protective effects of eupafolin
on cerebral I/R injury in rats and revealed that eupafolin at a
dose of 10, 20 and 50 mg/kg all exhibited significant protective
effects on cerebral I/R via inhibiting inflammation, oxidative
stress and apoptosis. Concurrently, 20 mg/kg eupafolin showed
nearly equivalent effects to nimodipine, which is used to improve
blood circulation in the recovery period of acute cerebrovascular
disease and has been proven to exert anti-inflammation and
anti-apoptosis effects (31–33).
These results provided in vivo data for the therapeutic
effect of low-dose eupafolin in treating cerebral I/R injury.
Eupafolin may exert its actions via targeting
multiple pathways, including NF-κB, PI3K/AKT and MAPK (11,34,35).
It is widely implicated that TLR4-MyD88 association may activate
NF-κB, which may activate neurons to secrete numerous
pro-inflammatory mediators, including TNF-α, IL-1β and IL-6,
ultimately resulting in ischemic injury (36). The results of the present study
demonstrated that cerebral I/R injury may significantly increase
MyD88, TRAF6, TAK1, p-IKKα and p-p65 expression, and TNF-α, IL-1β
and IL-6 levels. Following ligand binding, the toll/interleukin-1
receptor domain of TLR4 interacts with MyD88, thereby binding to
TRAF6, resulting in the activation of TAK1. Activated TAK1
continues to signal via MAPK or NF-κB (37). The present data indicated that
eupafolin may prevent the activation of TLR4/NF-κB signaling. In
addition, the neurological deficit score, brain edema and cerebral
infarct size were decreased compared with the model group,
indicating that the blockade of the TLR4/NK-κB signaling pathway in
cerebral I/R may have a protective effect. Furthermore, the
presence of LPS, which is publicly considered as the ligand of TLR4
and activates TLR4 signaling (38), markedly blunted the protective
effect of 20 mg/kg eupafolin on neurological functions, brain edema
and infarct volume of rats that underwent cerebral I/R injury,
implicating that the actions of eupafolin may be at least partially
dependent on the blocking of the TLR4/NF-κB signaling pathway.
However, the activation of TLR4 signaling is not
only associated with the NF-κB pathway but is also associated with
other processes, including the MAPK pathway. Therefore, the
protective effects of eupafolin against cerebral I/R injury may not
only be associated with the activation of the NF-κB pathway, but
also with other mechanisms, which require further investigation. In
addition, whether the inhibitory effect of eupafolin on
inflammation, oxidative stress and apoptosis during cerebral I/R
were dependent on blocking TLR4 signaling remain to be
investigated. Meanwhile, the employment of TLR4-knockout or
-knockdown mice is necessary to further confirm the results of the
present study and to identify the underlying molecular mechanisms,
and this will be performed in future experiments.
In conclusion, the results of the present study
provided novel evidence that eupafolin exerted protective effects
against cerebral I/R injury in rats exposed to MCAO followed by
reperfusion. This may be associated with inhibiting the TLR4/NF-κB
signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and XC contributed toward study conception and
design; XC, ZY, XP and LW contributed toward acquisition of data;
HW and YO contributed toward analysis and interpretation of data;
XC drafted the initial manuscript and JL revised it critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experiments involving animals were approved by
the Animal Studies Ethics Committees of the Shenzhen Hospital of
Integrated Traditional Chinese and Western Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bustamante A and Montaner J: Author
response: Usefulness of ADAMTS13 to predict response to
recanalization therapies in acute ischemic stroke. Neurology.
91:8992018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kahl A, Blanco I, Jackman K, Baskar J,
Mohan HM, Rodney-Sandy R, Zhang S, Iadecola C and Hochrainer K:
Cerebral ischemia induces the aggregation of proteins linked to
neurodegenerative diseases. Sci Rep. 8:27012018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang J, Wang T, Yu D, Fang X, Fan H and
Liu Q, Yi G, Yi X and Liu Q: l-homocarnosine attenuates
inflammation in cerebral ischemia-reperfusion injury through
inhibition of nod-like receptor protein 3 inflammasome. Int J Biol
Macromol. 118:357–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Park JH, Maharjan S, Park JA,
Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, et al: Sac-1004,
a vascular leakage blocker, reduces cerebral ischemia-reperfusion
injury by suppressing blood-brain barrier disruption and
inflammation. J Neuroinflammation. 14:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ya BL, Liu Q, Li HF, Cheng HJ, Yu T, Chen
L, Wang Y, Yuan LL, Li WJ, Liu WY and Bai B: Uric acid protects
against focal cerebral ischemia/reperfusion-induced oxidative
stress via activating Nrf2 and regulating neurotrophic factor
expression. Oxid Med Cell Longev. 2018:60691502018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Cheng S, Hu H, Zhang X, Xu J, Wang R
and Zhang P: Progranulin protects against cerebral
ischemia-reperfusion (I/R) injury by inhibiting necroptosis and
oxidative stress. Biochem Biophys Res Commun. 521:569–576. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui Y, Wang JQ, Shi XH, Wang YY, Liu HY,
Li Z, Dong Y, Mang J and Xu ZX: Nodal mitigates cerebral
ischemia-reperfusion injury via inhibiting oxidative stress and
inflammation. Eur Rev Med Pharmacol Sci. 23:5923–5933.
2019.PubMed/NCBI
|
|
8
|
Lee CW, Lin ZC, Hsu LF, Fang JY, Chiang
YC, Tsai MH, Lee MH, Li SY, Hu SC, Lee IT and Yen FL: Eupafolin
ameliorates COX-2 expression and PGE2 production in particulate
pollutants-exposed human keratinocytes through ROS/MAPKs pathways.
J Ethnopharmacol. 189:300–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maas M, Deters AM and Hensel A:
Anti-inflammatory activity of Eupatorium perfoliatum L.
Extracts, eupafolin, and dimeric guaianolide via iNOS inhibitory
activity and modulation of inflammation-related cytokines and
chemokines. J Ethnopharmacol. 137:371–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Chen MK, Li K, Hu C, Lu MH and
Situ J: Eupafolin nanoparticle improves acute renal injury induced
by LPS through inhibiting ROS and inflammation. Biomed
Pharmacother. 85:704–711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Zhang Y and Fan Y: Eupafolin
ameliorates lipopolysaccharide-induced cardiomyocyte autophagy via
PI3K/AKT/mTOR signaling pathway. Iran J Basic Med Sci.
22:1340–1346. 2019.PubMed/NCBI
|
|
12
|
Sung HC, Liang CJ, Lee CW, Yen FL, Hsiao
CY, Wang SH, Jiang-Shieh YF, Tsai JS and Chen YL: The protective
effect of eupafolin against TNF-α-induced lung inflammation via the
reduction of intercellular cell adhesion molecule-1 expression. J
Ethnopharmacol. 170:136–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan X, Tao J, Cai Z, Fredimoses M, Wu J,
Jiang Z, Zhang K and Li S: Eupafolin suppresses esophagus cancer
growth by targeting T-LAK cell-originated protein kinase. Front
Pharmacol. 10:12482019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang H, Wu D, Xu D, Yu H, Zhao Z, Ma D
and Jin J: Eupafolin exhibits potent anti-angiogenic and antitumor
activity in hepatocellular carcinoma. Int J Biol Sci. 13:701–711.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han MA, Min KJ, Woo SM, Seo BR and Kwon
TK: Eupafolin enhances TRAIL-mediated apoptosis through cathepsin
S-induced down-regulation of Mcl-1 expression and AMPK-mediated Bim
up-regulation in renal carcinoma Caki cells. Oncotarget.
7:65707–65720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu K, Park C, Chen H, Hwang J,
Thimmegowda NR, Bae EY, Lee KW, Kim HG, Liu H, Soung NK, et al:
Eupafolin suppresses prostate cancer by targeting
phosphatidylinositol 3-kinase-mediated Akt signaling. Mol Carcinog.
54:751–760. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lester SN and Li K: Toll-like receptors in
antiviral innate immunity. J Mol Biol. 426:1246–1264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hua F, Ma J, Ha T, Xia Y, Kelley J,
Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH and
Li C: Activation of toll-like receptor 4 signaling contributes to
hippocampal neuronal death following global cerebral
ischemia/reperfusion. J Neuroimmunol. 190:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Delft MA, Huitema LF and Tas SW: The
contribution of NF-κB signalling to immune regulation and
tolerance. Eur J Clin Invest. 45:529–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang W, Lin C, Yuan L, Chen L, Guo P, Li
P, Wang W and Zhang X: Preactivation of Notch1 in remote ischemic
preconditioning reduces cerebral ischemia-reperfusion injury
through crosstalk with the NF-κB pathway. J Neuroinflammation.
16:1812019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Auvin S, Shin D, Mazarati A and Sankar R:
Inflammation induced by LPS enhances epileptogenesis in immature
rat and may be partially reversed by IL1RA. Epilepsia. 51 (Suppl
3):S34–S38. 2010. View Article : Google Scholar
|
|
22
|
Zhao R, Jiang J, Li H, Chen M, Liu R, Sun
S, Ma D, Liang X and Wang S: Phosphatidylserine-microbubble
targeting-activated microglia/macrophage in inflammation combined
with ultrasound for breaking through the blood-brain barrier. J
Neuroinflammation. 15:3342018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sommer CJ: Ischemic stroke: Experimental
models and reality. Acta Neuropathol. 133:245–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li P, Zhang Y and Liu H: The role of
Wnt/β-catenin pathway in the protection process by dexmedetomidine
against cerebral ischemia/reperfusion injury in rats. Life Sci.
236:1169212019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Z, Weian C, Susu H and Hanmin W:
Protective effects of mangiferin on cerebral ischemia-reperfusion
injury and its mechanisms. Eur J Pharmacol. 771:145–151. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong L, Tang Y, An R, Lin M, Chen L and Du
J: RTN1-C mediates cerebral ischemia/reperfusion injury via ER
stress and mitochondria-associated apoptosis pathways. Cell Death
Dis. 8:e30802017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jing H, Liu L, Jia Y, Yao H and Ma F:
Overexpression of the long non-coding RNA Oprm1 alleviates
apoptosis from cerebral ischemia-reperfusion injury through the
Oprm1/miR-155/GATA3 axis. Artif Cells Nanomed Biotechnol.
47:2431–2439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamashita T and Abe K: Recent progress in
therapeutic strategies for ischemic stroke. Cell Transplant.
25:893–898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hensel A, Maas M, Sendker J, Lechtenberg
M, Petereit F, Deters A, Schmidt T and Stark T: Eupatorium
perfoliatum L.: Phytochemistry, traditional use and current
applications. J Ethnopharmacol. 138:641–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanz JM, Chiozzi P, Colaianna M, Zotti M,
Ferrari D, Trabace L, Zuliani G and Di Virgilio F: Nimodipine
inhibits IL-1β release stimulated by amyloid β from microglia. Br J
Pharmacol. 167:1702–1711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang GH, Liu Y, Wu XB, Lu Y, Liu J, Qin
YR, Li T and Duan HF: Neuroprotective effects of human umbilical
cord-derived mesenchymal stromal cells combined with nimodipine
against radiation-induced brain injury through inhibition of
apoptosis. Cytotherapy. 18:53–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan B, Sun Y, Zeng J, Chen Y, Li C, Song
P, Zhang L, Yang X, Wu Y and Ma P: Combined use of vitamin E and
nimodipine ameliorates dibutyl phthalate-induced memory deficit and
apoptosis in mice by inhibiting the ERK 1/2 pathway. Toxicol Appl
Pharmacol. 368:1–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ko HH, Chiang YC, Tsai MH, Liang CJ, Hsu
LF, Li SY, Wang MC, Yen FL and Lee CW: Eupafolin, a skin whitening
flavonoid isolated from Phyla nodiflora, downregulated
melanogenesis: Role of MAPK and Akt pathways. J Ethnopharmacol.
151:386–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen CC, Lin MW, Liang CJ and Wang SH: The
anti-inflammatory effects and mechanisms of eupafolin in
lipopolysaccharide-induced inflammatory responses in RAW264.7
macrophages. PLoS One. 11:e01586622016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo SY, Li R, Le ZY, Li QL and Chen ZW:
Anfibatide protects against rat cerebral ischemia/reperfusion
injury via TLR4/JNK/caspase-3 pathway. Eur J Pharmacol.
807:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Groen RAL, Schrader AMR, Kersten MJ,
Pals ST and Vermaat JSP: MYD88 in the driver's seat of B-cell
lymphomagenesis: From molecular mechanisms to clinical
implications. Haematologica. 104:2337–2348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Araya EI, Barroso AR, Turnes JM, Radulski
DR, Jaganaught JA, Zampronio AR and Chichorro JG: Toll-like
receptor 4 (TLR4) signaling in the trigeminal ganglion mediates
facial mechanical and thermal hyperalgesia in rats. Physiol Behav.
226:1131272020. View Article : Google Scholar : PubMed/NCBI
|